Abstract
The fungal genus Candida encompasses numerous species that inhabit a variety of hosts, either as commensal microbes and/or pathogens. Candida species are a major cause of fungal infections, yet to date there are no vaccines against Candida or indeed any other fungal pathogen. Our knowledge of immunity to Candida mainly comes from studies on C. albicans, the most frequent species associated with disease. However, non-albicans Candida (NAC) species also cause disease and their prevalence is increasing. Although research into immunity to NAC species is still at an early stage, it is becoming apparent that immunity to C. albicans differs in important ways from non-albicans species, with important implications for treatment, therapy and predicted demographic susceptibility. This review will discuss the current understanding of immunity to NAC species in the context of immunity to C. albicans, and highlight as-yet unanswered questions.
Introduction
Fungi constitute a poorly understood and comparatively under-studied (and under-funded) form of infectious disease. To date, there are no vaccines to any fungal pathogens, and the correlates of immunity are not well defined. However, fungal infections are on the rise, in part due to increasing populations of immunocompromised individuals [1]. Candida species comprise the second most frequent cause of fungal infections worldwide. The Candida genus contains multiple species that show considerable phylogenetic and phenotypic variation. Our knowledge of immunity to Candida has almost exclusively been gleaned from studies on C. albicans, the most common disease-causing species. However, the prevalence of disease caused by non-albicans Candida (NAC) species is on the rise, and our understanding of immunity to these species is the subject of this review.
Immunity to C. albicans has been studied intensively over the last decade, and a general picture of the essential components is now accepted [2]. Broadly, C. albicans is initially detected by C-type lectin receptors (CLRs), such as dectin-1, expressed dominantly on myeloid antigen presenting cells. In addition, an important antifungal contribution of epithelial cells is becoming appreciated, particularly during mucosal infection. Following fungal encounter, responding cells produce innate immune cytokines such as TNFα and IL-1β. These cytokines drive innate antifungal effector responses and trigger skewing of adaptive T cells to dominantly Th17 and Th1 populations. The Th1 and Th17 hallmark cytokines, IFNγ and IL-17, in turn act on neutrophils and macrophages to further amplify antifungal responses. Although this model is well substantiated for C. albicans, it is much less clear whether a similar picture is true of immunity to NAC species.
Infections caused by Candida species
Fungi belonging to the genus Candida are normally found as commensal organisms on mucosal and cutaneous surfaces throughout the human body. Only a subset of species are associated with disease, which include C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. krusei and C. dubliniensis [3, 4]. Mucocutaneous Candida infections are often mild or self-limiting, such as oral and vaginal candidiasis/thrush. However, these superficial infections can be associated with significant morbidity, such as in chronic mucocutaneuous candidiasis (CMC) and recurrent vaginal candidiasis. Additionally, Candida species cause potentially fatal systemic infection, where mortality rates are reported up to 80%. Candida species have also been associated with inflammatory bowel disease and asthma, though the link is not directly causal and is likely to be an exacerbating effect.
Although C. albicans remains the most frequently isolated species, the prevalence of NAC species is on the rise [4]. Risk factors for candidiasis vary by species. For example, C. glabrata is particularly associated with oral thrush in the elderly and denture wearers, whereas C. dubliniensis is frequently isolated from HIV+/AIDS individuals with oral thrush [5]. Neonates, transplant recipients and patients receiving parenteral nutrition are at increased risk of C. parapsilosis infection compared to other Candida species. Furthermore, geographical differences in Candida species prevalence are apparent. C. albicans and C. glabrata are prominent in North America and Europe, while C. tropicalis is typically the most frequently isolated Candida species in India and Latin America [6].
With the worldwide rise in fungal infections comes an increase in antifungal drug resistance [7]. Worryingly, antifungal drug resistance has been detected for all clinically relevant Candida species to some degree [6]. Moreover, the pattern of antifungal drug resistance differs among Candida species, making effective treatment with appropriate antifungal drugs challenging. A particular problem is present with C. glabrata, which is resistant to the most common drug classes, azoles and echinocandins [6–9].
The reasons underlying differences in Candida species prevalence and antifungal drug resistance are unclear. However, Candida species are heterogeneous, so understanding their phylogenetic differences may help to explain, and ultimately address, these disparities. The most closely related species are C. albicans, C. dubliniensis and C. tropicalis whereas C. glabrata is more closely related to Saccharomyces cerevisiae [10]. Accordingly, the agglutinin-like sequence (Als) cell wall virulence genes are found in C. albicans, C. dubliniensis and C. tropicalis but not C. glabrata [11]. Substantial differences exist even among the most related Candida species. For example, Als3 is specifically expressed by C. albicans but not C. dubliniensis [12]. Genomic divergence among Candida species thus results in considerable phenotypic variation within the Candida genus.
Phenotypic differences among Candida species and consequences for immunity
Although Candida species cause grossly similar infections, multiple phenotypic variations exist, including include morphology, cellular size, cell wall composition, growth requirements and virulence factor composition (Table 1) [13, 14]. Each of these alterations may contribute to the development of a distinct immune response. Therefore, it cannot be assumed that a ‘one size fits all’ immune response to Candida species is operative.
Table 1. Phenotypic differences among Candida species.
Clinically relevant Candida species vary in morphological capacity, fermentative growth ability, yeast cell size and virulence factor composition.
| Candida species | Morphology | Fermentation | Yeast cell size | Sap virulence factors | ||||
|---|---|---|---|---|---|---|---|---|
| Yeast | Pseudohyphae | Hyphae | Glucose | Maltose | Galactose | Diameter (μM) | Number | |
| albicans | + | + | + | + | + | V | 4–10 | 10 |
| dubliniensis | + | + | + | + | + | V | 4–10 | 8 |
| tropicalis | + | + | + | + | + | + | 5–11 | 4 |
| parapsilosis | + | + | − | + | − | V | 3–9 | 3 |
| krusei | + | + | − | + | − | − | 3–10 | ? |
| glabrata | + | +/− | − | + | − | − | 2–4 | ? |
Yeast cell size is approximate.
Sap = secreted aspartyle proteinase. V = variable.
Cell wall composition
The Candida cell wall is composed of an inner layer of chitin and β-1,3-glucan polysaccharides and an outer layer of mannans covalently associated with proteins [15, 16]. Many of the known pathogen-associated molecular patterns (PAMPs) derive from the cell wall [17], highlighting the importance of this structure in defense against Candida. Indeed, differences in cell wall composition and Candida recognition are known to impact the immune response. For example, variations in antigenic cell wall-associated proteins were detected among C. albicans, C. tropicalis and C. guilliermondii [18]. Moreover, a recent study identified a novel antigenic cell wall-associated protein of C. tropicalis, Kgd2p [19]. Although not tested in this study, the presence of species-specific antigenic proteins indicates that Candida species promote distinct immune responses. Ultrastructure analysis of C. glabrata versus C. albicans cell walls revealed ~50% more proteins, higher amounts of mannan and lower levels of total glucan in C. glabrata cell walls [20]. Conversely, C. albicans, C. tropicalis and C. parapsilosis contain higher chitin content than C. glabrata and C. krusei [21]. It has yet to be determined how or whether these cell wall differences among Candida species impact immunity.
Candida species morphology
C. albicans is polymorphic, existing in a unicellular yeast cells form, pseudohyphae and/or filamentous hyphae, and the transition between morphologies is a key virulence trait [17]. However, not all Candida species are polymorphic. Growth as true filamentous hyphae is usually associated only with C. albicans and C. dubliniensis. Some strains of C. tropicalis can also form true hyphae (Table 1), although many do not in vitro [22, 23]. Similarly, in vitro studies have shown that C. parapsilosis, C. krusei and C. glabrata do not form hyphae, though they have been reported to form pseudohyphae [24–27]. C. albicans can also switch between normal white yeast cell morphology and mating-competent opaque cell growth. Although white-opaque switching has been documented in other Candida species such as C. dubliniensis and C. tropicalis, this morphological switch is poorly understood in NAC species [28, 29].
Pattern Recognition
C. albicans is recognized by different classes of PRRs. The C-type lectin receptors (CLRs) are the most important, and include dectin-1, dectin-2, dectin-3, mannose receptor (MR) and Mincle [30]. Although little is known about the PRRs involved in recognition of NAC species, several insights have begun to emerge.
Dectin-1 is a key antifungal receptor in host defense against C. albicans infection. Dectin-1 recognition of C. albicans triggers phagocytosis, cytokine and chemokine production, reactive oxygen species (ROS) production and neutrophil extracellular trap (NET) formation [31, 32]. Dectin-1−/− mice display heightened susceptibility to disseminated and mucosal C. albicans infection, although this varies by strain of fungus and genetic background of the host [33–36]. Similarly, loss-of-function DECTIN1 mutations in humans are associated with increased Candida species colonization at mucosal surfaces and higher risk of Candida infection, as well as susceptibility to CMC [37, 38]. Some evidence supports a protective role for dectin-1 against C. tropicalis at mucosal and systemic sites. Dectin1−/− mice are more susceptible to colitis induced by dextran sulfate sodium (DSS) colitis, which was associated with resident C. tropicalis overgrowth and tissue invasion [39]. We recently demonstrated that Dectin1−/− mice were more susceptible to disseminated C. tropicalis infection than WT mice [22]. Little is known about whether dectin-1 participates in immunity to other NAC species, although phagocytosis of C. parapsilosis by neutrophils was not impaired following dectin-1 blockade in vitro [40]. Similarly, no difference in binding of C. glabrata was detected between WT and dectin-1−/− bone marrow macrophages [41].
Dectin-2 is another CLR that functions in many ways similarly to dectin-1. Dectin2−/− mice display increased susceptibility to disseminated C. albicans infection [42]. Concerning other Candida species, disseminated C. glabrata infection in Dectin2−/− mice was associated with a transient increase in kidney fungal burden and concomitant decreases in splenic TNFα, IFNγ and IL-17A production [43]. Additionally, Dectin2−/− macrophages and neutrophils were impaired in phagocytosis of C. glabrata, although killing was not affected [43]. However, given that WT or Dectin2−/− mice infected with C. glabrata do not succumb to disseminated infection, it is difficult to ascertain the importance of this CLR in protection against lethal C. glabrata infection. In contrast, Dectin2−/− mice were not impaired in their ability to survive a disseminated C. tropicalis infection [22]. Therefore, recognition of Candida species is far from uniform.
Another PRR garnering attention in the context of C. albicans infection is galectin-3. This soluble lectin receptor is found in many cell types, and possesses direct antifungal activity [44]. Lgals3−/− mice display increased mortality following disseminated C. albicans infection [45], demonstrating a role in antifungal immunity in vivo. Galectin-3 also appears to be involved in immunity to several NAC species. Both C. albicans and C. tropicalis were shown to induce secretion of galectin-3 by human gingival epithelial cells [46]. Moreover, galectin-3 directly kills C. albicans and C. glabrata in vitro. However, not all Candida species are targeted, since no effects were detected on C. guilliermondii [44]. In a model of disseminated C. parapsilosis infection, no difference in mortality was detected between WT and Lgals3−/− mice. However, Lgals3−/− mice had elevated kidney fungal burdens, suggesting this lectin receptor is required for control of C. parapsilosis in vivo [45]. The role of galectin-3 in defense against additional NAC species remains to be determined.
Several different PRRs recognize microbes simultaneously in the context of the immune response to a large organism. Indeed, an interaction between dectin-1 and galectin-3 on macrophages in response to C. albicans appears to be required for optimal TNFα production [47]. Dectin-1 also cooperates with TLR2 to induce maximal downstream responses following zymosan stimulation [48]. One study reported that dectin-2 and dectin-3 synergize to trigger enhanced NF-κB activation following C. albicans stimulation [49]. Together, these in vitro studies indicate that signaling through multiple PRRs mediates optimal antifungal immunity in vivo. In this regard, mice lacking downstream signaling molecules used by several PRRs tend to be profoundly more susceptible to C. albicans infection than mice deficient in individual receptors. This concept is exemplified by CARD9, an adaptor activated by numerous CLRs, including dectin-1, dectin-2, dectin-3 and Mincle [49, 50]. CARD9−/− mice are severely susceptible to disseminated C. albicans infection [51]. Moreover, humans with mutations in CARD9 present with severe CMC and systemic candidiasis, as well as other fungal infections [52–54]. Several Candida species were found responsible for candidiasis in these patients, including C. albicans, C. dubliniensis and C. glabrata. Along these lines, we observed that Card9−/− mice were profoundly more susceptible to disseminated C. tropicalis infection than Dectin1−/− mice [22], providing evidence that PRR cooperation is a requirement for immunity to NAC species.
The influence of Candida morphology on recognition
Fungal morphogenesis involves changes in cell wall composition, and therefore C. albicans morphotypes expose different putative recognition factors. For example, Als3 and hyphally regulated protein 1 (Hyr1) are hypha-specific cell wall proteins that contribute to C. albicans resistance to host defense mechanisms. Als3 is involved in adhesion and invasion of host cells and is also a receptor for ferritin, thus mediating iron acquisition [55]. Hyr1 helps resist phagocyte killing [56]. Moreover, both factors are vaccine targets, as vaccination of mice with recombinant Als3 or Hyr1 proteins improves clearance of C. albicans [57, 58]. Indeed, an experimental vaccine in clinical trials for vulvo-vaginal candidiasis (VVC) is based on Als3 [59, 60].
Not surprisingly, different C. albicans morphotypes induce altered downstream immune responses. For example, C. albicans yeast cells induce IL-12 in dendritic cells (DCs), whereas hyphae promote IL-4 production [61]. In macrophages, C. albicans yeast but not hyphae induce IFNγ production [62]. In another study, hyphae but not yeast-locked forms of C. albicans triggered IL-1β production by macrophages, which was associated with reduced mannan fibrils expression in hyphae compared to yeast cells [63]. Epithelial cells also respond differently to C. albicans yeast and hyphae. Yeast cells promote a tolerogenic epithelial cell response, whereas inflammatory response stimulated upon recognition of invasive hyphae [64, 65]. Moreover, C. albicans yeast cells and hyphae are differentially recognized by dectin-1 and dectin-2 [35, 66, 67]. White-opaque switching is another morphological switch that impacts fungal recognition. One study demonstrated that neutrophils phagocytose white but not opaque cells in vitro [68].
The ability of different C. albicans morphologies to influence the immune response is controversial [34, 69–71]. There are contrasting reports on the ability of yeast and hyphal growth forms to activate downstream dectin-2 responses or promote Th17 responses [42, 63, 69, 71, 72], which may be explained by differences in fungal strains. Alternatively differences between in vitro and in vivo experimental conditions may be important, since the fungal cell wall is dynamic and the availability of C. albicans PAMPs differs markedly in vitro compared to in vivo settings [34, 70].
While our knowledge on the impact of C. albicans morphology on immunity is expanding, this area is yet to be probed with respect to other Candida species. However, it is plausible that the varying morphologies of NAC species also drive altered immune responses. Given the importance of morphogenesis in C. albicans pathogenicity, understanding the impact of other Candida species morphotypes on immune responses may prove an important avenue of research.
Cellular immunity to Candida species: the first line of defense
Neutrophils
Neutrophils are crucial components of immunity to both mucosal and systemic C. albicans infection [73, 74]. They are the first cell type to be recruited to sites of C. albicans infection and are regarded as the most potent cell type in killing the fungus. In humans, neutropenia is a major risk factor for systemic candidiasis and individuals with dysfunctional neutrophils are defective in C. albicans killing [75]. Depleting neutrophils renders mice highly susceptible to oral and disseminated C. albicans infection [74, 76]. Furthermore, neutrophils are involved in preventing dissemination of C. albicans from the gut [77].
Neutropenia is also a risk factor for invasive candidiasis caused by NAC species, such as C. tropicalis and C. krusei [78] [79–81]. Strikingly, invasive C. tropicalis infection is associated with higher mortality rates compared to C. albicans infection, though the basis for this is unclear [82]. A crucial role for neutrophils in host defense against disseminated C. tropicalis has been confirmed in mouse models [22, 83]. Moreover, reduced neutrophil responses during intra-abdominal C. glabrata infection were associated with increased peritoneal fluid fungal burden [84]. Therefore, neutrophils appear to be key components of antifungal immunity against most Candida species.
Numerous studies have investigated neutrophil phagocytosis and downstream responses of different Candida species, primarily in vitro. Several NAC species appear to be killed more efficiently than C. albicans [40, 85–87]. For example, killing of C. tropicalis, C. parapsilosis, C. krusei and C. glabrata by human neutrophils was higher than C. albicans killing [85, 86]. In this regard, C. albicans induced more neutrophil cell death compared to C. glabrata [88], suggesting that the observed differences may be due in part to an enhanced capacity of C. albicans to kill neutrophils. Indeed, increased phagocytosis of C. dubliniensis relative to C. albicans uptake by human neutrophils was associated with reduced neutrophil damage, as well as elevated expression of neutrophil killing mechanisms such ROS and lactoferrin [89]. However, not all studies demonstrated a difference in neutrophil phagocytosis and killing of Candida species. For example, phagocytosis of serum-opsonized C. albicans, C. tropicalis, C. parapsilosis and C. glabrata by neutrophils was similar [90]. Another study showed that C. krusei was phagocytosed less efficiently than C. albicans by human neutrophils [91]. Interestingly, C. parapsilosis may be more resistant to damage by neutrophils than C. albicans in some settings [87]. Overall, it is clear that neutrophils respond to Candida species differently, though the mechanisms responsible for these differences still remain poorly understood.
Monocytes/Macrophages
Monocytes/macrophages can directly kill C. albicans, and these cells produce cytokines and chemokines required for immune defense. Mice deficient in monocytes or tissue-resident macrophages display increased susceptibility to disseminated C. albicans infection [92–94].
Moreover, individuals with mutations in CX3CR1, the signature chemokine receptor for tissue resident macrophages, were shown to be at increased risk of systemic candidiasis [95]. More efficient phagocytosis and killing of certain NAC species compared to C. albicans by macrophages has been reported, similar to neutrophils. C. parapsilosis is killed more efficiently than C. albicans, a process that involves production of oxygen radicals [96, 97]. Similarly, C. glabrata is phagocytosed at higher rates by macrophages than C. albicans, which was more lethal to macrophages [88, 97, 98]. In this regard, macrophage phagocytosis rate of C. albicans is dependent on fungal morphology, and C. albicans hyphae can lyse macrophages [98–100]. Therefore, differences in phagocytosis and killing of Candida species by macrophages may partly depend on Candida morphogenesis. Interestingly, C. glabrata can survive and replicate within macrophages, and be released intact [101]. This survival strategy of C. glabrata is based on intrinsic stress resistance and nutrient acquisition, and illustrates differences in the interaction of different Candida species with immune cells [102].
Not much is known regarding the physiological requirement of monocytes/macrophages in controlling NAC species. We observed that monocyte or macrophage depletion with clodronate liposomes increased the susceptibility of WT mice to disseminated C. tropicalis infection [22]. However, the effects were less profound than neutrophil depletion, suggesting that neutrophils are the dominant cell type required for protection against systemic infection. Notably, depletion of both neutrophils and monocytes by anti-Gr1 Ab treatment significantly increased susceptibility to infection compared to depletion of neutrophils or monocytes/macrophages alone. Therefore, the combined actions of neutrophils and monocytes are likely to be central to antifungal immunity against systemic C. tropicalis infection (Figure 1).
Figure 1. Model of immunity to disseminated C. tropicalis infection.
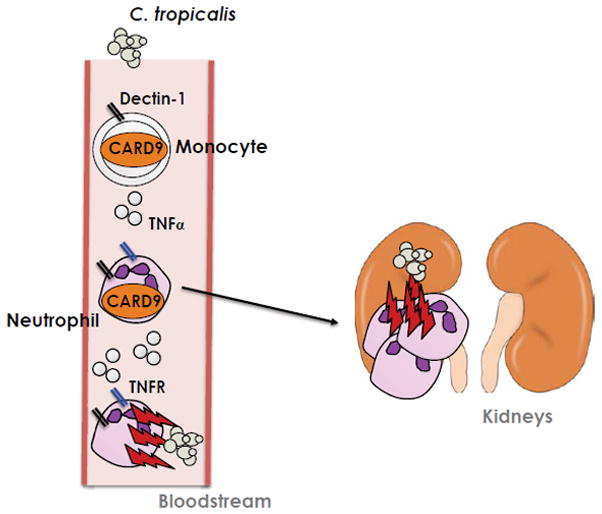
C. tropicalis is rapidly recognized by neutrophils and monocytes following invasive infection. Recognition by dectin-1 and other PRRs activates CARD9, which is crucial for host defense against C. tropicalis. CARD9 activation triggers the production of TNFα by both neutrophils and monocytes, which acts upon neutrophils to augment antifungal killing of C. tropicalis. TNFR = TNFα receptor.
Dendritic cells
Although DCs can phagocytose and kill C. albicans, their primary role in antifungal immunity is to direct adaptive immune responses [103]. DCs produce cytokines involved in helper Th cell differentiation in response to C. albicans, which is dependent on C. albicans morphology and DC subset [61, 71]. A crucial role for DCs in immunity to C. albicans was recently demonstrated using CD11c-specific deletion of Syk. Syk is a kinase activated by CLRs acting upstream of CARD9, and its loss in DCs rendered mice more susceptible to disseminated C. albicans infection. Notably, this study indicated that DC cooperation with NK cells and neutrophils was required for protective immunity against C. albicans [104], suggesting that DCs perform important antifungal functions aside from their ability to promote adaptive immune responses.
With respect to NAC species, one report showed that C. albicans, C. dubliniensis and C. glabrata induced IFNβ expression by BMDCs, with C. glabrata inducing the highest levels [105]. Moreover, differences in generation of the DC “fungipod”, a dorsal pseudopodial protrusion involved in DC function, were demonstrated among Candida species. C. parapsilosis displayed strong induction of fungipods compared to C. albicans and C. tropicalis [106]. However, little else is known about the activities of DCs in response to NAC species.
Epithelial cells
Epithelial cells are increasingly being appreciated as key components of immune responses. They are of particular significance for mucocutaneous Candida infections, where fungi normally reside as commensal microbes. Epithelial cells can phagocytose C. albicans. However, this does not result in killing of the fungus, and in fact has been shown to damage endothelial cells [107]. Oral and vaginal epithelial cells possess candidastatic capacity, which is cell-contact dependent [108–111]. Epithelial cells can also produce cytokines, chemokines and antimicrobial proteins, such as IL-6, IL-8, TNFα, CCL2 and S100A9, in response to C. albicans [112–114]. Moreover, epithelial cells can augment neutrophil antifungal activity in vitro [115], suggesting that these cell types are important in promoting optimal antifungal immunity, particularly at mucosal surfaces.
In general, NAC species induce weak cytokine and chemokine responses in epithelial cells. For example, C. albicans was able to induce efficient expression of IL-6, IL-8, CCL2 and adhesion molecules by endothelial cells in vitro, whereas C. tropicalis and C. glabrata did not [113]. Similarly, human oral epithelial cells produced GM-CSF and other pro-inflammatory cytokines in response to C. albicans, but to a much lower degree or not at all in response to C. tropicalis or C. glabrata [116]. In contrast, another group documented GM-CSF production by oral epithelial cells in response to C. glabrata rather than C. albicans. In the same study, oral epithelial cells were more resistant to killing by C. glabrata compared to C. albicans [117]. Clearly, much remains to be learned on the interaction between epithelial cells and NAC species.
Adaptive immunity to Candida species: call in the reinforcements
T lymphocytes
CD4+ T cells are vital players in the response to C. albicans, particularly Th17 cells, as demonstrated dramatically by both knockout mice and humans with mutations in components of the IL-17 pathway. Deficiency in CARD9, IL-17RA, IL-17RC, Act1, IL-17A IL-23 and STAT3 drive susceptibility to a variety of C. albicans infections, including oral, cutaneous and disseminated candidiasis [118]. HIV+/AIDS patients not only have reduced CD4+ cell counts but lose Th17 cells disproportionally to other subsets [119, 120]. AIDS patients are exquisitely susceptible to OPC, with over 95% of patients experiencing oral thrush [121]. In humans, memory T cells specific for C. albicans are of the Th17 subset [122, 123]. A similar scenario is observed in mice subjected to recall C. albicans infections [124, 125] Furthermore, protective vaccine responses are associated with robust Th1 and Th17 responses [58, 126] Protection against oral and cutaneous candidiasis is more selectively associated with specific Th17 immunity, whereas both Th1 and Th17 responses participate against systemic infection [124, 127–130].
Evidence for an involvement of CD4+ T cell responses in immunity to NAC species also exists. Sepsis caused by C. parapsilosis in an infant with ectodermal dysplasia and thymic hypoplasia was associated with reduced T cell numbers and reduced T cell proliferative capacity [131]. Both cross-reactive and distinct T cells are generated in response to different Candida species. Human T cells generated following stimulation with C. albicans cellular extract displayed cross-reactivity with C. tropicalis but not C. glabrata [132]. Despite the generation of CD4+ T cell responses with distinct specificity, it seems that induction of IL-17A by CD4+ T cells is a common feature of Candida species. C. albicans and C. dubliniensis, which are the most closely related phylogenetically, were found to trigger the most IL-17A, whereas the distantly related C. glabrata induced the least [125]. Given the protective role of IL-17 responses in immunity to C. albicans, it would be predicted that IL-17 immunity is similarly involved in responses to NAC species. However, IL-17-dependent responses were dispensable for protection against a mouse model of disseminated C. tropicalis infection. Rather, CARD9-dependent TNFα responses were crucial for protection [22]. Therefore, the dogma that IL-17 immunity is central to host defense against Candida may not hold true for all Candida species.
Innate lymphocytes
The recent recognition of various innate lymphocyte populations [133] has prompted reassessment of innate vs. adaptive immune responses in antifungal immunity to Candida. Such cell types include TCR-expressing subsets (NKT, γδ-T, ‘natural’ T cells) and TCR-negative cell types (NK, ILC1, ILC2, ILC3) [134]. Depletion of γδ T cells increased susceptibility to C. albicans infection, and γδ T cells enhanced macrophage nitric oxide production and candidacidal activity in vitro [135]. Since then, several other studies have confirmed a key role for γδ T cells in host defense against C. albicans infection, with a principle mechanism involving IL-17 production [136–138]. Additionally, we showed that “natural” Th17 cells protect against acute oral C. albicans infection in conjunction with γδ T cells [136]. Although ILC3 cells were suggested to be involved in antifungal immunity against C. albicans [139], they were not evident in other analyses [136]. NK cells possess anti-Candida killing ability and have been implicated in protection against disseminated C. albicans infection [140, 141]. However, a protective role for NK cells against C. albicans infection is controversial and may depend on host immune status [142]. More recently, reduced numbers of NKT and mucosal-associated invariant T (MAIT) cells that showed a selective defect in IL-17 production were documented in individuals with mutations in STAT3 [143]. STAT3 mutations are consistently associated with CMC [118], implicating these poorly understood T cell populations in antifungal immunity to Candida.
Again, little is known about the role of innate lymphocyte populations and NAC species immunity. However, emerging data hints at differences. For example, nTh17 cells were not induced during oral C. glabrata exposure, in contrast to C. albicans [136]. Furthermore, we saw no apparent role for innate T cells, ILCs or NK cells in protection against disseminated C. tropicalis infection, based on the observation that Rag2−/−Il2rg−/− mice did not display increased susceptibility to systemic infection [22].
Antifungal mechanisms: soluble factors
Cytokines and chemokines
A myriad of cytokines and chemokines are associated with protection against C. albicans infection. In addition to IL-17 discussed above, these include factors that promote development and recruitment of neutrophils, such as GM-CSF, G-CSF, CXCL1 and CXCL2 [130, 144]. Similarly, cytokines that promote phagocyte killing and recruitment, such as TNFα, IL-6, IL-1β and IFNγ, are key in host defense against C. albicans. Indeed, recombinant GM-CSF and IFNγ therapy have been used in the clinic to protect against mucosal and systemic C. albicans infections, though the utility of this approach is still under investigation [145–147].
Similarities in the induction of cytokines and chemokines by different Candida species have been reported, at least in vitro. However, C. glabrata is generally associated with the activation of weak cytokine and chemokine responses. For example, C. albicans, C. tropicalis and C. krusei induced IL-1β production by BMDMs, whereas C. glabrata did not [148]. Similarly, C. albicans but not C. glabrata promoted epithelial cell production of IL-8 and IL-1α [117]. However, several studies suggest that C. glabrata may favor GM-CSF production, as this NAC species induced production of GM-CSF by both epithelial cells and BMDMs in vitro [101, 117].
In contrast to the above in vitro studies, disseminated C. glabrata infection is associated with the production of TNFα, IL-12p35 and IFNγ [43, 149]. TNFα appears to be central in controlling C. glabrata growth, as Ab blockade of TNFα but not other cytokines increased kidney fungal burden [149]. We recently showed that depletion of TNFα by etanercept treatment renders mice more susceptible to disseminated C. tropicalis infection compared to controls [22]. Therefore, TNFα may be a broadly applicable antifungal mechanism.
Certain cytokines enhance phagocyte killing of Candida species. G-CSF augments neutrophil damage of C. albicans, C. parapsilosis and C. tropicalis. Interestingly however, IFNγ enhances neutrophil damage of C. albicans and C. parapsilosis but not C. tropicalis [87]. Overall, the impact of cytokines and chemokines in response to Candida are not identical.
Antimicrobial peptides and reactive chemical species
Other important antifungal events include the production of antimicrobial peptides (AMPs) and reactive chemical species, such as ROS. These soluble effectors directly kill C. albicans, and are primarily produced by phagocytic cells and epithelial cells. A major AMP associated with oral candidiaiss in mice is β-defensin 3 (BD3) [124, 130]. Moreover, deficiencies in BD1, S100A8 and S100A9 lead to heightened susceptibility to mucosal and systemic C. albicans infection [150–152]. C. albicans dissemination from the GI tract occurs in mice deficient in components of the ROS and RNS pathways [153]. In humans, Chronic Granulomatous Disease (CGD) patients that have defects in the NADPH oxidase system are at increased risk of invasive candidiasis and neutrophils from CGD patients are defective in killing opsonized C. albicans [154, 155].
Antimicrobial peptides including β-defensins, histatins, H1 histones and lactoferrin display antifungal activity against multiple Candida species [156–158]. Synthetic peptides can also kill C. albicans, C. tropicalis and C. glabrata strains in vitro [159] [160] [161]. In general, the antifungal activity of AMPs appears to vary among specific Candida strains rather than species. However, C. glabrata displays increased resistance to human BD2, BD3 and histatin compared to other species [162, 163].
Reactive chemical species are also implicated in immunity to NAC species. For example, p47phox−/− mice are significantly more susceptible to disseminated C. glabrata infection [164]. Moreover, myeloperoxidase (MPO)−/− mice were impaired in clearance of C. albicans and C. tropicalis from the lungs [165]. In contrast, clearance of lung C. glabrata was comparable between MPO−/− and WT mice. Therefore, although AMPs and reactive chemical species are involved in host defense against NAC species, considerable differences exist among Candida species.
Challenges to studying NAC species
It is clear that our understanding of immunity to NAC species is still immature. This is partly because C. albicans remains the dominant Candida species isolated in the Western world. However, other hurdles have made studying immunity to NAC species difficult. One issue is the paucity of NAC species-specific tools. As the Candida field has largely focused on C. albicans, a wealth of genetic mutants exist, such as morphotype-locked mutants, fluorophore-expressing reporters (e.g., GFP, Luciferase), and epitope-tagged strains for in vivo tracking [138, 166]. A parallel collection of genetic mutants does not yet exist for NAC species, although progress is being made for C. glabrata [167].
Another roadblock is that many NAC species are not usually pathogenic in mouse models of candidiasis, in contrast to C. albicans. For example, in a mouse model of disseminated candidiasis, WT mice did not succumb to C. parapsilosis, C. krusei or C. glabrata infection [168]. Similarly, C. dubliniensis is far less pathogenic than C. albicans in mouse models of disseminated and gastrointestinal infection [169–171]. In a model of oral candidiasis, even WT mice immunosuppressed with high dose cortisone were able to clear C. tropicalis, C. dubliniensis and C. glabrata without any signs of disease (NW and SLG, unpublished observations). These differences in susceptibility to Candida infection may reflect phenotypic variation among Candida species discussed above, but could also be explained by immune differences between mice and humans. Regardless of the reason, the field lacks tractable animal models with which to study these important pathogens. However, advances have been made with a newly described murine model of intra-abdominal C. glabrata infection, which closely mimics human disease [84]. Nevertheless, the difficulty in studying immune responses to NAC species in vivo means that most information to date has been gleaned from in vitro approaches which do not necessarily recapitulate the physiological environment. For instance, strains of C. tropicalis that do not form hyphae in vitro have been identified, yet it is unknown whether this holds true in vivo [23]. In this regard, we observed that a C. tropicalis clinical isolate did not form filamentous hyphae in vitro, yet formed invasive hyphae in kidneys [22]. Developing faithful models to understand immunity to NAC species is key for future studies in this area.
Consequences for emerging biologics
The first biologics to be approved for clinical use targeted TNFα, and have been successful in treating rheumatoid arthritis and other autoimmune conditions for the last 2 decades. Although cases have been reported, anti-TNFα therapy is not commonly associated with heightened risk of Candida infections [172]. However, meta-analyses have indicated that candidiasis may be an under-recognized opportunistic infection associated with this therapy [173]. We recently showed that immunity to systemic C. tropicalis infection is TNFα-dependent [22]. One reason this infection is not commonly reported may be that, although anti-TNFα therapy is widely used in the Western world, this is not true in developing countries due to high costs. Given the geographical differences in prevalence between C. albicans and C. tropicalis, it is conceivable that the risk of Candida infection associated with anti-TNFα therapy is underestimated.
Antibodies targeting IL-17 (secukinumab) or IL-12/IL-23 (ustekinumab) have shown impressive effects for the treatment of psoriasis and were recently approved for clinical use [174]. Given the importance of IL-17 responses in immunity to C. albicans infection, an obvious potential risk factor is increased susceptibility to this fungus. Indeed, early reports have documented increased mucosal Candida infections in patients receiving sekukinumab, although the frequency of particular Candida species was low [175]. However, given our finding that the IL-17 pathway is not required for protection against disseminated C. tropicalis infection in mice [22], IL-17 pathway biologics may not increase susceptibility to all Candida species.
Perspective
The rise in resistance to antifungal drugs and the lack of new medications or vaccines against Candida has prompted interest in development of novel treatment strategies. One example is immunotherapies that could be used alone or in combination with current treatments. The dogma on immunity to Candida infection is based almost entirely on our knowledge of immunity to C. albicans. As described here, important differences exist in immunity to Candida species. Given the rise in NAC species infections, it is pertinent to understand immunity to these emerging pathogens. Unraveling the similarities and differences in immunity to C. albicans and other Candida species will pave the way for appropriate immunotherapies and vaccines.
Acknowledgments
SLG was supported by the NIH (AI107825, DE022550, DE023815).
Abbreviations
- AMP
antimicrobial peptide
- ILC
innate lymphoid cell
- CARD9
caspase recruitment domain family member 9
- CLR
C-type lectin receptor
- OPC
oropharyngeal candidiasis
- NAC
non-albicans Candida species
- CMC
chronic mucocutaneous candidiasis
- PRR
pattern recognition receptor
- VVC
vulvo-vaginal candidiasis
Footnotes
Conflicts of interest: SLG has received research grants from Novartis and Janssen. She consults or has received honoraria and travel reimbursements from Novartis, Janssen, Pfizer, Eli Lilly and Amgen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–35. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merseguel KB, Nishikaku AS, Rodrigues AM, Padovan AC, RCEF, Salles de Azevedo Melo A, da Silva Briones MR, Colombo AL. Genetic diversity of medically important and emerging Candida species causing invasive infection. BMC Infect Dis. 2015;15:57. doi: 10.1186/s12879-015-0793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. 2013;9:e1003550. doi: 10.1371/journal.ppat.1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–34. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller MA, Messer SA, Jones RN, Castanheira M. Antifungal susceptibilities of Candida, Cryptococcus neoformans and Aspergillus fumigatus from the Asia and Western Pacific region: data from the SENTRY antifungal surveillance program (2010–2012) J Antibiot (Tokyo) 2015 doi: 10.1038/ja.2015.29. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125:S3–13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller MA, Jones RN, Castanheira M. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006–2011. Mycoses. 2014;57:602–11. doi: 10.1111/myc.12206. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009) J Clin Microbiol. 2011;49:396–9. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, Agrafioti I, Arnaud MB, Bates S, Brown AJ, Brunke S, Costanzo MC, Fitzpatrick DA, de Groot PW, Harris D, Hoyer LL, Hube B, Klis FM, Kodira C, Lennard N, Logue ME, Martin R, Neiman AM, Nikolaou E, Quail MA, Quinn J, Santos MC, Schmitzberger FF, Sherlock G, Shah P, Silverstein KA, Skrzypek MS, Soll D, Staggs R, Stansfield I, Stumpf MP, Sudbery PE, Srikantha T, Zeng Q, Berman J, Berriman M, Heitman J, Gow NA, Lorenz MC, Birren BW, Kellis M, Cuomo CA. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–62. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001;9:176–80. doi: 10.1016/s0966-842x(01)01984-9. [DOI] [PubMed] [Google Scholar]
- 12.Jackson AP, Gamble JA, Yeomans T, Moran GP, Saunders D, Harris D, Aslett M, Barrell JF, Butler G, Citiulo F, Coleman DC, de Groot PW, Goodwin TJ, Quail MA, McQuillan J, Munro CA, Pain A, Poulter RT, Rajandream MA, Renauld H, Spiering MJ, Tivey A, Gow NA, Barrell B, Sullivan DJ, Berriman M. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res. 2009;19:2231–44. doi: 10.1101/gr.097501.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunke S, Hube B. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol. 2013;15:701–8. doi: 10.1111/cmi.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parnanen P, Meurman JH, Nikula-Ijas P. A novel Candida glabrata cell wall associated serine protease. Biochem Biophys Res Commun. 2015;457:676–80. doi: 10.1016/j.bbrc.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Gozalbo D, Roig P, Villamon E, Gil ML. Candida and candidiasis: the cell wall as a potential molecular target for antifungal therapy. Curr Drug Targets Infect Disord. 2004;4:117–35. doi: 10.2174/1568005043341046. [DOI] [PubMed] [Google Scholar]
- 16.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 17.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10:112–22. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil ML, Casanova M, Martinez JP, Sentandreu R. Antigenic cell wall mannoproteins in Candida albicans isolates and in other Candida species. J Gen Microbiol. 1991;137:1053–61. doi: 10.1099/00221287-137-5-1053. [DOI] [PubMed] [Google Scholar]
- 19.Lee PY, Gam LH, Yong VC, Rosli R, Ng KP, Chong PP. Immunoproteomic analysis of antibody response to cell wall-associated proteins of Candida tropicalis. J Appl Microbiol. 2014;117:854–65. doi: 10.1111/jam.12562. [DOI] [PubMed] [Google Scholar]
- 20.de Groot PW, Kraneveld EA, Yin QY, Dekker HL, Gross U, Crielaard W, de Koster CG, Bader O, Klis FM, Weig M. The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell. 2008;7:1951–64. doi: 10.1128/EC.00284-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa-de-Oliveira S, Silva AP, Miranda IM, Salvador A, Azevedo MM, Munro CA, Rodrigues AG, Pina-Vaz C. Determination of chitin content in fungal cell wall: an alternative flow cytometric method. Cytometry A. 2013;83:324–8. doi: 10.1002/cyto.a.22250. [DOI] [PubMed] [Google Scholar]
- 22.Whibley N, Jaycox J, Reid D, Garg A, Taylor J, Clancy C, Nguyen M, Biswas P, MJM, Brown G, Gaffen SL. Delinking CARD9 and IL-17: CARD9 protects against Candida tropicalis infection through a TNFα-dependent, IL-17-independent mechanism. J Immunol. 2015 doi: 10.4049/jimmunol.1500870. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin MV, White FH. A microbiologic and ultrastructural investigation of germ-tube formation by oral strains of Candida tropicalis. Am J Clin Pathol. 1981;75:671–6. doi: 10.1093/ajcp/75.5.671. [DOI] [PubMed] [Google Scholar]
- 24.Thompson DS, Carlisle PL, Kadosh D. Coevolution of morphology and virulence in Candida species. Eukaryot Cell. 2011;10:1173–82. doi: 10.1128/EC.05085-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lachke SA, Joly S, Daniels K, Soll DR. Phenotypic switching and filamentation in Candida glabrata. Microbiology. 2002;148:2661–74. doi: 10.1099/00221287-148-9-2661. [DOI] [PubMed] [Google Scholar]
- 26.Csank C, Haynes K. Candida glabrata displays pseudohyphal growth. FEMS Microbiol Lett. 2000;189:115–20. doi: 10.1111/j.1574-6968.2000.tb09216.x. [DOI] [PubMed] [Google Scholar]
- 27.Brunke S, Seider K, Fischer D, Jacobsen ID, Kasper L, Jablonowski N, Wartenberg A, Bader O, Enache-Angoulvant A, Schaller M, d’Enfert C, Hube B. One small step for a yeast--microevolution within macrophages renders Candida glabrata hypervirulent due to a single point mutation. PLoS Pathog. 2014;10:e1004478. doi: 10.1371/journal.ppat.1004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pujol C, Daniels KJ, Lockhart SR, Srikantha T, Radke JB, Geiger J, Soll DR. The closely related species Candida albicans and Candida dubliniensis can mate. Eukaryot Cell. 2004;3:1015–27. doi: 10.1128/EC.3.4.1015-1027.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett RJ. Coming of age--sexual reproduction in Candida species. PLoS Pathog. 2010;6:e1001155. doi: 10.1371/journal.ppat.1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 31.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–25. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37:97–106. doi: 10.1007/s00281-014-0462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho A, Giovannini G, De Luca A, D’Angelo C, Casagrande A, Iannitti RG, Ricci G, Cunha C, Romani L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol Immunol. 2012;9:276–86. doi: 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, Maccallum DM, Wheeler R, Munro CA, Gow NA, Cramer RA, Brown AJ, Brown GD. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013;9:e1003315. doi: 10.1371/journal.ppat.1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 36.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–8. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, Netea MG. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–32. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 39.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DP, Brown GD, Underhill DM. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–7. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linden JR, Maccani MA, Laforce-Nesbitt SS, Bliss JM. High efficiency opsonin-independent phagocytosis of Candida parapsilosis by human neutrophils. Med Mycol. 2010;48:355–64. doi: 10.1080/13693780903164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn DM, Vyas VK. The Candida glabrata adhesin Epa1p causes adhesion, phagocytosis, and cytokine secretion by innate immune cells. FEMS Yeast Res. 2012;12:398–414. doi: 10.1111/j.1567-1364.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- 42.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Ifrim DC, Bain JM, Reid DM, Oosting M, Verschueren I, Gow NA, van Krieken JH, Brown GD, Kullberg BJ, Joosten LA, van der Meer JW, Koentgen F, Erwig LP, Quintin J, Netea MG. Role of Dectin-2 for host defense against systemic infection with Candida glabrata. Infect Immun. 2014;82:1064–73. doi: 10.1128/IAI.01189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–26. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- 45.Linden JR, De Paepe ME, Laforce-Nesbitt SS, Bliss JM. Galectin-3 plays an important role in protection against disseminated candidiasis. Med Mycol. 2013;51:641–51. doi: 10.3109/13693786.2013.770607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamai R, Kiyoura Y. Candida albicans and Candida parapsilosis rapidly up-regulate galectin-3 secretion by human gingival epithelial cells. Mycopathologia. 2014;177:75–9. doi: 10.1007/s11046-013-9725-1. [DOI] [PubMed] [Google Scholar]
- 47.Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci U S A. 2011;108:14270–5. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X. C-Type Lectin Receptors Dectin-3 and Dectin-2 Form a Heterodimeric Pattern-Recognition Receptor for Host Defense against Fungal Infection. Immunity. 2013;39:324–34. doi: 10.1016/j.immuni.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Vautier S, da Sousa MG, Brown GD. C-type lectins, fungi and Th17 responses. Cytokine Growth Factor Rev. 2010;21:405–12. doi: 10.1016/j.cytogfr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–6. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 52.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, Janssen H, van den Berg TK, Kuijpers TW. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121:2385–92. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- 54.Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, Denis B, Brunel AS, Martin S, Loop M, Peeters J, de Selys A, Vanclaire J, Vermylen C, Nassogne MC, Chatzis O, Liu L, Migaud M, Pedergnana V, Desoubeaux G, Jouvion G, Chretien F, Darazam IA, Schaffer AA, Netea MG, De Bruycker JJ, Bernard L, Reynes J, Amazrine N, Abel L, Van der Linden D, Harrison T, Picard C, Lortholary O, Mansouri D, Casanova JL, Puel A. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.12.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell. 2011;10:168–73. doi: 10.1128/EC.00279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010;201:1718–28. doi: 10.1086/652407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo G, Ibrahim AS, French SW, Edwards JE, Jr, Fu Y. Active and passive immunization with rHyr1p-N protects mice against hematogenously disseminated candidiasis. PLoS One. 2011;6:e25909. doi: 10.1371/journal.pone.0025909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr, Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, Edwards JE, Jr, Hennessey JP., Jr NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine. 2012;30:7594–600. doi: 10.1016/j.vaccine.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fidel PL, Jr, Cutler JE. Prospects for development of a vaccine to prevent and control vaginal candidiasis. Curr Infect Dis Rep. 2011;13:102–7. doi: 10.1007/s11908-010-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.d’Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–74. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–64. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, Kanneganti TD, van der Meer JW, Kullberg BJ, Joosten LA, Gow NA, Netea MG. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90:357–66. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moyes DL, Naglik JR. Mucosal immunity and Candida albicans infection. Clin Dev Immunol. 2011;2011:346307. doi: 10.1155/2011/346307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, Naglik JR. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–35. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bi L, Gojestani S, Wu W, Hsu YM, Zhu J, Ariizumi K, Lin X. CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem. 2010;285:25969–77. doi: 10.1074/jbc.M110.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. Embo j. 2005;24:1277–86. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sasse C, Hasenberg M, Weyler M, Gunzer M, Morschhauser J. White-opaque switching of Candida albicans allows immune evasion in an environment-dependent fashion. Eukaryot Cell. 2013;12:50–8. doi: 10.1128/EC.00266-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–51. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 2008;4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, Drummond RA, Zurawski SM, Zurawski G, Berman J, Iwasaki A, Brown GD, Kaplan DH. Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity. 2015;42:356–66. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonifazi P, Zelante T, D’Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, Fallarino F, Puccetti P, Romani L. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362–74. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- 73.Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med. 2010;207:923–31. doi: 10.1084/jem.20092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huppler AR, Conti HR, Hernandez-Santos N, PSB, Darville T, Gaffen SL. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol. 2014;192:1745–52. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shoham S, Levitz SM. The immune response to fungal infections. Br J Haematol. 2005;129:569–82. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 76.Fulurija A, Ashman RB, Papadimitriou JM. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology. 1996;142 (Pt 12):3487–96. doi: 10.1099/13500872-142-12-3487. [DOI] [PubMed] [Google Scholar]
- 77.Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008;4:e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24:1122–8. doi: 10.1086/513663. [DOI] [PubMed] [Google Scholar]
- 79.Wingard JR, Merz WG, Saral R. Candida tropicalis: a major pathogen in immunocompromised patients. Ann Intern Med. 1979;91:539–43. doi: 10.7326/0003-4819-91-4-539. [DOI] [PubMed] [Google Scholar]
- 80.Martin MV, Al-Tikriti U, Bramley PA. Yeast flora of the mouth and skin during and after irradiation for oral and laryngeal cancer. J Med Microbiol. 1981;14:457–67. doi: 10.1099/00222615-14-4-457. [DOI] [PubMed] [Google Scholar]
- 81.Walsh TJ, Merz WG. Pathologic features in the human alimentary tract associated with invasiveness of Candida tropicalis. Am J Clin Pathol. 1986;85:498–502. doi: 10.1093/ajcp/85.4.498. [DOI] [PubMed] [Google Scholar]
- 82.Dimopoulos G, Ntziora F, Rachiotis G, Armaganidis A, Falagas ME. Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth Analg. 2008;106:523–9. doi: 10.1213/ane.0b013e3181607262. table of contents. [DOI] [PubMed] [Google Scholar]
- 83.Wingard JR, Dick JD, Merz WG, Sandford GR, Saral R, Burns WH. Pathogenicity of Candida tropicalis and Candida albicans after gastrointestinal inoculation in mice. Infect Immun. 1980;29:808–13. doi: 10.1128/iai.29.2.808-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng S, Clancy CJ, Hartman DJ, Hao B, Nguyen MH. Candida glabrata intra-abdominal candidiasis is characterized by persistence within the peritoneal cavity and abscesses. Infect Immun. 2014;82:3015–22. doi: 10.1128/IAI.00062-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lehrer RI. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972;51:2566–72. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duggan S, Essig F, Hunniger K, Mokhtari Z, Bauer L, Lehnert T, Brandes S, Hader A, Jacobsen ID, Martin R, Figge MT, Kurzai O. Neutrophil activation by Candida glabrata but not Candida albicans promotes fungal uptake by monocytes. Cell Microbiol. 2015 doi: 10.1111/cmi.12443. [DOI] [PubMed] [Google Scholar]
- 87.Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Effects of granulocyte colony-stimulating factor and interferon-gamma on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J Leukoc Biol. 1995;57:651–6. doi: 10.1002/jlb.57.4.651. [DOI] [PubMed] [Google Scholar]
- 88.Dementhon K, El-Kirat-Chatel S, Noel T. Development of an in vitro model for the multi-parametric quantification of the cellular interactions between Candida yeasts and phagocytes. PLoS One. 2012;7:e32621. doi: 10.1371/journal.pone.0032621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Svobodova E, Staib P, Losse J, Hennicke F, Barz D, Jozsi M. Differential interaction of the two related fungal species Candida albicans and Candida dubliniensis with human neutrophils. J Immunol. 2012;189:2502–11. doi: 10.4049/jimmunol.1200185. [DOI] [PubMed] [Google Scholar]
- 90.Lyman CA, Walsh TJ. Phagocytosis of medically important yeasts by polymorphonuclear leukocytes. Infect Immun. 1994;62:1489–93. doi: 10.1128/iai.62.4.1489-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richardson MD, Donaldson F. Interaction of Candida krusei with human neutrophils in vitro. J Med Microbiol. 1994;41:384–8. doi: 10.1099/00222615-41-6-384. [DOI] [PubMed] [Google Scholar]
- 92.Qian Q, Jutila MA, Van Rooijen N, Cutler JE. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152:5000–8. [PubMed] [Google Scholar]
- 93.Lionakis MS, Netea MG. Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog. 2013;9:e1003079. doi: 10.1371/journal.ppat.1003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis. 2014;209:109–19. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, Wan W, Lee CC, Lim JK, Rivollier A, Yang JC, Laird GM, Wheeler RT, Alexander BD, Perfect JR, Gao JL, Kullberg BJ, Netea MG, Murphy PM. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013;123:5035–51. doi: 10.1172/JCI71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sasada M, Johnston RB., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980;152:85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toth R, Toth A, Papp C, Jankovics F, Vagvolgyi C, Alonso MF, Bain JM, Erwig LP, Gacser A. Kinetic studies of Candida parapsilosis phagocytosis by macrophages and detection of intracellular survival mechanisms. Front Microbiol. 2014;5:633. doi: 10.3389/fmicb.2014.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keppler-Ross S, Douglas L, Konopka JB, Dean N. Recognition of yeast by murine macrophages requires mannan but not glucan. Eukaryot Cell. 2010;9:1776–87. doi: 10.1128/EC.00156-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis LE, Bain JM, Lowes C, Gillespie C, Rudkin FM, Gow NA, Erwig LP. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012;8:e1002578. doi: 10.1371/journal.ppat.1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McKenzie CG, Koser U, Lewis LE, Bain JM, Mora-Montes HM, Barker RN, Gow NA, Erwig LP. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun. 2010;78:1650–8. doi: 10.1128/IAI.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seider K, Brunke S, Schild L, Jablonowski N, Wilson D, Majer O, Barz D, Haas A, Kuchler K, Schaller M, Hube B. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol. 2011;187:3072–86. doi: 10.4049/jimmunol.1003730. [DOI] [PubMed] [Google Scholar]
- 102.Seider K, Gerwien F, Kasper L, Allert S, Brunke S, Jablonowski N, Schwarzmuller T, Barz D, Rupp S, Kuchler K, Hube B. Immune evasion, stress resistance, and efficient nutrient acquisition are crucial for intracellular survival of Candida glabrata within macrophages. Eukaryot Cell. 2014;13:170–83. doi: 10.1128/EC.00262-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Newman SL, Holly A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect Immun. 2001;69:6813–22. doi: 10.1128/IAI.69.11.6813-6822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog. 2014;10:e1004276. doi: 10.1371/journal.ppat.1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bourgeois C, Majer O, Frohner IE, Lesiak-Markowicz I, Hildering KS, Glaser W, Stockinger S, Decker T, Akira S, Muller M, Kuchler K. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J Immunol. 2011;186:3104–12. doi: 10.4049/jimmunol.1002599. [DOI] [PubMed] [Google Scholar]
- 106.Neumann AK, Jacobson K. A novel pseudopodial component of the dendritic cell anti-fungal response: the fungipod. PLoS Pathog. 2010;6:e1000760. doi: 10.1371/journal.ppat.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fratti RA, Ghannoum MA, Edwards JE, Jr, Filler SG. Gamma interferon protects endothelial cells from damage by Candida albicans by inhibiting endothelial cell phagocytosis. Infect Immun. 1996;64:4714–8. doi: 10.1128/iai.64.11.4714-4718.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nomanbhoy F, Steele C, Yano J, Fidel PL., Jr Vaginal and oral epithelial cell anti-Candida activity. Infect Immun. 2002;70:7081–8. doi: 10.1128/IAI.70.12.7081-7088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steele C, Leigh J, Swoboda R, Fidel PL., Jr Growth inhibition of Candida by human oral epithelial cells. J Infect Dis. 2000;182:1479–85. doi: 10.1086/315872. [DOI] [PubMed] [Google Scholar]
- 110.Steele C, Leigh J, Swoboda R, Ozenci H, Fidel PL., Jr Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect Immun. 2001;69:7091–9. doi: 10.1128/IAI.69.11.7091-7099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steele C, Ozenci H, Luo W, Scott M, Fidel PL., Jr Growth inhibition of Candida albicans by vaginal cells from naive mice. Med Mycol. 1999;37:251–9. [PubMed] [Google Scholar]
- 112.Mostefaoui Y, Bart C, Frenette M, Rouabhia M. Candida albicans and Streptococcus salivarius modulate IL-6, IL-8, and TNF-alpha expression and secretion by engineered human oral mucosa cells. Cell Microbiol. 2004;6:1085–96. doi: 10.1111/j.1462-5822.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 113.Filler SG, Pfunder AS, Spellberg BJ, Spellberg JP, Edwards JE., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–17. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dongari-Bagtzoglou A, Kashleva H. Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb Pathog. 2003;34:169–77. doi: 10.1016/s0882-4010(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 115.Dongari-Bagtoglou A, Fidel P. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dent Res. 2005;84:966–77. doi: 10.1177/154405910508401101. [DOI] [PubMed] [Google Scholar]
- 116.Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118:652–7. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 117.Li L, Dongari-Bagtzoglou A. Oral epithelium-Candida glabrata interactions in vitro. Oral Microbiol Immunol. 2007;22:182–7. doi: 10.1111/j.1399-302X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 118.Milner J, Holland S. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol. 2013;13:635–648. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- 119.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–40. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fidel PL., Jr Candida-Host Interactions in HIV Disease: Implications for Oropharyngeal Candidiasis. Adv Dent Res. 2011;23:45–9. doi: 10.1177/0022034511399284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human T(H)17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 123.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 124.Hernández-Santos N, Huppler AR, Peterson AC, Khader SA, KCM, Gaffen SL. Th17 cells confer long term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bär E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, LeibundGut-Landmann S. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol. 2012;188:5636–43. doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- 126.Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JE., Jr The anti-Candida vaccine based on the recombinant N-terminal domain of Als1p is broadly active against disseminated candidiasis. Infect Immun. 2006;74:3039–41. doi: 10.1128/IAI.74.5.3039-3041.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Romani L, Zelante T, Palmieri M, Napolioni V, Picciolini M, Velardi A, Aversa F, Puccetti P. The cross-talk between opportunistic fungi and the mammalian host via microbiota’s metabolism. Semin Immunopathol. 2015;37:163–71. doi: 10.1007/s00281-014-0464-2. [DOI] [PubMed] [Google Scholar]
- 128.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 129.van de Veerdonk FL, Kullberg BJ, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, Netea MG. Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock. 2010;34:407–11. doi: 10.1097/SHK.0b013e3181d67041. [DOI] [PubMed] [Google Scholar]
- 130.Conti H, Shen F, Nayyar N, Stocum E, JNS, Lindemann M, Ho A, Hai J, Yu J, Jung J, Filler S, Masso-Welch P, Edgerton M, Gaffen S. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brooks EG, Klimpel GR, Vaidya SE, Keeney SE, Raimer S, Goldman AS, Schmalstieg FC. Thymic hypoplasia and T-cell deficiency in ectodermal dysplasia: case report and review of the literature. Clin Immunol Immunopathol. 1994;71:44–52. doi: 10.1006/clin.1994.1050. [DOI] [PubMed] [Google Scholar]
- 132.Tramsen L, Beck O, Schuster FR, Hunfeld KP, Latge JP, Sarfati J, Roger F, Klingebiel T, Koehl U, Lehrnbecher T. Generation and characterization of anti-Candida T cells as potential immunotherapy in patients with Candida infection after allogeneic hematopoietic stem-cell transplant. J Infect Dis. 2007;196:485–92. doi: 10.1086/519389. [DOI] [PubMed] [Google Scholar]
- 133.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 135.Jones-Carson J, Vazquez-Torres A, van der Heyde HC, Warner T, Wagner RD, Balish E. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552–7. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- 136.Conti H, Peterson A, Huppler A, Brane L, Hernández-Santos N, Whibley N, Garg A, Simpson-Abelson M, Gibson G, Mamo A, Osborne L, Bishu S, Ghilardi N, Siebenlist U, Watkins S, Artis D, McGeachy M, Gaffen S. Oral-resident ‘natural’ Th17 cells and γδ-T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–62. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–72. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gladiator A, Wangler N, Trautwein-Weidner K, Leibundgut-Landmann S. Cutting Edge: IL-17-Secreting Innate Lymphoid Cells Are Essential for Host Defense against Fungal Infection. J Immunol. 2013;190:521–5. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 140.Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–27. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 141.Gulay Z, Imir T. Anti-candidial activity of natural killer (NK) and lymphokine activated killer (LAK) lymphocytes in vitro. Immunobiology. 1996;195:220–30. doi: 10.1016/S0171-2985(96)80041-6. [DOI] [PubMed] [Google Scholar]
- 142.Quintin J, Voigt J, van der Voort R, Jacobsen ID, Verschueren I, Hube B, Giamarellos-Bourboulis EJ, van der Meer JW, Joosten LA, Kurzai O, Netea MG. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Eur J Immunol. 2014;44:2405–14. doi: 10.1002/eji.201343828. [DOI] [PubMed] [Google Scholar]
- 143.Wilson RP, Ives ML, Rao G, Lau A, Payne K, Kobayashi M, Arkwright PD, Peake J, Wong M, Adelstein S, Smart JM, French MA, Fulcher DA, Picard C, Bustamante J, Boisson-Dupuis S, Gray P, Stepensky P, Warnatz K, Freeman AF, Rossjohn J, McCluskey J, Holland SM, Casanova JL, Uzel G, Ma CS, Tangye SG, Deenick EK. STAT3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function. J Exp Med. 2015;212:855–64. doi: 10.1084/jem.20141992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saunus JM, Kazoullis A, Farah CS. Cellular and molecular mechanisms of resistance to oral Candida albicans infections. Front Biosci. 2008;13:5345–58. doi: 10.2741/3085. [DOI] [PubMed] [Google Scholar]
- 145.Vazquez JA, Gupta S, Villanueva A. Potential utility of recombinant human GM-CSF as adjunctive treatment of refractory oropharyngeal candidiasis in AIDS patients. Eur J Clin Microbiol Infect Dis. 1998;17:781–3. doi: 10.1007/s100960050185. [DOI] [PubMed] [Google Scholar]
- 146.Delsing CE, Gresnigt MS, Leentjens J, Preijers F, Frager FA, Kox M, Monneret G, Venet F, Bleeker-Rovers CP, van de Veerdonk FL, Pickkers P, Pachot A, Kullberg BJ, Netea MG. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect Dis. 2014;14:166. doi: 10.1186/1471-2334-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van de Veerdonk FL, Kullberg BJ, Netea MG. Adjunctive immunotherapy with recombinant cytokines for the treatment of disseminated candidiasis. Clin Microbiol Infect. 2012;18:112–9. doi: 10.1111/j.1469-0691.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- 148.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–81. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brieland J, Essig D, Jackson C, Frank D, Loebenberg D, Menzel F, Arnold B, DiDomenico B, Hare R. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect Immun. 2001;69:5046–55. doi: 10.1128/IAI.69.8.5046-5055.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tomalka J, Azodi E, Narra HP, Patel K, O’Neill S, Cardwell C, Hall BA, Wilson JM, Hise AG. beta-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J Immunol. 2015;194:1788–95. doi: 10.4049/jimmunol.1203239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yano J, Lilly E, Barousse M, Fidel PL., Jr Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78:5126–37. doi: 10.1128/IAI.00388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Balish E, Warner TF, Nicholas PJ, Paulling EE, Westwater C, Schofield DA. Susceptibility of germfree phagocyte oxidase- and nitric oxide synthase 2-deficient mice, defective in the production of reactive metabolites of both oxygen and nitrogen, to mucosal and systemic candidiasis of endogenous origin. Infect Immun. 2005;73:1313–20. doi: 10.1128/IAI.73.3.1313-1320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Espanol T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, Liese JG, van de Veerdonk FL, Roos D, van den Berg TK, Kuijpers TW. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood. 2014;124:590–7. doi: 10.1182/blood-2014-01-551473. [DOI] [PubMed] [Google Scholar]
- 156.Kashima M. H1 histones contribute to candidacidal activities of human epidermal extract. J Dermatol. 1991;18:695–706. doi: 10.1111/j.1346-8138.1991.tb03160.x. [DOI] [PubMed] [Google Scholar]
- 157.Nikawa H, Samaranayake LP, Tenovuo J, Pang KM, Hamada T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch Oral Biol. 1993;38:1057–63. doi: 10.1016/0003-9969(93)90167-k. [DOI] [PubMed] [Google Scholar]
- 158.Xu YY, Samaranayake YH, Samaranayake LP, Nikawa H. In vitro susceptibility of Candida species to lactoferrin. Med Mycol. 1999;37:35–41. doi: 10.1046/j.1365-280x.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 159.Burrows LL, Stark M, Chan C, Glukhov E, Sinnadurai S, Deber CM. Activity of novel non-amphipathic cationic antimicrobial peptides against Candida species. J Antimicrob Chemother. 2006;57:899–907. doi: 10.1093/jac/dkl056. [DOI] [PubMed] [Google Scholar]
- 160.Wang K, Yan J, Dang W, Xie J, Yan B, Yan W, Sun M, Zhang B, Ma M, Zhao Y, Jia F, Zhu R, Chen W, Wang R. Dual antifungal properties of cationic antimicrobial peptides polybia-MPI: membrane integrity disruption and inhibition of biofilm formation. Peptides. 2014;56:22–9. doi: 10.1016/j.peptides.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 161.Harris MR, Coote PJ. Combination of caspofungin or anidulafungin with antimicrobial peptides results in potent synergistic killing of Candida albicans and Candida glabrata in vitro. Int J Antimicrob Agents. 2010;35:347–56. doi: 10.1016/j.ijantimicag.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 162.Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005;84:445–50. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- 163.Helmerhorst EJ, Venuleo C, Beri A, Oppenheim FG. Candida glabrata is unusual with respect to its resistance to cationic antifungal proteins. Yeast. 2005;22:705–14. doi: 10.1002/yea.1241. [DOI] [PubMed] [Google Scholar]
- 164.Ju JY, Polhamus C, Marr KA, Holland SM, Bennett JE. Efficacies of fluconazole, caspofungin, and amphotericin B in Candida glabrata-infected p47phox−/− knockout mice. Antimicrob Agents Chemother. 2002;46:1240–5. doi: 10.1128/AAC.46.5.1240-1245.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, Maeda N, Koyama H. Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J Infect Dis. 2000;182:1276–9. doi: 10.1086/315843. [DOI] [PubMed] [Google Scholar]
- 166.Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42:590–8. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schwarzmuller T, Ma B, Hiller E, Istel F, Tscherner M, Brunke S, Ames L, Firon A, Green B, Cabral V, Marcet-Houben M, Jacobsen ID, Quintin J, Seider K, Frohner I, Glaser W, Jungwirth H, Bachellier-Bassi S, Chauvel M, Zeidler U, Ferrandon D, Gabaldon T, Hube B, d’Enfert C, Rupp S, Cormack B, Haynes K, Kuchler K. Systematic phenotyping of a large-scale Candida glabrata deletion collection reveals novel antifungal tolerance genes. PLoS Pathog. 2014;10:e1004211. doi: 10.1371/journal.ppat.1004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arendrup M, Horn T, Frimodt-Moller N. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection. 2002;30:286–91. doi: 10.1007/s15010-002-2131-0. [DOI] [PubMed] [Google Scholar]
- 169.Gilfillan GD, Sullivan DJ, Haynes K, Parkinson T, Coleman DC, Gow NA. Candida dubliniensis: phylogeny and putative virulence factors. Microbiology. 1998;144 (Pt 4):829–38. doi: 10.1099/00221287-144-4-829. [DOI] [PubMed] [Google Scholar]
- 170.Vilela MM, Kamei K, Sano A, Tanaka R, Uno J, Takahashi I, Ito J, Yarita K, Miyaji M. Pathogenicity and virulence of Candida dubliniensis: comparison with C. albicans. Med Mycol. 2002;40:249–57. doi: 10.1080/mmy.40.3.249.257. [DOI] [PubMed] [Google Scholar]
- 171.Stokes C, Moran GP, Spiering MJ, Cole GT, Coleman DC, Sullivan DJ. Lower filamentation rates of Candida dubliniensis contribute to its lower virulence in comparison with Candida albicans. Fungal Genet Biol. 2007;44:920–31. doi: 10.1016/j.fgb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 172.Strangfeld A, Listing J. Infection and musculoskeletal conditions: Bacterial and opportunistic infections during anti-TNF therapy. Best Pract Res Clin Rheumatol. 2006;20:1181–95. doi: 10.1016/j.berh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 173.Ford AC, Peyrin-Biroulet L. Opportunistic Infections With Anti-Tumor Necrosis Factor-alpha Therapy in Inflammatory Bowel Disease: Meta-Analysis of Randomized Controlled Trials. Am J Gastroenterol. 2013;108:1268–76. doi: 10.1038/ajg.2013.138. [DOI] [PubMed] [Google Scholar]
- 174.Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75:329–38. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- 175.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C, Group ES, Group FS. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]


