Abstract
While many studies in humans have investigated the effects of estrogen and hormone therapy on cognition, potential neurobiological correlates of these effects have been less well studied. An important site of action for estrogen in the brain is the cholinergic system. Several decades of research support the critical role of CNS cholinergic systems in cognition in humans, particularly in learning and memory formation and attention. In humans, the cholinergic system has been implicated in many aspects of cognition including the partitioning of attentional resources, working memory, inhibition of irrelevant information, and improved performance on effort-demanding tasks. Studies support the hypothesis that estradiol helps to maintain aspects of attention and verbal and visual memory. Such cognitive domains are exactly those modulated by cholinergic systems and extensive basic and preclinical work over the past several decades has clearly shown that basal forebrain cholinergic systems are dependent on estradiol support for adequate functioning. This paper will review recent human studies from our laboratories and others that have extended preclinical research examining estrogen-cholinergic interactions to humans. Studies examined include estradiol and cholinergic antagonist reversal studies in normal older women, examinations of the neural representations of estrogen-cholinergic interactions using functional brain imaging, and studies of the ability of selective estrogen receptor modulators such as tamoxifen to interact with cholinergic-mediated cognitive performance. We also discuss the implications of these studies for the underlying hypotheses of cholinergic-estrogen interactions and cognitive aging, and indications for prophylactic and therapeutic potential that may exploit these effects.
Keywords: Estradiol, acetylcholine, cognitive performance, memory, attention, brain imaging
INTRODUCTION
The prophylactic and therapeutic use of gonadal steroids remains among the most controversial areas in medicine. Large prospective trials such as the Women’s Health Initiative (Coker et al., 2010) have altered significantly what had been accepted about postmenopausal hormone treatment based on prior retrospective epidemiologic surveys (Maki and Hogervorst, 2003). While significant strides have been made in understanding the actions of these molecules on such target organs as bone and breast tissue, significant questions remain about their impact on the brain and brain function in humans. Cognitive symptoms reported by women at menopause include difficulties in memory, attention, and word-finding. A study of cognitive change across the menopause transition showed that performance on a number of cognitive tasks, including recognition speed, concept integration, and psychomotor skill declined at a faster rate immediately after menopause than would have been predicted by age alone, suggested an acceleration of decline due to loss of estradiol (Halbreich et al., 1995). There is some evidence that estrogen therapy (ET) results in the maintenance of a premenopausal level of cognitive functioning (i.e., (Duka et al., 2000; Jacobs et al., 1998; Resnick et al., 1997; Smith et al., 2001a; Zandi et al., 2002),
While many studies in humans have investigated the effects of estrogen and hormone therapy on cognition, few have proposed a mechanism of action for estrogen in the brain. One possible site of action for estrogen in the brain is the cholinergic system. Several decades of research support the critical role of CNS cholinergic systems in cognition in humans, particularly in learning and memory formation and attention. The activity of the CNS cholinergic system may be a primary determinant of the effectiveness of attentional, learning, and memory mechanisms (Sarter et al., 2005). In humans, the cholinergic system has been implicated in many aspects of cognition including the partitioning of attentional resources, working memory, inhibition of irrelevant information, and improved performance on effort-demanding tasks (see Newhouse et al. 2001 and Dumas and Newhouse, 2011 for a review). It is well accepted that cholinergic dysfunction is relevant to the cognitive and behavioral changes secondary to age-related dementias such as Alzheimer’s disease (Levey, 1996). Loss of p75NTR receptors that are modulated by estrogen (Bora et al., 2005) on basal forebrain cholinergic neurons is associated with early evidence of cognitive dysfunction even without evidence of cellular loss (Mufson et al., 2002). Studies support the hypothesis that estradiol helps to maintain aspects of attention and verbal and visual memory (LeBlanc et al., 2001; Sherwin, 1993; Sherwin, 2006), and may have positive effects on tasks mediated by the prefrontal cortex (Keenan et al., 2001) and hippocampus (Maki, 2005a) especially in younger postmenopausal women. Such cognitive domains are exactly those modulated by cholinergic systems and extensive basic and preclinical work over the past several decades has clearly shown that basal forebrain cholinergic systems are dependent on estradiol support for adequate functioning (Gibbs, 2010).
This paper will review recent human studies from our laboratories and others that have extended the pioneering animal research examining estrogen-cholinergic interactions to humans with a specific focus on cognitive changes that may occur with normal and pathologic aging, as cholinergic system integrity is critical for normal cognitive performance and deterioration of this system leads to age-related cognitive deficits (Dumas and Newhouse, 2011). Early studies involved classic cognitive neuropharmacology using cholinergic antagonists and focusing on specific cognitive domains thought to be sensitive to cholinergic systems. We also examine the potential effects of selective estrogen receptor modulators and their potential effects on cholinergic system-mediated cognitive performance. Later studies have extended this work using functional neuroimaging to examine the neural representation of cognitive operations sensitive to cholinergic blockade and the effect of estradiol treatment on brain activity. Finally, we discuss the implications of these studies for the underlying hypotheses of cholinergic-estrogen interactions, the relationship to cognitive aging, and approaches moving forward that will have the potential to exploit these interactions for prophylactic or therapeutic benefit.
Cognitive Changes with Aging
Research in cognitive aging has shown that older adults are impaired on tasks of working memory and episodic memory relative to younger adults (Verhaeghen and Cerella, 2002; Verhaeghen et al., 1993). Additionally, age differences in brain activation patterns during performance of working memory and episodic memory tasks have also been found in many studies (Cabeza et al., 2002; Rypma and D’Esposito, 2000). As will be described below there are many similarities in the patterns of age differences seen across these cognitive domains. There is strong empirical evidence that working memory, the ability to hold and manipulate information in mind over a short period of time (Baddeley, 1986) is affected by increased age (Verhaeghen et al., 1993). Age-related impairments in working memory have been hypothesized to be at the core of age differences in higher cognitive processes like problem solving and decision making (Labouvie-Vief, 2003). Thus, understanding how the neurobiology of aging influences working memory is important for understanding the mechanisms by which higher cognitive processes are affected by aging. Functional neuroimaging studies have shown that the age difference in working memory influences activation in frontal and posterior brain regions. For example on a task measuring reaction time during retrieval of information from working memory, faster younger adults showed less dorsolateral prefrontal cortex activation relative to slower younger adults (Rypma and D’Esposito, 2000). However, faster older adults showed increased activation relative to slower older adults. Thus, the increased activation in slower younger adults and faster older adults may represent compensation responses for difficulty in performing the task. In another study, older adults were less able than younger adults to inhibit irrelevant information during working memory encoding (Gazzaley et al., 2005). Younger adults showed enhanced posterior cortical activation during the presentation of task-relevant information and suppression in these same areas during the presentation of task-irrelevant information. Older adults showed similar task-relevant information enhancement in posterior regions, but did not show the same suppression as younger adults. This finding was interpreted as an age-related loss in top-down attentional control during working memory encoding. Thus, these prior data show age differences in working memory were seen in activation increases and decreases in frontal and occipital regions, respectively.
Research on age differences in episodic memory shows that older adults perform more poorly than younger adults on tests of episodic memory (Light, 1991). In addition, changes in episodic memory are the critical cognitive measure used to diagnose MCI and AD and older adults with episodic memory impairment in MCI are more likely to progress to AD (Albert et al., 2011). In recent years, studies have examined normal age differences in episodic memory during fMRI and older adults activated more bilateral frontal areas relative to younger adults during encoding. Cabeza and colleagues (Cabeza et al., 2002) have proposed the hemispheric asymmetry reduction for older adults (HAROLD) model of age differences in frontal cortex activation during memory tasks. This is an age-related modification of the hemispheric encoding/retrieval asymmetry (HERA; (Tulving et al., 1994) pattern of brain activation often seen in younger adults in which encoding processes activated left prefrontal cortex while retrieval processes activated right prefrontal cortex. Older adults showed a reduction in this asymmetry such that there was more bilateral activation in both episodic encoding and retrieval tasks. Morcom (Morcom et al., 2003) found encoding-related activity was left lateralized in younger adults as predicted by HERA and bilateral in older adults as predicted by HAROLD. Daselaar (Daselaar et al., 2003) found that poorer performing older adults had decreased frontal activity during episodic memory encoding relative good performing older adults. The good performing older adults had activation that was similar to that of younger adults.
This pattern of similar activation across working memory and episodic memory was summarized by Cabeza and colleagues (Cabeza et al., 2004). Older adults showed increased activation in the prefrontal cortex and decreased occipital activation during both tasks. Cabeza (Cabeza et al., 2004) interpreted these findings as evidence for task-independent age-related changes in brain activity representing sensory decline as well as compensation for sensory changes with recruitment of additional frontal cortical areas. Davis (Davis et al., 2008) named this phenomenon the posterior anterior shift in aging (PASA). Interestingly, frontal increases were positively correlated with performance and posterior decreases were negatively correlated with performance. Thus, the increased activation for older adults was related to improved performance on these cognitive tasks.
This data pattern suggests that adults who perform poorly on cognitive tasks relative to younger adults have difficulty controlling the focus of attention. This attentional control difficulty will affect memory processes that require the control of attention such as working memory and episodic memory. This difficulty may increase the need to recruit frontal brain regions. However, these data do not implicate the neurobiological processes that underlie the age differences in brain activation during working memory and episodic memory. Below evidence is presented relating the PASA pattern to age-related changes in the estrogen-cholinergic systems functioning.
Whether women show differential cognitive changes from men prior to menopause has not been clearly studied. However, as has been noted previously, women appear to show an inflection point at menopause with changes in cognitive performance in some domains that are greater than would be predicted by chronological age (Halbreich et al., 1995). Whether this is true across all cognitive domains is currently being studied in large cohort studies such as the Study of Women across the Nation (SWAN) (Greendale et al., 2009). Clearly however, women show higher risk for late life cognitive disorders such as Alzheimer’s disease(Carter et al., 2012), early menopause increases the risk for late life cognitive impairment and dementia (Coppus et al., 2010), and women may show more rapid cellular aging in association with high risk genotypes(Jacobs et al., 2013).
Estrogen Effects on Cholinergic Systems
Estradiol appears to modulate cholinergic neurotransmission in the brain (Gibbs, 1996; McMillan et al., 1996). Loss of estradiol support after ovariectomy in animals has been shown to decrease high affinity choline uptake, choline acetyltransferase (ChAT) activity, and ChAT mRNA levels (Gibbs et al., 1994; Luine et al., 1975; Luine et al., 1986; Singh et al., 1993). These effects can be reversed by exogenous estradiol (Yamamoto et al., 2007). Levels of NGF and BDNF mRNA also appear to decrease following ovariectomy and are restored following estradiol replacement (Singh et al., 1993; Singh et al., 1994; Singh et al., 1995). Short-term estradiol replacement in ovariectomized animals restores trkA mRNA levels to normal along with CAT levels in several nuclei (nucleus basalis, horizontal limb of the diagonal band) in the basal forebrain (McMillan et al., 1996), although longer-term (10 days) administration of estradiol results in a decrease in the levels of trkA mRNA, apparently commensurate with a restoration of cholinergic function. Estradiol increases BDNF and CREB expression in a regionally specific way, particularly in the hippocampus and amygdala (Zhou et al., 2005). While estradiol restores CAT levels in ovariectomized animals, the effects on NGF receptors may be regionally specific and time-linked to the restoration of cholinergic function (Gibbs, 1994; Gibbs, 2000). As over 90% of NGF-receptor containing neurons in the basal forebrain are cholinergic (Gibbs, 2000), this estradiol-related enhancement of NGF receptor mRNA presumably is related to increased NGF signaling, the increase in ChAT levels, and an improvement in cholinergic function.
Biologically active estradiol receptors are present in the basal forebrain cholinergic neurons with ERα and GPR30 being the predominant receptor subtypes (Hammond et al., 2011; Miettinen et al., 2002; Shughrue et al., 2000). Estradiol appears to be able alone to support the survival of transplanted cholinergic neurons (Tanaka et al., 1993), can directly modify the expression of muscarinic receptors (Cardoso et al., 2010) and receptor binding (Dohanich et al., 1982), as well as increasing acetylcholinesterase activity in the hypothalamus (Luine and Rhodes, 1983). Estradiol may have nicotinic effects as well, including increasing the density of hypothalamic α-bungarotoxin nicotinic receptors (Morley et al., 1983), and facilitating the response of neurons to the excitatory actions of acetylcholine (Kow and Pfaff, 1985). Estradiol-nicotinic receptor interactions appear potentially mediated by ERβ (Fernandez et al., 2013) and may be particularly relevant to depression (Kandi and Hayslett, 2011).
Recent studies have suggested that estradiol may produce important effects on cholinergic neurons through novel estrogen receptors such as GPR30 (Gibbs et al., 2014). GPR30 is a novel G protein-coupled estrogen receptor that is expressed in brain, particularly by cholinergic neurons in the basal forebrain and appears to be an important regulator of basal forebrain cholinergic functioning (Hammond and Gibbs, 2011). Stimulation of these receptors may produce significant increases in acetylcholine signaling in the hippocampus via direct effects on the basal forebrain (Hammond et al., 2011).
In rodents, ovariectomy reduces performance on learning and memory tasks and this decline in performance parallels a decline in cholinergic activity and choline acetyltransferase (ChAT) levels in several brain regions, while estradiol replacement prevents this decline (Singh et al., 1994). Estradiol administration in ovariectomized rats counteracted the negative learning effects of the cholinergic antagonist scopolamine on alternation learning (Dohanich et al., 1994). Estradiol replacement in ovariectomized rats enhanced acquisition of memory tasks and partially or completely blunted the effects of the anticholinergic scopolamine (Fader et al., 1999; Gibbs, 1999; Tanabe et al., 2004). In ovariectomized mice, estradiol administration increases the expression of choline acetyltransferase (ChAT), which correlated with improved performance (Miller et al., 1999). Even single subthreshold doses of estradiol can potentiate the effects of the cholinergic or glutamatergic agonist on avoidance learning when administered directly to the hippocampus (Farr et al., 2000). When cholinergic systems are experimentally destroyed or blocked, estradiol administration is ineffective in enhancing learning (Daniel et al., 2005; Gibbs, 2001), suggesting the cholinergic systems are critical for estradiol to have cognitively enhancing effects. Interestingly, high doses of ethinyl estradiol a synthetic form of 17-β estradiol, the most common estrogen in hormonal contraceptives, appears to negatively impact working memory performance in rodents and this correlates with a decline in measures of cholinergic integrity (Mennenga et al., 2015).
Long-term loss of estrogen function produces a decrease in the functional status of basal forebrain cholinergic neurons, particularly projecting to the hippocampus and cortex (Gibbs, 1998) and treatment with estradiol restored ChAT mRNA in the medial septum and trkA mRNA in the nucleus basalis (Yamamoto et al., 2007). Estradiol effects on cholinergic neurons appear mediated particularly through ERα via the MAPK pathway (Szego et al., 2006). Estradiol effects on visual-spatial attention appear to be modulated by cholinergic muscarinic receptors in primates (Tinkler and Voytko, 2005), but the ability of estradiol replacement to enhance cholinergic function may also be age-related. Savonenko and Markowska (Savonenko and Markowska, 2003) found that the ability of estradiol to decrease behavioral vulnerability to scopolamine was limited to younger ovariectomized animals and was not seen in older rats. The ability of estradiol to enhance basal forebrain cholinergic function appears to decline with age and with the time since loss of endogenous hormonal support (Daniel et al., 2006). Accumulating evidence suggests that the ability of estradiol to enhance both cholinergic functioning and cognitive performance is attenuated if a significant delay follows loss of endogenous hormonal support. This seems to also correlate with loss of ERα resulting from long-term hormone deprivation. Taken together these factors may suggest a reason for the so-called “critical period” (Daniel, 2013). Even the rapidity of how quickly estrogen is lost may impact memory and cholinergic functioning (Acosta et al., 2009). However some animal studies have shown that estradiol benefits cognitive performance even when administration is delayed after ovariectomy or natural menopause for several years (Foster et al., 2003; Lacreuse, 2006; Lacreuse et al., 2002; Rapp et al., 2003a). The reason for this discrepancy is not clear, but the length of the “window” or “critical period” may be quite different depending on the natural lifespan of the animal (Voytko et al., 2008).
In humans, the length of hormone treatment in postmenopausal women has been shown to be correlated with vesicular acetylcholine transporter binding in a variety of brain regions (Smith et al., 2001b) and greater muscarinic receptor density in hippocampus and frontal cortex as well as some cortical areas was found in hormone users compared to nonusers (Norbury et al., 2007). Using a positron emission tomography ligand of acetylcholinesterase as a marker of cholinergic integrity, Smith and colleagues (Smith et al., 2011) were able to show that women who had begun postmenopausal hormone therapy shortly after menopause showed significantly higher levels of acetylcholinesterase in the hippocampus and posterior cingulate cortex compared to women who had not taken any hormone therapy, consistent with a preservation of cholinergic neuronal integrity in the early hormone treatment group.
Estrogen Studies in Human Cognition
Studies of the cognitive effects of estradiol have strongly suggested that estradiol levels are directly relevant to cognitive function. An important study showed that performance on a number of cognitive tasks, including recognition speed, concept integration and psychomotor skill declined at a faster rate immediately after menopause than would have been predicted by age alone, suggesting an acceleration of decline due to loss of estradiol (Halbreich et al., 1995).
Studying estradiol replacement after surgical oophorectomy, Sherwin (Sherwin, 1988) found that an estradiol treated group with three months of hormone treatment after oopherectomy showed preservation of verbal learning and memory performance compared to placebo-treated women who showed a decline (Phillips and Sherwin, 1992). Other investigators have also shown similar experimental results in women after surgery (Kimura, 1995; Verghese et al., 2000) Beneficial effects of hormone treatment on cognition after natural menopause have been shown in a number of other studies (Jacobs et al., 1998; Krug et al., 2006; Resnick et al., 1997; Shaywitz et al., 2003; Smith et al., 2001a; Stevens et al., 2005a; Duka et al., 2000). Some other studies have not shown positive effects (Alhola et al., 2007; Barrett-Connor and Kritz-Silverstein, 1993; Binder et al., 2001; Ditkoff et al., 1991; Polo-Kantola et al., 1998) and studies examining estradiol therapy specifically in older women (70+) have not shown significant benefit (Almeida et al., 2006; Buckwalter et al., 2004; Espeland et al., 2004). A meta-analysis (Hogervorst et al., 2000) reviewing 32 studies of hormone treatment concluded that the majority showed positive cognitive benefit(s) of hormone treatment and a meta-analysis of studies since 2000 (Maki, 2005b) demonstrated that most trials showed cognitive benefit in younger women but no benefit or small negative effects in older women. Overall, studies support the hypothesis that estradiol helps to maintain aspects of attention, verbal and visual memory (LeBlanc et al., 2001; Sherwin, 1993; Sherwin, 2006), and may have positive effects on tasks mediated by the prefrontal cortex (Keenan et al., 2001) and hippocampus (Maki, 2005a) especially in younger postmenopausal women.
Estrogen effects on cholinergic neurons may be important for the risk of developing pathologic late life cognitive dysfunction or neurodegeneration such as Alzheimer’s disease (AD) (Ábrahám et al., 2009). Women appear to be at higher risk for AD, particularly if they carry the APOE4 allele (Bretsky et al., 1999). Furthermore, certain ER polymorphisms appear to be associated with the risk of developing cognitive impairment (Yaffe et al., 2002). Both prospective and case-control studies have shown that estradiol use in postmenopausal women may decrease the risk of the development and/or expression of AD (Kawas et al., 1997; Paganini-Hill and Henderson, 1994; Panidis et al., 2001; Tang et al., 1996; Zandi et al., 2002) with an overall odds ratio of 0.66 (LeBlanc et al., 2001). Estradiol treatment alone may improve cognitive functioning in some female AD patients (Fillit, 1994; Ohkura et al., 1994) but large controlled trials of estradiol alone as a therapy for mild to moderate AD have been negative, showing no significant effect on cognitive measures of long-term progression (Henderson et al., 2000; Mulnard et al., 2000), although estradiol appears to enhance the effect of anticholinesterase medication in AD (Schneider et al., 1996). It may be that estradiol alone cannot significantly prevent progression of an already established disease, perhaps due to the progressive deterioration in basal forebrain cholinergic systems (Craig et al., 2011) necessary for estrogen to have salutary effects on cognition. In addition, studies published as part of the Women’s Health Initiative study (WHI) showed that the relative risk of diagnosis of dementia in the active treatment (Premarin plus medroxyrogesterone acetate) group was approximately twice that of the placebo group (Shumaker et al., 2003) and that in women not diagnosed with dementia, no overall cognitive improvement was seen (Rapp et al., 2003b). However, the average age of the women in the study at treatment onset was 63.3 years, with two thirds between the ages of 60–70, far past menopause (Henderson et al., 2005). Thus, the question of whether gonadal steroid hormone therapy may have a role in the prevention of AD or in maintaining cognitive function in older women has not been answered by these studies (Yaffe, 2003).
Brinton and others have proposed the so-called “healthy cell bias of estrogen benefit” (Brinton, 2004; Harman et al., 2004), suggesting that estrogen may benefit healthy neurons but neurologically deficient neuronal systems may be compromised by long-term treatment. The human extension of this has been defined as the “critical period hypothesis” (Maki, 2006; Resnick and Henderson, 2002; Sherwin, 2006, 2007) suggesting that estrogen has maximal protective benefits on cognition in women when it is initiated closely in time to the menopause but may be ineffective, due to neuronal systems being refractory to estrogen (Silva et al., 2003) when initiated many years or decades after menopause (however see (Rapp et al., 2003a). This point is supported by the findings of the Cache County Study (Zandi et al., 2002), which found a linear association between the length of prior use of estrogen (but not current use) and risk reduction for the diagnosis of dementia in older women.
As studies of the effects of estrogen on cognition have become more selective, a pattern of results is emerging that suggests that initiation of hormone therapy improves cognitive performance particularly in younger postmenopausal women, but produces either no benefit or worsening cognitive performance in older postmenopausal women (Maki, 2005b). Studies examining estradiol therapy specifically initiated in older women (70+) have not shown significant benefit (Almeida et al., 2006; Buckwalter et al., 2004; Espeland et al., 2004; Pefanco et al., 2007; Yaffe et al., 2006), whereas studies of hormone treatment in women initiating at midlife or in the early postmenopausal period, tend to show improvement (MacLennan et al., 2006; Shaywitz et al., 2003; Stevens et al., 2005a; Stevens et al., 2005b). This has been formulated as the “critical period hypothesis” (Maki, 2005b; Pinkerton and Henderson, 2005; Sherwin, 2007) suggesting that there is a limited window of time to initiate hormone treatment for an undefined period after menopause if beneficial effects on brain function are to be seen. Dumas and colleagues (Dumas et al., 2008a) have shown that estrogen effects on enhancing cholinergic-related performance effects on episodic memory may be limited to younger postmenopausal women. These data also suggest that cholinergic system activity may be a major player in the “critical period”.
Experimental Estrogen-Cholinergic Interaction Studies in Humans
Many of the studies reviewed here create a temporary neurochemical “lesion” utilizing cholinergic antagonist drugs. This approach simulates the effects of age- or disease-related neuroreceptor and/or neuronal dysfunction by temporarily blocking pre- and postsynaptic muscarinic and nicotinic cholinergic receptors. This model reliably produces mild and quantifiable but rapidly reversible cognitive impairment and has proved valuable in understanding the role of the cholinergic system and its impairment on human cognitive functioning. This model has been successfully used to establish the effects of the loss of muscarinic and nicotinic cholinergic receptors in aging and neurodegenerative disorders (Newhouse et al., 1988; Sunderland et al., 1986; Sunderland et al., 1988) and show that cholinergic blockade alters task-related brain activity in a similar fashion as normal aging (Dumas et al., 2008b). This model has now been extended to demonstrate the effects of estradiol treatment on cholinergic function and cognitive performance in normal aging.
The effects of cholinergic modulation on cognitive processing and related brain circuitry have been examined in neuroimaging studies (see (Newhouse et al., 2011) and (Thiel, 2003) for reviews). In general, anticholinergic and procholinergic drugs show opposite patterns of brain activation across a range of cognitive tasks. However, the patterns of activation after anticholinergic drugs may differ in older subjects. For example, Dumas and colleagues (Dumas et al., 2012; Dumas et al., 2010b) found that scopolamine increased activation in frontal brain areas during both working memory and episodic memory tasks relative to placebo in older women and increased frontal activation after the nicotinic cholinergic antagonist mecamylamine during an N-back working memory test in older women. Dumas et al. (Dumas et al., 2010b) also found increased frontal and hippocampal activation and decreased occipital activation after mecamylamine compared to placebo during an episodic encoding task in older women.
Overall, these and cholinergic agonist studies suggest evidence for two related functions of the cholinergic system in information processing. The first is that the cholinergic system modulates stimulus-specific processing of sensory information in extrastriate cortical areas (Bentley et al., 2004; Furey et al., 2000). Second, brain regions involved in memory processing such as the hippocampus and frontal lobe are influenced by cholinergic modulation (Dumas et al., 2008b; Sperling et al., 2002; Thiel et al., 2003).
Newhouse, Dumas and colleagues have utilized anticholinergic models in humans to examine the potential for estrogen to produce salutary effects on cholinergic system integrity that would convey greater resistance to the effects of a given dose of an anticholinergic agent, possibly via trophic effects on cholinergic neurons, leading to increased synaptogenesis, increase in receptors, etc.
An initial study (Dumas et al., 2006) demonstrated that relatively short periods of estradiol administration produced quantifiable and significant changes in the effects of cholinergic antagonists on cognitive performance. In a within-subjects crossover design 15 non-smoking normal postmenopausal women (mean age 60.1±10.1) received either oral 17β-estradiol (1.0 mg/day) or placebo for 3 months. Subjects then took part in five challenge sessions including the cholinergic antagonists scopolamine at doses of 2.5 and 5 μg/kg administered intravenously and mecamylamine at 10 and 20 mg administered orally, or placebo. Subjects were blindly crossed over to the alternate treatment for an additional three months followed by a second set of pharmacological challenges. There were no baseline or pre-challenge differences between treatment phases.
Results showed that three months of treatment with estradiol resulted in a blunting or reduction of the effects of cholinergic antagonists on specific cognitive domains. In the domain of attention and vigilance, analysis of the Critical Flicker Fusion (CFF) showed performance was significantly less impaired after three months of estradiol following the muscarinic antagonist scopolamine challenge. Analysis with age group and years since menopause as the covariates showed a significant effect of estrogen in each model with better performance on the estrogen treatment relative to the placebo treatment.
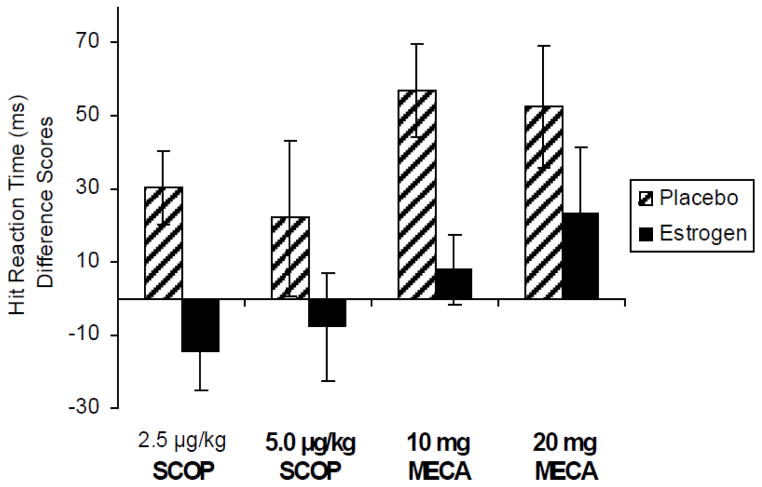
Estradiol treatment significantly reduced the effects of a challenge with mecamylamine on the Choice Reaction Time Task (CRT), a measure of attention and psychomotor speed. Motor reaction time showed a significant reduction following anticholinergic drug challenge when subjects were treated with three months of estradiol compared to three months of placebo treatment (Figure 1).
Figure 1. Choice Reaction Task.
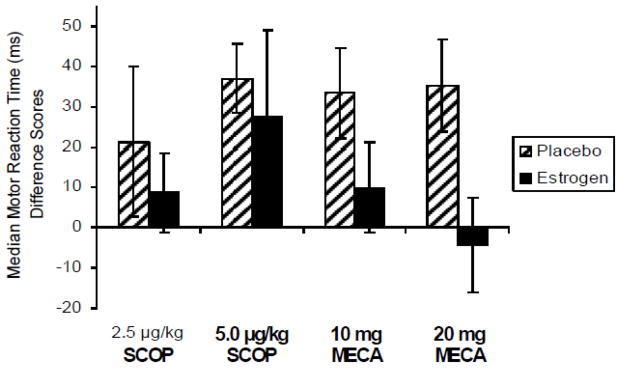
Median motor reaction time difference scores (in milliseconds) following 3 months of 1 mg oral estradiol or placebo treatment and after exposure to anti-cholinergic challenge. Paired bars represent reaction time difference from placebo challenge following each drug challenge by long-term treatment (estradiol or placebo). Positive difference score indicates a slowing of RT (impaired performance) after drug compared to placebo challenge. SCOP = scopolamine; MECA = mecamylamine. Doses are μg/kg for intravenous scopolamine and oral milligram dosage for mecamylamine. F(1,12) = 7.71, p < .05 for main effect of estradiol treatment. Reproduced with permission from (Dumas et al., 2006).
Performance on the Continuous Performance Task (CPT) showed that estradiol treatment reduced the effects of both cholinergic antagonists on performance with similar patterns for the scopolamine and mecamylamine challenge days (Figure 2). There were main effects of estradiol treatment resulting in shorter hit reaction times compared to placebo treatment, thus attenuating the impairing effects of the anticholinergic drugs. Estradiol treatment also resulted in an improvement in throughput on the CPT task as the speed of hit performance was improved without affecting accuracy, thus there was no speed-accuracy tradeoff. Thus for the attention tasks, the effect of estradiol treatment was attenuation of the negative effects of anti-cholinergic drugs on performance and improved performance during anti-cholinergic drug challenge relative to placebo treatment.
Figure 2. Continuous Performance Task.
Hit reaction time difference scores (in milliseconds) following 3 months of 1 mg oral estradiol or placebo treatment and after exposure to anti-cholinergic challenge. Paired bars represent reaction time difference from placebo challenge following each drug challenge by long-term treatment (estradiol or placebo). Positive difference score indicates a slowing of RT (impaired performance) after drug compared to placebo challenge. SCOP = scopolamine; MECA = mecamylamine. Doses are μg/kg for intravenous scopolamine and oral milligram dosage for mecamylamine. F(1,14) = 7.69, p < .05 for main effect of estradiol treatment during scopolamine challenge and F(1,14) = 9.71, p < .01 for main effect of estradiol treatment during mecamylamine challenge. Reproduced with permission from (Dumas et al., 2006).
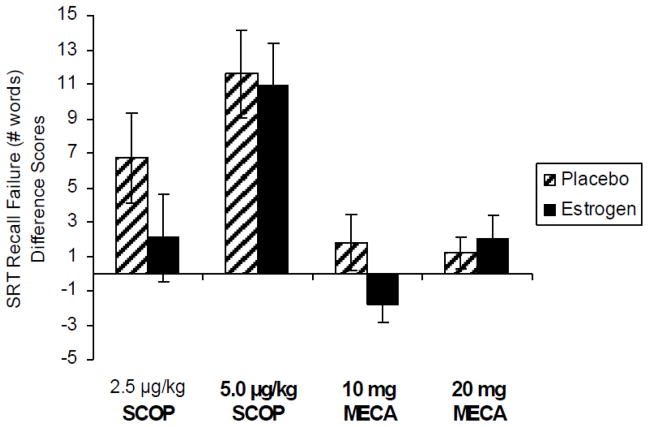
By contrast, effects on verbal episodic memory were less strong. The Selective Reminding Task (SRT) showed that estradiol treatment resulted in a reduction in recall failure after scopolamine challenge compared to placebo treatment, although this did not quite reach statistical significance. On the same measure, there was an interaction of estrogen treatment and the dose of mecamylamine, consistent with a small improvement in performance with a small improvement (negative difference score) after low dose mecamylamine challenge during estrogen treatment (Figure 3).
Figure 3. Selective Reminding Task.
Recall failure difference scores following 3 months of 1 mg oral estradiol or placebo treatment and after exposure to anti-cholinergic challenge. Paired bars represent total recall failure difference from placebo challenge following each drug challenge by long-term treatment (estradiol or placebo). Greater impairment indicated by greater positive difference score. SCOP = scopolamine; MECA = mecamylamine. Doses are μg/kg for intravenous scopolamine and oral milligram dosage for mecamylamine. F(1,14) = 4.82, p < .05 for treatment by mecamylamine challenge dosage interaction. Reproduced with permission from (Dumas et al., 2006).
Overall, estradiol treatment attenuated the impairing effects of the anticholinergic drugs on tests of attention and speed. There was less attenuation of the anticholinergic-induced impairment on a test of verbal episodic memory. Intriguingly, the human estrogen therapy literature would predict that estradiol would influence verbal memory performance, while the animal literature on the interaction of estrogen and the cholinergic system would predict that estrogen would influence attentional performance.
In a further study (Dumas et al., 2008a), Dumas and colleagues examined whether dose and age may be factors in determining how estrogen affects cholinergic system responsivity. Twenty-two cognitively normal postmenopausal women were evenly divided into two age groups (50–60 and 70+) and treated with either 17β-estradiol 1 mg/day for 1 month and 2 mg/day for a further 2 months (to lead to typical late follicular-phase levels of estradiol) or placebo. They then underwent three challenges (2.5 μg/kg scopolamine, 10 mg oral mecamylamine, or placebo).
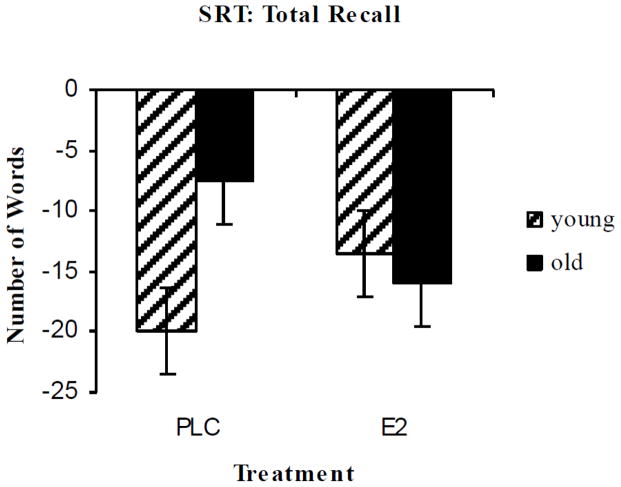
By contrast with the prior 1 mg estradiol study, stronger but selective effects of 2 mg estradiol were seen on verbal episodic memory after anticholinergic challenge. For the SRT there were interactions of age and treatment for the dependent measures total recall, recall failure, and recall consistency following scopolamine challenge. There were significant estradiol treatment by age interactions on total recall, recall consistency, and recall failure (Figure 4). In all cases, these interactions showed that estradiol improved performance for the younger group and impaired performance for the older group on the SRT measures compared to placebo treatment following scopolamine challenge.
Figure 4. Selective Reminding Task.
Total recall difference scores following 3 months of 2 mg oral estradiol or placebo treatment and after exposure to anticholinergic (scopolamine) challenge vs placebo (greater impairment indicated by greater negative score). Paired bars represent 8-trial total recall difference from placebo challenge following scopolamine challenge by age group (Young = 50–60; Old = 70+). F(1,20) = 10.73, p = .004 for age group by estradiol treatment interaction. Reproduced with permission from (Dumas et al., 2008a).
Thus, the beneficial effects of estrogen on attenuating anticholinergic effects on verbal episodic memory were seen for the younger group (mean age 55.8) of postmenopausal women but not for the older group (mean age 74.3) of subjects. This study provides experimental evidence supporting the epidemiologic finding that younger postmenopausal women may benefit from estradiol therapy while older women may not.
Importantly, support for the critical period hypothesis was not seen the effect of estrogen therapy on memory performance was examined alone without the cholinergic challenge. These data suggest that prior studies have not seen significant effects on cognitive performance in the early postmenopausal period with estradiol therapy because the lack of significant cholinergic impairment at this age. Thus, if a significant part of estradiol effects on cognitive performance are mediated through cholinergic system stimulation or maintenance, the lack of significant cholinergic dysfunction at the age that most women go through menopause means that it will be difficult to demonstrate cognitive improvement. However, cholinergic blockade studies model cholinergic dysfunction, thus the estradiol effect can be detected.
Tamoxifen-Cholinergic Interactions
Tamoxifen (TMX) is a selective estrogen receptor modulator that is used as an peripheral estrogen receptor antagonist for the treatment of breast cancer but may have ER agonist properties in the human brain (Haun et al., 2001; Haynes and Dowsett, 1999). Newhouse and colleagues examined how TMX affects cognitive performance in older women using anticholinergic drug-induced cognitive dysfunction (both muscarinic and nicotinic) to probe whether TMX would blunt or reverse anticholinergic impairment in attention and verbal memory. In this study (Newhouse et al., 2013), 18 normal postmenopausal women were administered 20 mg of oral TMX or placebo for three months. Subjects then took part in five drug challenges using the anticholinergics mecamylamine and scopolamine and were assessed cognitively. After a three-month washout, they were crossed over to the alternate treatment for three months and completed another five anticholinergic challenge days.
Results showed that TMX appears to act as an estrogen agonist by reducing the effects of anticholinergics on attention, speed, and episodic memory. For example, when subjects were on TMX treatment, effects of low dose cholinergic antagonists, particularly the effects of the nicotinic antagonist mecamylamine, were significantly less than on placebo treatment. Episodic memory performance showed that long-term recall was enhanced with significant reduction of impairment after cholinergic blockade while subjects were treated with TMX compared to placebo treatment (Figure 5).
Figure 5. Selective Reminding Task.
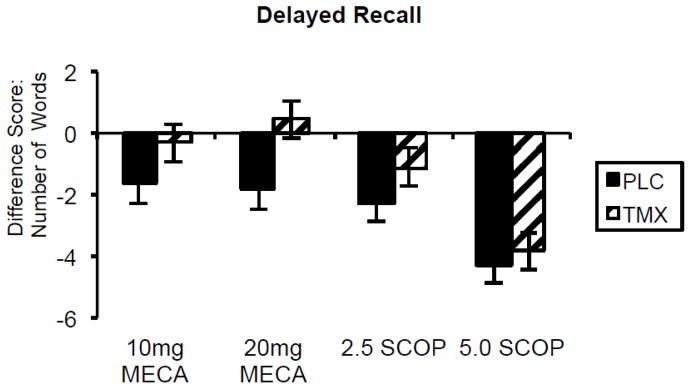
Delayed recall difference scores following 3 months of 10 mg daily oral tamoxifen or placebo treatment and after exposure to anticholinergic (scopolamine and mecamylamine) challenge vs placebo (greater impairment indicated by greater negative score). Paired bars represent delayed word recall difference from placebo challenge following each drug challenge by long-term treatment tamoxifen (TMX) or placebo (PLC).. SCOP = scopolamine; MECA = mecamylamine. Doses are μg/kg for intravenous scopolamine and oral milligram dosage for mecamylamine. F(1,17) = 5.0, p=.03 for main effect of tamoxifen treatment (Newhouse et al., 2013).
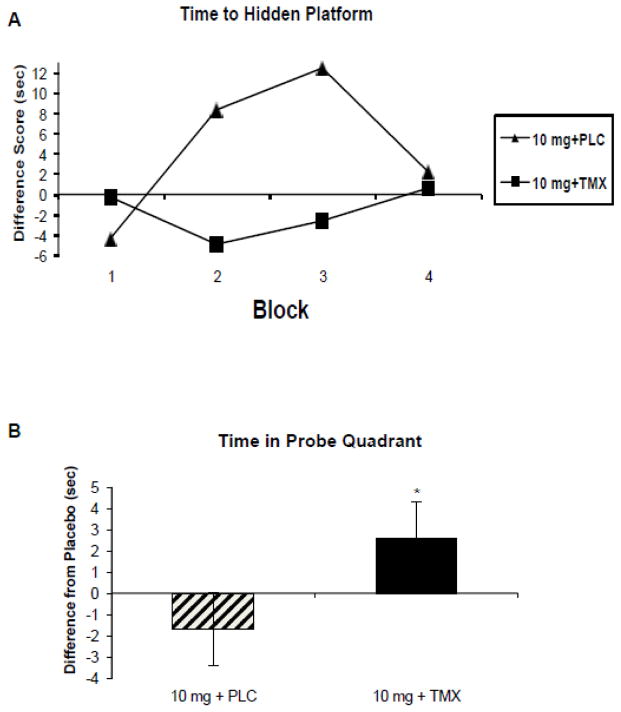
To further examine hippocampal-dependent cognitive processes, this study added analysis of spatial learning and memory skills, utilizing the computerized Virtual Morris Water task (VMWM) for measuring spatial navigation (Astur et al., 2002; Newhouse et al., 2007). TMX treatment attenuated the impairment produced by nicotinic cholinergic blockade with mecamylamine on hidden platform latency during training trials (Figure 6a) and also improved correct quadrant time during the probe trial (Figure 6b). Similar though less robust effects were seen on training trials after scopolamine. These findings support the hypothesis that TMX treatment may blunt anticholinergic-induced impairment in visuospatial learning by improving cholinergic system integrity and/or input to the hippocampus and suggests cholinergic system integrity and/or hormonal status may have direct influence on hippocampally-mediated visuospatial learning.
Figure 6. Virtual Morris Water Maze.
(modified from Newhouse et al., 2013):
A. Platform latency during learning trials after mecamylamine 10 mg challenge. Filled triangle represents long-term placebo treatment. Filled squares represent long-term tamoxifen 10 mg treatment. F(1,14) = 6.6, p=.024 for main effect of tamoxifen treatment. B. Time spent in correct quadrant during the probe trial; difference from placebo challenge during mecamylamine challenge (10 mg). Crosshatch bar represents placebo long-term treatment and dark bar represents tamoxifen 10 mg oral long-term treatment. F(1,67) = 6.28, p<.01 for main effect of tamoxifen treatment.
It is of interest that our results are somewhat at variance with some studies in breast cancer patients that have suggested that long-term treatment with TMX may have negative cognitive consequences (Boele et al., 2015; Schilder et al., 2009). However, our studies were performed in normal postmenopausal women. Previous studies with TMX have been performed in cancer patients, many of whom may have had chemotherapy, which is known to produce cognitive deficits (Janelsins et al., 2014). Administered alone, TMX may act as a weak estrogen agonist in the brain compared to its use in premenopausal breast cancer patients in which, (in the presence of estradiol), TMX will act as an antagonist.
Gene-Hormone Treatment Interactions
The APOE4 genotype is a known risk factor for the development of late life cognitive dysfunction and Alzheimer’s disease (Risacher et al., 2013; Roses, 1994). An unanswered question is to what extent APOE genotype may influence estrogen-cholinergic interactions. Newhouse and colleagues (Newhouse et al., 2013) also performed a reanalysis of the TMX data examining interactions between genotype (APOE4 + or −) and TMX treatment in normal women with at least one APOE4 allele (APOE4+). Numerous interactions with genotype were seen in the direction of a greater TMX benefit in APOE4+ individuals. For example, APOE4+ subjects showed reduction or reversal of the mecamylamine-induced slowing of RT following TMX treatment compared to APOE4- subjects. TMX treatment in APOE4+ subjects during the VMWM showed significant improvement in hidden platform latency compared to APOE4- subjects. Thus there is evidence for a significant APOE4 genotype effects on how ER stimulation modulates cholinergic system function. These data support the hypothesis that cholinergic impairment is necessary to reveal estrogen-like stimulation effects on cognitive functioning in normal women and suggests that there may be important genetic differences in how women respond to estrogen-like stimulation or blockade on cognitive systems.
Neural Representations of Estrogen-Cholinergic Interactions
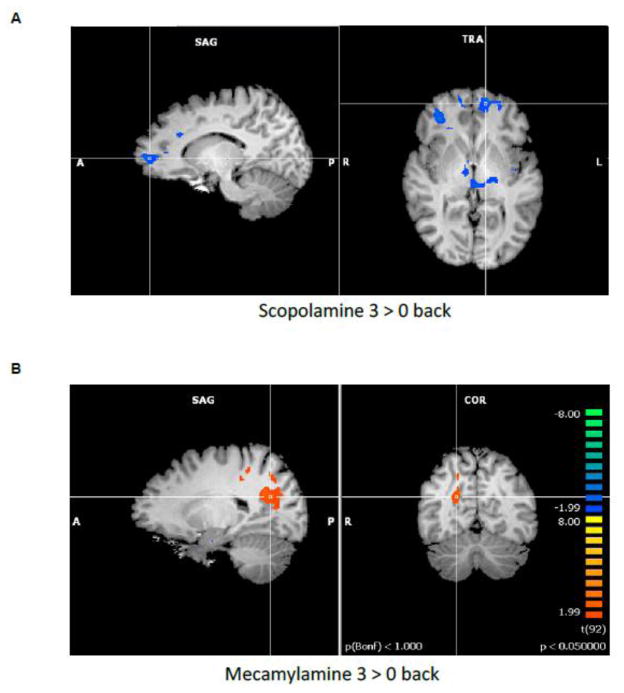
To assess how estradiol and cholinergic manipulations affected cognitive processes and related brain activation, Dumas and colleagues examined the effects of the estradiol-cholinergic interaction on functional brain activity during a working memory task in postmenopausal women. It has been shown previously that cholinergic antagonists (Dumas et al., 2010b; Dumas et al., 2008b) and estradiol (Dumas et al., 2010a; Joffe et al., 2006; Shaywitz et al., 1999; Smith et al., 2006) can separately modulate frontal lobe functioning during working memory in postmenopausal women. Dumas and colleagues examined activation patterns on the N-back working memory task to establish the anticholinergic patterns of brain activation without the influence of estradiol treatment. Increased activation during scopolamine was seen in the left medial frontal gyrus (BA 10 and BA 6), right inferior parietal lobule (BA 40), left cingulate gyrus (BA 23), right precuneus (BA 7), and right medial dorsal nucleus of the thalamus. Increased activation for mecamylamine compared to placebo challenge was found in right inferior frontal gyrus (BA 46), right middle temporal gyrus (BA 21), and right superior temporal gyrus (BA 38).
To examine the estradiol-cholinergic interaction, three months of estradiol or placebo were administered to 25 cognitively normal postmenopausal women, ages 51–71 years (Dumas et al., 2012). Brain activation during working memory performance was evaluated for both the estradiol and placebo treatment groups after scopolamine challenge. The estradiol group had less activation compared to the placebo treatment group in the left medial frontal gyrus (BA 10), right anterior cingulate gyrus (BA 24), left inferior parietal lobule (BA 40), right insula (BA 13), and left superior temporal gyrus (BA 22; see Figure 7). Decreased activation for the estradiol group compared to the placebo treatment group was seen in the right parahippocampal gyrus (BA 34). Estradiol treatment eliminated the significant relationship between brain activation and performance (d′) in the right inferior parietal lobule (BA 40) seen after scopolamine alone. Thus, in a region involved in working memory performance, estradiol treatment altered the negative relationship between activation and performance seen following scopolamine challenge.
Figure 7. N-Back Working Memory Task.
(Reproduced with permission from Dumas et al., 2012):
A. Activation map for estradiol treatment minus placebo treatment for the 3-back minus 0-back conditions of the N-back task for subjects on the scopolamine challenge day (p < .05). Blue colors represent activation that is greater for the placebo treatment group relative to the estradiol treatment group.
B. Activation map for estradiol treatment minus placebo treatment for the 3-back minus 0-back conditions of the N-back task for subjects on the mecamylamine challenge day (p < .05). Orange colors represent activation that is greater for the estradiol treatment group relative to the placebo treatment group.
Muscarinic and nicotinic blockade alone increased frontal lobe activity during working memory, but three months of estradiol treatment resulted in decreased frontal activation for the estradiol group after muscarinic blockade and increased precuneus activation after nicotinic blockade. These data demonstrate that the ability of estradiol to alter cholinergic-related brain activity associated with cognitive processing. While estradiol by itself has been shown to modulate working memory-related brain activation (e.g., (Dumas et al., 2010a; Joffe et al., 2006), it also clearly interacts with the cholinergic system-related cognitive processes. The estradiol effect in this estradiol-cholinergic interaction study was specific to modifying activation associated with the cholinergic manipulations. Thus, consistent with the preclinical data described above, estradiol affected muscarinic- and nicotinic-related cognitive processes specifically.
The current data showed that anticholinergic blockade resulted in an “older” pattern of brain activation (Davis et al., 2008) for postmenopausal women and estradiol treatment appeared to modify this pattern. Estradiol decreased this frontal activation during antimuscarinic challenge and increased posterior activation during antinicotinic challenge. Thus estradiol treatment appeared to alter this pattern to one that is more consistent with the pattern observed in younger adults. A number of rodent (see for a review) and human cognitive studies (Dumas et al., 2008a; Dumas et al., 2006) have shown the importance of the cholinergic system in observing effects of estradiol on cognition, this study was the first to show that working memory-related brain regions are specifically modulated by the estradiol-cholinergic interaction.
Implications and Discussion
A number of conclusions can be drawn from this work thus far. Estradiol appears to have a substantial trophic effect on neuronal growth, development, and survival. These effects are particularly significant in cholinergic neurons of the basal forebrain and regions of the hippocampus important in memory formation. These neurons have critical relevance for the development of age-related cognitive changes as well as dementing disorders such as AD. Estradiol loss impairs cholinergically-mediated performance in animals and estradiol administration can reverse these effects. Changes in estradiol levels after surgical and natural menopause are associated with negative changes in cognitive function and these changes are preventable by estradiol administration. Loss of estradiol support is a risk factor for the development of AD, perhaps particularly in vulnerable, at risk women.
Hormone treatment initiated close to the menopause may provide neuroprotection and reduce risk of neurodegenerative disorders. It appears to be the case that following the loss of hormonal support (e.g. menopause) there is a reappearance of the growth-promoting properties of estradiol on neurons including increased dendritic density and synapse formation which is not seen in the adult animal (Gould et al., 1990; Woolley and McEwen, 1993). Estradiol may influence cell growth and differentiation directly by inducing and regulating growth-related genes (e.g. tubulin or tau) or through interactions with growth factors and their receptors such as the NGF group of peptides (NGF, BDNF, etc) (Lee and McEwen, 2001; Toran-Allerand, 1996). Estradiol may exert permissive effects (Chalbos et al., 1994) on neurotrophin actions by the facilitating expression of neurotrophin-induced intracellular protein kinases whose nuclear targets may also be E2-responsive growth-related genes (Toran-Allerand, 1996).
Salutary effects of estradiol in humans are mediated at least in part through actions on cholinergic systems. These studies strongly suggest that administration of estradiol in humans produces significant changes in cholinergic neurotransmission that manifest themselves in alterations in cognitive performance and related brain activity. As cholinergic dysfunction represents the most widely understood neurochemical mechanism related to cognitive aging and dementia (Dumas and Newhouse, 2011) and is particularly important in the mediation of attentional function and hippocampally-mediated memory processes (Hasselmo and Sarter, 2011), estradiol support of basal forebrain cholinergic function may be critical to maintenance of cognition into old age. We hypothesize that early administration of estradiol may promote the ability of the cholinergic system to compensate for age- or disease-related cortical changes leading to cognitive impairment. Prior data support this thesis and suggest that the linkage between the cholinergic system and estrogen support may be a critical variable in determining the cognitive benefits or lack thereof of postmenopausal hormone treatment.
As studies of the effects of estrogen on cognition have become more selective, a pattern of results is emerging that suggests that initiation of hormone therapy (hormone treatment) improves cognitive performance particularly in younger postmenopausal women, but produces either no benefit or worsening cognitive performance in older postmenopausal women (Maki, 2005b). Studies examining estradiol therapy specifically initiated in older women (70+) have not shown significant benefit (Almeida et al., 2006; Buckwalter et al., 2004; Espeland et al., 2004; Pefanco et al., 2007; Yaffe et al., 2006), whereas studies of hormone treatment in women initiating at midlife or in the early postmenopausal period, tend to show improvement (MacLennan et al., 2006; Shaywitz et al., 2003; Stevens et al., 2005a; Stevens et al., 2005b). This has been formulated as the “critical period hypothesis” (Maki, 2005b; Pinkerton and Henderson, 2005; Sherwin, 2007) suggesting that there is a limited window of time to initiate hormone treatment for an undefined period after menopause if beneficial effects on brain function are to be seen (Singh et al., 2013). Dumas and colleagues have shown (Dumas et al., 2008a) that estrogen effects on enhancing cholinergic-related performance effects on episodic memory may be limited to younger postmenopausal women. These results provide experimental support for the concept of a “critical period” in the early postmenopausal years when hormone administration may have salutary effects on brain function, but that this ability of estrogen to enhance brain function is lost after an indeterminate period of time. These data also suggest that cholinergic system activity may be a major player in the “critical period”. It may be possible that cognitive complaints or performance may be an important proxy marker and may turn out to be a better predictor of response to estradiol then age per se. Thus it may be possible to reformulate the “critical period hypothesis” to be a “critical window hypothesis” with less a window of time, but rather a window of adequate cognitive performance.
Women who notice significant cognitive disruption during the perimenopause or early postmenopausal periods may have a cholinergic system or other brain structures that are more vulnerable due to biological characteristics, early neurodegeneration, or other factors. Thus, the loss of estrogen support with its negative effects on cholinergic function may be more noticeable in terms of cognitive processes, particularly attention and episodic memory. Data from the Seattle Midlife Women’s Health Study (Woods et al., 2000) suggest that nearly half of postmenopausal women report noticeable cognitive symptoms including attention, concentration, and memory problems. Approximately a third of women reported these problems as at least moderate in severity. In terms of the potential for benefits on short and long-term brain function, if the cholinergic system is already damaged, it is possible that these individuals may be less likely to benefit from estradiol as suggested by the “healthy cell bias effect” hypothesis (Brinton, 2008) that suggests that intact neuronal systems are more likely to be benefited by estradiol treatment than damaged ones. However, relatively little work has been done in this area and thus we know little about what characteristics may be important in predicting cognitive improvement with estrogen treatment. While the presence of autonomic symptomatology and sleep disturbance has been examined in terms of its relationship to the cognitive effects of estradiol (LeBlanc et al., 2007; Weber et al., 2013), less is known about the relationship between cognitive symptoms or characteristics, brain morphology, or genetics. Furthermore, relatively little is known in humans about the relationship between cholinergic system integrity and postmenopausal estrogen treatment with regard to these above factors.
Cognitive complaints (“subjective cognitive decline”) in the postmenopausal period may be a useful index of developing cognitive and/or brain dysfunction (Jessen et al., 2014). Older adults with subjective cognitive complaints but normal cognitive performance have been shown to convert to dementia at higher rates than those without complaints (Reisberg et al., 2010) and to have morphologic and functional changes in the brain that may presage developing neurodegenerative disorders (Rodda et al., 2011; Rodda et al., 2009; Saykin et al., 2006). Cognitive complaints have also been associated with the menopause transition (e.g. (Weber and Mapstone, 2009) and the presence of complaints was related to poorer working memory and encoding performance. Data showing objective cognitive impairments after menopause are inconsistent (see (Hogervorst and Bandelow, 2010) for a meta-analysis). Dumas, Newhouse, and colleagues have recently examined the cognitive and neural representation of cognitive complaints in postmenopausal women (Dumas et al., 2013). Middle-aged postmenopausal women with subjective cognitive complaints showed increased activation relative to women with no complaints in working memory-related brain regions as working memory load increased during an N-back task. There were no group differences in working memory performance and thus this increased activation made be a compensation response in these otherwise healthy postmenopausal women with significant cognitive complaints after loss of estradiol.
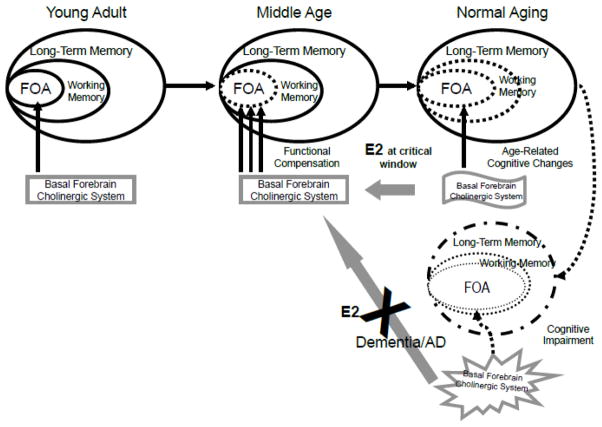
Results of these studies are informative for estradiol-cholinergic interactions. We have proposed that age- and disease-related changes in cholinergic functioning are responsible for cognitive brain activity changes such as those observed in these studies (Dumas and Newhouse, 2011). Our model suggests that cholinergic system activity initially increases in response to developing age-related changes in bottom-up sensory-related (attentional) activity by increasing cortical activity and that neural compensation seen in older adults is related to increased recruitment of the cholinergic system. Administration of estradiol during the “critical window” may serve to support cholinergic functioning in the Dumas and Newhouse model, and may serve to maintain the configuration of cognitive functioning in a more “middle-aged” conformation (Figure 8).
Figure 8. Cholinergic functional compensation model of age-related cognitive dysfunction with the proposed role of estrogen.
The functional compensation model illustrating the effects of aging and cholinergic dysfunction on the focus of attention (FOA). Dotted and grayed lines indicate impairments. When younger adults (left panel) perform attention and memory tasks they show cholinergic modulation of the FOA. Normal cognitive aging (middle panel) may degrade the control processes of the focus of attention thereby affecting working memory and long-term memory. Functional compensation will recruit further activity of the cholinergic system and associated cortical areas to maintain adequate performance on a task. Estradiol (estradiol) treatment during the critical window may help maintain a middle-aged configuration of cognitive performance by maintaining cholinergic basal forebrain functioning. By contrast, estradiol treatment of dementia patients is ineffective because the basal forebrain cholinergic system is severely damaged and does not respond positively to estradiol treatment. Modified from Dumas and Newhouse, 2011.
Pines (Pines, 2007) has suggested that we are already in the “third era” of postmenopausal hormone therapy with individualization of treatment, stressing the prognostic importance of individual parameters, compared to the prior eras, when “one-size-fits-all” was more of the standard. Furthermore, the risks of unselected hormone therapy are better understood. However, for optimization of brain function in older women, continuing studies will be necessary to improve our understanding of the particular cognitive operations that postmenopausal estradiol can be expected to influence, to begin to establish what potential predictors of positive or negative cognitive responses to estradiol treatment will be useful in clinical studies. It will be necessary to examine whether age is the most important factor in determining postmenopausal estradiol-cholinergic responsiveness or whether other factors such as early signs of cognitive dysfunction such as increased cognitive complaints or subtle decreases in cognitive performance might be more useful as guides to therapeutic response or nonresponse. Other potential biomarkers including APOE genotype, markers of amyloid accumulation in CSF or brain amyloid PET imaging may also turn out to be useful tools for determining the potential brain benefits of selective estradiol supplementation.
Pro-cholinergic agents remain the mainstay of cognitive enhancing therapy in aging, mainly directed at patients with Alzheimer’s disease. Whether estradiol supplementation can enhance cholinergic therapies directly to ameliorate normal or pathologic cognitive aging remains a subject for future study. Analysis of some of the initial cholinesterase inhibitor trials revealed that patients taking estradiol supplementation had stronger responses to these agents (Schneider et al., 1996), but negative results from estradiol-alone studies (Mulnard et al., 2000) and studies from the Women’s Health Initiative have discouraged estrogen supplementation in older women with Alzheimer’s disease. Whether combining estradiol supplementation and cholinergic treatment in younger postmenopausal women with early signs of cognitive dysfunction might be beneficial is as yet untested, in part because this phenotype is just beginning to be recognized, and thus studies of a positive potential interaction between estrogen and pro-cholinergic drugs may be useful.
Highlights.
Cholinergic systems are critical for learning and memory formation and attention.
Cognitive changes that occur in aging are related to cholinergic system impairment.
Basal forebrain cholinergic systems depend on estradiol for adequate functioning.
Estradiol enhances cholinergic-mediated cognitive performance in humans.
Combining estrogen and cholinergic treatment may improve cognitive performance.
Acknowledgments
This work was supported by NIA 2R01 AG021476 and AA IIRG-99-1811 to PN, NIA K01 AG 030380 to JD, NCRR-00109, and DoE SC 0001753.
Preparation of this work was supported by 2R01 AG021476 to PN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ábrahám IM, Kőszegi Z, Tolod-Kemp E, Szegő ÉM. Action of estrogen on survival of basal forebrain cholinergic neurons: promoting amelioration. Psychoneuroendocrinology. 2009;34:S104–S112. doi: 10.1016/j.psyneuen.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Tsang CWS, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional Versus Surgical Menopause in a Rodent Model: Etiology of Ovarian Hormone Loss Impacts Memory and the Acetylcholine System. Endocrinology. 2009;150:4248–4259. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P, Erkkola R, Portin R. Estrogen therapy and cognition: A 6-year single-blind follow-up study in postmenopausal women. Neurology. 2007;67:706–709. doi: 10.1212/01.wnl.0000230135.10179.86. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition, and quality of life. Neurobiology of Aging. 2006;27:141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behavioural Brain Research. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Clarendon Press; Oxford: 1986. [Google Scholar]
- Barrett-Connor E, Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. Journal of the American Medical Association. 1993;269:2637–2641. [PubMed] [Google Scholar]
- Bentley P, Husain MK, Dolan RJ. Effects of Cholinergic Enhancement on Visual Stimulation, Spatial Attention, and Spatial Working Memory. Neuron. 2004;41:969–982. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38:137–146. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- Boele FW, Schilder CMT, de Roode ML, Deijen JB, Schagen SB. Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause. 2015;22:17–25. doi: 10.1097/GME.0000000000000271. [DOI] [PubMed] [Google Scholar]
- Bora SH, Liu Z, Kecojevic A, Merchenthaler I, Koliatsos VE. Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Experimental Neurology. 2005;194:506–522. doi: 10.1016/j.expneurol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bretsky PM, Buckwalter JG, Seeman TE, Miller CA, Poirier J, Schellenberg GD, Finch CE, Henderson VW. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Disease & Associated Disorders. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Impact of Estrogen Therapy on Alzheimer’s Disease: A Fork in the Road? CNS Drugs. 2004;18:405–422. doi: 10.2165/00023210-200418070-00001. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends in Neuroscience. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JG, Crooks VC, Robins SB, Petitti DB. Hormone Use and Cognitive Performance in Women of Advanced Age. Journal of the American Geriatric Society. 2004;52:182–186. doi: 10.1111/j.1532-5415.2004.52053.x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic memory retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cardoso CC, Ricardo VP, Frussa-Filho R, Porto CS, Abdalla FMF. Effects of 17β-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor α in rat hippocampus. European Journal of Pharmacology. 2010;634:192–200. doi: 10.1016/j.ejphar.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A. Sex and gender differences in Alzheimer’s disease: recommendations for future research. J Womens Health (Larchmt) 2012;21:1018–1023. doi: 10.1089/jwh.2012.3789. [DOI] [PubMed] [Google Scholar]
- Chalbos D, Philips A, Rochefort H. Genomic cross-talk between the estrogen receptor and growth factor regulatory pathways in estrogen target tissues. Cancer Biology. 1994;5:361–368. [PubMed] [Google Scholar]
- Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the Women’s Health Initiative Memory Study (WHIMS) J Steroid Biochem Mol Biol. 2010;118:304–310. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppus AM, Evenhuis HM, Verberne GJ, Visser FE, Eikelenboom P, van Gool WA, Janssens AC, van Duijn CM. Early age at menopause is associated with increased risk of dementia and mortality in women with Down syndrome. Journal of Alzheimer’s disease: JAD. 2010;19:545–550. doi: 10.3233/JAD-2010-1247. [DOI] [PubMed] [Google Scholar]
- Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neuroscience & Biobehavioral Reviews. 2011;35:1397–1409. doi: 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: The impact of timing. Hormones and Behavior. 2013;63:231–237. doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats whien initiated immediately after ovariectomy but not after a lone-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts S, Raaijmakers J, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The Posterior Anterior Shift in Aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditkoff EC, Crary WG, Cristo M, Lobo RA. Estrogen improves psychological function in asymptomatic postmenopausal women. Obstetrics and gynecology. 1991;78:991–995. [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behavioral Neuroscience. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Witcher JA, Weaver DR, Clemens LG. Alteration of muscarinic binding in specific brain areas following estrogen treatment. Brain Research. 1982;241:347–350. doi: 10.1016/0006-8993(82)91075-7. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: Evidence for the critical period hypothesis. Hormones and Behavior. 2008a;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Hancur-Bucci C, Naylor M, Sites C, Newhouse PA. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal post-menopausal women. Neuropsychopharmacology. 2006;31:2065–2078. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, McDonald BC, Naylor MR, Pfaff AC, Saykin AJ, Newhouse PA. Increased working memory-related brain activity in middle-aged women with cognitive complaints. Neurobiology of aging. 2013;34:1145–1147. doi: 10.1016/j.neurobiolaging.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010a doi: 10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol treatment altered anticholinergic-related brain activation during working memory in postmenopausal women. Neuroimage. 2012;60:1394–1403. doi: 10.1016/j.neuroimage.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol reversal of anticholinergic-related brain activation in postmenopausal women. Neuroimage. 2012;60:1394–1403. doi: 10.1016/j.neuroimage.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, McDonald BC, Saykin AJ, McAllister TW, Hynes ML, West JD, Newhouse PA. Cholinergic modulation of hippocampal activity during episodic memory encoding in postmenopausal women: a pilot study. Menopause. 2010b;17:852–859. doi: 10.1097/gme.0b013e3181e04db9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Newhouse PA. The cholinergic hypothesis of cognitive aging revisited again: Cholinergic functional compensation. Pharmacology, Biochemistry & Behavior. 2011;99:254–261. doi: 10.1016/j.pbb.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Saykin AJ, McDonald BC, McAllister TW, Hynes ML, Newhouse PA. Nicotinic versus muscarinic blockade alters verbal working memory-related brain activity in older women. American Journal of Geriatric Psychiatry. 2008b;16:272–282. doi: 10.1097/JGP.0b013e3181602a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker S, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogen and global cognitive function in postmenopausal women. Journal of the American Medical Assocation. 2004;291:2959–2967. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacology Biochemistry and Behavior. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Estradiol potentiates acetylcholine and glutamate-mediated post-trial memory processing in the hippocampus. Brain Research. 2000;864:263–269. doi: 10.1016/s0006-8993(00)02184-3. [DOI] [PubMed] [Google Scholar]
- Fernandez JW, Grizzell JA, Wecker L. The role of estrogen receptor β and nicotinic cholinergic receptors in postpartum depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40:199–206. doi: 10.1016/j.pnpbp.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Fillit H. Estrogens in the pathogenesis and treatment of Alzheimer’s disease in postmenopausal women. Annals of the New York Academy of Sciences. 1994:233–238. doi: 10.1111/j.1749-6632.1994.tb55795.x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiology of Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic Enhancement and Increased Selectivity of Perceptual Processing During Working Memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen and Nerve Growth Factor- related systems in brain. Annals of the New York Academy of Sciences. 1994;743:165–196. doi: 10.1111/j.1749-6632.1994.tb55792.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. Journal of Neuroscience. 1996;16:1049–1055. doi: 10.1523/JNEUROSCI.16-03-01049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Experimental Neurology. 1998;151:289–302. doi: 10.1006/exnr.1998.6789. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones and Behavior. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen enhancement of learning requires intact basal forebrain cholinergic neutrons. Society of Neuroscience Abstracts. 2001;31:312, 312. [Google Scholar]
- Gibbs RB. Estrogen Therapy and Cognition: A Review of the Cholinergic Hypothesis. Endocrine Reviews. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Hammond R. Role of GPR30 in mediating estradiol effects on acetylcholine release in the hippocampus. Hormones and Behavior. 2014;66:339–345. doi: 10.1016/j.yhbeh.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetytransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Experimental Neurology. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. Journal of Neuroscience. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. Possible acceleration of age effects on cognition following menopause. J Psychiatr Res. 1995;29:153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Research. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Clarkson T, Heward CB, Hecht HS, Karas RH. Is the WHI relevant to HRT started in the perimenopause? Endocrine. 2004;24:195–202. doi: 10.1385/ENDO:24:3:195. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun F, Drott J, Ruben A, Kolb B. Tamoxifen both reduces and prevents cognitive deficits and increases synaptic space in the cortex of aged female rats. Society for Neuroscience. 2001;31:247.210. [Google Scholar]
- Haynes B, Dowsett M. Clinical pharmacology of selective estrogen receptor modulators. Drugs & Aging. 1999;14:323–336. doi: 10.2165/00002512-199914050-00001. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer’s disease in women. Randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas. 2010;66:56–71. doi: 10.1016/j.maturitas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognition function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Tang MX, Stern Y, Sano M, Marder K, Bell KL, Schofield P, Dooneief G, Gurland B, Mayeux R. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- Jacobs EG, Kroenke C, Lin J, Epel ES, Kenna HA, Blackburn EH, Rasgon NL. Accelerated Cell Aging in Female APOE-ε4 Carriers: Implications for Hormone Therapy Use. PLoS ONE. 2013;8:e54713. doi: 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, Pentzek M, Riedel-Heller SG, Luck T, Fuchs A, Weyerer S, Werle J, van den Bussche H, Scherer M, Maier W, Wagner M. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimer’s & Dementia. 2014;10:76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kandi P, Hayslett RL. Nicotine and 17β-estradiol produce an antidepressant-like effect in female ovariectomized rats. Brain Research Bulletin. 2011;84:224–228. doi: 10.1016/j.brainresbull.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s affect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Kimura D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Hormones and Behavior. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Estrogen effects on neural responsiveness to electrical and neurotransmitter stimulation: an in vitro study on the ventromedial nucleus of the hypothalamus. Brain Research. 1985;347:1–10. doi: 10.1016/0006-8993(85)90883-2. [DOI] [PubMed] [Google Scholar]
- Krug R, Born J, Rasch B. A 3-day estrogen treatment improves prerontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology. 2006;31:965–975. doi: 10.1016/j.psyneuen.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G. Dynamic integration: Affect, cognition, and the self in adulthood. Current Directions in Psychological Science. 2003;12:201–206. [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiology of Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. Journal of the American Medical Association. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause. 2007;14:191–202. doi: 10.1097/01.gme.0000230347.28616.1c. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annual Review of Pharmacology & Toxicology. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- Levey AI. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proceedings of the National Academy of Sciences. 1996;93:13541–13546. doi: 10.1073/pnas.93.24.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual review of psychology. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Luine VN, Khylchevskaya RI, McEwen BS. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Research. 1975;86:293–306. doi: 10.1016/0006-8993(75)90704-0. [DOI] [PubMed] [Google Scholar]
- Luine VN, Renner KJ, McEwen BS. Sex-dependent differences in estrogen regulation of choline acetyltransferase are altered by neonatal treatments. Endocrinology. 1986;119:874–848. doi: 10.1210/endo-119-2-874. [DOI] [PubMed] [Google Scholar]
- Luine VN, Rhodes JC. Gonadal hormone regulation of MAO and other enzymes in hypothalamic areas. Neuroendocrinology. 1983;36:235–241. doi: 10.1159/000123461. [DOI] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathia J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Maki P, Hogervorst E. HRT and cognitive decline. Best Practice and Research Clinical Endocrinology and Metabolism. 2003;17:105–122. doi: 10.1016/s1521-690x(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Maki PM. Estrogen effects on the hippocampus and frontal lobes. International Journal of Fertility and Women’s Medicine. 2005a;50:67–71. [PubMed] [Google Scholar]
- Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Annals of the New York Academy of Science. 2005b;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, Singer CA, Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female sprague-dawley rat. Journal of Neuroscience. 1996;16:1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga SE, Gerson JE, Koebele SV, Kingston ML, Tsang CWS, Engler-Chiurazzi EB, Baxter LC, Bimonte-Nelson HA. Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: Tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrinology. 2015;54:1–13. doi: 10.1016/j.psyneuen.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen RA, Kalesnykas G, Koivisto EH. Estimation of the total number of cholinergic neurons containing estrogen receptor-á in the rat basal forebrain. Journal of Histochemistry and Cytochemistry. 2002;50:891–902. doi: 10.1177/002215540205000703. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder M, Assag R, Panarella R, Tousignant P, Franklin KBJ. Estrogen modulates spontaneous alternation and to the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RSJ, Rugg MD. Age effects on the neural correlates of sucessful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Rodriguez-Sierra JF, Clough RW. Increase in hypothalamic nicotine acetylcholine receptors in prepuberal female rats administered estrogen. Brain Research. 1983;278:262–265. doi: 10.1016/0006-8993(83)90250-0. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Dills J, Cochran EJ, Leurgans S, Wuu J, Bennett DA, Jaffar S, Gilmor ML, Levey AI, Kordower JH. Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer’s disease. Journal of Comparative Neurology. 2002;443:136–153. doi: 10.1002/cne.10122. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. Journal of the American Medical Association. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Albert K, Astur R, Johnson J, Naylor M, JAD Tamoxifen improves cholinergically-modulated cognitive performance in postmenopausal women. Neuropsychopharmacology. 2013;38:2632–2643. doi: 10.1038/npp.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Newhouse C, Astur RS. Sex differences and visuospatial learning using a virtual maze in pre-pubertal children. Behavioral Brain Research. 2007 doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter AS, Dumas JA, Thiel CM. Functional brain imaging of nicotinic effects on higher cognitive processes. Biochemical Pharmacology. 2011;82:943–951. doi: 10.1016/j.bcp.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thomason K, Mellow AM, Cohen RM, Murphy DL. The effects of acute scopolamine in geriatric depression. Archives of General Psychiatry. 1988;45:906–912. doi: 10.1001/archpsyc.1988.01800340028004. [DOI] [PubMed] [Google Scholar]
- Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, Murphy DGM. Estrogen Therapy and brain muscarinic receptor density in healthy females: A SPET study. Hormones and Behavior. 2007;51:249–257. doi: 10.1016/j.yhbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akawaz K. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer’s type. Endocrine Journal. 1994;41:361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. American journal of epidemiology. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Panidis DK, Matalliotakis IM, Rousso DH, Kourtis AI, Koumantakis EE. The role of estrogen replacement therapy in Alzheimer’s disease. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2001;95:86–91. doi: 10.1016/s0301-2115(00)00373-0. [DOI] [PubMed] [Google Scholar]
- Pefanco MA, Kenny AM, Kaplan RF, Kuchel G, Walsh S, Kleppinger A, Prestwood K. The effect of 3-year treatment with 0.25 mg/day of micronized 17beta-estradiol on cognitive function in older postmenopausal women. Journal of the American Geriatrics Society. 2007;55:426–431. doi: 10.1111/j.1532-5415.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Pines A. Postmenopausal hormone therapy: The way ahead. Maturitas. 2007;57:3–5. doi: 10.1016/j.maturitas.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Henderson VW. Estrogen and cognition, with a focus on Alzheimer’s disease. Seminars in Reproductive Medicine. 2005;23:172–179. doi: 10.1055/s-2005-869485. [DOI] [PubMed] [Google Scholar]
- Polo-Kantola P, Portin R, Polo O, Helenius H, Irjala K, Erkkola R. The effect of short-term estrogen replacement therapy on cognition: a randomized, double-blind, crossover trial in postmenopausal women. Obstetrics & Gynecology. 1998;91:459–466. doi: 10.1016/s0029-7844(97)00700-x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic Estrogen Replacement Improves Cognitive Function in Aged Overiectomized Rhesus Monkeys. The Journal of Neuroscience. 2003a;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. Journal of the American Medical Association. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: A critical time. Journal of the American Medical Association. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory: a possible protective effect. Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Kim S, Shen L, Nho K, Foroud T, Green RC, Petersen RC, Jack CR, Jr, Aisen PS, Koeppe RA, Jagust WJ, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ Alzheimer’s Disease Neuroimaging Initiative d. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI) Front Aging Neurosci. 2013;5:11. doi: 10.3389/fnagi.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda J, Dannhauser T, Cutinha DJ, Shergill SS, Walker Z. Subjective cognitive impairment: Functional MRI during a divided attention task. European psychiatry: the journal of the Association of European Psychiatrists. 2011;26:457–462. doi: 10.1016/j.eurpsy.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Rodda JE, Dannhauser TM, Cutinha DJ, Shergill SS, Walker Z. Subjective cognitive impairment: increased prefrontal cortex activation compared to controls during an encoding task. Int J Geriatr Psychiatry. 2009;24:865–874. doi: 10.1002/gps.2207. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E affects the rate of Alzheimer disease expression: b-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. Journal of Neuropathology and Experimental Neurology. 1994;53:429–437. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature neuroscience. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, van Dam FSAM, Schagen SB. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncologica. 2009;48:76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Farlow MR, Henderson VW, Pogoda JM. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology. 1996;46:1580–1584. doi: 10.1212/wnl.46.6.1580. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Naftolin F, Zelterman D, Marchione KE, Holahan JM, Palter SF, Shaywitz BA. Better oral reading and short-term memory inmidlife, postmenopausal women taking estrogen. Menopause. 2003;10:420–426. doi: 10.1097/01.GME.0000060241.02837.29. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Hormones, mood, and cognitive functioning in postmenopausal women. Obstetrics and gynecology. 1993;87:20S–26S. doi: 10.1016/0029-7844(95)00431-9. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature. Journal of Neuroendocrinology. 2007;19:88–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. Journal of the American Medical Association. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Silva I, Mello LE, Freymüller E, Haidar MA, Baracat EC. Onset of estrogen replacement has a critical effect on synaptic density of CA1 hippocampus in ovariectomized adult rats. Menopause. 2003;10:406–411. doi: 10.1097/01.GME.0000064816.74043.E9. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Huang FS, Millard WJ, Simpkins JW. Ovariectomy reduced ChAT activity and NGF mRNA levels in the frontal cortex and hippocampus of the female Sprague-Dawley rat. Society for Neuroscience Abstracts. 1993;19:514.511. [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Research. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor mRNA expression in brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Singh M, Simpkins JW, Bimonte-Nelson HA, Brinton RD. Window of opportunity for estrogen and progestin intervention in brain aging and Alzheimer’s disease. Brain research. 2013;1514:1–2. doi: 10.1016/j.brainres.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Bowen L, Love TM, Berent-Spillson A, Frey KA, Persad CC, Reame NK, Koeppe RA, Zubieta JK. Early initiation of hormone therapy in menopausal women is associated with increased hippocampal and posterior cingulate cholinergic activity. The Journal of Clinical Endocrinology & Metabolism. 2011;96:E1761–E1770. doi: 10.1210/jc.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Giordani B, Lajiness-O’Neill R, Zubieta J. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertility and Sterility. 2001a;76:1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nicols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. Journal of Clinical Endocrinology & Metabolism. 2006;91:4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Minoshima S, Kuhl DE, Zubieta JK. Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 2001b;86:679–684. doi: 10.1210/jcem.86.2.7222. [DOI] [PubMed] [Google Scholar]
- Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Cocchiaro A, Rosen B, Lake S, Lange N, Routledge C, Albert M. Functional MRI detection of pharmacologically induced memory impairment. Proceeds of the National Academy of Science. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens Clark, Prestwood Low-dose estradiol alters brain activity. Psychiatry Research: Neuroimaging. 2005a;139:199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Clark VP, Prestwood KM. Low-dose estradiol alters brain activity. Psychiatry Research. 2005b;139:199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Tariot P, Weingartner H, Murphy D, Newhouse P, Mueller E, Cohen R. Pharmacologic modeling of Alzheimer’s disease. Progress in Neuropsychopharmacology and Biological Psychiatry. 1986;10:599–610. doi: 10.1016/0278-5846(86)90030-8. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Tariot PN, Newhouse PA. Differential responsivity of mood, behavior, and cognition to cholinergic agents in elderly neuropsychiatric populations. Brain Research Reviews. 1988;13:371–389. doi: 10.1016/0006-8993(88)91227-9. [DOI] [PubMed] [Google Scholar]
- Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. Journal of Neuroscience. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe F, Miyasaka N, Kubota T, Aso T. Estrogen and progesterone improve scopolamine-induced impairment of spatial memory. Journal of Medical & Dental Sciences. 2004;51:89–98. [PubMed] [Google Scholar]
- Tanaka K, Tamura T, Kawashima M, Ueda S, Matsumoto Y, Kawata M, Ogino Y, Yamamoto T, Honjo H, Okada H. The histochemical study of the effects of estrogen on the forebrain cholinergic neurons of fetal female rats transplanted into the anterior eye chamber of adult female rats. Folia Endocrinologica Japonica. 1993;69:534–540. doi: 10.1507/endocrine1927.69.5_534. [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of estrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Thiel CM. Cholinergic modulation of learning and memory in the human brain as detected with functional neuroimaging. Neurobiology of Learning and Memory. 2003;80:234–244. doi: 10.1016/s1074-7427(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cholinergic modulation of visuospatial attention: an event-related fMRI study with pharmacological challenge. 2003 doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. The estrogen/neurotrophin connection during neural development: is co-localization of estrogen receptors with the neurotrophins and their receptors biologically relevant? Developmental Neuroscience. 1996;18:36–48. doi: 10.1159/000111393. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proceedings of the National Academy of Science. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Katz MJ, Sliwinski M, Crystal HA, Buschke H, Lipton RB. Cognitive performance in surgically menopausal women on estrogen. Neurology. 2000;55:872–874. doi: 10.1212/wnl.55.6.872. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neuroscience Biobehavioral Reviews. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: a quantitative integration of research findings. Journal of Gerontology. 1993;48:P157–P171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience. 2008;154:1205–1217. doi: 10.1016/j.neuroscience.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Mapstone M. Memory complaints and memory performance in the menopausal transition. Menopause. 2009;16:1–7. doi: 10.1097/gme.0b013e318196a0c9. [DOI] [PubMed] [Google Scholar]
- Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013 doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257–265. [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yaffe K. Hormone therapy and the brain: déjà vu all over again? Journal of the American Medical Association. 2003;289:2717–2719. doi: 10.1001/jama.289.20.2717. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui L, Grady D, Massa S, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biological Psychiatry. 2002;51:677–682. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, Grady D. Effects of ultra-low dose transdermal estradiol on cognition and health-related quality of life. Archives of Neurology. 2006;63:945–950. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kitawaki J, Kikuchi N, Okubo T, Iwasa K, Kawata M, Honjo H. Effects of estrogens on cholinergic neurons in the rat basal nucleus. The Journal of Steroid Biochemistry and Molecular Biology. 2007;107:70–79. doi: 10.1016/j.jsbmb.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women. Journal of the American Medical Association. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of BDNF and CREB expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]