Abstract
Memory processing is presumed to depend on synaptic plasticity, which appears to have a role in mediating the acquisition, consolidation, and retention of memory. We have studied the relationship between estrogen, recognition memory, and dendritic spine density in the hippocampus and medial prefrontal cortex, areas critical for memory, across the lifespan in female rodents. The present paper reviews the literature on dendritic spine plasticity in mediating both short and long term memory, as well as the decreased memory that occurs with aging and Alzheimer's Disease. It also addresses the role of acute and chronic estrogen treatment in these processes.
Keywords: Estrogen, estradiol, dendritic spines, memory, hippocampus, prefrontal cortex, synaptic plasticity
1. Introduction
The complex mechanisms underlying the formation and retention of memories are assumed to involve synaptic plasticity in the hippocampus and cerebral cortex. Although dendritic spines are sites of synaptic contact and turnover of dendritic spines is an important aspect of synaptic plasticity, the precise role that dendritic spines play in immediate memory formation and long-term storage remains unclear. Given that the focus of our studies has been the relationship between estradiol, memory, and dendritic spines in rodents, the present chapter will review the literature on dendritic spines and memory function and highlight the association(s) between estradiol, spines, and memory.
2. Dendritic spines
First described by Ramon Y Cajal, using Golgi impregnation in purkinje cells of the bird cerebellum (reviewed by Garcia Lopez, 2007 and Yuste, 2010), most dendrites are covered with dendritic spines, which were initially assumed to increase the surface area for neurotransmission (Nimchinsky et al., 2002). Spine density varies from extremely spiny pyramidal neurons in the cortex and hippocampus (Nimchinsky et al., 2002; von Bohlen und Halbach, 2009) to the relatively sparse spine density of neurons in the hypothalamus (Frankfurt et al., 1990). In addition to identifying spines on many different neurons, Ramon Y Cajal also described subtypes of spines and noted that the density of dendritic spines increased with increased innervation. Although spine subtype classification varies, dendritic spines generally consist of a protrusion and may have a bulbous termination (in the case of mushroom spines). One may also distinguish between thin spines with a smaller head and stubby spines, which lack any terminal enlargement and have extremely thin filapodia. This latter type are presumed to be the precursors to mature spines (Bourne and Harris, 2008; von Bohlen Und Halbach, 2009; Urbanska, et al., 2012).
The cytoskeleton of dendritic spines consists mostly of filamentous actin, which extends from the base of the spine to the postsynaptic density (Bosch and Hayashi, 2012; Penzies and Rafalovich, 2012; Koleske, 2013). The vast majority of dendritic spines have an excitatory synapse at their termination suggesting that their role is to increase the surface area for synaptic contact. However, the observation that adjacent dendritic shafts are devoid of excitatory synapses combined with studies that have demonstrated that Ca++ is concentrated in spines has led to the hypothesis that dendritic spines play a critical role in the formation and plasticity of specific functional neuronal circuits rather than increasing the surface area for synaptic contact (Yuste, 2010; Yuste, 2011). Filopodia, which lack a bulbous termination, also seem to lack synapses, promoting the idea that filopodia may be an immature/transient spine species. The synapse localized to most dendritic spines is an excitatory, glutamatergic one and modulation of that excitatory input by other neurotransmitters, especially inhibitory GABA, is assumed to be indirect. However, recent studies have shown that GABA neurons synapse directly on a subset of dendritic spines adjacent to a glutamatergic synapse (Higley, 2014) demonstrating a more direct relationship between inhibitory and excitatory inputs.
2a. Dendritic spine plasticity
It has become increasingly clear that the nervous system is capable of plasticity and that the dendritic spine is poised to be the major site of this activity (Bourne and Harris, 2008; Bosch and Hayashi, 2012; Penzies and Rafalovich, 2012; Urbanska et al., 2012). There exists a developmental increase in the number of dendritic spines followed by pruning, which appears to depend on many factors (Urbanska et al., 2012). Perhaps more importantly, it is clear that after the connectivity is initially established in the brain, dendritic spine turnover continues (reviewed by Koleske, 2013). As part of the process of establishing neural networks, dendrites and dendritic spines develop, mature, and are retracted as needed. In the adult brain, however, plasticity appears to involve primarily the dendritic spine rather than the dendritic tree. In addition, alterations in dendritic spines are now known to accompany brain trauma, underlie addiction and are implicated in neurological and psychiatric disease (see below) as well as learning and memory.
Until more sophisticated techniques were developed, fluctuations of dendritic spine number and type had been demonstrated in response to many different types of stimuli at different developmental stages and over different periods of time using Golgi impregnation to label spines (See Figures 1 and 2). Early experiments demonstrated the importance of afferent input to the maintenance of spine density. In 1967, Valverde demonstrated that mice raised in total darkness had significantly fewer dendritic spines on apical dendrites of pyramidal cells in layer V of the striate cortex compared to control animals. In the hippocampus, deafferentation of granule cells, following lesions of the entorhinal cortex, resulted in a decrease in the number dendritic spines present on the granule cells, an effect that was reversed with reinnervation (Parnavelas et al., 1974). As described below, many different types of stimuli have been demonstrated to influence dendritic spine plasticity since these early studies.
Figure 1.
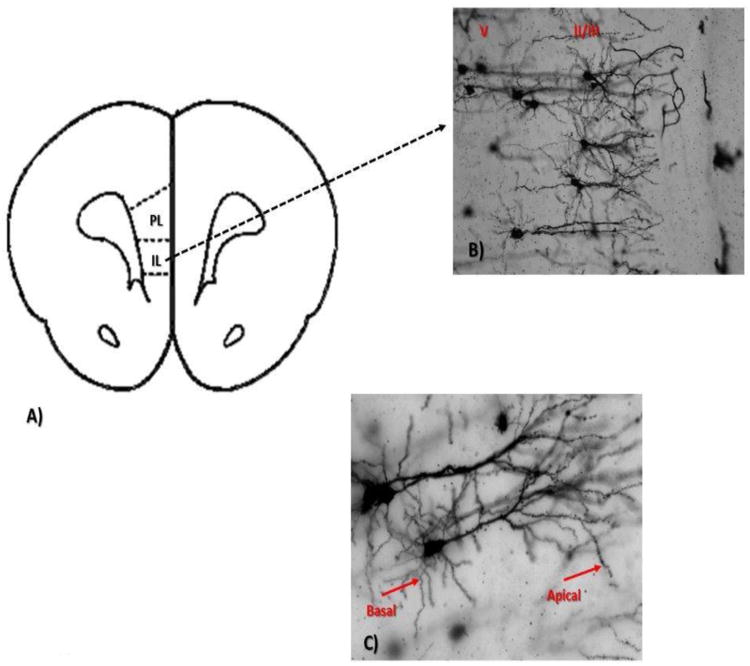
A. Schematic coronal section illustrating the region of the medial prefrontal cortex of the rat examined in Golgi preparations. B. Golgi impregnated pyramidal neurons from layer ll/lll and V of the medial prefrontal cortex (10×). C. Representative pyramidal cells from medial prefrontal cortex. Arrows denote the secondary basal and tertiary apical dendrites used for analysis (20×).
Figure 2.
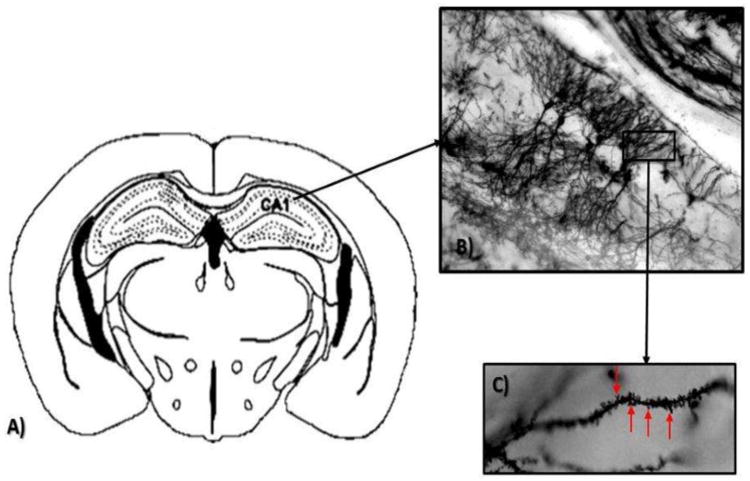
A: Schematic coronal section illustrating the CA1 region of the rat hippocampus examined in Golgi preparations. B. Golgi impregnated pyramidal neurons from CA1 (10×). C. Representative dendrite used to count spines (denoted by arrows, 100× under oil).
2a1. Estrogens and dendritic spine plasticity
Estrogen receptors in the brain were initially found to be highly concentrated in the hypothalamus and other brain regions known to be involved in reproductive function (Pfaff, 1968). Following this observation, it became increasingly apparent that estrogen receptors were widely distributed in the brain and had important functions related to cognition. Moreover, dendritic spines in brain regions related to cognition, such as the hippocampus and cortex, were highly sensitive to estrogen fluctuations. Chronic changes in circulating estradiol have been shown to alter dendritic spine density on pyramidal cells in the hippocampus (Gould et al., 1990; Woolley et al., 1990; Luine and Frankfurt, 2013) and cortex (Tang et al., 2004; Chen et al., 2009; Khan et al., 2013; Inagaki et al, 2012; Luine and Frankfurt, 2013), as well as neurons in the hypothalamus (Frankfurt et al., 1990) and amygdala (de Castilhos et al., 2008; Rasio-Filho et al., 2012) of the rat brain. Fluctuations of approximately 30% occur in dendritic spine density on neurons in the ventromedial hypothalamic nucleus (VMN, Frankfurt et al, 1990) and pyramidal cells in the CA1 region of the hippocampus (CA1) during the 4-5 day estrous cycle of the rat (Woolley et al., 1990). In both these regions, spine density is greatest when estrogen levels are highest. In ovariectomized (OVX) female rats, dendritic spine density in the VMN (Frankfurt, et al., 1990), CA1 (Gould et al, 1990; Woolley et al 1990; Wallace et al., 2006), and the medial prefrontal cortex (mPFC, Wallace et al., 2006) is decreased as compared to gonadally intact rats. Administration of exogenous estradiol following OVX in rats restores the levels of dendritic spines in the VMN (Frankfurt et al., 1990; Calizo and Flanagan-Cato, 2003), CA1 (Gould, et al., 1990; Luine and Frankfurt 2013) and the amygdala (de Castilhos et al., 2008) as well as in spine synapses in the hippocampi of OVX monkeys (Leranth et al., 2002). Spine synapses in CA1 were increased by 38% when estradiol was administered to OVX female rats as compared to oil and decreased 32% in the period between proestrus and estrous (Woolley and McEwen, 1992). Inhibition of the enzyme, aromatase, responsible for converting testosterone into estrogen, causes dendritic spine loss in the hippocampus of mice (Zhou, et al., 2010). Similarly, estradiol has been shown to increase both dendritic spine and spine synapse density in CA1 and the mPFC of non-human primates (Tang et al., 2004; Leranth et al., 2008; Hajszan and Leranth, 2010).
In addition to the estrous cycle and OVX data, a relationship between estrogen and dendritic spines has been shown with pregnancy, a period in the female life span when circulating levels of estrogen are very high. Kinsey et al. (2006) found that in late pregnancy and during lactation in rats, dendritic spine density in CA1 pyramidal cells is greater than at any time in the estrous cycle.
Phytoestrogens, weak estrogens found in plants, also alter spine density in OVX rats. OVX rats fed a diet low in phytoestrogens compared to a group fed ordinary rat chow which contains soy meal (Patisol, 2005) and is, therefore, rich in phytoestrogens, had significantly lower dendritic spine density on pyramidal cell dendrites in both the mPFC and CA1 as compared to rats fed the high phytoestrogen diet (Luine et al., 2006). In more recent studies, the effects of the synthetic, non-steroidal compound, bisphenol-A (BPA) (a weak estrogen that also has anti-estrogen properties) on dendritic spine density in pyramidal cells of the mPFC and CA1 have been assessed. Chronic administration of 50 μg/kg/day of BPA in OVX monkeys for 28 days completely blocked estradiol induced synaptogenesis in both the hippocampus and the mPFC (Leranth, et al., 2008). Administration of a low dose of BPA in adolescent rats for seven days resulted in a 21% decrease in dendritic spine density in the mPFC of males and females when they were examined at seven weeks (adolescence) or 11 weeks (adulthood) of age. (Bowman et al., 2014). In CA1, the BPA induced decrease in dendritic spine density was 24 % in both sexes at both adolescence and adulthood as compared to control. These decreases in spine density were also present at 13 weeks of age in CA1 but not in the mPFC (Bowman et al., 2015). These studies suggest that adolescent exposure to compounds that interact with estrogen receptors lead to enduring changes in dendritic spine density in both the hippocampus and mPFC.
Acute effects of BPA on neurons have also been demonstrated. A single injection of 40 μg/kg of BPA reduces dendritic spine density within 40 min in the mPFC and CA1 of adult male rats (Eilam-Stock et al., 2012). In female rats, the interaction between BPA and estradiol is complex. Following administration, spine density was assessed at times of memory consolidation (30 min) and retention (4 h) after 17β estradiol, BPA, or both (Inagaki et al., 2012). In CA1, BPA blocked estradiol induced increases in basal spines of pyramidal cells at 4 h and was additive with estradiol at 30 min. In the mPFC, 40 μg/kg of BPA had no effect at either 30 min or 4h (Inagaki et al., 2012). Although BPA -estrogen interactions are complex, these studies clearly support the idea that estrogen influences dendritic spine density in a number of brain regions and in a number of physiological contexts.
Reviewing the data on changes in spine density during the estrous cycle, estrogen replacement after OVX, during pregnancy and following phytoestrogen and BPA treatments leads to the conclusion that, in general, greater spine density in the mPFC and CA1 is related to higher levels of estrogen. There is increasing evidence that estrogen influences spine assembly (see below) which provides a mechanistic explanation of how estrogen increases spines and may also provide a bridge for understanding the relationship(s) between estrogen, spines and memory.
3. Memory and dendritic spines
The process of memory formation, which involves obtaining, storing, and eventually retrieving information, relies on a complex series of still poorly understood events. Memory is essentially an adaptive process by which neuronal connections process many different types of stimuli. Memory initially appears to affect intrinsic plasticity, which is an alteration in the excitability of one cell and therefore non-synaptic in nature (Kasai, 2010; Sehgal et al., 2013) as well as synaptic plasticity, which involves more than one cell and is the focus of the present review. Synaptic plasticity encompasses both structural changes, including dendritic spine plasticity, and the resulting physiological plasticity: long-term potentiation (LTP) and long term depression (LTD), considered the foundations of learning and memory. Although these phenomena have been most closely studied in the hippocampus, both the hippocampus and the mPFC are involved in memory function (Ennaceur and Aggleton, 1994; Dumitriu et al., 2010; Funahashi, 2013; Luine, 2015).
A strong relationship between dendritic spine density in the hippocampus and memory has been demonstrated using different behavioral assessments. The acquisition of new memories in a conditioning paradigm is associated with increased spine density in CA1 pyramidal cells in adult male rats (Leuner et al., 2003; Jedlicka et al., 2008) and female rats (Beltran-Campos et al., 2011). Performance on two different spatial memory tasks, the Morris water maze and object placement, is associated with a higher dendritic spine density on pyramidal cells in CA1 (Moser et al., 1994; Conrad et al., 2012; Eilam-Stock et al., 2012) suggesting that there is a morphological substrate for memory In addition, existing spines in the hippocampus undergo structural alterations that result in LTP (Jedlicka et al., 2008; Leuner et al., 2003). In the hippocampus, it has been demonstrated that increased dendritic spine size/number is associated with LTP (Matsuzaki et al. 2004) and decreased dendritic spine/number is associated with LTD (Muller et al., 2000; Bosch and Hayashi, 2012). In addition, LTP induces the formation of new dendritic spines in the rat dentate gyrus (Yuste, 2010). Therefore, there is a strong interaction between LTP, dendritic spine density and memory in the hippocampus. It must be noted, however, that memory may increase both spines and LTP making it impossible at present to determine whether increased dendritic spine density is the cause or result of improved hippocampal dependent memory in the aforementioned animal studies.
Data accumulated from Alzheimer's disease models and post mortem brains support the role of impaired synaptic plasticity in memory loss. Increased levels of amyloid, an integral part of the plaques seen in post mortem brains, are known to interfere with neurotransmission in the hippocampus in Alzheimer's disease. In addition, the decline in cognitive function in Alzheimer's disease is associated with a decrease in the number of dendritic spines in the hippocampus and cortex (Koleske, 2013; Lazcano et al., 2014). There appears to be a relationship between increased amyloid deposition and decreased spine density (for review see Koffie et. al., 2011), and proteins that are concentrated in dendritic spines are decreased in the brains of Alzheimer's patients. Moreover, increased amyloid levels in mouse models of Alzheimer's disease results in decreased LTP and increased LTD (Palop and Mucke, 2010). In the mouse, infusion of beta amyloid into the hippocampus results in memory impairment, and when cultured neurons are treated with soluble beta amyloid, there are decreases in dendritic filopodia (Chen et al., 2014).
Choi et al. (2014) examined neuritin, a trophic factor important to dendritic growth and maturation of dendritic circuits, in Alzheimer's disease patients and Tg2576 mice, a transgenic Alzheimer's disease model. They found that neuritin was decreased in the hippocampus and cortex of patients and that the low dendritic spine density in the hippocampal cells of Tg2576 mice, as well as poor performance on the Morris water maze, was reversed by neuritin treatment. Taken together, these studies demonstrate that one mechanism underlying memory loss in Alzheimer's disease involves a loss of dendritic spines in hippocampal pyramidal cells and supports the concept that impaired memory is the result rather than the cause of spine loss.
Examination of the neural pathways involved in drug addiction, craving, and relapse, also supports the involvement of dendritic spines in memory and learning because these pathways are similar and both require synaptic plasticity (Kelley, 2004, Russo et al., 2010). Drug addiction may be considered an aberrant form of learning and memory. Dendritic spines in many brain regions respond to drug administration during development and in adulthood. Daily administration of 30 mg/kg cocaine to pregnant rats from day two of gestation until birth increases dendritic spine density by 13-23% on pyramidal cell dendrites in CA1 and the mPFC and by 33% on the medium spiny neurons of the striatum of 21-day-old offspring (Frankfurt et al., 2009). In adult rats, both chronic amphetamine and cocaine administration increase dendritic spine density in the mPFC and nucleus accumbens (Robinson and Kolb, 2004). Administration of 30 mg/kg cocaine for 12 days to pregnant or virgin female rats increased dendritic spine density on pyramidal cell dendrites of the mPFC and CA1 as well as on dendrites of cells in the medial amygdala, compared to control, 24 hours after the last injection (Frankfurt et al., 2011). In contrast to stimulants, which have been shown to increase dendritic spine density, morphine administration decreases dendritic spine density in the cortex and nucleus accumbens of adult rats (Robinson and Kolb, 1999) and cultured hippocampal neurons (Liao, et al., 2005). The alterations in neuronal structure that accompany initial exposure to the drugs induce changes in neural networks that increase susceptibility during subsequent exposure (Russo et al, 2010), a form of learning.
Thus, evidence from both basic studies in rodents and clinical studies in Alzheimer's patients and animal models of the disease, suggest dendritic spine plasticity as an important mechanism underlying learning and memory. Importantly, these studies also demonstrate that plasticity is not limited to hippocampal areas. Lastly, both the drug studies and the Alzheimer's data clearly support the idea that spine plasticity induces learning and memory changes and not vice versa.
3a. Memory tasks in rodents
In rodents, spatial memory is assessed by a variety of tasks including the radial arm maze, Morris water maze, and recognition memory tasks. We have chosen to use recognition memory tasks, which have been recently reviewed in Luine (2015), to assess memory, but a brief overview is appropriate here. Recognition memory tasks are advantageous for studying memory because rats are naturally curious and will explore new objects when presented and because learning is minimal. Importantly, recognition tasks require neither positive nor negative rewards, which could inadvertently alter performance through stress or other influences. In the object placement (OP) task, animals examine two identical objects for a training period (T1) of three minutes followed by a delay of varying times (1 to 4 h) and then examine the same objects with one object moved to a different location for another three minutes (T2). Memory is demonstrated when an animal spends greater time with the object in the new location than the old (Ennaceur et al., 1997). For object recognition (OR), the T1 portion and the inter-trial delays of the task are the same as that for object placement. However, during T2, one of the objects is replaced with a novel object. Memory is demonstrated by more time spent exploring at the new object than the old object. Once the animal is trained, new information requires consolidation, which occurs within 1-2 h immediately after training. It has been shown that these tasks require input from the PFC as well as the hippocampus (Ennaceur and Aggleton, 1994; Ennaceur et al., 1997; Broadbent et al., 2009).
3a1 Memory and estrogen: chronic/subchronic treatments
That estrogen has an impact on memory has been demonstrated repeatedly in rodents. Initial studies used chronic or subchronic estrogen treatments (reviewed by Luine and Frankfurt 2012), which will be reviewed first, while more recent studies focus on acute estrogen treatments. Performance on the radial arm maze, water maze (Daniel et al., 1999), and T- maze (Gibbs, 1999) is improved when OVX rats are given estradiol. OR memory is greater when estrogen levels are high in intact, cycling rats and when OVX rats are given estrogen, progesterone, or both (Walf, et al., 2006). Administration of 50 μg/kg estradiol benzoate (EB) to OVX rats for two days results in improved OP and OR memory when assessed 48 h after the last EB injection (Jacome, et al., 2010). Similar results were obtained for OVX mice given 1 μg of EB daily for 5 days and tested for OP 24 h after the last EB injection (Li et al., 2004). Direct infusion of EB into the dorsal hippocampus results in enhanced performance 24 h later in the water maze (Packard, 1998) and in OP and OR (Boulware et al., 2013). During pregnancy, high levels of hormones, including estrogens, also enhance OP and OR memory (Macbeth and Luine, 2010).
Altered dendritic spine density in both CA1 and the mPFC accompany the changes in memory resulting from exposure to increased or decreased circulating estrogens. When female rats are OVX, memory performance declines and dendritic spine density is decreased in CA1 and the mPFC by 17-53%, seven weeks later (Wallace et al., 2006). An estradiol induced increase in OP is accompanied by an increase in dendritic spine density in CA1 in OVX rats (Luine and Frankfurt, 2013; see Table 1) and mice (Li et al., 2004). Estradiol enhanced performance on the Y maze is also associated with increased dendritic spine density in the mPFC (Velázquez-Zamora et al., 2012). OVX rats fed a low phytoestrogen diet have impaired memory and lower spine density in pyramidal cells of CA1 and the mPFC than OVX rats fed normal rat chow, which is high in phytoestrogens (Luine et al., 2006).
Table 1. Temporal Differences in Estradiol effects on dendritic spines in brain regions.
| Time sacrificed after Estradiol Treatment | ||||||
|---|---|---|---|---|---|---|
| Brain Region | S.C. 30 min | S.C. 4 h | S.C. 2 days | Hippocampal 30min | Hippocampal 2h | |
| Medial prefrontal cortex pyramidal cells layer II/III. | Apical | 16% | No change | No change | No change | 18% |
| Basal | 27% | 12% | No change | No change | No change | |
| Hippocampus pyramidal cells CA1 | Apical | No change | No change | No change | 15% | 30% |
| Basal | 29% | 33% | 12% | 30% | 27% | |
| Dentate Gyrus Granule | No change | No change | Not analyzed | No change | No change | |
| Hypothalamus Ventromedial nucleus | No change | 20%* | 15% | No change | No change | |
Rats received either a subcutaneous injection (S.C.) of estrogen or a bilateral intrahippocampal infusion of E2 (2-hydroxypropyl-β-cyclodextrin) and were sacrificed at the indicated times after injections. For 30 min and 4h points, rats received 20 ug/kg of E2. Data adapted from Inagaki et al., 2012. For the 2 day treatment, rats received 50ug/kg of 17β-estradiol benzoate. Data adapted from Luine and Frankfurt, 2013 and Luine, 2014. For intrahippocampal infusion, 5 ug/side were given. Data adapted from Tuscher et al, 2014.
Frankfurt, unpublished.
Aged rats (Markowska, 1999; Luine et al., 2014), mice (Frick et al., 2000, Von Bohlen und Halbach et al., 2006), and monkeys (Dumitriu et al., 2010) exhibit a decline in estrogen levels, recognition memory, and spine density as compared to young animals. Aged female Fischer 344 rats have memory impairments that are accompanied by decreased circulating estrogen and a marked decrease in dendritic spine density in pyramidal cells in both CA1 (Luine et al., 2011) and the mPFC (Wallace et al., 2007). Administration of estradiol to retired OVX Long Evans breeders for two days or 28 days improved their memory in the acquisition stage of the Morris water maze (Markham et al., 2002).
Clinical data indicates that the risk for women developing Alzheimer's disease rises after menopause when estradiol drops (Lan et al., 2015). Although there is disagreement as to the beneficial effects of hormone replacement therapy in Alzheimer's patients, some animal studies suggest that estradiol has a neuroprotective effect (Carroll and Pike, 2008; Srivastava and Penzes, 2011). Administration of estradiol or an estrogen receptor (ER) α agonist, propylpyrazole triol (PPT), to 3xTg-AD mice, a transgenic model for Alzheimer's disease reduced the amount of OVX induced amyloid accumulation in CA1 and several other brain regions and improved memory (Carroll and Pike, 2008). In this model, diarylpropionitrile (DPN), an ER β agonist had no effect on OVX induced accumulation of beta amyloid in the hippocampus but did reduce the amount of amyloid in the amygdala. Given that increased amyloid correlates with decreased spine density (Koffie et. al., 2011), these results suggest that the neuroprotective effect of estradiol occurs via dendritic spines.
Experiments with BPA, which were introduced above (“Estrogens and neural plasticity”) support the role of chronic estrogen in memory and also suggest a possible role for dendritic spines in the process. Exposure of adolescent rats to BPA for seven days leads to impaired OR and OP in adolescent male and female rats (Diaz-Weinstein et al., 2013) and to impaired OR in adult male rats (Bowman et al., 2015). In these studies, decreases in dendritic spine density of 21 to 24% in CA1 and the mPFC were seen in both adolescence and adulthood (Bowman et al, 2014; Bowman et al., 2015).
Thus, across the lifespan of female rodents, positive relationships are seen between estrogens and memory and between estrogens and dendritic spines in the mPFC and CA1. Examination of the estradiol effects in our animal studies in conjunction with the data obtained from Alzheimer's disease and drug induced plasticity supports the concept that increases in estradiol lead to increases in spines, which then enhance memory consolidation. While definitive evidence for this relationship is currently lacking, results of molecular studies are providing new insights (see section 4).
3a2 Memory and estrogen: acute treatments
Alterations in dendritic spine density, in addition to other actions of estrogen following chronic exposure, are due to estrogen-induced increases in protein synthesis through genomic mechanisms. The observation that estrogen could enhance memory rapidly, within several hours, (Luine et al., 2003) indicated that membrane estrogen receptors, could mediate rapid effects on spine plasticity. Thus, experiments with shorter estrogen treatment times were undertaken. When OVX rats were injected with 15 μg/kg of 17α- or 17β-estradiol 30 min before exploration of objects in T1 and the T2 recognition/retention trial was given 4 h later, both 17α-and 17β-estradiol enhanced memory (Inagaki et al., 2010). Moreover, the effective dose of 15 μg/kg was far lower than that used in chronic study where estradiol enhanced memory (50 μg/kg/day, Jacome et al., 2010). The demonstration that acute estradiol could even be given immediately following the T1 trial, indicated that estradiol is promoting the consolidation of memory (Luine 2015). Further support for the rapid effects of estradiol comes from a study in which a dose dependent increase in the postsynaptic spine density (PSSD), which is a marker for synapses, was observed within 30 min in CA1, when OVX rats were given injections of either 17α- or 17β- estradiol (MacLusky et al., 2005). Agonists for both ER α and β have been shown to differentially affect spine density on pyramidal cell dendrites in CA1 in the rodent. In hippocampal slices from male rats, application of 1 nM estradiol increased dendritic spine density in CA1 neurons in two hours as did PPT, an ER α agonist (Murikami et al., 2006). However, in this study the ER β agonist, DPN, had no effect. In the mouse, PPT increased dendritic spine density in the stratum radiatum and lacunosum-moleculare within 40 minutes, whereas DPN, decreased spine density (Phan et al., 2011). In the latter study, PPT enhanced both OP and OR while DPN had no effect on OP but improved OR. However, in cortical neuron cultures, WAY-200070, an ERβ agonist, increased spinogenesis, spine size, and PSD-95 within 30 min (Srivastava et al., 2010). The results of these studies show that estrogens rapidly promote both memory consolidation and spine density and that both ER subtypes are involved in mediating rapid estradiol effects on spinogenesis, but these effects vary depending on location.
In order to determine whether estrogen dependent increases in dendritic spines in the mPFC and CA1 contribute to the rapid enhancements in recognition memory by estrogens, OVX female rats were given a recognition memory sample trial and an immediate injection of 20μg/kg 17β-estradiol. Subjects were sacrificed 30 min after the T1 trial, during which time consolidation of memory for the OP task is occurring (Inagaki et al, 2010; Luine 2015). As summarized in Table 1, in pyramidal cells in CA1 and the mPFC, spine density was increased by 16 to 29% by estradiol, depending on the dendritic branch, compared to control, suggesting that enhanced OP in this paradigm is related to an increase in dendritic spine density. No rapid estradiol effects were seen in the dentate gyrus. In addition there was no change in dendritic spine density in the VMN at 30 minutes post injection, but spine density was increased by 20% four hours after the injection (Frankfurt, unpublished, see Table 1). The rapid increase, 30 min following estradiol administration, in spines in the mPFC and CA1, areas that are involved in memory, but not in other brain areas, provides further evidence that increases in spine density may contribute to estrogen's ability to enhance memory.
Recent studies by Frick and co-workers (reviewed in Frick, 2012) have shown that direct infusion of estradiol into the dorsal hippocampus is sufficient to rapidly enhance object and place memory consolidation in mice and that this effect is associated with rapid activation, within 5 min, of NMDA receptors as well as multiple upstream and downstream cell signaling pathways including those contributing to spine formation such as the extracellular signal-regulated kinase/mitogen activated protein kinase (ERK/MAPK) pathway, cyclic AMP response element binding protein (CREB), and activation of the mammalian target of rapamycin (mTOR) protein synthesis pathway (Fortress and Frick, 2013; Fortress and Frick, 2014; Luine and Frankfurt, 2013). Moreover, memory consolidation can be blocked by inhibition of these pathways (Fortress and Frick, 2013; Fortress and Frick 2014). Thus, it was of interest to investigate whether bilateral infusion of 5 ug of 17β-estradiol-cyclodextrin, which does not pass through cell membranes, directly into the hippocampus, would also increase spine density.
Consistent with peripheral estradiol injections, a large increase in the spine density on apical (15%) and basal (30%) dendrites in pyramidal cells in CA1was present 30 min after estradiol injection (Tuscher et al., 2014; Table 1). Thus, estradiol, acting only in the hippocampus, activates both memory and spines suggesting that increases in spines are part of the process for enhancing memory consolidation. At 30 min post estradiol, there was no increase in dendritic spine density in the mPFC; however, when examined two h after the bilateral 17β estradiol infusion, an 18% increase in spine density on basal mPFC dendrites was found as well as a further increase, 27% and 30%, in spine density on basal and apical CA1 dendrites (Tuscher et al., 2014; Table 1). The temporal pattern of the estrogen dependent changes in spine density, first in CA1 and then in the mPFC, supports the idea that following an initial increase in dendritic spine density in CA1, neurons in CA1 subsequently relay information to the mPFC which then shows an increase in spine density. Although it could be argued that the delayed increase in the mPFC dendritic spines was due to diffusion of estradiol from the hippocampus to the mPFC, this possibility is not supported because spine density in the VMN, which responds to estradiol, was not increased at either point (Table 1). It should be noted that in previous experiments, peripheral administration of estradiol increased dendritic spine density on both branches of pyramidal neurons in the mPFC within 30 minutes (Inagaki et al, 2012). Comparing the results of intrahippocampal estradiol with peripheral estradiol administration suggests that there may be multiple (temporal) mechanisms that estrogen influences with respect to recognition memory (Table 1). Indeed, it has been demonstrated that the hippocampus interacts with the mPFC for spatial encoding, retrieval, and delayed spatial working memory (Churchwell and Kesner, 2011).
Taken together, these changes in spines suggest that estradiol effects on memory are multifaceted and that an initial process in the hippocampus may be subsequently projected to the mPFC but that the mPFC alone can also be rapidly altered by estradiol. Future studies will be required to address the differential, perhaps sequential roles of the mPFC relative to the hippocampus and to correlate where specific component(s) of memory processing occur in the brain.
From the aforementioned results, it appears that both chronic and acute exposure to estrogen induces alterations in dendritic spines in two key brain areas responsible for memory. Table 1 summarizes results from several studies conducted in our laboratories and shows a temporal comparison of increases in dendritic spine density in several brain regions after acute and chronic peripheral treatments and intrahippocampal infusions of estradiol. Estradiol administration, whether peripheral (acute or chronic) or intrahippocampal, increases dendritic spine density on the basal dendrites of pyramidal cells in CA1. The very rapid responses after both peripheral and intrahippocampally administered estradiol point to the importance of increased dendritic spine density to memory consolidation while the chronic increase in dendritic spine density following estradiol suggests that an increased dendritic spine number may prime the cells for better memory. Srivastava and Penzies (2011) have shown that 17 σ estradiol induces a transient increase in functional dendritic spines in cortical neurons in vitro within 30 minutes. Therefore, the population of spines that is increased by acute vs chronic estradiol administration may differ.
The effects of estradiol in the mPFC, as noted above, may be direct following peripheral administration at 30 min and also indirect, following intrahippocampal administration. The role of the mPFC in mediating memory and also enhancing memory following estradiol treatment as well as the relative importance of chronic versus rapid estrogen effects on memory requires further elucidation.
4. What happens at the level of the spine?
Dendritic spine plasticity refers to the generation of “new” spines and the process of maturation from thin filipodial projections to more complex structures that can make synapses (Ziv and Smith 1996) as well as to changes that existing spines may undergo (Kasai et. Al., 2010, Koleske, 2013, Sehgal et al., 2013). This process requires the mobilization of many proteins, particularly actin (Penzies and Rafalovich, 2012), which is highly concentrated in dendritic spines (Matus et al., 1982), and actin associated proteins, which project into the postsynaptic density (Fortin et al, 2012). Cellular actin is present two forms: globular, G-actin, or filamentous, F-actin. The cycling between the two forms is an essential part of spine plasticity and requires interaction with the actin-associated proteins, including cofilin that inhibits actin depolymerization (Lamprecht, 2014). The relationship between postsynaptic actin dynamics and LTP has also been established. Repetitive firing of synapses, such as that occurring during high-frequency synaptic stimulation to induce LTP, promotes actin polymerization within the spine, causing the spine to enlarge. Conversely, treatment that weakens synaptic efficacy, such as low-frequency stimulation that results in LTD causes actin loss and dendritic spine shrinkage (Matzusaki et al, 2004; Koleske, 2013).
If synaptic rearrangement is the basis for learning and memory, what is the relationship between spine dynamics and memory processes? Nelson et al. (2012) have shown a dose dependent inhibition of OP in female rats when latrunculin A, a drug that inhibits actin assembly, is injected into the dorsal hippocampus. Synaptopodin is an actin-associated protein found in dendritic spines, and in synaptopodin knock out mice, hippocampal cells lack dendritic spines, LTP is decreased and animal learning is impaired in young but not old animals (Deller et al, 2003). As recently reviewed by Lamprecht (2014), inhibition of actin assembly impairs all types of memory in different brain areas and this effect is achieved via alterations in actin-associated proteins.
In relation to what is known about changes in spines and memory function, estradiol increases the magnitude of LTP at hippocampal synapses in hippocampal slices (Foy et al., 1999; Kramár et al., 2009). For example, in hippocampal slices, inhibition of estrogen synthesis by letrazole, an aromatase inhibitor, decreased LTP and the number of dendritic spines (Vierk et al., 2012). In addition, consistent with estrogen's activation of LTP and latrunculin A's effects on actin polymerization, estradiol induced LTP is inhibited by latrunculin (Babayan and Kramar 2013). Estrogens also increase many synaptic proteins, including PSD-95 and spinophilin (Lee et al., 2004). Inhibition of the enzyme aromatase, that converts testosterone into estrogen, induces a loss of synaptophysin in the hippocampus of mice (Zhou et al., 2010). Lastly, estradiol has been shown to inactivate cofilin, which is responsible for disassembly of actin, which may explain how estradiol promotes F-actin and spine assembly (Kramar et al., 2009). These studies provide a mechanism for understanding how estradiol can influence the proteins involved in spine dynamics and increase dendritic spine density.
Recent studies, using hippocampal cultures in the absence of ovarian derived steroids, have demonstrated that estradiol is synthesized within the hippocampus (Vierk et al., 2014) and that intrahippocampally-derived estradiol is needed for maintaining spine stability in female rodents. Determination of estradiol levels during the rat estrous cycle reveals that estradiol levels within the hippocampus are far higher than can be accounted for by ovarian release and that levels are maintained after OVX (Kato et. al., 2013). In addition, in this study, dendritic spine density in CA1 fluctuated over the estrous cycle with the highest dendritic spine density occurring when estradiol and progesterone levels were greatest. When letrozole, which inhibits the final step in synthesis of estradiol, is given to hippocampal cultures, synaptogensis is inhibited and spine synapses in CA1 decrease (Prange-Keil et al., 2006; Vierk et al., 2012). These findings suggest that, regardless of the many studies that have manipulated estrogen levels by OVX and observed alteration in dendritic spine density and memory, local estradiol biosynthesis may an important source of estradiol. In our previous studies with OVX rats, it took up to five weeks to see a decrease in OP (Wallace et al., 2006), which may have been due to intraneuronal synthesis of estrogen. With respect to this observation, it is important to note that Kato et al. (2013) have demonstrated that two weeks after OVX the amount of estradiol in the rat hippocampus was similar to that seen at diestrus, but when 40μg/kg of estradiol was administered subcutaneously to OVX rats five hours before the hippocampi were examined, intrahippocampal levels of estradiol were the same as those seen in proestrous. Thus, there appears to be an interaction between peripheral and intrahippocampal estradiol, but future studies are required to provide a more detailed temporal relationship between OVX and intrahippocampal estrogen and to explain how this relationship may relate to memory function.
5. Conclusion
Dendritic spine density alterations in both the hippocampus and the prefrontal cortex appear to underlie memory function. The fact that dendritic spines are altered within minutes may correspond to consolidation of initial memories whereas increased dendritic spine density after longer periods of time may be part of a type of continued plasticity necessary for storage of long-term memories. Some of the short- vs. long-term differences may be related to specific brain regions involved. It is well known that the formation of memories occurs in the hippocampus and that long-term storage requires the neocortex. The data available on the relationship between dendritic spine density and memory fits well with the results on estrogen involvement in memory, in that rapid changes are membrane receptor mediated and long term changes require nuclear–transcription mechanisms.
A pattern emerges where the formation of a memory induces an initial electrical alteration in a cell, probably in the hippocampus, which then initiates a series of structural changes that lead to strengthening of synaptic function and LTP. Over time this initial change is translated into further, more persistent structural changes. Estrogen seems to influence many aspects of these processes, from the electrophysiological to the short and long term morphological alterations. Further, detailed study of the interconnected circuits is needed to better understand the morphological underpinnings of memory and how estradiol modulates it.
Highlights.
Dendritic spine plasticity underlies memory formation
Aging and Alzhiemer's Disease decrease memory and dendritic spine density
Chronic estrogen increases dendritic spine density improves memory
Acute estrogen induces rapid changes enhancements in memory consolidation and increases dendritic spine density
Acknowledgments
Recent experimental work discussed in this review was supported by The City University of New York, PSC-CUNY, NIH grants GM60654 (VNL), GM60665 (VNL) and RR03037 (HC). We would like to thank Dr. A. Bornstein and Lauren Tesoriero, Hofstra North Shore-LIJ School of Medicine for help with the figures and editing, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babayan AH, Kramár EA. Rapid effects of oestrogen on synaptic plasticity: interactions with actin and its signaling proteins. J Neuroendocrinol. 2013;11:1163–72. doi: 10.1111/jne.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Campos V, Pado-Alcala RA, Leon-Jacinto U, Aguilar-Vazquez A, Quirarte GL, Ramirez-Amaya V, Diaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Res. 2011;1369:119–130. doi: 10.1016/j.brainres.2010.10.105. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2009;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–8. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Ann Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Luine V, Khandaker H, Villafane JJ, Frankfurt M. Adolescent bisphenol-A exposure decreases dendritic spine density: role of sex and age. Synapse. 2014;68:498–507. doi: 10.1002/syn.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Luine V, Weinstein SD, Khandaker H, DeWolf S, Frankfurt M. Bisphenol-A exposure during adolescence leads to enduring alterations in cognition and dendritic spine density in adult male and female rats. Horm Behav. 2015;69:89–97. doi: 10.1016/j.yhbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Flanagon-Cato LM. Hormonal-neural integration in the female rat ventromedial hypothalamus: triple labeling for estrogen receptor-alpha, retrograde tract tracing from the periaqueductal gray, and mating-induced Fos expression. Endocrinology. 2003;144:5430–40. doi: 10.1210/en.2003-0331. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607–2611. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Yan YT, Wang TJ, Chen LJ, Wang WJ, Tseng GF. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb Cortex. 2009;19:2719–27. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- Chen X, Hu J, Jiang L, Xu S, Zheng B, Wang C, Zhang J, Wei X, Chang L, Wang Q. Brilliant Blue G improves cognition in an animal model of Alzheimer's disease and inhibits amyloid-β-induced loss of filopodia and dendrite spines in hippocampal neurons. Neurosci. 2014;279:94–101. doi: 10.1016/j.neuroscience.2014.08.036. [DOI] [PubMed] [Google Scholar]
- Choi Y, Lee K, Ryu J, Kim HG, Jeong AY, Woo R, Lee J, Hyun J, Hahn S, Kim K, Kim H. Neuritin attenuates cognitive function impairments in Tg2576 Mouse Model of Alzheimer's Disease. PLoS ONE. 2014;9(8):e104121. doi: 10.1371/journal.pone.0104121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: Interactions and independent parallel processing. Behavioural Brain Research. 2011;225:389–395. doi: 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: relationship with hippocampal CA1 spine density and dendritic complexity. Behav Neurosci. 2012;126:142–56. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiology and Behavior. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- de Castilhos J, Forti CD, Achaval M, Rasia-Filho AA. Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: a Golgi study. Brain Res. 2008;1240:73–81. doi: 10.1016/j.brainres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Diaz Weinstein S1, Villafane JJ, Juliano N, Bowman RE. Adolescent exposure to Bisphenol-A increases anxiety and sucrose preference but impairs spatial memory in rats independent of sex. Brain Res. 2013;1529:56–65. doi: 10.1016/j.brainres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. PNAS. 2003;100:10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara1 Y, Kaufmann J, Janssen WGM, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–15. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam-Stock T, Serrano P, Frankfurt M, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav Neurosci. 2012;126:175–185. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Srivastava T, Soderling TR. Structural modulation of dendritic spines during synaptic plasticity. Neuroscientist. 2012;50:496–501. doi: 10.1177/1073858411407206. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–55. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Heisler JD, Frick KM. The mTOR and canonical Wnt signaling pathways mediate the mnemonic effects of progesterone in the dorsal hippocampus. Hippocampus. 2014 Dec 8; doi: 10.1002/hipo.22398. 2014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–9. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendo. 1990;51:530–5. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Salas-Ramirez K, Friedman E, Luine V. Cocaine alters dendritic spine density in cortical and subcortical brain regions of the postpartum and virgin female rat. Synapse. 2011;65:955–961. doi: 10.1002/syn.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Wang HY, Marmolejo N, Bakshi K, Friedman E. Prenatal Cocaine increases dendritic spine density in cortical and subcortical brain regions of the rat. Dev Neurosci. 2009;31:71–75. doi: 10.1159/000207495. [DOI] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety, and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Funahashi S. Space representation in the prefrontal cortex. Progress in Neurobiology. 2013;103:131–155. doi: 10.1016/j.pneurobio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- García-López P, García-Marín V, Freire M. The discovery of dendritic spines by Cajal in 1888 and its relevance in the present neuroscience. Progress in Neurobiology. 2007;83:110–130. doi: 10.1016/j.pneurobio.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–91. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Front Neuroendocrinol. 2010;31:519–30. doi: 10.1016/j.yfrne.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ. Localized GABAergic inhibition of_dendritic_Ca(2+) signalling. Nat Rev Neurosci. 2014;15:567–72. doi: 10.1038/nrn3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;53:3357–67. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;4:488–98. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Behav Brain Res. 2008;192:12–9. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–9. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circ. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Khan MM, Dhandapani KM, Zhang Q, Brann DW. Estrogen regulation of spine density and excitatory synapses in rat prefrontal and somatosensory cerebral cortex. Steroids. 2013;78:614–623. doi: 10.1016/j.steroids.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;26:63–72. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;8:536–50. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Chen CY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–93. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R. The actin cytoskeleton in memory formation. Prog Neurobiol. 2014;117:1–19. doi: 10.1016/j.pneurobio.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Lan YL, Zhao J, Li S. Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer's disease. J Alzheimers Dis. 2015;43:1137–48. doi: 10.3233/JAD-141875. 2015. [DOI] [PubMed] [Google Scholar]
- Lazcano Z, Solis O, Bringas ME, Limón D, Diaz A, Espinosa B, García-Peláez I, Flores G, Guevara J. Unilateral injection of Aβ25-35 in the hippocampus reduces the number of dendritic spines in hyperglycemic rats. Synapse. 2014;68:585–594. doi: 10.1002/syn.21770. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience. 2004;127:983–8. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. PNAS. 2008;105:14187–91. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Li C, Brake W, Romeo R, Dunlop J, Gordon M, Buzescu R, Magarinos A, Allen P, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory tasks in female mice. PNAS. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. PNAS. 2005;102:1725–1730. doi: 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Falduta J, Shors TA. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol and cognition function: Past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Recognition memory tasks in neuroendocrine research. Behavioural Brain Research, SI: Object Recognition Memory in Rats and Mice edited by Abdel Ennaceur. 2015;285:158–164. doi: 10.1016/j.bbr.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–7. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and spine density changes. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neurosci. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Wallace ME, Frankfurt M. Age-related deficits in spatial memory and hippocampal spines in virgin, female Fischer 344 rats. Curr Gerontol Geriatr Res. 2011;316386 doi: 10.1155/2011/316386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–67. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–93. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–33. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A, Ackerman M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. PNAS. 1982;79:7590–4. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Anderson P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. PNAS. 1994;9:12673–5. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Toni N, Buchs PA. Spine changes associated with long-term potentiation. Hippocampus. 2000;10:596–604. doi: 10.1002/1098-1063(2000)10:5<596::AID-HIPO10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, Hojo Y, Kominami S, Yamazaki T, Kimoto T, Kawato S. Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun. 2006;351:553–8. doi: 10.1016/j.bbrc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- Nelson BS, Witty CF, Williamson EA, Daniel JM. A role for hippocampal actin rearrangement in object placement memory in female rats. Neurobiology of Learning and Memory. 2012;98:284–290. doi: 10.1016/j.nlm.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Ann Rev Physiol. 2002;64:313–53. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;7:812–8. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravalas JG, Lynch G, Brecha N, Cotman CW, Globus A. Spine loss and regrowth in hippocampus following deafferentiation. Nature. 1974;248:71–73. doi: 10.1038/248071a0. [DOI] [PubMed] [Google Scholar]
- Patisol HB. Phytoestrogen action in the adult and developing brain. J Neuroendocrinol. 2005;17:57–64. doi: 10.1111/j.1365-2826.2005.01268.x. [DOI] [PubMed] [Google Scholar]
- Penzies P, Rafalovich I. Regulation of the Actin Cytoskeleton in Dendritic Spines. Advances in Experimental Medicine and Biology. 2012;970:81–95. doi: 10.1007/978-3-7091-0932-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW. Autoradiographic localization of radioactivity in the rat brain after injection of tritiated sex hormones. Science. 1968;161:1355–6. doi: 10.1126/science.161.3848.1355. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endo. 2011;152:1492–502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carrétero J, Rune GM. Inhibition of hippocampal estrogen synthesis causes region-specific down regulation of synaptic protein expression in hippocampal neurons. Hippocampus. 2006;16:464–471. doi: 10.1002/hipo.20173. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Dalpian F, Menezes IC, Brusco J, Moreira JE, Cohen RS. Dendritic spines of the medial amygdala: plasticity, density, shape, and subcellular modulation by sex steroids. Histol Histopathol. 2012;27:985–1011. doi: 10.14670/HH-27.985. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–2. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Song C, Ehlers VL, Moyer JR., Jr Learning to learn - intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Mem. 2013;105:186–99. doi: 10.1016/j.nlm.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Penzes P. Rapid estradiol modulation of neuronal connectivity and its implications for disease. Front Endocrinol (Lausanne) 2011 Nov;22:2–77. doi: 10.3389/fendo.2011.00077. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes PJ. Estrogen receptor ß activity modulates synaptic signaling and structure. Neurosci. 2010;30:13454–60. doi: 10.1523/JNEUROSCI.3264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Frankfurt M, Luine V, Frick K. Program No 451.13 2014Neuroscience Meeting Planner. Washington D.C: Society for Neuroscience; 2014. Dorsal hippocampal infusion of 17β-estradiol increases dendritic spine density in the CA1 subfield of the hippocampus in ovariectomized female mice. 2014. Online. [Google Scholar]
- Urbanska M, Swiech L, Jaworski J. Developmental plasticity of the dendritic compartment: focus on the cytoskeleton. Adv Exp Med Biol. 2012;970:265–84. doi: 10.1007/978-3-7091-0932-8_12. [DOI] [PubMed] [Google Scholar]
- Valverde F. Apical dendritic spines of the visual cortex and light deprivation in the mouse. Exp Brain Res. 1967;3:337–52. doi: 10.1007/BF00237559. 1967. [DOI] [PubMed] [Google Scholar]
- Velázquez-Zamora DA, Garcia-Segura LM, González-Burgos I. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm Behav. 2012;61:512–7. doi: 10.1016/j.yhbeh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Vierk R, Brandt N, Rune GM. Hippocampal estradiol synthesis and its significance for hippocampal synaptic stability in male and female animals. Neurosci. 2014;274:24–32. doi: 10.1016/j.neuroscience.2014.05.003. Epub 2014 May 15. [DOI] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–26. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O. Structure and function of dendritic spines within the hippocampus. Ann Anat. 2009;19:518–31. doi: 10.1016/j.aanat.2009.08.006. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–31. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats, Neurobiol. Learn Mem. 2006;1:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellano A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in hippocampus and prefrontal cortex. Brain Research. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V. Impaired Recognition Memory and Decreased Prefrontal Cortex Spine Density in Aged Female Rat. Imaging and the Aging Brain Annals NYAS. 2007;1097:54–57. doi: 10.1196/annals.1379.026. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–9. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;25:49–54. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Dendritic spines. MIT press; 2010. [Google Scholar]
- Yuste R. Dendritic spines and distributed circuits. Neuron. 2011;71:772–81. doi: 10.1016/j.neuron.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–60. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ, S S. Evidence for a role in dendritic filopodia in synaptogensis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]


