Abstract
Objective
Patients with obsessive-compulsive disorder (OCD) often only partially respond to serotonin reuptake inhibitors (SRIs). In such cases, American Psychiatric Association practice guidelines suggest augmenting SRIs with cognitive behavioral therapy consisting of exposure and response prevention (EX/RP) or antipsychotic medication (i.e., risperidone). We examined moderators and predictors of these two augmentation strategies.
Method
Data came from a randomized controlled trial that compared adding EX/RP or risperidone to SRIs in adults with OCD. Patients entered the study on a stable SRI dosage and were randomized to EX/RP (N= 40), risperidone (N= 40) or placebo (N = 20). Data were analyzed using multilevel modeling.
Results
Pre-treatment OCD severity, age and depression were significant moderators. Although OCD severity was unrelated to EX/RP response, individuals with more severe OCD had poorer outcome and slower improvement with risperidone. Increasing age predicted better response to risperidone, but not EX/RP. Increased depression predicted poorer response to placebo, but not EX/RP or risperidone. Poorer functioning predicted worse outcome across all three conditions. Together, these moderators and predictor accounted for 33% of the variance in outcomes, above and beyond the 30.8% accounted for by treatment condition.
Conclusions
SRI augmentation with EX/RP was more effective than risperidone across all of the demographic and clinical variables tested. EX/RP’s superiority over risperidone increased with baseline OCD severity and with younger age. These data indicate that EX/RP should be the recommended SRI augmentation strategy, even for severe OCD. What determines the degree of EX/RP response in individual patients deserves further study.
Keywords: Obsessive compulsive disorder (OCD), Exposure and response prevention (EX/RP), SRI Augmentation
Obsessive-compulsive disorder (OCD) is a significant global cause of non-fatal illness burden (Ayuso-Mateos, 2006). Two evidence-based OCD treatments include cognitive behavioral therapy (CBT) involving exposure and response prevention (EX/RP) and pharmacotherapy with serotonin reuptake inhibitors (SRIs), both of which are first-line treatment options according to American Psychiatric Association (APA)’s practice guidelines (Koran et al., 2007; Koran & Simpson, 2013; CBT is the first treatment choice in NICE guidelines; NICE, 2005). For patients receiving SRI treatment first, most will continue to have significant residual symptoms. For these patients, APA’s practice guidelines suggest adding an antipsychotic (i.e., risperidone) or EX/RP (Koran et al., 2007; Koran & Simpson, 2013). As yet, it remains unclear how to match these two augmentation strategies to individual patients.
Our recent randomized controlled trial compared the acute outcomes from these two augmentation strategies (Simpson, Foa, et al., 2013). OCD patients who remained symptomatic despite receiving an SRI at a maximally tolerated dose for at least 12 weeks were randomized to risperidone (RIS; N=40), EX/RP (N = 40), or pill placebo (PBO; N=20). After 8 weeks of treatment, patients randomized to EX/RP had significantly greater reduction in OCD severity, while patients receiving risperidone did not differ from placebo. Many more patients had a treatment response (≥25% symptom reduction) in the EX/RP group (80%) as compared to risperidone (23%) and placebo (15%).
These results demonstrate that EX/RP can outperform risperidone as an SRI augmentation strategy in adults with OCD. However, not all patients respond to the combination of SRIs with EX/RP; moreover, some patients do respond to the combination of SRIs and risperidone, both in our study (Simpson, Foa, et al., 2013) and in prior antipsychotic augmentation studies (Bloch et al., 2006; Veale et al., 2014). Further, although EX/RP outperformed risperidone on average in our study, it is possible that some individuals with certain characteristics might have particularly good risperidone response. Identifying what predicts differential response to these augmentation strategies could help clinicians decide which treatment to initiate, enabling more personalized care.
About one-third of adults with OCD respond to an antipsychotic (Bloch et al., 2006, Dold et al., 2013; Skapinakis, Papatheodorou, & Mavreas, 2007), but what predicts response is less clear. In 40 OCD adults randomized to quetiapine augmentation, Carey et al. (2012) found that the total number of failed SRI trials (a putative marker of treatment resistance) predicted poorer response. In 10 OCD adults randomized to risperidone augmentation, Hollander et al. (2003) reported that greater improvement was significantly correlated with better insight as assessed by an item on the Yale Brown Obsessive-Compulsive Scale (YBOCS; Goodman et al., 1989a; 1989b). Two studies reported that individuals with comorbid tic disorders responded especially well to SRI augmentation with an antipsychotic (specifically pimozide and haloperidol) (McDougle, et al., 1990; McDougle, et al., 1994), though not all antipsychotic trials (specifically those using risperidone) have replicated this finding (McDougle, et al., 2000). A meta-analysis suggests that overall, patients with tics demonstrate more favorable response to antipsychotic response than patients without tics (Bloch et al., 2006).
Studies have also investigated predictors of EX/RP response (for review see Keeley et al., 2008; Knopp et al., 2013). Perhaps the most consistently reported predictor of poor response to EX/RP is the presence of prominent hoarding symptoms (Abramowitz et al., 2003; Mataix-Cols et al., 2002; Rufer et al., 2006), consonant with the recent separation of hoarding disorder from OCD in DSM-5 (Mataix-Cols et al., 2010). Although other findings vary, two relatively consistent predictors of poor EX/RP outcome are higher initial OCD symptom severity (Franklin et al., 2000; Keijsers, Hoogduin, & Schaap, 1994; Mataix-Cols, Marks, Greist, Kobak, & Baer, 2002) and severe comorbid depression (Abramowitz, Franklin, Street, Kozak, & Foa, 2000; Abramowitz & Foa, 2000; Buchanan et al., 1996; Foa, 1979). However, two recent meta-analyses found no consistent relationship between depressive severity and EX/RP effectiveness (Olatunji et al. 2013; Knopp et al., 2013); moreover, although one found a link between OCD symptom severity and worse outcome (Knopp et al., 2013), the other did not (Olatunji et al., 2013). Methodological differences between the two meta-analyses (e.g., inclusion criteria, analytic technique) may account for some of these discrepancies; importantly, both included CBT treatments other than those strictly involving EX/RP. Methodological differences between individual EX/RP studies also exist, including which individual variables are examined as possible predictors, and how outcome is measured (e.g., post-treatment symptoms versus change in symptoms).
Even less is known about predictors of EX/RP augmentation of SRI pharmacotherapy. In an open trial (N = 20) of EX/RP augmentation, Tolin et al. (2004) reported that better insight significantly predicted greater reduction in OCD symptoms following treatment. Maher et al. (2010) investigated predictors and moderators of treatment response in OCD patients randomized to SRI augmentation with either EX/RP (N=54) or stress management training (N = 54). Women had worse outcome than men in EX/RP but not in stress management, and baseline OCD severity predicted outcome of stress management therapy, but not outcome of EX/RP. Irrespective of the therapy received, individuals with more comorbid disorders, worse quality of life, and more past SRI trials showed less symptom improvement.
In summary, relatively few studies have investigated predictors of response to SRI augmentation with either antipsychotics or EX/RP, and no study has investigated what moderates outcome differences between these two augmentation strategies. To address this gap in the literature, the present study uses data from the first randomized controlled trial comparing EX/RP, risperidone, and pill placebo augmentation of SRI pharmacotherapy. This design allowed us to examine whether key variables act as moderators (differentially related to outcome in one treatment condition vs. another) or predictors (associated with response irrespective of treatment type). To address the fact that most prior studies examined only a subset of possibly relevant variables and only one outcome (either post-treatment symptoms or change in symptoms pre-post), we chose the analytic approach described by Fournier and colleagues (2009; described below). This allowed us to investigate a wide range of individual characteristics, from demographics to baseline psychological characteristics, to OCD features, while limiting the risk of both Type I and Type II error. In addition, this approach uses both severity of symptoms at post-treatment and change in symptoms over time as outcomes.
The Fournier approach is hypothesis-generating, rather than hypothesis-driven. At the same time, based on the literature above, we hypothesized that higher initial OCD symptom severity and number of past SRI trials (a potential marker of treatment-resistance) would predict worse outcome across all groups and that hoarding symptoms would be related to worse outcome for EX/RP specifically. Given the mixed results with depression and the fact that some but not all studies have linked insight to outcome (risperidone augmentation, Hollander et al., 2003; EX/RP augmentation, Tolin et al., 2004), we explored whether depression or insight was related to outcome in this sample.
Method
Description of the parent study
Data came from a two-site randomized controlled trial of SRI augmentation described elsewhere (Simpson, Foa et al., 2013). Eligible patients were enrolled at academic outpatient clinics in Philadelphia and New York City. Institutional Review Boards at both sites approved the study protocol, and patients provided written informed consent. Patients entered the study on a stable SRI dose that their study psychiatrist maintained throughout the trial. Exclusion criteria were: 1) diagnosis of bipolar or psychotic disorder; 2) substance abuse or dependence in the past 3 months; 3) clinically significant suicidal ideation; 4) severe depression (≥ 25 on the 17-item Hamilton Depression Rating Scale [HDRS; Hamilton, 1960]); 5) hoarding as the sole OCD symptom; 6) previous trial of risperidone (≥ 0.5 mg/day for 8 weeks) or EX/RP (≥ 8 sessions over 2 months) while taking an SRI.
For patients receiving EX/RP augmentation, medication visits with their psychiatrist occurred every four weeks (weeks 0, 4, and 8). Patients randomized to pill augmentation (risperidone or pill placebo) met with their study psychiatrist weekly for the first four weeks and then biweekly (weeks 0, 1, 2, 3, 4, 6, and 8). Risperidone and placebo were dispensed in identical capsules with the same dosing schedule. Initial psychiatrist visits lasted 60 minutes; subsequent visits were 30 minutes. Patients randomized to EX/RP received 17 therapy sessions delivered twice weekly. EX/RP sessions were 90 minutes long and consisted of 2 introductory sessions followed by 15 exposure sessions, daily homework assignments (self-directed exposures), and phone check-ins between each session (Kozak & Foa, 1997).
Sample for the present study
Of 100 randomized patients, 86 completed the acute treatment phase (Risperidone: 32 of 40; EX/RP: 37 of 40; placebo: 17 of 20). Attrition did not differ by treatment group, χ2(2)=2.62, p=.27. Since we used multilevel modeling to analyze the data (see below), all randomized patients were included in our analyses. Details of the sample, including demographics and baseline clinical characteristics appear in Table 1. We first examined the data for presence of outliers (more than 3 SD from the mean) because such outliers can bias the estimates of the regression coefficients (Tabachnick & Fidell, 2013); this resulted in the removal of one subject.1
Table 1.
Descriptive Statistics
| Domain | Risperidone (N = 40) | EX/RP (N = 40) | Pill Placebo (N = 20) |
|---|---|---|---|
| DEMOGRAPHIC VARIABLES | |||
|
| |||
| Age (in years), mean (S.D.) | 33.78 (10.77) | 34.28 (12.67) | 33.4 (10.42) |
| Female, n (%) | 21 (52.5) | 21 (52.5) | 6 (30.0) |
| Married–partnered, n (%) | 10 (25.0) | 12 (30.0) | 4 (20.0) |
| Non-Hispanic white, n (%) | 35 (87.5) | 36 (90.0) | 19 (95.0) |
| Years of education, mean (S.D.) | 15.9 (2.06) | 15.35 (3.13) | 15.65 (1.87) |
|
| |||
| OCD FEATURES | |||
|
| |||
| Insight (BABS), mean (S.D.) | 5.73 (4.03) | 6.13 (4.62) | 5.25 (3.78) |
| OCD duration (years), mean (S.D.) | 16.18 (11.36) | 16.18 (11.13) | 15.78 (9.88) |
| OCD onset age (years), mean (S.D.) | 17.26 (8.98) | 17.74 (9.26) | 18.28 (7.73) |
| OCD-related Beliefs (OBQ-44), mean (S.D.) | 168.98 (61.1) | 175.75 (57.36) | 169.15 (54.09) |
| YBOCS severity, mean (S.D.) | 26.13 (4.32) | 27.17 (3.94) | 25.9 (4.56) |
|
| |||
| OCD SYMPTOM DIMENSION | |||
|
| |||
| YBOCS Cleanliness dimension | .38 (.31) | .34 (.31) | .31 (.28) |
| YBOCS Doubts/Checking dimension | .29 (.25) | .26 (.21) | .27 (.21) |
| YBOCS Hoarding dimension | .40(.47) | .33 (.45) | .35 (.49) |
| YBOCS Symmetry dimension | .26 (.19) | .36 (.26) | .33 (.26) |
| YBOCS Taboo Thoughts dimension | .15 (.16) | .14 (.16) | .17 (.27) |
|
| |||
| COMORBIDITY AND BASELINE FUNCTIONING | |||
|
| |||
| Comorbid Axis I disorders, n (%) having ≥ 1 | 23 (57.5) | 13 (32.5) | 12 (60) |
| Depression severity (HDRS), mean (S.D.) | 9.82 (5.56) | 7.8 (6.09) | 7.65 (5.93) |
| Functioning (SAS-SR), mean (S.D.) | 2.25 (0.42) | 2.25 (0.49) | 2.23 (0.67) |
| Quality of Life (QLESQ), mean (S.D.) | 52.28 (14.14) | 57.83 (16.22) | 56.1 (16.18) |
|
| |||
| MEDICATION FEATURES | |||
|
| |||
| Number of past SRI trials, mean (S.D.) | 2.40 (1.30) | 2.55 (1.28) | 2.45 (1.19) |
| Weeks receiving SRI dose, mean (S.D.) | 50.39 (88.14) | 65.67 (101.61) | 50.39 (88.14) |
| Past antipsychotic trial, n (%) having ≥ 1 | 6 (15) | 8 (20) | 3 (15) |
Note. YBOCS = Yale Brown Obsessive-Compulsive Scale; EX/RP = exposure and ritual prevention; BABS = Brown Assessment of Beliefs Scale; HDRS = Hamilton Depression Rating Scale; SAS-SR = Social Adjustment Scale; OBQ = Obsessive Beliefs Questionnaire-44; QLESQ = Quality of Life Satisfaction Scale. Gender and YBOCS severity were included in each domain of the regression analyses as control variables.
Outcome Measure
The Yale-Brown Obsessive Compulsive Scale (YBOCS; Goodman et al. 1989a, b) was the primary outcome measure. Independent evaluators blinded to treatment condition assessed patients’ OCD symptoms using the YBOCS at baseline (week 0), mid-treatment (week 4) and post-treatment (week 8).
Potential Moderators and Predictors
Potential moderator/predictor variables (see Table 1) were assessed at baseline (week 0), prior to randomization. Variables were grouped into conceptually-related categories (domains) as in Fournier et al. (2009) described below.
Demographics
This category included age, gender, years of education, race/ethnicity, and current relationship status (i.e., single or divorced/separated=0, married or living with partner=1). Because the sample was mainly non-Hispanic Caucasian (90%), race/ethnicity was dichotomized into “non-Hispanic Caucasian” and “Other” categories.
OCD Features
This domain included OCD illness duration, assessed by SCID (First et al., 1996); baseline symptom severity as assessed by the YBOCS (Goodman et al., 1989a; 1989b); degree of insight, measured with the Brown Assessment of Beliefs Scale (BABS; Eisen, et al., 1998); and degree of maladaptive beliefs (considered important in the development and maintenance of OCD), measured with the Obsessive Beliefs Questionnaire-44 (OBQ-44; OCCWG, 2005). Age of onset of OCD was initially included in this domain, but because it was exactly multi-collinear with “current age” and “duration of OCD”, age of onset was dropped from the analyses. 2
OCD Symptom Dimensions
To balance category size and reduce the likelihood of Type II error (see Data Analysis section), OCD symptom dimensions were analyzed in their own domain, separate from other OCD features. Scores for individual OCD symptom dimensions were computed by calculating the proportion of current symptoms reported at baseline in each of five dimensions (Taboo Thoughts, Contamination/Washing, Symmetry/Exactness, Doubts/Checking, and Hoarding) as in previous studies employing the YBOCS (Pinto et al., 2007; 2008).
Comorbidity and Baseline Functioning
This category included the number of comorbid Axis I disorders3, as determined using the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2001), and depression as measured by the HDRS (Hamilton, 1960). In addition, patients’ baseline functioning was measured by the Quality of Life Satisfaction Scale (QLESQ; Endicott, Nee, Harrison & Blumenthal, 1993) and Social Adjustment Scale (SAS-SR; Weissman & Bothwell, 1976). Only five individuals had a lifetime history of tic disorder (1 in risperidone group, 2 in EX/RP, 2 placebo), precluding using it as a predictor in the analyses.
Medication History
This domain included information about prior medication use, including number of past SRI trials (including the current SRI), weeks on the current SRI dosage before augmentation, and history of a past antipsychotic trial (of at least one week duration).
Data Analysis
We used the Fournier approach (Amir, Taylor, & Donohue, 2011; Fournier et al., 2009, Smits et al., 2013, Powers et al., 2014) to identify significant predictors and moderators of outcome. This stepwise procedure extracts significant variables related to post-treatment YBOCS and rate of YBOCS change from each of the five domains identified above, and then uses these variables in a final model predicting post-treatment YBOCS. This approach represents a balance between (1) examining multiple predictor/moderator variables separately, one variable at a time, which increases Type I error and enhances the likelihood that other third-variables may alternatively explain the relations, and (2) including all variables simultaneously in the prediction equation, which could violate guidelines for the ratio of variables to subjects and would likely produce high Type II error (low power).
The stepwise procedure for each domain is conducted as follows: In Step 1, all variables within a domain are included in one analysis both as predictors (main effects) and as moderators (interacting with treatment group) of outcome. In Step 2, only the variables (main effects and interactions) with a significance level p<.20 in Step 1 of the analysis are included in the second analysis. In Step 3, the analysis includes only all variables from Step 2 that were p<.10. In Step 4, the analysis includes variables from Step 3 that were significant at p<.05. Finally, the final model includes each variable that was significant at p<.05 in Step 4 from each domain, allowing the testing of the effects of each of these variables while controlling for the effects of the others. In keeping with the previous reports utilizing the Fournier approach (Amir, Taylor, & Donohue, 2011; Fournier et al., 2009, Smits et al., 2013, Powers et al., 2014), we only interpret effects that are significant in the Final Model, since some variables that are significant in Step 4 of a domain are not necessarily significant in the final model.
We used multilevel modeling (MLM) to analyze moderators/predictors of the change in YBOCS scores during treatment (from baseline to post-treatment). MLM is an intent-to-treat approach that uses all data from all randomized patients to estimate both the slope of change and intercept for each individual. Primary outcomes for the randomized controlled trial are described elsewhere (Simpson, Foa et al; 2013). In this previous report, YBOCS scores were found to decrease linearly over time. Thus, our “Time” variable was number of weeks from baseline, and our Time variable was “centered” (“zeroed”) at post-treatment (e.g., Time was coded -8 for baseline, -4 for mid-treatment, and 0 for post-treatment). When Time is “zeroed” at post-treatment in this way, the intercept represents the level of YBOCS at post-treatment, and predictors of the intercept reflect the effect of those predictors on the post-treatment level of YBOCS (Singer and Willett, 2003).
Level 1 of the MLM (which describes the growth curve of YBOCS within individuals over time) was comprised of the intercept (at post-treatment) and Time. Level 2 was comprised of individual characteristics that were used to predict the intercept (post-treatment score) and the slope of improvement over time. The level 2 variables included our potential moderator and predictor variables plus treatment condition, and the interaction of the moderators with treatment condition. Treatment condition was included as a control variable in all analyses, as was baseline YBOCS. We included baseline YBOCS as a control variable because it was previously found to moderate EX/RP augmentation (Maher et al., 2010), and because moderators (e.g., depressive symptoms) may affect YBOCS merely because they are related to initial YBOCS severity. Thus, variables identified from each domain were related to post-treatment YBOCS over-and-above baseline YBOCS.
The three treatment conditions were coded using two dummy variables. As EX/RP proved superior to both risperidone and placebo (Simpson, Foa et al., 2013), we were primarily interested in moderators that either increase or decrease (or reverse) the superiority of EX/RP relative to RIS and/or PBO. Thus, the two dummy variables were coded such that each compared a pill group to EX/RP. Hence, moderators of each dummy variable would indicate that the difference between a pill group (RIS or PBO) and EX/RP depended on the level of the moderator. Following standard practice (Jaccard & Turrisi, 2003), when a moderator interacted with one dummy variable coding treatment group differences (either RIS or PBO), hence prompting its inclusion in the next step of the Fournier analysis, the moderator’s interaction with the other “treatment group dummy variable” was also included in the next step of the analysis (i.e., if RIS x age was included in the next step of the Fournier analysis, PBO x age was also included in the next step since both dummy variables are required to code treatment groups). All predictors (except Time) were converted to z-scores to facilitate comparison among them and to center them at their means for the interactions.
Following the recommendations of Fournier et al. (2009) and Smits et al. (2013), we identified variables as predictors if they significantly predicted both the slope and the outcome (at post-treatment) irrespective of study condition, as evidenced by a significant interaction with Time (e.g., HDRS x Time interaction) and a significant main effect (e.g., HDRS main effect). Similarly, moderator variables had to predict differences in slope and outcome between treatment conditions. As such, a moderator is evidenced by a significant interaction between the moderator, treatment condition, and Time (slope effect) and a significant moderator x treatment condition interaction (for outcome at post-treatment) (Fournier et al., 2009).
Significant interactions were explored and interpreted in the Final Model. Specifically, significant moderators of treatment effects were examined following Aiken and West (1991) by investigating the effect of each significant moderator within each treatment condition, and by comparing the predicted effects of treatment condition for high and low levels of the moderator.
Power analyses using the program PinT 2.12 (Power in Two-Level Models; Snijders and Bosker, 1993), revealed that we would have greater than .80 power to detect an effect size of d=.33 (which is midway between a small [d=.20] and a medium [d=.50] effect size).
Results
Preliminary analysis
Missing data for the predictors/moderators were minimal, with five patients missing information on duration of OCD and one missing baseline HDRS. The only variable that differed significantly by group was the proportion with at least one co-morbid Axis I diagnosis, χ2(2)=6.45, p=.04, with the EX/RP group having fewer comorbid disorders than either pill group.
Results of the Step-Wise Analyses within each Domain
Below we report significant results of Step 4 from the MLM analysis for each domain. Table 2 summarizes these results.
Table 2.
Significant variables from Step 4 of each Domain and the Final Model
| Domain/Predictor | Post-Treatment Main Effects | Slope Effects | ||||
|---|---|---|---|---|---|---|
| DOMAIN: DEMOGRAPHIC VARIABLES | ||||||
|
| ||||||
| Variable | b | t | p | b | t | p |
| Age | −.96 | −1.80 | .073 | −.08 | −.92 | .358 |
| Risperidone X Age | −1.56 | −3.11 | .002 | −.20 | −2.34 | .020 |
| Placebo X Age | −.46 | −.73 | .464 | −.05 | −.51 | .613 |
| Non-Hispanic White ethnicity | −1.28 | −2.54 | .012 | −.16 | −1.95 | .052 |
| Married–partnered | −1.53 | −2.97 | .003 | −.20 | −2.31 | .022 |
|
| ||||||
| BASELINE OCD FEATURES | ||||||
|
| ||||||
| Baseline YBOCS | 2.02 | 4.16 | <.001 | −.19 | −2.31 | .022 |
| Risperidone X Baseline YBOCS | 2.22 | 4.19 | <.001 | .31 | 3.49 | .001 |
| Placebo X Baseline YBOCS | −.30 | −.56 | .576 | −.01 | −.11 | .916 |
| BABS | 1.13 | 2.37 | .019 | .11 | 1.41 | .160 |
| Risperidone X BABS | −.89 | −1.76 | .081 | −.08 | −.89 | .378 |
| Placebo X BABS | 1.34 | 2.50 | .013 | .18 | 1.89 | .060 |
| OCD Duration | −.72 | −2.17 | .033 | −.11 | 1.40 | .163 |
|
| ||||||
| OCD SYMPTOM DIMENSIONS | ||||||
|
| ||||||
| Hoarding dimension | −1.45 | 3.10 | .002 | −.21 | −2.63 | .009 |
| Risperidone X hoarding dimension | −2.02 | −3.52 | .001 | −.22 | −2.48 | .014 |
| Placebo X hoarding dimension | −.76 | −1.52 | .130 | −.07 | −.81 | .419 |
|
| ||||||
| COMORBITITY AND BASELINE FUNCTIONING | ||||||
|
| ||||||
| SAS-SR | 1.63 | 2.87 | .005 | .23 | 2.42 | .017 |
| HDRS | .37 | .64 | .524 | .01 | .07 | .943 |
| Risperidone X HDRS | .41 | .74 | .463 | .02 | .24 | .809 |
| Placebo X HDRS | 2.01 | 2.94 | .004 | .24 | 2.13 | .034 |
|
| ||||||
| MEDICATION FEATURES | ||||||
|
| ||||||
| Past SRI trials | .83 | 1.98 | .051 | .07 | 2.29 | .010 |
|
| ||||||
| FINAL MODEL | ||||||
|
| ||||||
| Risperidonea | 5.14 | 10.74 | <.001 | .68 | 8.11 | <.001 |
| Placeboa | 4.65 | 9.48 | <.001 | .61 | 6.98 | <.001 |
| Baseline YBOCS | 2.20 | 4.94 | <.001 | −.17 | −2.14 | .033 |
| Risperidone X Baseline YBOCS | 2.02 | 4.12 | <.001 | .29 | 3.34 | .001 |
| Placebo X Baseline YBOCS | .31 | .63 | .528 | .07 | .76 | .448 |
| Age | −.48 | −.93 | .354 | −.04 | −.46 | .643 |
| Risperidone X Age | −1.38 | −2.95 | .003 | −.17 | −2.04 | .043 |
| Placebo X Age | 1.32 | 1.93 | .054 | .15 | 1.28 | .204 |
| Hoarding dimension | −1.85 | −3.96 | <.001 | −.26 | −3.30 | .001 |
| Risperidone X hoarding dimension | −1.24 | −2.59 | .010 | −.11 | −1.27 | .205 |
| Placebo X hoarding dimension | −2.10 | −3.60 | <.001 | −.23 | −2.34 | .020 |
| HDRS | 1.09 | 1.95 | .052 | .09 | .97 | .334 |
| Risperidone X HDRS | .35 | .69 | .489 | .01 | 0.09 | .932 |
| Placebo X HDRS | 3.22 | 4.44 | <.001 | .40 | 3.33 | .001 |
| SAS-SR | 1.43 | 2.75 | .006 | .22 | 2.39 | .018 |
Note.
“Risperidone” refers to the dummy variable coding the contrast between those in the Risperidone condition and those in EX/RP. “Placebo” refers to the dummy variable contrasting those in the Placebo condition and those in EX/RP. Placebo, Risperidone, and baseline YBOCS (as well as their interaction terms) were included in all domains but only reported in the final model. All variables were z-scored before computing interactions. Hence, main effects for all predictors/moderators reflected their effects for the mean of the present sample. Variables that did not reach significance were omitted from the table. Full results are available upon request from the corresponding author.
YBOCS = Yale Brown Obsessive-Compulsive Scale; EX/RP = exposure and ritual prevention; BABS = Brown Assessment of Beliefs Scale; HDRS = Hamilton Rating Scale for Depression; SAS-SR = Social Adjustment Scale.
Demographic Characteristics
Step 4 of the Fournier analysis showed that non-Hispanic Caucasian participants had lower post-treatment YBOCS than other race/ethnicities (b=−1.28, t(252)=2.54, p=.012). Married/partnered patients had lower YBOCS at post-treatment, (b=−1.53, t(244)=−2.97, p<.003), and had faster rates of reduction in YBOCS (b=−.20, t(196)=−2.31, p=.022) than those single or divorced/separated. Finally, age significantly moderated the difference between RIS and EX/RP (effect on post-treatment YBOCS: b=1.56, t(249)=−3.11, p<.002; effect on rate of YBOCS change: b=−.20, t(193)=−2.34, p<.020). Details of this moderator effect (and all subsequent moderator effects in the analyses of the individual domains) are reported under the Final Model to avoid repetition.
Baseline OCD features
Although baseline YBOCS was included in every analysis, we report the results for baseline YBOCS under this domain since it is a “baseline OCD feature.” Step 4 of the analysis of this domain indicated that baseline YBOCS moderated the difference between RIS and EX/RP (effect on post-treatment YBOCS: b=2.22, t(231)=4.19, p<.001; effect on rate of YBOCS change: b=.31, t(184)=3.49, p=.001).
Step 4 of this domain also indicated that “insight” predicted outcome across treatments (b=1.13, t(233)=2.37, p=.019), and that the difference between EX/RP and PBO on post-treatment YBOCS depended on insight (b=1.34, t(230)=2.50, p<.013). Finally, OCD duration was a significant predictor in this domain, as patients with longer OCD duration exhibited lower YBOCS scores across all conditions (b=−.72, t(94)=−2.17, p<.033).
OCD Symptom Dimensions
Hoarding was the only OCD symptom dimension related to YBOCS in Step 4 of the analysis of this domain. Hoarding symptoms moderated the difference between RIS and EX/RP (effect on post-treatment YBOCS: b=−2.02, t(251)=3.52, p<.001; effect on rate of YBOCS change: b=−.22, t(193)=−2.48, p=.014).
Comorbidity and Baseline Functioning
Worse functional impairment at baseline as assessed by the SAS-SR was related to higher post-treatment YBOCS and slower improvement during treatment (b=1.63, t(245)=2.87, p=.005 and b=.23, t(191)=2.42, p=.017, respectively). In addition, HDRS moderated the differences between PBO and EX/RP (effect on post-treatment YBOCS: b=2.01, t(246)=2.94, p=.004; effect on rate of YBOCS change: b=.24, t(196)=2.13, p=.034).
Medication Features
Patients who had undergone a greater number of past SRI trials had marginally higher post-treatment YBOCS scores (b=.83, t(94)=1.98, p=.051) and slower rates of improvement over the course of the treatment (b=.07, t(43)=2.69, p<.010) than those with fewer past SRI trials.
Final Model with All Significant Variables
The final model included dummy-coded treatment condition and all variables found significant in Step 4 of the analyses of each domain (predictors of outcome: non-Hispanic Caucasian ethnicity, relationship status, OCD duration, number of past SRI trials, and functioning; moderators of outcome: age, baseline YBOCS, insight, hoarding symptoms, and depressive severity).
Table 2 presents the significant results for the final model. As reported previously (Simpson, Foa et al., 2013), patients randomized to EX/RP had lower post-treatment YBOCS and faster rates of improvement than patients randomized to either risperidone (b=5.14, t(241)=10.74, p<.001 and b=.68, t(183)=8.11, p<.001, respectively) or placebo (b=4.65, t(240)=9.48, p<.001 and b=.61, t(176)=6.98, p<.001, respectively). In addition to treatment condition, the final model revealed four significant moderators (OCD severity, age, hoarding symptoms and depression) and one predictor (functioning). These are discussed in more detail below.
Adding the significant moderators/predictors to treatment condition produced an increase in R2 from 30.8% for treatment condition only, to R2=63.8% in the final model, an R2 increase of 33.0%. Of that, 16.3% was attributable to baseline YBOCS, with the remaining 16.7% due to the other predictors and moderators.
Moderators
Baseline OCD severity
Baseline YBOCS severity moderated the difference in outcome between the EX/RP and RIS (effect on post-treatment YBOCS: b=2.02, t(240)=4.12, p<.001; effect on rate of YBOCS change: b=.29, t(180)=3.34, p<.001). Baseline YBOCS severity did not moderate the effect of EX/RP compared to PBO (for outcome and rate of change: p’s>.631).
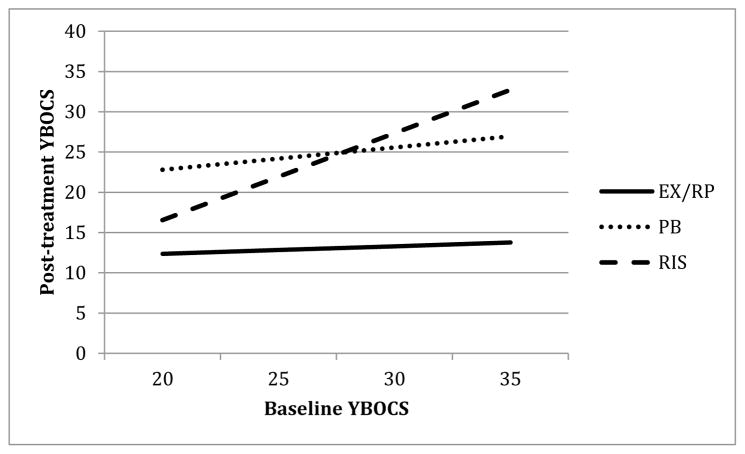
Figure 1 depicts the moderating effect of baseline OCD severity across the range of baseline YBOCS scores. As can be seen, baseline OCD severity was not related to outcome for either EX/RP or placebo (b=.40, t(239)=.56, p=.576 and b=1.16, t(239)=1.18, p=.239, respectively). For risperidone, however, higher baseline YBOCS scores were associated with higher post-treatment YBOCS (b=4.52, t(241)=6.39, p<.001).
Figure 1. Effect of Baseline YBOCS on post-treatment YBOCS.
Note. YBOCS = Yale Brown Obsessive-Compulsive Scale; EX/RP = exposure and ritual prevention; PB = Placebo; RIS = Risperidone.
To further probe the effects of baseline severity on the difference between RIS and EX/RP, we compared the model-predicted post-treatment YBOCS scores for individuals high on baseline YBOCS (1 SD above the mean of baseline YBOCS: YBOCS=30.7) to individuals low on baseline YBOCS (1 SD below the mean: YBOCS=22.3) (following the recommendations of Aiken and West, 1991). Among patients with high baseline YBOCS, those receiving EX/RP were predicted to have much lower post-treatment YBOCS (by 14.6 points) than patients receiving RIS (b=14.62, t(240)=10.21, p<.001). For patients with low baseline YBOCS, those receiving EX/RP still had significantly lower post-treatment YBOCS than those in RIS, but the difference (6.4 points) was significantly smaller (b=6.37, t(241)=4.67, p<.001).
Age
The final model also showed that age moderated the difference in outcome between RIS and EX/RP (effect on post-treatment YBOCS: b=−1.38, t(245)=2.95, p=.003; effect on YBOCS rate of change: b=−.17, t(184)=2.04, p=.043). Age was not a significant moderator of the difference between PBO and EX/RP (for outcome and rate of change: p’s>.054)
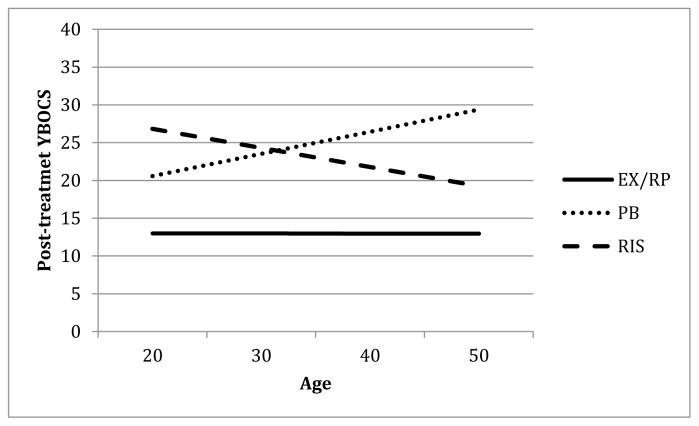
Figure 2 depicts post-treatment YBOCS as a function of patient age for the three groups. As can be seen, age was not related to post-treatment YBOCS for those assigned to EX/RP (b=−.01, t(233)=−.02, p=.988). However, for RIS, greater age was related to lower post-treatment YBOCS (b=−2.83, t(244)=3.77, p<.001) while for PBO, greater age was associated with higher post-treatment YBOCS (b=3.28, t(234)=2.05, p=.042)4.
Figure 2. Effect of age on post-treatment YBOCS.
Note. YBOCS = Yale Brown Obsessive-Compulsive Scale; EX/RP = exposure and ritual prevention; PB = Placebo; RIS = Risperidone.
To further probe the effects of age on the difference between RIS and EX/RP, we compared the model-predicted post-treatment YBOCS scores for younger patients (1SD below the mean in age, about 22.5 years old) to older patients (1SD above the mean age, about 45 years old). For younger patients, the difference between those in RIS and those in EX/RP on post-treatment YBOCS was large (over 13 points) (b=13.32, t(246)=9.77, p<.001), while for those who were older, patients in RIS still had higher post-treatment YBOCS than those in EX/RP, but the difference was smaller (about 7.7 points) (b=7.67, t(239)=5.60, p<.001).
Hoarding symptoms
In the final model, hoarding symptoms moderated the difference between EX/RP and PBO (effect on post-treatment severity: b=−2.10, t(238)=−3.60, p<.001; effect on rate of YBOCS change: b=−.23, t(200)=−2.34, p=.020). In addition, the difference between EX/RP and RIS for post-treatment YBOCS was affected by hoarding (b=−1.24, t(245)=−2.59, p=.010), but there was not a significant interaction with rate of YBOCS change (p=.205).
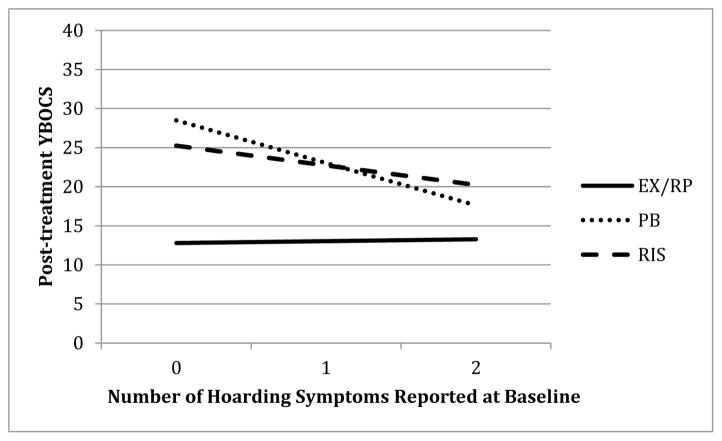
Figure 3 shows how post-treatment YBOCS varied as a function of hoarding symptoms for the three groups. As can be seen, number of hoarding symptoms endorsed did not relate to post-treatment YBOCS for those assigned to EX/RP (b=.22, t(243)=.32 p=.751). However, more hoarding symptoms predicted lower post-treatment YBOCS in both RIS and PBO (b=−2.32, t(247)=−3.31, p=.001 and b=−5.02, t(236)=−3.89, p<.001, respectively).
Figure 3.
Effect of hoarding symptoms on post-treatment YBOCS
Note. YBOCS = Yale Brown Obsessive-Compulsive Scale; EX/RP = exposure and ritual prevention; PB = Placebo; RIS = Risperidone.
To further probe the effect of hoarding symptoms on the difference between EX/RP and PBO, we compared the model-predicted post-treatment YBOCS scores for individuals reporting both hoarding symptoms (hoarding obsessions and hoarding compulsions) to those with no hoarding symptoms. For patients reporting no baseline hoarding symptoms, those assigned to PBO had much higher post-treatment YBOCS scores than those receiving EX/RP (16 points higher, b=15.72, t(238)=8.68, p<.001). Among patients endorsing both hoarding symptoms at baseline, those in the PBO condition still fared worse than those in EX/RP (b=4.31, t(239)=1.98, p=.049), but the difference was smaller (4.3 points).
Since hoarding symptoms did not affect the difference between RIS and EX/RP on both post-treatment YBOCS and rate of change in YBOCS over treatment, it was not considered a moderator and its effects were not analyzed.
Depression
Finally, depressive severity (measured by the HDRS) moderated the difference between EX/RP and PBO (effect on post-treatment YBOCS: b=3.22, t(236)=4.44, p<.001; effect on rate of YBOCS change: b=.40, t(197)=3.33, p=.001). Depression did not moderate the differences between EX/RP and RIS (for outcome and rate of change: p’s>.48).
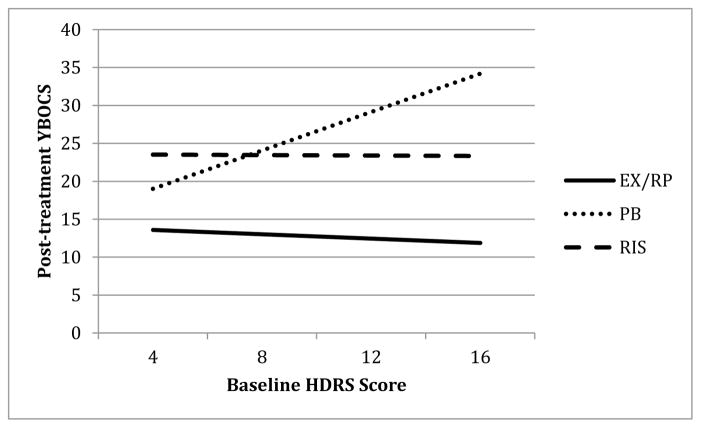
Figure 4 depicts post-treatment severity as a function of baseline depressive symptoms for the three groups. As can be seen in Figure 4, depression scores were not related to outcome for individuals receiving either EX/RP or RIS (b=−.80, t(239)=−1.10, p=.271 and b=−.09, t(237)=−.12, p=.906). However, for those receiving PBO, higher depression scores were related to higher post-treatment YBOCS scores (b=7.25, t(235)=4.34, p<.001).
Figure 4.
Effect of baseline depression on post-treatment YBOCS
Note. YBOCS = Yale Brown Obsessive-Compulsive Scale; EX/RP = exposure and ritual prevention; PB = Placebo; RIS = Risperidone; HDRS = Hamilton Depression Rating Scale.
To further probe the effect of depressive severity on the difference between PBO and EX/RP, we compared the model-predicted post-treatment YBOCS scores for participants with moderate baseline depression (HDRS = 16) and low baseline depression (HDRS = 4, within the normal range (0–7) of HDRS symptoms; Zimmerman, Posternak & Chelminski, 2005). For those with moderate baseline depression symptoms, patients in EX/RP had much lower post-treatment YBOCS scores than those in PBO (21.8 points lower on YBOCS) (b=21.83, t(236)=7.62, p<.001). For those with low levels of depression, patients in EX/RP again had significantly lower post-treatment YBOCS scores than those in PBO (b=5.34, t(238)=3.29, p=.001), but the difference was smaller (5.3 points).
Predictors
There was only one significant predictor in the final model. Greater problems in functioning (higher SAS-SR) were related to higher post-treatment YBOCS scores (b=1.43, t(240)=2.75, p=.006) and slower rates of improvement (b=.22, t(181)=2.39, p=.018), regardless of treatment condition. Neither ethnicity, nor relationships status, nor OCD duration, nor number of past SRI trials were significantly related to either slope or intercept in the final model.
Discussion
In a randomized controlled trial comparing the effects of augmenting SRIs with EX/RP, risperidone, or pill placebo, we found that pre-treatment OCD severity, age, baseline depression, and hoarding moderated outcome after acute treatment, while baseline level of functioning predicted outcome and rate of change across all three augmentation strategies. Together these moderators and predictors accounted for 33% in the variance of post-treatment OCD severity, above and beyond the 30.8% of the variance explained by treatment condition.
Our hypothesis that baseline symptom severity would predict treatment outcome was partially supported: higher baseline YBOCS was predictive of worse outcome in the risperidone group, but not in EX/RP or placebo. The finding that pre-treatment severity was unrelated to post-treatment outcome for EX/RP is consistent with two previous studies examining predictors of EX/RP augmentation of SRIs (Maher et al., 2010; Tolin et al., 2004) as well as a recent meta-analysis (Olantunji et al., 2013) indicating that severe OCD does not preclude patients from benefitting from EX/RP augmentation. In contrast, greater pre-treatment OCD severity predicted worse treatment outcome for patients randomized to risperidone. Thus, while EX/RP proved a more effective augmentation strategy overall for all patients, this was especially true for patients with more severe OCD, who on average benefitted much more from EX/RP than risperidone. This finding is contrary to the clinical intuition that severe OCD may require an antipsychotic as opposed to behavior therapy. Our data instead suggest that EX/RP should be the treatment of choice, even for severe OCD.
Age moderated the difference between EX/RP and risperidone. Whereas age was unrelated to response for EX/RP, older individuals had a better response with risperidone augmentation. It is unclear why the efficacy of risperidone should depend on patient age. This result has not previously been reported in other studies of risperidone augmentation, and therefore needs replication. Again, these results suggest that EX/RP should be the treatment of choice because it is equally effective with younger and older patients.
Depressive severity moderated the difference between EX/RP and placebo. Whereas depressive severity was unrelated to EX/RP response, those with higher depressive severity had a lower placebo response. This finding was not a consequence of participants with high depressive severity having high initial OCD severity, since baseline OCD severity was controlled in our analyses. This result with placebo is in line with previous findings in the depression literature. For example, Khan et al. (2002) analyzed data from 45 studies in the Food and Drug Administration database and found that higher baseline depression scores predicted less improvement with pill placebo and that placebo response was greatest among patients with less severe depression (Khan et al., 2002). Similarly, although placebo response is rare in OCD (Huppert et al., 2004; Mavissakalian, Jones, & Olson, 1990), our findings suggest that it will be more common in patients with low levels of depression. However, it should be noted that for patients with nonclinical levels of depression, EX/RP still performed significantly better than placebo. It should also be noted that patients with very severe depression were excluded from this trial, so these results may not extend to severely depressed OCD patients.
Hoarding symptoms also moderated the difference between EX/RP and placebo. There was no relationship between hoarding symptoms and EX/RP outcome, which contradicts some previous studies reporting that hoarding symptoms predict worse EX/RP response (Abramowitz et al., 2003; Mataix-Cols et al., 2002; Rufer et al., 2006). At the same time, more hoarding symptoms predicted better outcome within the placebo condition, an unexpected result. In addition, the difference in outcome between EX/RP and risperidone depended on hoarding symptoms. Specifically, within the risperidone group, increasing hoarding symptoms were associated with lower levels of post-treatment OCD symptoms (although hoarding symptoms did not similarly impact differences in rate of change). It is important to emphasize that the parent study excluded participants with primary hoarding symptoms. In addition, we assessed hoarding using the YBOCS checklist, in lieu of a hoarding-specific measure (such as the Saving Inventory Revised; Frost, Steketee, & Grisham, 2004), which would have been preferable because it provides a continuous measure of symptoms. Thus, these findings need replication, although they suggest to us that the effects of antipsychotics in patients with primary hoarding disorder may be worth investigating.
Only one variable predicted improvement across the three augmentation strategies: baseline psychosocial functioning as measured by the SAS-SR. This finding is consistent with a previous study showing poor functioning to be a predictor of negative outcome for EX/RP (Steketee, 1993). A previous study by our group found quality of life as measured by the QLESQ, but not functioning as measured by the SAS-SR, predictive of EX/RP outcome (Maher et al., 2010). Importantly these two questionnaires are often highly correlated, possibly explaining why one measure might appear more important in one sample than the other. Impaired functioning may make it difficult to comply with treatment, and functional impairment has been associated with poorer treatment outcomes broadly. For example, in the depression literature, worse psychosocial functioning has been predictive of poorer treatment response with both psychotherapy and pharmacotherapy (Elkin et al., 1995). This result highlights the importance of assessing and addressing impairments in psychosocial functioning regardless of which strategy is used to augment SRIs.
Several variables we had anticipated might predict outcome based on prior studies were not significant in our final model (e.g., number of SRI trials [Carey et al. 2010] and insight [Hollander et al. 2003, Tolin 2004]. For both, restriction of range may explain some of the discrepancy with past results, as in our sample, most patients had good to fair insight (88% of the sample) and the majority of patients (80%) reported three or fewer SRI trials. An alternative possibility, supported by the fact that these variables were significant in the domain analyses but non-significant in the Final Model, is that the variability in results was better accounted for by the other variables included in our analyses. Therefore factors such as insight and past SRI trials might emerge as significant predictors in studies that employ a limited number of control variables; this possibility supports the use of methods like the current Fournier approach, which tests the robustness of predictors over and above a wide range of other variables.
Similarly, some demographic variables were significantly associated with outcome at the level of individual domains (e.g., at the domain level, unmarried/divorced patients benefitted less from all three interventions and non-Hispanic Caucasian participants tended to have better outcomes). However, none of these effects persisted in the final model. Previous studies of EX/RP have also not reported differences in effectiveness based on race/ethnicity (Williams et al., 2010). At the same time, improving minority enrollment in future OCD clinical trials is necessary to further explore the issue of treatment equivalence across ethnic/racial groups (Williams et al., 2010).
Variation between some of our findings and the results of previous investigations of SRI augmentation may reflect differences not only the breadth of predictors examined but also the relatively modest effects for individual predictors. In all studies, including the present one, the effects of individual variables tend to be small to medium, and hence may be sensitive to sampling variability. Notably, our results suggest that in aggregate, the moderators and predictors account for a substantial amount of variance in post-treatment severity scores. It follows that rather than focusing on individual variables, a better approach might be to investigate the combination of factors that characterize patients at risk for poor outcome with a given augmentation strategy. Notably, aside from the measure of psychosocial functioning, which was a general predictor across interventions, none of the other variables significantly predicted response to EX/RP augmentation. On the one hand, this result suggests that EX/RP was effective regardless of the level of any of the other potential predictors and moderators. On the other hand, it highlights the need for more research to identify variables that do predict response to EX/RP, since not all patients benefit equally from this treatment (Olatunji et al., 2013).
Limitations
The results of this study require interpretation in light of study limitations. First, although this trial was the largest conducted to date, the sample size may have limited our power to detect small effects, and risk of type I error may have been inflated by the number of potential moderators we examined. However, by adopting conservative criteria for identifying significant moderators, testing variables simultaneously and requiring them to be significant in Step 4 of both the Domain Model and the Final Model, the inflation of Type I error was limited. Second, characteristics of our sample limited our ability to examine some variables. For example, few patients in our sample had a history of tic disorders and severe depression was an exclusion criterion, limiting our examination of these features. Primary hoarding problems were also an exclusion criterion, and so it is unknown whether our results related to hoarding symptoms can be generalized to patients with hoarding disorder. Also, our sample was mainly non-Hispanic Caucasian, which may limit the generalizability of our results. Third, our study found a more modest response to risperidone augmentation relative to three prior trials (Erzegovesi et al., 2005; McDougle et al., 2000; Hollander et al., 2003). Finally, the parent study did not include a measure of baseline anxiety severity, precluding us from examining it.
Conclusions and Clinical Implications
In summary, poorer functioning at baseline predicted worse response across SRI augmentation strategies (EX/RP, RIS, PBO). Pre-treatment OCD and depressive severity, age, and hoarding symptoms moderated treatment differences: none was related to EX/RP outcome, but greater OCD severity and younger age predicted worse risperidone response and lower depression and more hoarding predicted better placebo response. Although the final model of predictors and moderators explained a large proportion of the variability in post-treatment scores, the effectiveness of EX/RP did not change with the potential moderator variables. This suggests EX/RP is a robust treatment across multiple clinical characteristics and should be recommended as a primary SSRI augmentation strategy. In particular, whereas some clinical intuition would suggest that severe OCD may require augmentation with an antipsychotic, the superiority of EX/RP over risperidone increased with baseline OCD severity, suggesting that EX/RP should be the treatment of choice, even for severe OCD. At the same time, given that no variable other than baseline functioning predicted outcomes with EX/RP, identifying markers of EX/RP response requires further research.
Public health significance.
We examined factors associated with treatment outcome in the first randomized controlled trial comparing risperidone versus cognitive-behavioral therapy (CBT) augmentation of serotonin reuptake inhibitors (SRIs) for patients with OCD. CBT was more effective, and we found that the superiority of CBT over risperidone increased with baseline OCD severity. This suggests that CBT should be the SRI augmentation strategy of choice, even for severe OCD.
Footnotes
One subject in the placebo group scored 4.25 SD above the SAS-SR mean (corresponding to a p of 1 in 100,000). That subject was dropped from the analyses. Including this patient in the analyses does not change significant results for any analysis.
If we instead dropped “current age” from analyses, and included “age of onset”, the final results are identical, except that any results involving “current age” would not be present.
As suggested during the review process, we also investigated whether separating the number of comorbid Axis I disorders into presence of comorbid anxiety disorders and presence of comorbid depressive disorders would impact our results. Separating these disorders did not change any of the results of our analyses.
Although age was related to outcome in PBO but not related to outcome in EX/RP, age was not a moderator of the difference between PBO and EX/RP because the interaction terms for both outcome and slope did not reach significance.
Disclosures: This study was funded by National Institute of Mental Health grants R01 MH045436 (Dr. Simpson) and R01 MH45404 (Dr. Foa). Medication was provided at no cost by Janssen Scientific Affairs LLC. During the study period Dr. Simpson received research funds for clinical trials Janssen Pharmaceuticals (2006–2012), Transcept Pharmaceuticals (2011–2013) and Neuropharm Ltd (2009), served on a Scientific Advisory Board for Pfizer (for Lyrica, 2009–2010) and Jazz Pharmaceuticals (for Luvox CR [controlled release], 2007–2008), consulted for Quintiles Inc. (on the therapeutic needs for OCD, September, 2012) and received royalties from Cambridge University Press and UpToDate Inc. Dr. Foa was a consultant to Jazz Pharmaceuticals (for Acetelion) and received royalties from Bantam and Oxford University Press for book sales, including a manual of cognitive behavioral therapy for OCD. No other disclosures were reported.
References
- Abramowitz JS, Foa EB. Does comorbid major depressive disorder influence outcome of exposure and response prevention for OCD? Behavior Therapy. 2000;31:795–800. [Google Scholar]
- Abramowitz JS, Franklin ME, Schwartz SA, Furr JM. Symptom presentation and outcome of cognitive-behavioral therapy for obsessive-compulsive disorder. Journal of Consulting and Clinical Psychology. 2003;71(6):1049–1057. doi: 10.1037/0022-006X.71.6.1049. [DOI] [PubMed] [Google Scholar]
- Abramowitz JS, Franklin ME, Street GP, Kozak MJ, Foa EB. Effects of comorbid depression on response to treatment for obsessive-compulsive disorder. Behavior Therapy. 2000;31:517–528. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- Amir N, Taylor CT, Donohue MC. Predictors of response to an attention modification program in generalized social phobia. Journal of consulting and clinical psychology. 2011;79(4):533. doi: 10.1037/a0023808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Ayuso-Mateos J. Global Burden of obsessive-compulsive disorder in the year 2000. World Health Organization; Geneva: 2006. [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Molecular psychiatry. 2006;11(7):622–632. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- Buchanan AW, Meng KS, Marks IM. What predicts improvement and compliance during behavioral treatment of obsessive compulsive disorder? Anxiety. 1996;2:22–27. doi: 10.1002/(SICI)1522-7154(1996)2:1<22::AID-ANXI3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Carey PD, Lochner C, Kidd M, Van Ameringen M, Stein DJ, Denys D. Quetiapine augmentation of serotonin reuptake inhibitors in treatment-refractory obsessive–compulsive disorder: is response to treatment predictable? International clinical psychopharmacology. 2012;27(6):321–325. doi: 10.1097/YIC.0b013e3283576881. [DOI] [PubMed] [Google Scholar]
- Dold M, Aigner M, Lanzenberger R, Kasper S. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. The International Journal of Neuropsychopharmacology. 2013;16(03):557–574. doi: 10.1017/S1461145712000740. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown assessment of beliefs scale: reliability and validity. American Journal of Psychiatry. 1998;155(1):102–108. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- Elkin I, Gibbons RD, Shea MT, Sotsky SM, Watkins JT, Pilkonis PA, Hedeker D. Initial severity and differential treatment outcome in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1995;63(5):841. doi: 10.1037//0022-006x.63.5.841. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology bulletin. 1993;29:321–326. [PubMed] [Google Scholar]
- Erzegovesi S, Guglielmo E, Siliprandi F, Bellodi L. Low-dose risperidone augmentation of fluvoxamine treatment in obsessive-compulsive disorder: a double-blind, placebo-controlled study. European neuropsychopharmacology. 2005;15(1):69–74. doi: 10.1016/j.euroneuro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Foa EB. Failure in treating obsessive–compulsive disorder. Behaviour Research and Therapy. 1979;17:169–176. doi: 10.1016/0005-7967(79)90031-7. [DOI] [PubMed] [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, Tu X. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. American Journal of Psychiatry. 2005;162(1):151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- Franklin ME, Abramowitz JS, Kozak MJ, Levitt JT, Foa EB. Effectiveness of exposure and ritual prevention for obsessive–compulsive disorder: Randomized compared with nonrandomized samples. Journal of Consulting and Clinical Psychology. 2000;68:594–602. [PubMed] [Google Scholar]
- Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behaviour Research and Therapy. 2004;42(10):1163–1182. doi: 10.1016/j.brat.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. Journal of Consulting and Clinical Psychology. 2009;77(4):775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Charney DS. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Archives of general psychiatry. 1989a;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown obsessive compulsive scale: II. Validity. Archives of general psychiatry. 1989b;46(11):1012. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23(1):56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Rossi NB, Sood E, Pallanti S. Risperidone augmentation in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. The International Journal of Neuropsychopharmacology. 2003;6(4):397–401. doi: 10.1017/S1461145703003730. [DOI] [PubMed] [Google Scholar]
- Huppert JD, Schultz LT, Foa EB, Barlow DH, Davidson JR, Gorman JM, Woods SW. Differential response to placebo among patients with social phobia, panic disorder, and obsessive-compulsive disorder. American Journal of Psychiatry. 2004;161(8):1485–1487. doi: 10.1176/appi.ajp.161.8.1485. [DOI] [PubMed] [Google Scholar]
- Jaccard J, Turrisi R. Interaction Effects in Multiple Regression. Thousand Oaks, CA: Sage Publications, Inc; 2003. [Google Scholar]
- Keeley ML, Storch EA, Merlo LJ, Geffken GR. Clinical predictors of response to cognitive-behavioral therapy for obsessive–compulsive disorder. Clinical Psychology Review. 2008;28(1):118–130. doi: 10.1016/j.cpr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Keijsers GP, Hoogduin CA, Schaap CP. Predictors of treatment outcome in the behavioural treatment of obsessive–compulsive disorder. British Journal of Psychiatry. 1994;165:781–786. doi: 10.1192/bjp.165.6.781. [DOI] [PubMed] [Google Scholar]
- Khan A, Leventhal RM, Khan SR, Brown WA. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. Journal of clinical psychopharmacology. 2002;22(1):40–45. doi: 10.1097/00004714-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Knopp J, Knowles S, Bee P, Lovell K, Bower P. A systematic review of predictors and moderators of response to psychological therapies in OCD: Do we have enough empirical evidence to target treatment? Clinical psychology review. 2013;33:1067–1081. doi: 10.1016/j.cpr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2007;164(suppl):5–53. [PubMed] [Google Scholar]
- Koran L, Simpson H. APA Practice Guidelines. American Psychiatric Publishing, Inc; 2013. Guideline Watch (March 2013): Practice Guideline for the Treatment of Patients with Obsessive-Compulsive Disorder. [Google Scholar]
- Kozak MJ, Foa EB. Mastery of obsessive-compulsive disorder: A cognitive-behavioral approach. Graywind Publications; 1997. [Google Scholar]
- Maher MJ, Huppert JD, Chen H, Duan N, Foa EB, Liebowitz MR, Simpson HB. Moderators and predictors of response to cognitive-behavioral therapy augmentation of pharmacotherapy in obsessive–compulsive disorder. Psychological medicine. 2010;40(12):2013–2023. doi: 10.1017/S0033291710000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavissakalian MR, Jones B, Olson S. Absence of placebo response in obsessive-compulsive disorder. The Journal of nervous and mental disease. 1990;178(4):268–270. doi: 10.1097/00005053-199004000-00010. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Frost RO, Pertusa A, Clark LA, Saxena S, Leckman JF, Wilhelm S. Hoarding disorder: a new diagnosis for DSM-V? Depression and anxiety. 2010;27(6):556–572. doi: 10.1002/da.20693. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Marks IM, Greist JH, Kobak KA, Baer L. Obsessive–compulsive symptom dimensions as predictors of compliance with and response to behaviour therapy: Results from a controlled trial. Psychotherapy and Psychosomatics. 2002;71:255–262. doi: 10.1159/000064812. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Goodman WK, Leckman JF, Lee NC, Heninger GR, Price LH. Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder: a double-blind, placebo-controlled study in patients with and without tics. Archives of general psychiatry. 1994;51(4):302. doi: 10.1001/archpsyc.1994.03950040046006. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Goodman WK, Price LH, Delgado PL. Neuroleptic addition in fluvoxamine-refractory obsessive-compulsive disorder. The American journal of psychiatry. 1990;147:652–654. doi: 10.1176/ajp.147.5.652. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Archives of General Psychiatry. 2000;57(8):794. doi: 10.1001/archpsyc.57.8.794. [DOI] [PubMed] [Google Scholar]
- NICE. Obsessive-compulsive Disorder: Core Interventions in the Treatment of Obsessive-compulsive Disorder and Body Dysmorphic Disorder. 2005 NICE clinical guideline 31. Available at http://guidance.nice.org.uk/CG31 [NICE guideline] [PubMed]
- Obsessive Compulsive Cognitions Working Group (OCCWG) Psychometric validation of the Obsessive Beliefs Questionnaire and the Interpretation of Intrusions Inventory--Part 2: Factor analyses and testing of a brief version. Behaviour Research and Therapy. 2005;43:1527–1542. doi: 10.1016/j.brat.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Davis ML, Powers MB, Smits JA. Cognitive-behavioral therapy for obsessive-compulsive disorder: a meta-analysis of treatment outcome and moderators. Journal of psychiatric research. 2013;47(1):33–41. doi: 10.1016/j.jpsychires.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Pinto A, Eisen JL, Mancebo MC, Greenberg BD, Stout RL, Rasmussen SA. Taboo thoughts and doubt/checking: a refinement of the factor structure for obsessive–compulsive disorder symptoms. Psychiatry research. 2007;151(3):255–258. doi: 10.1016/j.psychres.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, III, Samuels JF, Murphy DL, Nestadt G. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry research. 2008;160(1):83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Warren AM, Rosenfield D, Roden-Foreman K, Bennett M, Reynolds MC, Davis ML, Foreman ML, Petrey LB, Smits JAJ. Predictors of PTSD symptoms in adults admitted to a Level I trauma center: A prospective analysis. Journal of Anxiety Disorders. 2014;28:301–309. doi: 10.1016/j.janxdis.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer M, Fricke S, Moritz S, Kloss M, Hand I. Symptom dimensions in obsessive–compulsive disorder: prediction of cognitive-behavior therapy outcome. Acta Psychiatrica Scandinavica. 2006;113(5):440–446. doi: 10.1111/j.1600-0447.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Foa EB, Liebowitz MR, Huppert JD, Cahill S, Maher MJ, Campeas R. Cognitive-behavioral therapy vs risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2013;70(11):1190–1199. doi: 10.1001/jamapsychiatry.2013.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis. New York: Oxford University Press; 2003. [Google Scholar]
- Skapinakis P, Papatheodorou T, Mavreas V. Antipsychotic augmentation of serotonergic antidepressants in treatment-resistant obsessive–compulsive disorder: a meta-analysis of the randomized controlled trials. European neuropsychopharmacology. 2007;17(2):79–93. doi: 10.1016/j.euroneuro.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Hofmann SG, Rosenfield D, DeBoer LB, Costa P, Simon NM, MH D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: prognostic and prescriptive variables. Journal of Consulting & Clinical Psychology. 2013;81:1100–1112. doi: 10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TA, Bosker RJ. Standard errors and sample sizes for two-level research. Journal of Educational Statistics. 1993;18:237–259. [Google Scholar]
- Steketee G. Social support and treatment outcome of obsessive compulsive disorder at 9-month follow-up. Behavioural and Cognitive Psychotherapy. 1993;21(02):81–95. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 6. Boston, MA: Pearson; 2013. [Google Scholar]
- Tolin DF, Maltby N, Diefenbach GJ, Hannan SE, Worhunsky P. Cognitive-behavioral therapy for medication nonresponders with obsessive-compulsive disorder: a wait-list-controlled open trial. Journal of Clinical Psychiatry. 2004;65:1169–1175. doi: 10.4088/jcp.v65n0708. [DOI] [PubMed] [Google Scholar]
- Veale DM, Miles SK, Smallcombe N, Ghezai H, Goldacre B, Hodsoll J. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis. BMC psychiatry. 2014;14(1):317. doi: 10.1186/s12888-014-0317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of general psychiatry. 1976;33(9):1111–115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Williams M, Powers M, Yun YG, Foa E. Minority participation in randomized controlled trials for obsessive–compulsive disorder. Journal of anxiety disorders. 2010;24(2):171. doi: 10.1016/j.janxdis.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Posternak MA, Chelminski I. Is the cutoff to define remission on the Hamilton Rating Scale for Depression too high? The Journal of nervous and mental disease. 2005;193:170–175. doi: 10.1097/01.nmd.0000154840.63529.5d. [DOI] [PubMed] [Google Scholar]