Abstract
Estrogens are becoming well known for their robust enhancement on cognition particularly for learning and memory that relies upon functioning of the hippocampus and related neural systems. What is also emerging is that estrogen modulation of cognition is not uniform, at times enhancing yet at other times impairing learning. This review explores the bidirectional effects of estrogens on learning from a multiple memory systems view, focusing on the hippocampus and striatum, whereby modulation by estrogens sorts according to task attributes and neural systems engaged during cognition. We highlight our findings that show the ability to solve hippocampus-sensitive tasks typically improves under relatively high estrogen status while the ability to solve striatum-sensitive tasks degrades with estrogen exposures. Though constrained by dose and timing of exposure, these opposing enhancements and impairments of cognition can be observed following treatments with different estrogenic compounds including the hormone estradiol, the isoflavone genistein found in soybeans, and agonists that are selective for specific estrogen receptors, suggesting that activation of a single receptor type is sufficient to produce the observed shifts in learning strategies. Using this multi-dimensional framework will allow us to extend our thinking of the relationship between estrogens and cognition to other brain regions and cognitive functions.
Introduction
Estrogens belong to a class of steroid hormones most commonly recognized for their roles in. reproductive physiology and behavior. However, as evidenced by the topic of this special issue, estrogens also have powerful effects on cognition, acting to modulate many aspects of brain structure and function. Estrogens’ roles in the brain are especially meaningful for women’s health, as many women experience marked changes in cognition and affect following natural or surgical menopause (Morrison et al., 2006).
The potential “non-reproductive” actions of estrogens on learning and memory gained particular interest among basic scientists following findings that the hippocampus, a brain structure critical for certain types of cognition, undergoes substantial changes in neuron morphology and neurotransmission in response to 17β-estradiol, the principal circulating estrogen in women prior to menopause (Gould et al., 1990; Woolley et al., 1990). Estradiol was also found to induce structural and functional changes in other neural systems, including the striatum, amygdala, and cortex, aligning well with earlier reports of estradiol modulation of the hypothalamus (Carrer and Aoki, 1982; Dohanich et al., 2009).
Behavioral studies in laboratory animals confirmed that changes in hormone levels lead to altered performance on memory tasks that engage these different brain areas (McEwen and Alves, 1999; Dohanich et al., 2009). Importantly, estradiol does not uniformly alter cognition, as it can enhance, impair, or have no effect on learning and memory processes depending on the cognitive demands of a given task (Gold and Korol, 2010; Korol, 2004). Further studies showed that mnemonic outcomes following treatment with estrogens are sensitive to many other variables such as dose, timing, age, the type of estrogen used, and the duration of hormone deprivation prior to treatment (Dohanich et al., 2009). Adding another layer of complexity, estrogens can be locally synthesized in the brain (Hojo et al., 2004; Remage-Healey et al., 2011) and may act like neuromodulators or neurotransmitters to up-or down-regulate information flow through the brain. Finally, estrogens are also known to exert their effects in the brain through novel signaling mechanisms, acting through membrane-associated cascades that alter neuronal signaling themselves and that may also influence the transcriptional activities of classical nuclear hormone receptors (Mani et al., 2012; Vasudevan and Pfaff, 2008) or transactivate neurotransmitter receptor systems (Meitzen and Mermelstiein, 2011).
Shifting viewpoints on estrogenic regulation of learning and memory
Until relatively recently, estrogen replacement with and without progesterone was a popular hormone therapy to protect against the symptoms of hormone loss, including changes in cognitive function, as women transition through menopause. However the health benefits of estrogens were challenged by the findings from the Women’s Health Initiative (WHI) reports, and the subsequent fallout from and the media surrounding the reports, suggesting that conjugated equine estrogens and synthetic progestins found in the commonly prescribed hormone replacement treatment Premarin® and Prempro® can increase risk for various cancers, stroke, and potentially Alzheimer’s disease (Espeland et al., 2004; Rapp et al., 2003; Rossouw et al., 2002; Shumaker et al., 2003). These findings were used to support the view that ovarian steroids may be detrimental to health and to brain health in particular. However, the generalizability of the WHI findings have since been put into question because of participant selection based on age, time since menopause, and severity of menopausal symptoms, selection of hormone treatments that are not made specifically from the endogenous hormones estradiol and progesterone, and the validity of the cognitive battery used to test memory that is instead a screen for dementia (Sherwin, 2009). In essence, the results failed to address the important question of whether estrogens given at or around the time of reproductive senescence protect against decline in learning, memory, and brain health.
Estrogens regained their status for neural protection and beneficial actions on cognition and related brain functions through direct tests of these design flaws. Substantial progress has been made using a variety of experimental models to demonstrate that there is a window of opportunity around the perimenopausal period for estrogens to protect the brain against declines in cognitive function and related neurodegenerative diseases (Daniel, 2012; Gibbs, 2000; Resnick and Henderson, 2002). When administered to middle-aged female rodents close to the time of hormone deprivation, estrogens improve memory for several different cognitive tasks (Bimonte-Nelson et al., 2006; Daniel et al., 2006; Fernandez and Frick, 2004; Markham et al., 2002; Neese et al., 2014; Rodgers et al., 2010; Talboom et al., 2008). In contrast, estrogen-induced memory enhancements are not seen following prolonged periods of hormone deprivation (Daniel et al., 2006; Gibbs, 2000), a loss of responsiveness that extends to measures of neuronal plasticity and transmission (Daniel, 2012). This decreased sensitivity appears to become more robust with age, as elderly rats appear to either lose cognitive responses to treatment (Gresack et al., 2007; Savonenko and Markowska, 2003; Talboom et al., 2008) or require higher doses of estrogens (Foster et al., 2003; Frick et al., 2002) and increased task difficulty (Korol et al., 2007) to detect estrogen-related memory improvements. Notwithstanding these enhancements, not all estrogens are equally effective even when given around the time of hormone deprivation.
Classic estrogens are C18 steroid hormones present endogenously as 17β-estradiol, estrone, and estriol, plus their sulfate and glucuronide conjugates. Estradiol is the most potent naturally occurring estrogen, followed by estrone and estriol, and is also the main circulating estrogen in humans of reproductive age (Kuhl, 2005; Rannevik et al., 1995). While levels of all estrogens decline precipitously at menopause, estrone becomes the predominant species following reproductive senescence (Rannevik et al., 1995). Interestingly, until now, the most widely-used postmenopausal estrogen replacement therapy did not involve administration of estradiol, but was instead a complex cocktail of estrogenic compounds purified from the urine of pregnant horses that is taken orally and results in increased levels of estrone and reduced 17β-metabolites (Bhavnani, 1998; Bhavnani, 2003).
Studies examining the use of these conjugated equine estrogens in postmenopausal women, including the Women’s Health Initiative, mostly report null, mixed, or negative cognitive effects of estrogens whereas the majority of studies utilizing estradiol have found cognitive benefits (Sherwin and Henry, 2008). In rodent studies, conjugated equine estrogens both impair (Barha and Galea, 2013) and enhance (Acosta et al., 2009b) spatial memory, outcomes that appear to be dose-dependent and correlated to relative serum levels of estradiol and estrone (Engler-Chiurazzi et al., 2011). In contrast to the nuances of conjugated equine estrogens, a wide body of work demonstrates that estradiol generally improves hippocampus-sensitive cognition in rodents (see Dohanich et al., 2009).
Indeed, estrogen treatment may not be the long sought after silver bullet for cognition, but not because the hormones are detrimental to the overall health of the post-reproductive female. Rather, elevated levels of estrogens not only enhance cognition but also impair cognition even in healthy young adult females depending upon the task attributes, which memory systems are engaged, and the physiological state of the organism (for review see Dohanich et al., 2009). These often overlooked but robust impairing effects suggest that estrogens, and perhaps more generally other steroids, have opposing actions on cognition, shifting abilities across different types of learning strategies and memory processes.
Estrogens regulate learning and memory in a dissociable manner based on attributes of the behavioral task and neural systems engaged. For example, increased levels of estradiol, either endogenously or through exogenous administration, enhance performance on spatially-driven, working memory, or explicit tasks that rely on the hippocampus, such as the radial arm maze (Holmes et all, 2002; Fader et al., 1999; Luine et al., 1998; Daniel et al., 1997), swim task (Kiss et al., 2012; Bimonte and Denenberg, 1999; Packard and Teather, 1997), delayed matching-to-position (Gibbs, 2000), place task (Zurkovsky et al., 2007; Zurkovsky et al., 2006; Davis et al., 2005; Korol and Kolo, 2002), and object placement (Frye et al., 2007; Luine et al., 2003). Improvements in hippocampus-sensitive learning and memory are not immutable, however, as magnitude and direction of effects are sensitive to many factors such as age of the subjects, task difficulty, stress level, enrichment, estradiol dose, and period of hormone depletion (for review see Dohanich et al., 2009).
Although estradiol largely promotes the use of cognitive strategies that engage the hippocampus, elevations in hormone levels impair the use of processes that engage the dorsal striatum. Learning that requires stimulus-response strategies or egocentric navigation rules such as cued win-shift on a radial arm maze (Galea et al., 2001), response learning (Zurkovsky et al., 2011; Zurkovsky et al., 2007; Davis et al., 2005; Korol and Kolo 2002), and cued swim task (Daniel and Lee, 2004; Pleil et al., 2011) becomes impaired with estradiol exposure. Furthermore, estrogens are detrimental to the performance of operant tasks such as delayed alternation (Neese et al., 2010a; 2010b; Neese et al., 2012; Wang et al., 2009).
The opposing effects of estrogens on different types of cognition resonate well with the theory of multiple memory systems purporting that different brain structures act as parallel processors for a given memory task, each optimized to handle particular types of information and relationships (White and McDonald, 2002; White et al., 2013). While many brain areas are likely to be engaged during cognitive activity, there may be specific regions that are selectively required for effective task solution; the physiological status of the respective neural systems predicts the ability to solve tasks with specific cognitive demands. For example, findings from studies that disrupt learning and memory with functional or structural lesions (Packard et al., 1989; Packard and McGaugh, 1996; White and McDonald, 2002; Chang and Gold, 2003a, 2004; Featherstone and McDonald, 2004; Hallock et al., 2013) and that correlate cognitive performance with functional (Bohbot et al., 2004; Iaria et al., 2003), neurochemical (Chang and Gold, 2003b; Gold et al., 2013), and molecular signatures (Columbo et al., 2003; Colombo, 2004) reveal the importance of hippocampal function in allocentric or place learning and striatal function in egocentric or response learning. Tasks with specific cognitive attributes such as these can engage the canonical memory systems, i.e. hippocampus or striatum, depending on which neural structure provides optimal processing of the task features.
Estrogenic shifts in cognition: differential activation of multiple memory systems
Estradiol does not modulate learning and memory capacity in a singular manner, enhancing all or some types of cognition. Much of our work on estrogens and cognition dissociates the effects of estrogens on hippocampus- and striatum-sensitive learning strategies to create a conceptual framework in which estrogens modulate cognition by shifting the pattern of engagement of multiple memory systems that optimally process different kinds of information. The magnitude and possibly direction of effects of estrogens on cognition vary by age, reproductive status, and even reproductive experience (Barha et al., 2015; Gatewood et al., 2005; Workman et al., 2013). Moreover, we recently characterized age-related shifts from place to response learning strategies in males (Korol et al., 2014), suggesting that the interaction of age and estrogen status on multiple memory systems is that much more complex. Thus, the majority of studies described herein were conducted using virgin young adult female rats to test first how estrogens modulate learning and memory across memory systems before extending questions to contexts of aging, hormone timing, parity, and menopause etiology (Acosta et al., 2013; Daniel, 2012; Frick, 2009; Galea et al., 2014). It is important to note, however, estradiol-induced enhancements in place learning originally observed in young adults are also seen in middle aged (Neese et al., 2014) and elderly (Korol et al., 2007) multiparous rats, suggesting that our behavioral tasks and treatments are useful in assessing cognitive function across the lifespan.
To test the effects of estrogens on learning across multiple memory systems, we have used place and response learning tasks. These maze-based tasks are ideal for targeting hippocampus-or striatum-sensitive cognitive processes as their demands are well matched in all areas except the strategy needed to obtain a food reward. The place task requires rats to learn the spatial location of the reward using extra-maze cues by always going towards the same place in the testing room, while the response task requires the rat to adopt a consistent turning strategy to reach the goal (Tolman et al., 1946). These tasks use the same apparatus, appetitive reinforcement, and pre-training handling regimen and thus have similar levels of stress and cognitive difficulty (Korol and Kolo, 2002). The balanced nature of the place and response tasks is very important, as stress and aversive or appetitive conditions can differentially affect the outcomes of estrogen memory modulation (Dohanich et al., 2009). As estrogens have non-mnemonic effects on motor function, motivation, and food drive (McEwen and Alves, 1999), the comparable features of the tasks used here obviate the confounds introduced by these variables. In sum, the place and response tasks serve as good models for examining the selective cognitive actions of estrogens.
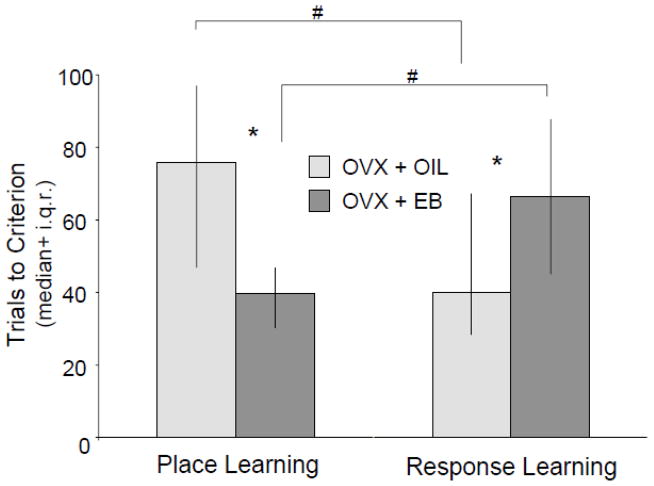
Using these place and response learning tasks, we have generated several sets of results (Korol and Kolo, 2002; Pisani et al., 2012b; Zurkovsky et al., 2006; 2007) demonstrating that estrogens enhance place learning by increasing accuracy across training trials and by decreasing the trials it takes to reach a predetermined criterion. Mirroring these improvements, the same doses of estrogens impair response learning by decreasing accuracy across acquisition periods and by increasing the number of trials to reach criterion (Figure 1). Because estrogens shift the ability to solve these tasks in opposite directions and because non-cognitive behaviors such as choice time and exploration speed are relatively unaffected by treatment, we propose that estrogens not only alter how much we learn but also, and more importantly for our conceptual and experimental approaches, the manner by which we learn.
Figure 1.
Young adult rats were ovariectomized for 21 days before training on a place learning or on a response learning task in a plus-shaped maze. Estradiol-benzoate (EB; 10 μg in 0.1 ml sesame oil) was injected (s.c.) once daily for two days before training. Rats treated with estradiol take significantly fewer trials to reach criterion on place learning but significantly more trials on response learning. Rats with low hormone levels show the opposite effects of slow acquisition for the place learning task and fast acquisition for response learning. * = p < 0.05 between treatments within task; # = p < 0.05 within treatments across tasks using Mann-Whitney analyses. Adapted from Korol and Kolo, 2002.
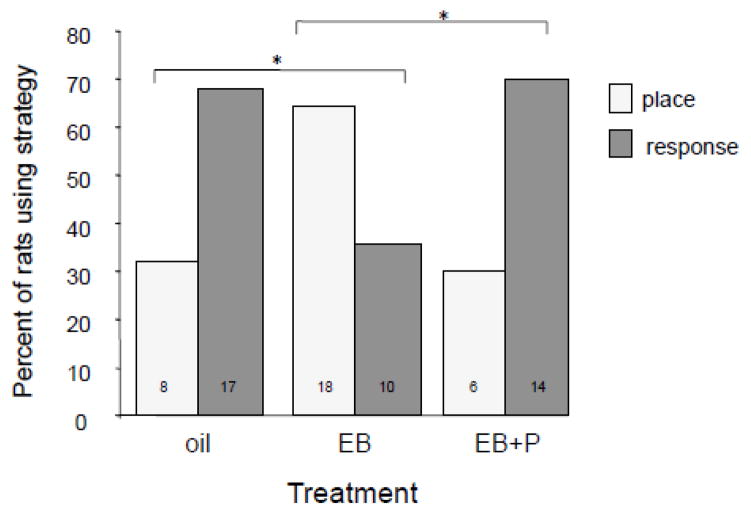
These estrogen-induced cognitive shifts are highlighted by the use of behavioral tasks that can be solved in multiple ways, e.g. dual-solution tasks. When young adult female rats were trained to find food in a T-maze where place and response strategies provided equally effective solutions, significantly more rats with high estradiol levels (proestrus) selected place strategies compared to response strategies while rats with low estradiol (estrus) showed the converse, i.e. choosing response strategies over place strategies. The different strategies were evident when examined across the estrous cycle (Korol et al., 2004) or when examined in ovariectomized rats treated with two days of 10 μg of estradiol benzoate (Figure 2), which results in serum estradiol levels similar to those found in proestrus (Korol and Kolo, 2002). An important finding in the dual-solution task was that rates of learning measured by trials to meet a criterion of ninety percent accuracy were the same for rats with high and low estradiol levels, showing that hormone status shifted the strategy used but not necessarily learning ability in general or the strength of memory formation. The consensus from multiple reports is that rats with relatively high estradiol levels are biased towards hippocampus-sensitive place strategies and away from striatum-sensitive response or cued strategies and hormone-deprived rats demonstrate the opposite: they select response strategies over place strategies (Daniel and Lee, 2004; Korol et al., 2004; Quinlan et al., 2008).
Figure 2.
Rats were ovariectomized then trained and tested for strategy on the dual solution task 21 days later. 48 and 24 hours prior to training rats were treated for two days with oil vehicle (s.c.), or estradiol benzoate (EB; 10 μg g/kg, s.c.). A separate group of rats received EB plus progesterone (500 ug/kg) 4 hours prior to training. The data show that EB for two days produces a place bias in learning strategy that is reversed with progesterone treatment. * = significant (p < 0.05) shifts in learning strategy between oil and EB and between EB and EB+P treatments,
What has grown out of these initial experiments is that our place and response learning tasks may serve as behavioral assays for the function of or plasticity in hippocampus and striatum. If so, the direction of estrogen effects on cognition will generalize to other tasks that sort according to the memory system predominating during the task, such as hippocampus or striatum. Delayed alternation in operant conditioning tasks is believed to rely on cortico-striatal processing and is impaired by estrogens including estradiol and genistein in young adult, middle-aged, and elderly rats (Neese et al., 2010a; 2010b; Neese et al., 2012; Wang et al., 2009; Neese et al. 2014). For example, chronic treatments of estradiol initiated at the time of ovariectomy in 12-month-old female rats significantly impair working memory in a spatial alternation task (Figure 3) believed to rely on intact prefrontal cortex function. Performance decrements due to estradiol are particularly robust as memory load increases, i.e. at delays greater than 0 seconds but shorter than 18 seconds. Thus, estrogens interfere with information processing that relies on the function of the striatum, cortical-striatal connections, and related circuits.
Figure 3.
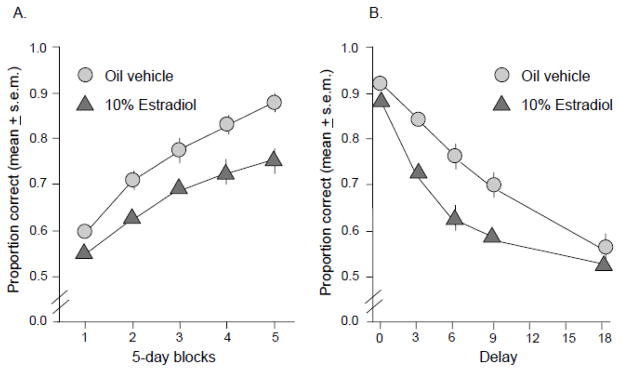
Effects of chronic estradiol treatment on spatial alternation in an operant conditioning task in middle aged ovariectomized female rats. (A) Proportion correct on the DSA task across 5-day blocks of testing for the vehicle control and estradiol-treated groups. aEstradiol treated<vehicle treated, (p < 0.05). (B) Proportion correct on the DSA across 5 delays for the vehicle control and estradiol treated groups. aEstradiol treated<vehicle treated, (p<0.05).
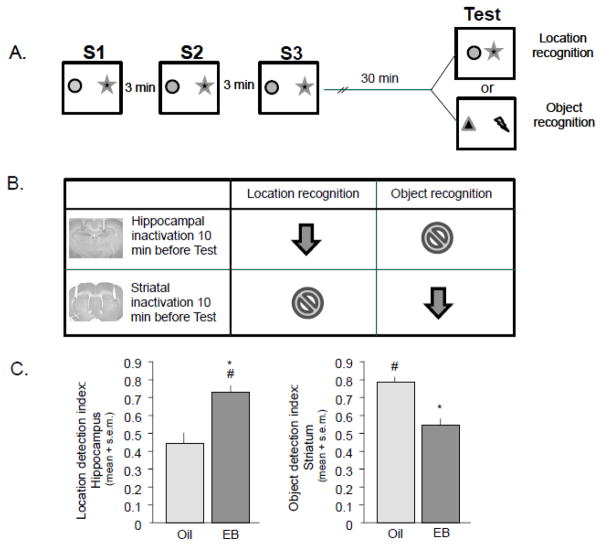
We recently tested the opposing actions of estrogens in two novel recognition tasks that are differentially sensitive to hippocampus and striatum manipulations, tested by disrupting the function of each structure with lesions and chemical inactivation.. In both recognition tasks, rats are allowed to explore two objects placed near opposite ends of an arena over three study sessions (S1-S3) followed after a specific delay by a subsequent test trial. In the location recognition task, the two familiar objects are moved closer together for the test trial. In the object recognition task, both familiar objects are replaced by novel objects and positioned in the same spatial locations (Figure 4A). The rats’ memory for the original object patterns is expressed as time spent exploring the novel object arrangement relative to time spent exploring the familiar object arrangement in the last study session (S3). Hippocampal manipulations such as lidocaine inactivation disrupt recognition of location change but not of object change (Figure 4B; Goodrich-Hunsaker et al., 2008). Conversely, striatal inactivation impairs object recognition but not location recognition.
Figure 4.
Procedure and results for location and object recognition tasks. A. Schematic of the general procedure for the recognition tasks. Rats explore arena for three 5-min study sessions (S1, S2, S3) after which they are tested 30 min later on a location recognition Test where same objects are moved closer together or an object recognition task where both objects are replaced with novel ones. B. Lidocaine infused into the hippocampus 20 min after S3 and 10 min prior to Test impairs location recognition but not object recognition, whereas infusions into the striatum impair object recognition but not location recognition. These findings reveal a brain structure X task double dissociation. C. Systemic treatments of estradiol-benzoate (EB) to young adult ovariectomized female rats enhance hippocampus-sensitive location recognition (left panel) and impair striatum sensitive object recognition (right panel) measured with a location detection index derived with the formula: T/S3+T.; * = p < 0.05 between treatments within task; # = p < 0.05 within treatments between tasks.
The tasks share the important quality of site specificity with our maze tasks yet they differ with regard to the apparatus used (arena vs maze), specifics of training paradigm (four 5-min sessions vs scores of 30-sec trials), and motivators (novelty vs food reward), to name a few. However, similar to findings in our maze-based tasks, elevated levels of estradiol, endogenously across the cycle or exogenously through systemic injections (45 μg/kg estradiol benzoate 48 and 24 h before testing to ovariectomized rats; Figure 4C), improved recognition in the hippocampus-sensitive location recognition task but blocked recognition in the striatum-sensitive object recognition task. Conversely, hormone deprivation produced good performance on the striatum-sensitive object recognition task but not the hippocampus-sensitive task (Figure 4C). These findings reveal a novel task-by-structure double dissociation that is sensitive to the opposing effects of estrogens, strengthening the idea that estrogens enhance hippocampal function at the expense of striatal function and vice versa: estrogen depletion can enhance striatal function at the expense of hippocampal function. Taken together, the sum of the results suggests that estrogens are not generally good or bad for brain and cognition, but instead are both, shifting the memory system engaged toward the hippocampus and away from striatum.
Estrogenic regulation of the balance of memory systems: independent or interactive?
As mentioned above, dissociations between the brain region manipulated and task performance reveal multiple memory systems that can operate independently as a single processor, giving rise to the notion of canonical memory systems and tasks. However, the tenet of independent memory systems is complicated by findings that memory systems can also interact in a cooperative or competitive manner whereby activity of one system respectively potentiates or decreases the contribution of a parallel neural system (White et al., 2013).
The hippocampus and striatum are two structures shown to interact competitively. Lesions or chemical inactivation of one system improves learning and memory in tasks that tap the intact structure (Chang and Gold, 2003a; White and McDonald, 2002). Furthermore, the corollary is also true: potentiation of one system may interfere with function of the noncanonical system, impairing cognitive performance that depends on that system. What follows, then, is the possibility that the facilitation of place learning and impairment of response learning by estrogens reflect the singular action of estrogens on either the hippocampus or the striatum that, through competition, leads to the opposing actions. For example, estrogens may improve hippocampal function, thereby impairing striatal function because of competitive inhibition of hippocampus on striatum. Conversely, estrogens may impair striatal function, thereby improving hippocampal function by releasing competitive inhibition of the striatum on hippocampus. Alternatively, estrogens may act on each structure independently, improving place learning through modulation of hippocampus and impairing response learning through modulation of the striatum, without modulating the interactions between memory systems.
Evidence from direct brain infusions of estradiol supports the latter possibility that estrogens modulate learning and memory through independent actions on hippocampal and striatal memory systems. Young adult ovariectomized rats received bilateral hippocampal infusions of estradiol 48 and 24 hours prior to training and three weeks following ovary removal, matching the systemic treatments given in previous work (Korol and Kolo 2002; Zurkovsky et al., 2006). The rats showed significant improvements in place learning with no apparent effects on response learning. Similar infusions into the striatum produced impairments in response learning with no measurable effects on place learning (Zurkovsky et al., 2007). Furthermore, blockade of classical estrogen receptors (ERs) in the hippocampus with hippocampal implants of 10% ICI 182,780, prevented place learning enhancements seen with two days of systemic estradiol treatment (Zurkovsky et al., 2006). Likewise, striatal implants of ICI 182,780 reversed the response learning impairments seen with systemic estradiol (Kent et al., 2005). Together the results suggest that estradiol in the canonical memory system is both necessary and sufficient to produce place learning enhancements and response learning impairments and seem to do so through estrogen receptor-mediated events at each structure.
Despite the clear dissociation in effects of central infusions of estradiol, inactivation experiments present a conundrum regarding how estrogens shift the balance of memory systems. When the hippocampus is inactivated with the GABAA agonist muscimol, rats at proestrus with endogenously high levels of estradiol shift from place to response strategy use in a dual solution task that allows both strategies during training (Figure 5). Importantly, estrous cycle stage interacts with muscimol dose such that increasing doses of muscimol are needed to produce increased shifts towards response learning as endogenous levels rise across the estrous cycle. Thus, the effective dose for response learning impairments is high at proestrus and intermediate at diestrus.
Figure 5.
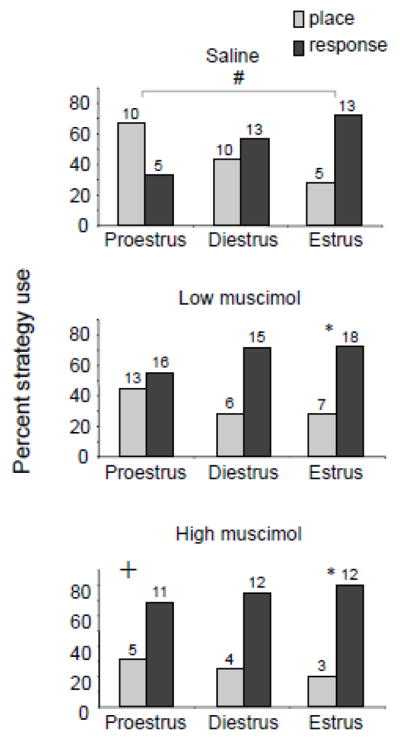
Strategy selection and the effects of muscimol treatment and estrous cycle stage. (Top) Strategy biases across the estrous cycle in saline treated rats. Note that significant differences between groups appeared with proestrous rats using a place strategy and estrous rats using a response strategy. Diestrous rats demonstrated no strategy bias. (Middle, bottom) Low (middle) and high (bottom) muscimol treatment shifted strategy use toward response. *p < 0.05 within estrous cycle group; #p < 0.05 between estrous cycle groups within muscimol treatment; +p < 0.05 relative to saline controls within estrous cycle group. Adapted from McElroy and Korol, 2005.
The results align well with theories suggesting that estradiol decreases inhibitory tone in the hippocampus through modulation of GABA signaling, perhaps through ERα activation (Huang and Woolley, 2012), and thus higher concentrations of the GABA agonist muscimol are needed to effectively block functional output (McElroy and Korol, 2005). However, the results also suggest that endogenous elevations in ovarian hormones shift memory systems through competitive interactions, presenting an alternative interpretation from that drawn by the findings from central infusions described above. If estradiol works independently to enhance hippocampus and to suppress striatum, under high hormone states of proestrus and in the presence of hippocampal muscimol, place and response strategies would both be inhibited and a random selection of place and response learning strategies, i.e. no observed strategy bias, and slower overall learning reflected in higher trials to reach criterion would be expected. Instead, we observed that under high hormone states without the function of the hippocampus rats reach criterion in the same number of trials and can effectively use response strategies, suggesting that the response learning impairment with high hormones is due to increased activation of the hippocampus. One possibility for the conflicting findings is that in the latter case rats were gonadally intact, while in the former, rats were ovariectomzed and replaced with estradiol alone; hormones other than estradiol, such as progesterone, may alter competitive interactions between hippocampus and striatum. Direct tests of this possibility have not yet been made, however we find that progesterone treatment in estradiol-primed rats shifts strategies towards striatum-sensitive response strategies (Figure 2).
Regardless of how the balance across brain regions is shifted, what comes into view through this multiple memory systems lens is that the opposing actions of estrogens on cognition sort by the brain systems engaged during task performance and that, by logical extension, estrogens can both up- and down-regulate the neural plasticity in brain areas that are respectively involved in cognition. The neurobiological mechanisms responsible for bidirectional regulation of cognition undoubtedly depend on many features of the canonical memory system related to estrogenic sensitivity, including receptor subtypes, distributions, and downstream signaling mechanisms.
The role of different estrogen receptor subtypes in learning strategy shifts
Estradiol acts through multiple types of estrogen receptors (ER) in the brain. Throughout the brain, ER subtypes ERα and ERβ are detected as classical nuclear receptors but also can be localized to the plasma membrane (Milner et al., 2005; Milner et al., 2001; Shughrue et al., 1997; McEwen et al., 2012; Meitzen and Mermelstein, 2011; Mhyre and Dorsa, 2006). The G-protein coupled estrogen receptor (GPER), formerly identified as an orphan receptor called GPR30, is a novel seven-transmembrane domain G protein-coupled receptor that binds estradiol with high affinity but is structurally and genetically unrelated to ERα and ERβ (Prossnitz and Barton, 2009). GPER can be located in the plasma membrane and densely expressed in other membrane-bound cellular structures, particularly the endoplasmic reticulum (Brailoiu et al., 2007; Matsuda et al., 2008; Prossnitz and Barton, 2009). ERα, ERβ, and GPER are all expressed in the hippocampus and striatum, but with distinct nuclear and extra-nuclear distributions and densities, activation through which will lead to different modes of signaling and downstream effects on neural function.
In the hippocampus, classical ERα and ERβ and GPER are detected in relatively high abundance in both nuclear and extranuclear membrane sites in neurons and glia (Brailoiu et al., 2007; Milner et al., 2005; Milner et al., 2001; Shughrue et al., 1997), allowing for both genomic-initiated transcriptional events and nongenomic- or membrane-initiated signaling pathways. Like estradiol, the highly-specific GPER agonist G1 (Bologa et al., 2006) activates ERK in neurons, increases calcium currents through L-type voltage gated calcium channels (Sun et al., 2010), and increases excitatory post-synaptic currents in hippocampal slices (Lebesgue et al., 2009). In the striatum, while levels of nuclear ERs are strikingly low (Shughrue, 1997), moderate to high levels of ERα, ERβ, and GPER are found at extranuclear sites (Almey et al., 2012; Grove-Strawser et al., 2010; Kuppers and Beyer, 1999; Schultz et al., 2009). Therefore, it is likely that the primary mode of signaling in the striatum is through membrane-associated ERs or transactivation of other receptors.
Because the mnemonic effects of estrogens are mediated through local actions in the hippocampus or striatum (Zurkovsky et al., 2006; 2007; Kent et al., 2005), the differential expression of ER subtypes across these structures may lead to different outcomes on learning when a specific receptor is targeted. Investigating the contributions of distinct ER subtypes to shifts in learning and memory is valuable because certain natural compounds and pharmaceuticals, such as phytoestrogens and selective estrogen receptor modulators (e.g. tamoxifen) bind to estrogen receptors with varying selectivity, and their cognitive effects are largely unknown. Acute treatments of the phytoestrogen genistein mimic the bidirectional effects of estradiol on place and response learning (Pisani et al. 2012b). Genistein demonstrates twenty-fold selectivity for ERβ over ERα (Kuiper et al., 1998), pointing to a possible role of ERβ in both hippocampus-sensitive and striatum-sensitive cognition.
Previous studies implicate the actions of ERβ as critical for the memory enhancing effects of estrogens on radial arm maze (Liu et al., 2008), object recognition (Jacome et al., 2010; Frick et al., 2010) and social transmission of food preference (Clipperton et al., 2008), but others point to signaling through ERα, especially when hormone treatments were given over short periods (Frye et al., 2007; Phan et al., 2011), or to activation of both receptors (Hammond et al., 2009; Qu et al., 2013). A small group of reports shows that activation of GPER can also enhance memory for tasks that engage the hippocampus (Ervin et al., 2013; Gabor et al., 2011; Hammond et al., 2009 Hawley et al., 2014).
Information regarding the roles of ERα, ERβ, or GPER in hippocampus- or prefrontal cortex-sensitive cognition is accumulating while relatively less is known about the effects of these receptor subtypes on striatum-sensitive learning. The distribution of ERα in extranuclear sites of cholinergic neurons in the striatum and the ability of striatal cholinergic neurons to regulate GABAergic signaling (Almey et al., 2012, Schultz et al. 2009) suggests that activation of ERα may mediate striatum-sensitive response learning impairments. Hormone loss with ovariectomy increases AMPA receptor density in the striatum (Cyr et al., 2001) that is attenuated by estradiol and ERα agonists but not ERβ agonists (Le Saux et al., 2006). The decrease in AMPA binding may reduce excitatory cortical glutamate input (Davis et al., 2005), decrease striatal synaptic plasticity, and obstruct striatum-sensitive information processing. Sensitive detection methods reveal expression of ERβ predominantly in dopamine afferents to the striatum even though ERα density is substantially higher in the striatum per se (Almey et al., 2012; Mitra et al., 2003; Creutz and Kritzer, 2002; Shughrue and Merchenthaler, 2001; Kuppers and Beyer, 1999). ERβ agonists may therefore produce impairments in response learning with expected dose-response functions shifted to the right compared to those for ERα agonists.
We recently conducted a large-scaled study in young adult female rats using a range of doses of ER-selective agonists to characterize the possible contributions of different ERs in the learning strategy shifts frequently observed with estradiol (Pisani et al. 2012a). Rats ovariectomized for three weeks were treated systemically with compounds selective for ERα (propyl pyrazole triol—PPT) or ERβ (diarylpropionitrile—DPN; or Br-ERb-041, a brominated analog of the non-steroidal compound also known as WAY-200070; Malamas et al., 2004) for two days before training on either place learning or response learning mazes. Each compound was tested at four different doses in separate groups of rats, ranging from 3.3 ug/kg to the highest dose of 1000 ug/kg.
These experiments were designed to maintain an appreciation for dose-response functions and to follow the guidelines proposed for the creation of mechanistically-based dose-response modeling (Andersen et al., 1999), including the use of multiple doses and the consideration of receptor binding profiles, pharmacokinetics, pharmacodynamics, and behavioral outcomes in dose selection. In featuring dose-sensitivity, we adopted a fairly unique approach for the estrogens and memory field, a perspective that may be useful for assessments of other estrogen-sensitive behaviors and for clinical applications.
We found that two days of systemic treatment with the ERα agonist PPT and ERβ agonists Br-ERb-041 or DPN led to place learning enhancements and response learning impairments but with dose-response functions that were specific to each agonist and task. Place learning enhancements emerged at a single optimal dose for the highly selective agonists PPT (333 ug/kg; range = 33–1000 ug/kg) and for Br-ERb-041 (100 ug/kg; range = 10–333 ug/kg), creating an inverted dose-response function. For the less-selective and lower-affinity DPN, place learning enhancements were observed across a broader range of doses DPN (100–1000 ug/kg; range = 33–1000 ug/kg). Response learning impairments were also sensitive to dose and compound, with the most selective effects observed for Br-ERb-041 at the same moderately low dose (100 ug/kg) that enhanced place learning. PPT and DPN both impaired response learning at the two highest doses of 333 and 1000 ug/kg. Thus, we failed to see a rightward shift in dose needed to detect ERβ-mediated impairments predicted from ER distributions across the striatum.
In separate experiments targeting the membrane-related estrogen receptor GPER (Pisani et al. 2013), the GPER agonist G1 failed to modulate place learning when administered 48 and 24 h before behavioral training but impaired response learning at the highest dose of 100 ug/kg. Interestingly, when an additional dose of G1 at 10 ug/kg was given 30 minutes prior to training in rats primed for two days with the same dose, G1 produced a robust enhancement in place learning. Collectively, these nuanced effects highlight the importance of considering dose and timing and also point to the convergence of slow, durable estrogen effects with more rapid signaling actions (Gold and Korol, 2010; Vasudevan and Pfaff, 2008).
When investigating the mnemonic effects of a compound, frequently only one dose is examined; the selected dose is sometimes chosen based on pharmacological or other properties but appears to often be picked arbitrarily. Importantly, conclusions may be drawn that a compound has no effect or unidirectional effects when in actuality its response is biphasic, simply because an insufficient number of doses were used. It is becoming clear that the mnemonic effects of estrogens vary by dose, even for established patterns of learning modulation, such as improving hippocampus-sensitive learning and impairing striatum-sensitive learning. Behavioral dose-effect functions are not monotonic, but rather demonstrate an inverted pattern in which cognitive changes are observed at an optimal dose of estrogens, while lower and higher doses have no effect or opposite effects. Estrogens generally enhance the performance of hippocampus-sensitive tasks at low to moderate levels but lose efficacy or impair performance at higher concentrations (Inagaki et al., 2010; Barha et al., 2010; McLaughlin et al., 2008; Holmes et al., 2002; Packard and Teather, 1997; Pisani et al. 2012b). Non-linear dose responses to estrogenic compounds are widely reported and occur across an array of physiological systems (Calabrese, 2001).
In addition to dose, the timing of estradiol effects may also reveal important information about the mechanisms by which hormones modulate cognition. In this regard, it is notable that the rapid actions of estrogens through membrane-initiated events appear to play a critical role in mediating the cognitive effects of estradiol. Estrogens impair response learning and enhance place learning within 2 hours of central administration (Korol et al., 2010; Zurkovsky et al., 2011) and improve object placement, object recognition, contextual fear conditioning, and radial arm maze performance with only 40 mins of exposure (Phan et al., 2012; Barha et al., 2010; Sinopoli et al., 2006). These treatment schedules are short enough to largely exclude genomic-level actions of estrogen receptors and thus point to rapid mechanisms of neuromodulation. Additionally, antagonizing GPER in cycling rats leads to impairments on the delayed matching-to-position task, indicating that membrane receptors play a key role in estrogen effects on spatial learning and memory (Hammond et al., 2012). Furthermore, rapid signaling mechanisms may be critical for estrogen-induced shifts in learning, as memory enhancements for hippocampus-sensitive tasks are abolished when the PKA (Lewis et al., 2008) or MAPK (Fan et al., 2010; Walf and Frye, 2008; Fernandez et al., 2008) pathways are blocked.
Concluding remarks
Our multiple memory systems approach can be used to clarify some of the conflicting reports of improved, impaired, or unchanged cognitive performance. Capitalizing on tasks that maximize the participation of different brain areas, the results described here, and summarized in Table 1, highlight the intricate relationship between estrogens and cognition; they are not solely “tried-and-true friends” or “fretful foes”, but clearly both. Moreover, the effects of estrogens on learning and memory can no longer be described simply as enhancements or impairments in function based on independent memory systems. Instead, these bidirectional effects are subject to modulation themselves by the ER systems that are activated, the dose and timing of exposures, the specific attributes of the tasks at hand, the potential for interactions across memory systems, and by the features of the individual such as age, reproductive status, and general health. Understanding the neural mechanisms determining when and what types of cognitive functions benefit from estrogen exposure may have important implications for understanding the changes in brain health that accompany menopause.
Table 1.
Summary of results from our lab demonstrating the opposing actions of estrogens on learning and memory across a variety of tasks. Studies were conducted in ovariectomized, young adult virgin Sprague-Dawley female rats and treatments administered 48 and 24 h before behavioral training unless otherwise specified.
| Behavioral task | Direction of learning modulation | Treatment or cycle stage | Dose, timing, other notes |
|---|---|---|---|
| Place task | ↑ | EB; systemic injection | 10 μg to young adult a,b,c and elderly ratsc, 4.5 μg/kg to young adultd and middle-agede LE rats; enhancement blocked by intrahippocampal ICI 182,780b |
| ↑ | ES; intracranial infusion | 0.5 μM infusion into dorsal hippocampus; infusion into dorsolateral striatum had no effect on place learningf | |
| ↑ | ES; intracranial infusion | A single 0.5 μM infusion into dorsal hippocampus enhanced place learning when given 2 h, but not 15 min, before trainingg | |
| ↑ | Genistein; oral pellets | Multiple daily oral dosings to LE rats beginning 48 h before trainingd | |
| ↑ | PPT; systemic injection | 333 μg/kg to LE rats; higher (1000 μg/kg) and lower (33, 100 μg/kg) doses not effectiveh | |
| ↑ | DPN; systemic injection | 100, 333, and 1000 μg/kg to LE ratsh | |
| ↑ | Br-Erb-041; systemic injection | 100 μg/kg to LE rats; higher (333 μg/kg) and lower (10, 33 μg/kg) doses not effectiveh | |
| ↑ | G1; systemic injection | 10 μg/kg to LE rats; only effective when given 30 min before training after 48 and 24 h of G1 primingi | |
| Metric change in object location | ↑ | Proestrus | LE rats in proestrus showed better recognition of a change in metric pattern than rats in estrusj |
| ↑ | EB; systemic injection | 45 μg/kg to LE ratsj | |
| Rewarded spontaenous alternation | ↑ | Proestrus, diestrus | Rats in proestrus and diestrus showed increased spatial alternation behavior compared to rats in estrusk |
| Response task | ↓ | EB; systemic injection | 10 μg to SD ratsa,l, 4.5 μg/kg and 45 μg/kg to LE ratsd; impairment blocked by intrastriatal ICI 182,780l |
| ↓ | ES; intracranial infusion | 0.5 μM infusion into dorsolateral striatum; infusion into dorsal hippocampus had no effect on response learningf | |
| ↓ | ES; intracranial infusion | A single 0.5 μM infusion into dorsolateral striatum imapired response learning when given 2 h, but not 15 min, before trainingm | |
| ↓ | Genistein; oral pellets | Multiple daily oral dosings to LE rats beginning 48 h before trainingd | |
| ↓ | PPT; systemic injection | 333 and 1000 μg/kg to LE ratsh | |
| ↓ | DPN; systemic injection | 333 and 1000 μg/kg to LE ratsh | |
| ↓ | Br-Erb-041; systemic injection | 100 μg/kg to LE rats; higher (333 μg/kg) and lower (10, 33 μg/kg) doses not effectiveh | |
| ↓ | G1; systemic injection | 100 μg/kg to LE rats 48 and 24 h before training; no acute dose requiredi | |
| Double object recognition | ↓ | EB; systemic injection | 45 μg/kg to LE ratsj |
| Operant delayed spatial alternation | ↓ | 17-β estradiol; silastic capsule | Chronic estradiol replacement impaired DSA performance in young adultn,o, middle-agedo,p, and elderlyo LE rats |
| ↓ | Genistein; oral pellets | Chronic daily oral dosings impaired DSA performance in middle-aged LE ratsq | |
| ↓ | DPN; systemic injection | Chronic daily injections of 20 μg/kg; higher (80, 200 μg/kg) doses not effectivep | |
| ↓ | PPT; systemic injection | Chronic daily injections of 200 μg/kg subtly impaired DSA performance late in testing under long delaysp | |
| Dual solution strategy | Place | Proestrus; EB, systemic injection | Proestrous rats showed preference for place over response strategies with no change in learning speedk,r |
| Response | Estrus; Oil; systemic injection | Estrous rats showed preference for response strategies with no change in learning speedk,r |
Abbreviations: EB: 17β-estradiol benzoate; ES: 17β-estradiol 3-sulfate; SD: Sprague-Dawley; LE: Long-Evans; OVX: ovariectomized; DSA: delayed spatial alternation.
Korol et al., 2007. Experiments were conducted using Fischer 344 x Brown Norway rats.
Zurkovsky et al., 2007; ES was infused directly into the dorsal hippocampus or dorsolateral striatum 48, 24, and 2 h before behavioral testing.
Highlights.
Estrogens are known to improve some types of learning and memory
A growing body of evidence suggests that estrogens including estradiol can robustly impair cognition
Bidirectional effects of estrogens on cognition sort by task attributes and memory system
Activation of classical and membrane-associated estrogen receptors produce both enhancements and impairments in cognition
Acknowledgments
This work was supported by NIH P50 AT006268, NIH P01 AG024387, NSF IBN 0081061, and NSF IOB 0520876. The views in this article are solely the responsibility of the authors and do not necessarily reflect those of the NSF, NCCAM, ODS, NCI, or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically-used hormone therapies in the female rat: Models, mazes, and mechanisms. Brain Res. 2013;1514:18–39. doi: 10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009a;150:4248–4259. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009b;55:454–464. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151:3795–3804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–5383. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ME, Conolly RB, Faustman EM, Kavlock RJ, Portier CJ, Sheehan DM, Wier PJ, Ziese L. Quantitative mechanistically based dose-response modeling with endocrine-active compounds. Environ Health Perspect. 1999;107(Suppl 4):631–638. doi: 10.1289/ehp.99107s4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2010;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Galea LA. The hormone therapy, Premarin, impairs hippocampus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiol Aging. 2013;34:986–1004. doi: 10.1016/j.neurobiolaging.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Chow C, Galea LA. Multiparity-induced enhancement of hippocampal neurogenesis and spatial memory depends on ovarian hormone status in middle age. Neurobiol Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. Pharmacokinetics and pharmacodynamics of conjugated equine estrogens: chemistry and metabolism. Proc Soc Exp Biol Med. 1998;217:6–16. doi: 10.3181/00379727-217-44199. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer’s. J Steroid Biochem Mol Biol. 2003;85:473–482. doi: 10.1016/s0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Iaria G, Petrides M. Hippocampal function and spatial memory: evidence from functional neuroimaging in healthy participants and performance of patients with medial temporal lobe resections. Neuropsychology. 2004;18:418–425. doi: 10.1037/0894-4105.18.3.418. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Estrogen and related compounds: biphasic dose responses. Crit Rev Toxicol. 2001;31:503–515. doi: 10.1080/20014091111785. [DOI] [PubMed] [Google Scholar]
- Carrer HF, Aoki A. Ultrastructural changes in the hypothalamic ventromedial nucleus of ovariectomized rats after estrogen treatment. Brain Res. 1982;240:221–233. doi: 10.1016/0006-8993(82)90218-9. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behav Brain Res. 2003a;144:19–24. doi: 10.1016/s0166-4328(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003b;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Inactivation of dorsolateral striatum impairs acquisition of response learning in cue-deficient but not cue-available conditions. Behavioral Neuroscience. 2004;118:383–388. doi: 10.1037/0735-7044.118.2.383. [DOI] [PubMed] [Google Scholar]
- Clipperton AE, Spinato JM, Chernets C, Pfaff DW, Choleris E. Differential effects of estrogen receptor alpha and beta specific agonists on social learning of food preferences in female mice. Neuropsychopharmacology. 2008;33:2362–2375. doi: 10.1038/sj.npp.1301625. [DOI] [PubMed] [Google Scholar]
- Colombo PJ. Learning-induced activation of transcription factors among multiple memory systems. Neurobiol Learn Mem. 2004;82:268–277. doi: 10.1016/j.nlm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Brightwell JJ, Countryman RA. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J Neurosci. 2003;23:3547–3554. doi: 10.1523/JNEUROSCI.23-08-03547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregioncal, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev. 2001;37:153–61. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: The impact of timing. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;10:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82:142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Korol DL, Shors TJ. Steroids, Learning and Memory. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. Academic Press; San Diego: 2009. pp. 539–576. [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32:680–697. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin KS, Phan A, Gabor CS, Choleris E. Rapid oestrogenic regulation of social and nonsocial learning. J Neuroendocrinol. 2013;25:1116–1132. doi: 10.1111/jne.12079. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J Women’s Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a stimulus-response-based instrumental discrimination task, while sparing conditioned place preference learning. Neuroscience. 2004;124:23–31. doi: 10.1016/j.neuroscience.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta. 2010;1800:1045–1055. doi: 10.1016/j.bbagen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor C, Lymer J, Systerova U, Phan A, Choleris E. Rapid effects of estrogen receptor GPER/GPR30 agonist G-1 on learning and memory in female mice. Society for Neuroscience Abstracts. 2011;37:728.03. [Google Scholar]
- Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26:641–648. doi: 10.1111/jne.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gold PE, Korol DL. Hormones and Memory. In: Koob GF, Le Moal M, Thompson RF, Dantzer R, editors. Encyclopedia of Behavioral Neuroscience. 2. Vol. 2. Oxford: Academic Press; 2010. pp. 57–64. [Google Scholar]
- Gold PE, Newman LA, Scavuzzo CJ, Korol DL. Modulation of multiple memory systems: From neurotransmitters to metabolic substrates. Hippocampus. 2013;23:1053–1065. doi: 10.1002/hipo.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Res. 2007;1160:91–101. doi: 10.1016/j.brainres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, Griffin AL. Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiol learn mem. 2013;100:108–116. doi: 10.1016/j.nlm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav. 2012;62:367–374. doi: 10.1016/j.yhbeh.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Moody NM, Dohanich GP, Vasudevan N. Activation of G-protein-coupled receptor 30 is sufficient to enhance spatial recognition memory in ovariectomized rats. Behav Brain Res. 2014;262:68–73. doi: 10.1016/j.bbr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α andP450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot V. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent MH, Zurkovsky L, Fornelli DC, Fell JA, Korol DL. Intra-striatal antiestrogen ICI 182,780 attenuates the impairing effects of peripheral estradiol treatment on response learning in young adult ovariectomized rats. 35th Annual meeting for the Society for Neuroscience, Society for Neuroscience Abstracts, 31, 883.3.2005. [Google Scholar]
- Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17beta-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res. 2012;227:100–108. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Korol DL, Scavuzzo CJ, Collier RL. Intrahippocampal infusions of estradiol can enhance or impair place learning depending on timing of treatment. 40th Annual meeting for the Society for Neuroscience, Society for Neuroscience; 2010. Abstracts 36, 296.12. [Google Scholar]
- Korol DL, Zurkovsky L, Serio SJ, Decker LA, Gold PE. Effects of age and task difficulty on estradiol enhancement of place learning. Society for Neuroscience Abstracts. 2007;33:95.20. [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276:95–98. doi: 10.1016/s0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith JC, Jr, Harris HA. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands. J Med Chem. 2004;47:5021–5040. doi: 10.1021/jm049719y. [DOI] [PubMed] [Google Scholar]
- Mani SK, Mermelstein PG, Tetel MJ, Anesetti G. Convergence of multiple mechanisms of steroid hormone action. Horm Metab Res. 2012;44:569–576. doi: 10.1055/s-0032-1306343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- McElroy MW, Korol DL. Intrahippocampal muscimol shifts learning strategy in gonadally intact young adult female rats. Learn Mem. 2005;12:150–158. doi: 10.1101/lm.86205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Bandara SB, Doerge DR, Helferich WG, Korol DL, Schantz SL. Effects of multiple daily genistein treatments on delayed alternation and a differential reinforcement of low rates of responding task in middle-aged rats. Neurotoxicol Teratol. 2012;34:187–195. doi: 10.1016/j.ntt.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Horm Behav. 2010;58:878–890. doi: 10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Pisani SL, Doerge DR, Helferich WG, Sepehr E, Chittiboyina AG, Rotte SC, Smillie TJ, Khan IA, Korol DL, Schantz SL. The effects of dietary treatment with S-equol on learning and memory processes in middle-aged ovariectomized rats. Neurotoxicol Teratol. 2014;41:80–88. doi: 10.1016/j.ntt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Wang VC, Doerge DR, Woodling KA, Andrade JE, Helferich WG, Korol DL, Schantz SL. Impact of dietary genistein and aging on executive function in rats. Neurotoxicol Teratol. 2010;32:200–211. doi: 10.1016/j.ntt.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. Low doses of 17beta-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Pisani SL, Katzenellenbogen JA, Korol DL. Effects of acute administration of ER-selective agonists on place and response learning in ovariectomized young adult female rats. Program Book; 16th Annual meeting of the Society for Behavioral Neuroendocrinology; 2012a. p. P1.55. [Google Scholar]
- Pisani SL, Neese SL, Doerge DR, Helferich WG, Schantz SL, Korol DL. Acute genistein treatment mimics the effects of estradiol by enhancing place learning and impairing response learning in young adult female rats. Horm Behav. 2012b;62:491–499. doi: 10.1016/j.yhbeh.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani SL, Neese SL, Schantz SL, Korol DL. Activation of the membrane estrogen receptor GPER regulates place and response learning in ovariectomized young adult rats according to dose and timing. Abstract Book; 8th Biennial Rapid Responses to Steroid Hormones meeting; 2013. p. 27. [Google Scholar]
- Pleil KE, Glenn MJ, Williams CL. Estradiol alters Fos-immunoreactivity in the hippocampus and dorsal striatum during place and response learning in middle-aged but not young adult female rats. Endocrinology. 2011;152:946–956. doi: 10.1210/en.2010-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009;89:89–97. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu N, Wang L, Liu ZC, Tian Q, Zhang Q. Oestrogen receptor alpha agonist improved long-term ovariectomy-induced spatial cognition deficit in young rats. Int J Neuropsychopharmacol. 2013;16:1071–1082. doi: 10.1017/S1461145712000958. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm Behav. 2008;53:185–191. doi: 10.1016/j.yhbeh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–113. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, Schlinger BA. Estradiol signaling and action at the synapse: Evidence for “synaptocrine” signaling. Front Endocrinol. 2011;2:28. doi: 10.3389/fendo.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Sinopoli KJ, Floresco SB, Galea LA. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol Learn Mem. 2006;86:293–304. doi: 10.1016/j.nlm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC, Ritchie BF, Kalish D. Studies in spatial learning; place learning versus response learning. J Exp Psychol. 1946;36:221–229. doi: 10.1037/h0060262. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Tunur T, Castelan L, Hawley WR, Gold PE, Korol DL. A tale of two memory systems: Differential involvement in two pattern separation tasks. 44th Annual meeting for the Society for Neuroscience, Society for Neuroscience; 2014. Abstracts40, 749.05. [Google Scholar]
- Tunur T, Zendeli L, Korol DL. Nuances of pattern separation determine modulation by estradiol. 42nd Annual meeting for the Society for Neuroscience, Society for Neuroscience; 2012. Abstracts 38, 92.08. [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm Behav. 2009;56:382–390. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Crozier T, Lieblich SE, Galea LA. Reproductive experience does not persistently alter prefrontal cortical-dependent learning but does alter strategy use dependent on estrous phase. Horm Behav. 2013;64:439–447. doi: 10.1016/j.yhbeh.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Serio SJ, Korol DL. Intra-striatal estradiol in female rats impairs response learning within two hours of treatment. Horm Behav. 2011;60:470–477. doi: 10.1016/j.yhbeh.2011.07.014. [DOI] [PubMed] [Google Scholar]