Abstract
We retrospectively analyzed time to detection of 3747 positive MGIT sputum cultures at a laboratory in a country with heavy burden of tuberculosis. Ninety-nine percent of diagnostic cultures turned positive within 28 days, suggesting that physicians may consider alternative diagnoses if sputum cultures remain negative after 4 weeks of incubation.
Keywords: Sputum culture, MTB complex, MGIT 960, TTD
Recent advances in molecular techniques [1, 2] have shortened the time needed to diagnose tuberculosis (TB), leading to improved case detection and management; however, culture is still essential for full drug susceptibility testing and improves the diagnostic yield for specimens that are smear or molecular diagnostic testing negative. A major limitation is the long culture-negative turnaround time – 6 to 8 weeks for solid media and 6 weeks for liquid media. This may delay physician decisions and limit laboratory capacity. Studies from Switzerland and the U.S., low TB burden countries, suggested that the incubation time of MGIT cultures could be shortened from six weeks [9, 10]. We sought to test this hypothesis in a setting with a high incidence of TB and HIV infection. We retrospectively analyzed MGIT time to detection (TTD) of sputum cultures at the Joint Clinical Research Centre's mycobacteriology laboratory in Kampala, Uganda. TTD is the time from the first incubation day of the MGIT culture until the time the instrument flags it as positive.
Sputum specimens were processed using standard procedures [3, 4], modified to achieve a final sodium hydroxide concentration of 1.5%. MGIT tubes with PANTA were inoculated with 0.5 ml of re-suspended pellet and incubated in the MGIT 960 instrument following the manufacturer's recommended procedures[3]. Auramine smears were made, examined, and graded using the CDC scheme [5]. Positive cultures were automatically flagged any time from the first day of incubation to 42 days while negative cultures were flagged after 42 days. Smears were made from positive cultures, stained by Ziehl-Neelsen (ZN) method and examined with bright-field microscopy for acid-fast bacilli (AFB). Cultures were considered positive only when ZN was positive. To rule out contamination, positive cultures were inoculated on blood agar (BA) plates and incubated at 37°C for 48 hours. Growth on BA indicated contamination. Contaminated cultures were excluded from this analysis. Positive cultures from diagnostic specimens and specimens collected after 4 or more months of anti-TB treatment underwent definitive identification for MTB complex by rapid MPT64 antigen testing [Capilia TB-Neo (TAUNS Laboratories, Numazu, Japan) and SD BIOLINE TB Ag MPT64 Rapid (Standard Diagnostics, Yongin, Republic of Korea)]. For TTD analysis, only cultures confirmed as MTB complex were included. Data was analyzed using STATA, Version 12 (StataCorp, College Station, Texas, USA).
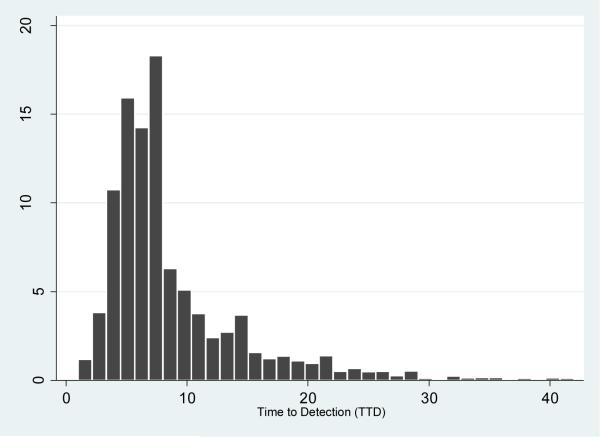
Twenty-nine thousand eight hundred and twelve (29,812) sputum specimens were cultured from July 1, 2006 to May 31, 2014 (Table 1). Of these, 28.1% (8380/29812) were flagged positive by MGIT and 8379 confirmed AFB-positive by ZN microscopy. Of the positive cultures, 49% (4098/8379) underwent identification, while 51% (4281/8379) were not identified because they were from visit intervals not requiring definitive identification. Of cultures identified, 91.4% (3747/4098) were MTB complex; the rest (8.6%) were non-tuberculous mycobacteria (NTM). NTMs were not identified to species level and were not included in the TTD analysis as this work focused on MTB complex. As shown in Table 2, the median TTD for all 3747 MTB complex cultures was 7 days (IQR 5-10 days). The median TTD for the 2617 diagnostic cultures was 6 days (IQR 5-9 days), with a range of 1 to 42 days: 90.3% (2364/2617) were detected within 14 days, 97.6% (2556/2617) within 21 days, and 99.2% (2594/2617) within 28 days. The median TTD for the 1130 follow-up cultures was 8 days (IQR 6-13 days), with a range of 1-40 days: 78.1% (882/1130) were detected within 14 days, 89.8%(1015/1130) within 21 days, and 97.3% (1100/1130) within 28 days. Overall, 87% (3246/3747) were detected within 14 days, 95% (3571/3747) within 21 days and 99% (3694/3747) were detected within 28 days. Figure 1 shows the overall TTD distribution of all 3747 MTB complex-positive cultures.
Table 1.
Sputum Specimen Characteristics
| Characteristic | N | Percent | |
|---|---|---|---|
| Primary AFB smear | 1+ | 1650 | 5.5 |
| 2+ | 1329 | 4.5 | |
| 3+ | 1735 | 5.8 | |
| 4+ | 1990 | 6.7 | |
| Total Positive | 6704 | 22.5 | |
| Negative | 23106 | 77.5 | |
| MGIT culture result | Negative | 21432 | 71.9 |
| Positive | 8380 | 28.1 | |
| Cultures confirmed | ZN Positive | 8379 | 99.99 |
| Culture not confirmed | ZN not done | 1 | 0.01 |
| Identification | Not identified | 4281 | |
| NTM | 351 | 8.6 | |
| MTB complex | 3747 | 91.4 | |
| Diagnostic specimens | 2785 | 68.0 | |
| Follow-up specimens | 1313 | 32.0 |
Table 2.
TTD for MTB complex-positive sputum cultures (diagnostic vs. follow-up)
| Interval | # Specimen | Median TTD in days (IQR; Range) | ≤14 days | 15-21 days | 22-28 days | 29-35 days | 36-42 days | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |||
| Diagnostic | 2617 | 6 (5-9; 1-42) | 2,364 | 90.33 | 192 | 7.34 | 38 | 1.45 | 13 | 0.5 | 10 | 0.38 |
| Follow-up | 1130 | 8 (6-13; 2-42) | 882 | 78.05 | 133 | 11.77 | 85 | 7.52 | 23 | 2.04 | 7 | 0.62 |
| Overall | 3747 | 7 (5-10; 1-42) | 3,246 | 86.63 | 325 | 8.67 | 123 | 3.28 | 36 | 0.96 | 17 | 0.45 |
TTD= time to detection
Figure 1.
Distribution of TTD for 3747 MTB complex-positive sputum cultures
Incubation in enriched liquid cultures significantly shortens TTD for MTB complex[6-8]. However, it remains standard practice to incubate them for as long as 42 days before a negative report, consequently delaying physician decision-making on the next course of action. Additionally, laboratories hold more cultures than they would if incubation time for negative cultures were shorter. This reduces laboratory capacity. In our analysis of the 3747 MTB complex positive sputum cultures, we found that 99.2% of diagnostic cultures and 97.3% of follow-up cultures were detected within 28 days. Pfyffer and Wittwer in a smaller study from Switzerland found that 50% of their MGIT cultures for MTB complex became positive within 14 days, concluding that a final report of a negative culture could be issued after 4 weeks of incubation [9]. In a multicenter study of 366 MTB complex positive MGIT cultures done at 9 U.S. laboratories, Tyrrell et al. reported that 99.4% of diagnostic specimens were detected by week 4, concluding that a negative culture could reliably be reported at 4 weeks for diagnostic specimens and 5 weeks for follow-up specimens [10]. Our analysis, based on a much larger number of MTB complex positive sputum cultures collected over 8 years in a high TB incidence country, confirms the findings of these studies. Diagnostic cultures with TTD greater than 28 days made up only 0.8% of the total. Also, follow-up cultures with TTD greater than 28 days likely reflect low mycobacterial burden of limited clinical significance. While we recommend more studies done in different settings, our findings suggest that 28 days are long enough to make a decision about a negative sputum culture for MTB complex. An interim report could be issued for all MTB complex cultures negative at 28 days, advising physicians to begin considering other diagnostic options for effective management of the patient. For efficient utilization of the MGIT instrument, these cultures could be removed to free space and incubated off-line in an ordinary incubator to be read only on days 35 and 42 before finally discarding them if they remain negative.
Highlights.
We analyzed 3747 positive MGIT cultures in a high TB burden country.
Ninety-nine percent of cultures turned positive within 28 days.
We conclude that MGIT cultures could be initially reported as presumptively negative after 28 days of incubation.
Acknowledgments
We acknowledge the staff of JCRC Mycobacteriology Laboratory, Kampala, Uganda for performing all testing, keeping accurate data and for their cooperation that made this work possible.
Funding:
Activities of the JCRC Mycobacteriology Laboratory where this work was done were supported, in part, by the Tuberculosis Research Unit at Case Western Reserve University, established with funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract numbers NO1-AI95383 and HHSN266200700022C/NO1-AI-70022. CMB is supported by the NIH/NCRR CTSA KL2TR000440. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement of authors: We have no conflicts of interest to declare.
Ethical approval: All procedures performed involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
References
- 1.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco-Alvarez de Luna F, Ruiz P, Gutierrez J, Casal M. Evaluation of the GenoType Mycobacteria Direct assay for detection of Mycobacterium tuberculosis complex and four atypical mycobacterial species in clinical samples. J Clin Microbiol. 2006;44(8):3025–3027. doi: 10.1128/JCM.00068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi SH, Rusch-Gerdes S. MGIT Procedure Manual for BACTEC MGIT 960 TB System. Foundation for Innovative New Diagnostics; Geneva, Switzerland: 2006. [Google Scholar]
- 4.CLSI . Laboratory detection and identification of mycobacteria; approved guideline. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. [Google Scholar]
- 5.Guler M, Unsal E, Dursun B, Aydln O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61(2):231–235. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ML, Stone BL, Hildred MV, Reves RR. Comparison of recovery rates for mycobacteria from BACTEC 12B vials, Middlebrook 7H11-selective 7H11 biplates, and Lowenstein Jensen slants in a public health mycobacteriology laboratory. J Clin Microbiol. 1995;33(9):2516–2518. doi: 10.1128/jcm.33.9.2516-2518.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp SE, Suarez CA, Lemes M, Poppiti RJ., Jr. Evaluation of the mycobacteria growth indicator tube compared to Septi-Chek AFB for the detection of mycobacteria. Diagn Microbiol Infect Dis. 1996;25(2):71–75. doi: 10.1016/s0732-8893(96)00084-3. [DOI] [PubMed] [Google Scholar]
- 8.Sharp SE, Lemes M, Erlich SS, Poppiti RJ., Jr. A comparison of the Bactec 9000MB system and the Septi-Chek AFB system for the detection of mycobacteria. Diagn Microbiol Infect Dis. 1997;28(2):69–74. doi: 10.1016/s0732-8893(97)89665-4. [DOI] [PubMed] [Google Scholar]
- 9.Pfyffer GE, Wittwer F. Incubation time of mycobacterial cultures: How long is long enough to issue a final negative report to the clinician? J Clin Microbiol. 2012;50(12):4188–4189. doi: 10.1128/JCM.02283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyrrell FC, Budnick GE, Elliott T, Gillim-Ross L, Hildred MV, Mahlmeister P, Parrish N, Pentella M, Vanneste J, Wang YF, Starks AM. Probability of negative mycobacterium tuberculosis complex cultures based on time to detection of positive cultures: a multicenter evaluation of commercial-broth-based culture systems. J Clin Microbiol. 2012;50(10):3275–3282. doi: 10.1128/JCM.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]