Abstract
Objective
Young men who have sex with men (YMSM) in the U.S. have a high HIV incidence with substantial racial disparities that are poorly understood. We use a data-driven simulation model to understand the impact of network-level mechanisms and STI infections on the spread of HIV among YMSM.
Methods
We designed and parameterized a stochastic agent-based network simulation model using results of a longitudinal cohort study of YMSM in Chicago. Within this model, YMSM formed and dissolved partnerships over time, and partnership-types were stratified by length of partnership, sex and age of the partner. In each partnership, HIV, gonorrhea and chlamydia could be transmitted. Counterfactual scenarios were run to examine drivers of HIV.
Results
Over a 15 year simulation, the HIV epidemic among YMSM continued to rise with Latino/White YMSM facing a steeper increase in the HIV burden compared to Black YMSM. YMSM in partnerships with older MSM, in particular Black YMSM with older Black MSM, were at highest risk for HIV and one infection prevented with an older partner would prevent 0.8 additional infections among YMSM. Additionally, racial disparities in HIV were driven by differences in the HIV prevalence of YMSM partners. Finally, of all HIV infections among YMSM, 14.6% were attributable to NG and CT infections.
Conclusion
Network-level mechanisms and STI infections play a significant role in the spread of HIV, and in racial disparities among YMSM. HIV prevention efforts should target YMSM across race, and interventions focusing on YMSM partnerships with older MSM might be highly effective.
Keywords: HIV, STI, Men who have sex with men, sexual behavior, sexual partners, Mathematical Models
Introduction
Between 2006 and 2009, the number of new HIV infections in the United States attributable to young men who have sex with men (YMSM) aged 13–29 years rose by 34%, resulting in 27% of all new HIV infections in 2009 attributed to YMSM1,2. In contrast, the overall number of new HIV infections has remained stable at an estimated 50,000 cases per year throughout the same period2. Additionally, there are substantial racial/ethnic disparities in HIV among YMSM, with Black YMSM accounting for more than 50% of new HIV infections among YMSM in 20091,2, and racial and ethnic minority YMSM have a higher incidence of HIV compared to White YMSM3. For example, Black YMSM are estimated to have a 3–6 fold increased annual HIV incidence compared to White YMSM3,4.
Our understanding of the high incidence and racial/ethnic disparities in HIV among YMSM is limited5,6. There is evidence that some individual-level mechanisms, such as sexually transmitted infections (STIs), contribute to an elevated HIV risk among YMSM5. In particular, rectal infections of gonorrhea (NG) and Chlamydia (CT) have received attention as possible drivers4,7 due to: 1) the biological evidence that NG and CT increase susceptibility and transmissibility of HIV8–11; 2) the empirical evidence of a 2 to 3 times greater rectal prevalence of NG and CT compared to urethral prevalence in YMSM4,12; and 3) the estimates of rectal testing rates being 7 and 9 times lower compared to urethral testing rates among MSM7. However, it remains unclear to what extent urethral and rectal NG and CT infections contribute to the high HIV incidence among YMSM13,14.
However, individual-level mechanisms alone do not adequately explain the observed racial/ethnic disparities in HIV incidence among YMSM13,15,16. Several network-level and contextual mechanisms have been hypothesized, but most evidence is inconclusive5. Among these, age-assortative and race-assortative mixing are commonly hypothesized to contribute to racial/ethnic disparities and high incidence among YMSM17–22. Particularly, partnerships of YMSM with older MSM are assumed to be significant drivers of racial/ethnic disparities and higher HIV incidence in YMSM because of the elevated HIV prevalence and differences in HIV prevalence among older MSM partners19,20,23. However, only a limited number of studies focus on understanding these network-level mechanisms19,20,24 and results are mixed and inconclusive5, thus underscoring the need for additional research.
Traditional epidemiological and statistical study designs may not be sufficient to fully explain and understand the complex HIV epidemic among YMSM4,25. Decomposing this complex problem into several distinct analyses of hypothesized mechanisms may result not only in an inaccurate estimate of single mechanisms, but also could potentially fail to detect important interactions between these mechanisms25. Epidemic modeling, in particular simulation-based approaches, provide the opportunity to study such complex systems and examine both main effects and interactions of hypothesized mechanisms4,25. Despite their utility for examining the HIV epidemic among YMSM, no epidemic model has yet been developed to study the impact of the broad range of hypothesized mechanisms and their interactions on the HIV spread among YMSM.
In this study, we developed a data-driven agent-based dynamic network simulation model to study HIV spread among YMSM. Using this novel model we studied the impact of age-assortative and race-assortative mixing, and NG and CT infections on HIV incidence and racial disparities among YMSM. We parameterized the simulation model using data of an ongoing longitudinal cohort study of YMSM in Chicago.
Methods
Data-driven simulation model
We developed a discrete-time stochastic agent-based dynamic network simulation model26,27 to study HIV spread among a YMSM population age 16 to 21.8 years over 15 years. The simulation model consisted of two major components: the partnership formation and dissolution model simulating the sexual partnership behavior of YMSM and the disease transmission model simulating the transmission of HIV, NG, and CT across sexual partnerships. The online Appendix provides a detailed description of the underlying empirical study (online Appendix section SDC 1), the design and parameterization of the simulation model (online Appendix section SDC 2 and section SDC 3), the design of the population size and race mix, the aging-in, death, and aging-out processes (online Appendix section SDC 4), the implementation and validation (online Appendix section SDC 5), the counterfactual scenarios (online Appendix section SDC 6), and further details on results presented in this paper (online Appendix section SDC 7).
Partnership formation and dissolution model
The design of the partnership formation and dissolution model was informed using data from the Crew 450 study28,29. We modeled partnerships by first splitting sexual partner-type by those who were one-night-partnerships (one-night partners) and those who were extended partners. Then we further divided extended partners into both partners who were also YMSM (within partners) and partners who were not YMSM (outside partners). Therefore, there are three major types of partnerships: one-night-partnerships, outside-partnerships, and within-partnerships (online Appendix section SDC 2). Despite being a study of YMSM, including outside partners (those older than 21.8 years at baseline or female) increases the accuracy of our model versus others30, as these relationships are prevalent within our sample and differ in associated risks. For example, 27.3% of the 421 YMSM within the Crew 450 cohort identified their sexual orientation as something other than “homosexual-only” or “mostly-homosexual,” and 11.3% of all sex-contacts named by YMSM at data collection waves T1 and T2 were female (online Appendix section SDC 1).
One-night-partnerships, outside-partnerships, and within-partnerships are each modelled in one of two ways within our simulation. Within-partnerships are modelled as a tie in the network of YMSM, but both one-night-partnerships and outside-partnerships are not. Instead, these partnerships are modelled as attributes of YMSM in the network, and are dependent on the individual attributes and the sexual momentary degree of the individual YMSM. We chose to model the networks of within-partners as ties because the strongest empirical data available to us was around YMSM and their partners who were also YMSM (online Appendix section SDC 2.2.1).
We assumed each YMSM had a sexual tendency shaped by their self-reported sexual orientation, their desired sex-role, and their desired sex-frequency (online Appendix section SDC 2.4, Figure 7). In our model, the sexual orientation of a YMSM impacts his choice of forming a partnership either with a man or a women. Additionally, because the desired sex-role and sex-frequency might differ from the actual sex-role behavior and sex-frequency in a partnership, a novel approach was used in which we modeled the desired sex-role and sex-frequency as latent variables which influence the actual sex-role behavior and sex-frequency in a partnership. Actual sexual behavior was then modelled as a function of the sexual tendency of each individual YMSM, the sexual tendency of his partner, and the overall sexual behavior among the YMSM cohort (online Appendix section SDC 2.4).
After the partnership formation, partnership attributes such as oral-sex only, seriousness, mean length and propensity of unprotected anal and vaginal intercourse are determined in sequence using probability estimates derived from multivariate regression models (online Appendix sections SDC 2.2.3, 2.2.4, and 2.2.5). We assumed that outside-partnerships and within-partnerships dissolve at each time step with a probability determined by the mean duration for each partnership31 (online Appendix section SDC 2.3).
Disease transmission model
We model simultaneous HIV, NG, and CT spread among YMSM (online Appendix section SDC 3) where sexually-active YMSM could become infected with HIV, NG, and CT having either penile-vaginal or insertive anal intercourse in female-male partnerships, or receptive or insertive anal intercourse in male-male partnerships. Transmission through oral sex was not considered due to the very low transmission risk for HIV (i.e., 0.04% per sex act32) and missing evidence about the pharyngeal-to-urethral transmission risk for NG and CT33.
The level of infectiousness of a HIV-positive individual differed by time since infection34, use of antiretroviral therapy (ART)35, and full or partial viral suppression30,36, all of which were stratified by race. HIV-infected YMSM initiated ART only if they tested positive and an appropriate amount of time had passed since their exposure to the HIV infection, reflecting current access to treatment and treatment levels30,37 (online Appendix section SDC 3.2). We assumed that on average all outside partners stratified by race and sex have the same HIV prevalence31,32. Thus, outside partners were randomly assigned to be HIV-positive or HIV-negative based on their race and gender. HIV, NG, and CT prevalence of outside male partners was updated over time due to aging-out of YMSM (online Appendix sections SDC 3.2.2 and SDC 7.1). The infectiousness of HIV-infected outside partners was also stratified by sex and race.
We assumed increased HIV susceptibility and HIV transmissibility in case of an infection with NG or CT, stratified by site of infection (urethra or rectum)8–11 (online Appendix section SDC 3.3.3). Due to missing or limited biological evidence, we assumed NG and CT infections to be independent of each other, as well as of HIV infection and ART, and that these factors would not impact the spread and course of NG and CT. YMSM and outside partners could have HIV, NG, and CT infections simultaneously. For NG and CT we assumed that only one site, i.e., urethra or rectum, could be infected because of the unknown pharyngeal-to-urethral transmission risk and the low prevalence of dual site infections in particular for CT7,38, and that the newly infected site is complementary to the infected body site of the sex-partner (i.e., having sex with a rectally infected can only result in an urethral infection). The course of rectal and urethral NG and CT infections was stratified by type of infection (symptomatic vs. asymptomatic), treatment-seeking behavior, and the decision to cease sex while being infected. Individuals with an asymptomatic infection could only receive treatment if they tested positive (online Appendix sections SDC 3.3.1 and 3.3.2).
Parameterization
Longitudinal cohort study: Crew 450
Empirical data were utilized from an ongoing longitudinal study of 450 Chicago YMSM, with study recruitment starting in December 2009 and ending in February 2013. After baseline (T1), data were collected every six months. Retention between waves was high, with 86.7% of participants completing the assessment at T2. An individual was eligible for participation if they were between the ages of 16 and 20 at baseline, birth sex male, spoke English, reported a sexual encounter with a male or an identity of gay/bisexual, and was available for 2 years of follow-up. Participants were recruited through a modified form of respondent-driven sampling28,39 (online Appendix section SDC 1).
Model parameterization
We simulate a YMSM population of size n=4484 YMSM over 15 years where the total population size increases by 0.264% each year40. Size and race-mix were chosen such that the simulated population is representative of the YMSM population age 16 to 21.8 years in Chicago. YMSM age-in, die or age-out of the simulated population over time (online Appendix section SDC 4.2). The partnership formation and dissolution model was parameterized using data of n=421 YMSM enrolled in the Crew 450 at T1 and 6 month follow-up (T2). The fixed age range of the simulated YMSM population was determined by the age range of these YMSM across T1 and T2. Multivariate regression analysis was performed and significant regression covariates were used to predict partnership formation rates and partnership attributes such as the seriousness or mean length of a partnership. Other partnership attributes including race and sex of the partner mixing probabilities (i.e., for one-night-partnerships and outside-partnership) were calculated using available partnership data from T1 and T2 (online Appendix sections SDC 2.2.3, 2.2.4, and 2.2.5).
We parameterized our disease transmission model using biomedical testing data from Crew 450, data from the Chicago Department of Public Health (CDPH)41,42 and other publicly available surveillance data12,43,44, as well as estimates published in literature (online Appendix section SDC 3).
Validation
To validate the model, we compared the biomedical findings of the Crew 450 study with our simulated results. Figure 18 in the online Appendix section SDC 5.2 shows the simulated HIV incidence per 100 person-years compared to the empirical estimates of the Crew 450 study after 3.5 years. The simulated results are within the 95% confidence intervals of the empirical results. Further details of the validation are available in online Appendix section SDC 5.2, where besides sensitivity analysis, the outcomes of the NG and CT transmission and partnership formation model are compared to estimates of the Crew 450 study and other published findings. The comparison of simulated biomedical, partnership formation and network topology measurements with empirical findings as well as the results of the sensitivity analysis show an appropriate validation of our simulation model.
Simulation studies
To determine the impact of age-assortative mixing on HIV spread among YMSM we first examine the overall HIV incidence per 100 person-years stratified by partnership-type. Second, we determine the HIV incidence per male-male partnership year since male-male partnerships are the main mode of transmission and HIV incidence per 100 person-years equals the HIV risk on the dyad-level, i.e. per partnership-year, multiplied with the actual number of partnerships (online Appendix section SDC 7.2). Race-assortative mixing is assumed to maintain and potentially increase racial disparities in HIV among YMSM because of significant differences in HIV prevalence among races17,45. To isolate the impact of the HIV risk of a partnership attributable to race-assortative mixing from the impact of racial differences in HIV prevalence, we use two counterfactual scenarios: one with no race-assortative mixing and one with no racial differences in HIV prevalence (online Appendix section SDC 6). Additionally, to quantify the impact of HIV transmission through older MSM, we use a counterfactual assuming no HIV transmission occurs in outside partnerships (i.e., partnerships with older MSM or females) and another assuming a 50% reduction in HIV transmission risk for YMSM in outside partnerships. Finally, we quantify the overall impact of NG and CT infections on HIV spread among YMSM by comparing the total HIV incidence of a counterfactual where there is no increased HIV transmissibility and susceptibility due to NG and CT infections to the base-case (online Appendix sections SDC 3.3.3 and SDC 5.4).
Results shown in the following section are statistically significant (i.e., non-overlapping 95% confidence intervals (CI)). Results are expressed as means. The half-width of the 95% CI are ≤ 1.5% of the mean, unless otherwise stated.
Results
HIV epidemic over time
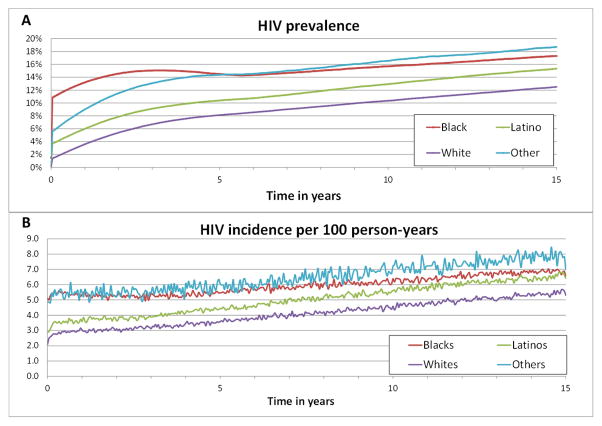
Black YMSM experienced the highest HIV prevalence and incidence compared to Latino and White YMSM (Figures 1A,1B), but only a moderate increase in HIV prevalence and incidence across the modeled 15 years (i.e., HIV incidence increased 1.59 fold). However, Latino YMSM and White YMSM experienced steeper increases in HIV prevalence and incidence (i.e., HIV incidence increased 1.97 fold for Latino YMSM and 2.03 fold for White YMSM). 3076 YMSM were newly infected with HIV over 15 years (i.e., 1220 Black YMSM vs. 836 Latino and 770 White YMSM) (see also Table 37 in SDC 5.2).
Figure 1.
Simulated HIV prevalence (Figure 1A) and incidence per 100 person-years (Figure 1B) stratified by race over 15 years. Figure 1B shows the mean HIV incidence per 100 person-years per time-step (i.e., 0.5 months) with the half-width of the 95% CI are ≤3.5% of the mean except for Other YMSM.
Age and race mixing
Overall, 44.4% of all new HIV infections among YMSM occurred in within-partnerships, 34.5% in outside-partnerships, and 21.1% in one-night-partnerships as shown in Figure 2. These proportions varied marginally across races and time, i.e. except for Other YMSM deviations to the above fractions were within 5 percentage-points.
Figure 2.
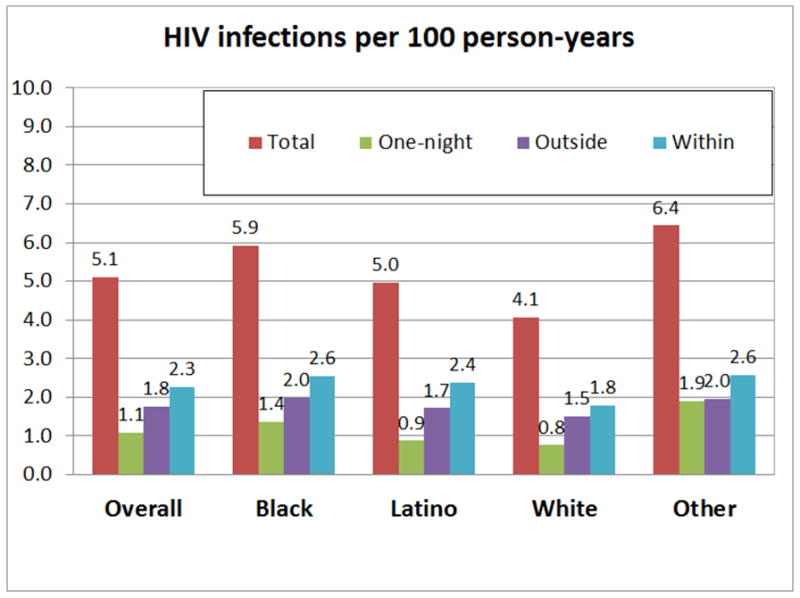
Simulated new HIV infections per 100 person-years over 15 years among YMSM stratified by race and relationship type. “Total “ shows the simulated number of total HIV infections that occurred in all sexual relations. “One-night “: simulated new HIV infections that occurred in one-night-partnerships; “Outside “: simulated new HIV infections that occurred in outside-partnerships, i.e. with older male or female partners. HIV infections from females was rare. In outside partnerships with females, 0.0049 HIV infections per 100 person-years (95% CI: 0.0040–0.0057) occurred. In one-night-partnerships with females, 0.0035 (95% CI: 0.0029–0.0041) occurred. “Within “: simulated new HIV infections that occurred in within-partnerships, i.e. partnerships with other YMSM.
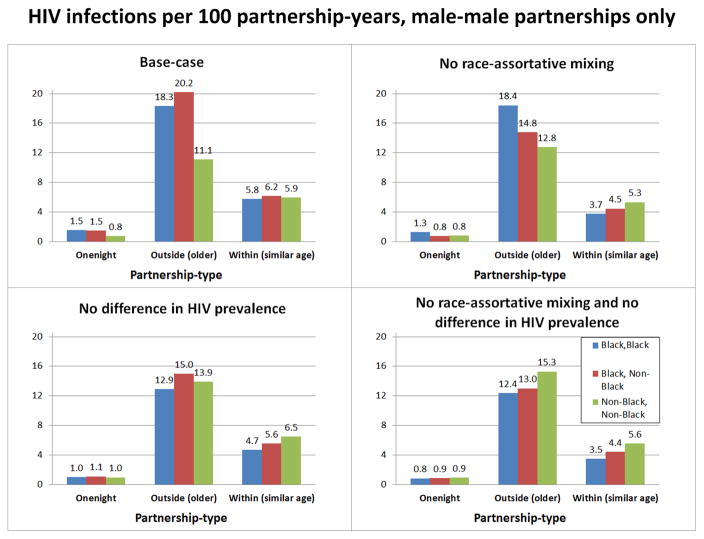
We examined male-male partnerships as the main mode of HIV transmission among YMSM with a specific focus on Black YMSM given their high incidence; HIV incidence per 100 male-male partnership-years was highest for Black-Black and NonBlack-(older)Black outside-dyads (Figure 3A). Without race-assortative mixing (i.e., partners are selected without regards to their race; Figure 3B), HIV incidence decreased for Black-NonBlack outside-partnerships as well as for all within-partnerships whereas HIV incidence for NonBlack-NonBlack outside-partnerships increased. Assuming the same HIV prevalence for all male outside partners (17.2%) and the same HIV prevalence for all YMSM (5.6%) at baseline t=0 (Figure 3C), differences in HIV incidence for male-male outside-partnerships across racial combinations almost vanished with the HIV incidence for Black-NonBlack outside-partnerships being marginally higher compared to Black-Black and NonBlack-NonBlack outside-partnerships. In a counterfactual scenario with both (Figure 3D), we observe an increase in HIV incidence for NonBlack-NonBlack outside-partnerships and a decrease in HIV incidence for all within-partnerships compared to the counterfactual shown in Figure 3C.
Figure 3.
HIV infections per 100 partnership-years, male-male partnerships only. A) Base-case scenario corresponding to Figures 1 and 2. HIV infections per 100 male-male partnership-years in case of one-night-partnerships denotes HIV infections per average number of one-night-partnerships per year. For within-partnerships the number of partnership-years is the sum of the number of susceptible-infected partnership-years plus two times the number of susceptible-susceptible partnership-years (see online Appendix section 7.2 for details). B) Counterfactual scenario with no race-assortative mixing, i.e. YMSM select partners independent of race. C) Counterfactual scenario with no difference by race in initial HIV prevalence (5.6% at baseline) and among outside partners (set to 17.2%). D) Counterfactuals of Figures B) and Figure C) combined. In case of outside partnerships, i.e. partnerships of YMSM with older MSM, Black-NonBlack partnerships denote both partnerships of Black YMSM with older NonBlack MSM and partnerships of NonBlack YMSM with older Black MSM (see also Figure 27 in online Appendix section SDC 7.2).
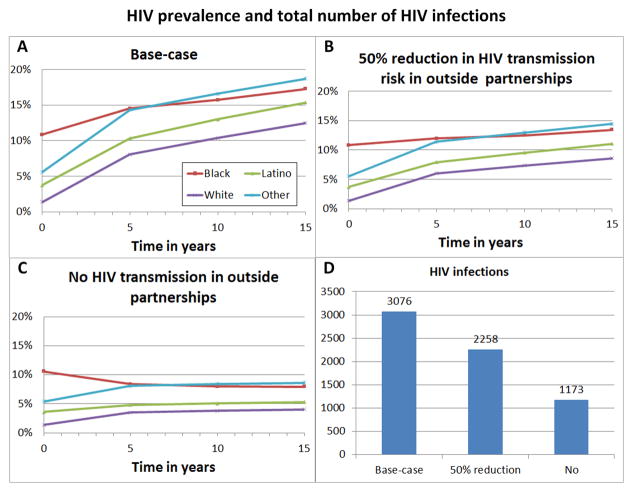
As shown in Figure 3A–D, HIV incidence in outside-partnerships was always higher than in within-partnerships. With no HIV transmissions occurring in outside-partnerships, total HIV incidence would decrease by 61.9% (95% CI 61.57%–62.19%) (Figure 4D) and HIV prevalence be close to steady-state after 5 years (Figure 4C). If HIV transmission risk is reduced by 50% in outside-partnerships, HIV infections would decrease by 26.6% (95% CI 26.13%–27.06%) (Figures 4B, 4D). In both counterfactuals scenarios, racial disparities decreased but remained significant.
Figure 4.
HIV prevalence stratified by race and total number of new HIV infections over 15 years (Figure 4D) for the base-case scenario (Figure 4A) corresponding to Figures 1 and 2, for a counterfactual scenario where no HIV transmission occurs in outside partnerships of YMSM with older MSM or females (Figure 4C); and for a counterfactual scenario where HIV transmission risk in outside partnerships is reduced by 50% compared to the base-case scenario (Figure 4B). HIV infections from females was rare, see also caption of Figure 2.
Gonorrhea and chlamydia
Using a counterfactual where NG and CT do not affect HIV transmission, we found that the fraction of HIV infections attributable to NG or CT was 14.6% (95% CI 14.1%–15.2%). In the base-case, 66.4% of these HIV infections were attributable to rectal NG or CT infections. Also, 41.7% of all HIV infections attributable to NG or CT in this scenario were attributable to increased HIV susceptibility due to NG or CT infection of a HIV-negative individual.
Discussion
We developed a novel data-driven simulation model of HIV spread among YMSM. Our study focused on the impact of age- and race-mixing and STIs on HIV incidence and racial disparities and was motivated by the limited understanding about the impact of these mechanisms on HIV spread among YMSM5.
Over 15 years, the HIV epidemic among YMSM continued to rise with an estimated 3076 new HIV infections. Racial disparities also continued to persist, but increases in HIV prevalence and incidence differed by race, with Latino YMSM and White YMSM facing greater increases. These data map onto YMSM specific data from the CDPH, that show an overall increase in HIV diagnoses among YMSM, and steeper increases in HIV diagnoses and prevalence46 in Latino/White YMSM vs. Black YMSM (online Appendix section SDC 5.2). Further, our estimates of the total number of HIV infections among YMSM are within the 95% CI of the CDPH HIV incidence estimates of 15 to 24 year old YMSM adjusted for age-range and the fraction of HIV infected YMSM being unaware of their HIV infection (online Appendix section SDC 5.2). These results alone suggest that HIV will continue to place a heavy burden on the YMSM population. While Black YMSM will continue to be disproportionately impacted, our model suggests that racial disparities in this group will decrease due to increasing incidence in White and Latino YMSM, but unfortunately not because of declining incidence among Black YMSM.
Our results indicate that approximately 45% of all HIV infections among YMSM occurred in partnerships between YMSM. While in a partnership, we find that the risk of HIV infection is highest across partnership-types for Black YMSM with an older Black MSM. This is consistent with findings from studies using other methods18,20,47,48. Differences in HIV prevalence among races, particularly the high HIV prevalence among Black MSM, drive the high HIV risk of a Black YMSM-Black(older)MSM partnership compared to other NonBlack-(older)NonBlack partnerships, confirming the hypotheses of multiple studies5,17,22,45. While 34.5% of all HIV infections among YMSM in the simulation occur in partnerships with older MSM or females, a hypothetical scenario where no HIV transmissions happen in such partnerships decreases HIV infections among YMSM by 61.9%. Thus, each YMSM transmission avoided from an older MSM partner will also prevent an additional 0.8 HIV infections among YMSM. Therefore, prevention should target the reduction of HIV transmission in age-disassortive partnerships.
Simulating the simultaneous spread of HIV, NG, and CT among YMSM, we determined the fraction of HIV infections attributable to NG or CT to be 14.6%, a proportion within the range of the few estimates reported. Chesson and Pinkerton49 used a simple modeling approach to estimate the fraction of HIV infections attributable to NG or CT in the adult heterosexual US population to be between 4.6% and 9.2%. Another modeling study among MSM in the Netherlands50 estimated the fraction of HIV infections solely attributable to CT to be 15.2%. Among the 14.6% of all HIV infections attributable to NG or CT in our study, 66.4% and 41.7% were due to rectal infections and increased susceptibility, respectively. Rectal testing of NG and CT is rare within the population7, and this data suggest further evaluation of HIV and STI testing policies is necessary to determine a holistic and cost-optimal testing strategy7,14.
This study has several limitations. First, we simulated the HIV spread among a cohort of Chicago YMSM over time, which limits the generalizability of our results to YMSM populations in other US cities or older age groups. However, both the comparability of the empirical estimates from the Crew 450 study to estimates of other studies3,4 and the validation of the model suggests the applicability of our findings to other YMSM populations. Further, as the disease transmission model input parameters are based on few data sources, they may be biased due to sampling error; especially in the case of NG and CT, parameter estimates were difficult to obtain and estimates varied widely51, highlighting the need for more accurate parameter estimates of NG, CT, and their interaction with HIV in this context. Furthermore, modeling only within-partnerships as a network did not allow us to examine whether potential network effects on HIV transmission observed in within-partnerships also apply to one-night-partnerships and outside-partnerships. Finally, to parameterize the partnership formation model we used multivariate regression analysis where only significant parameters were used to predict partnership formation rates and partnership attributes. Thus, certain effects hypothesized in other studies were observable but not significant, and therefore not included in the model. This could contribute to the fact that racial/ethnic disparities had a lower magnitude in our simulated results within the first 3.5 years and thus might also influence racial differences in the increase in HIV prevalence and incidence over 15 years.
Using an agent-based dynamic network simulation model of HIV spread among YMSM, we demonstrated: first, that the HIV epidemic among YMSM continues to rise especially among Latino and White YMSM; second, racial disparities in HIV risk per partnership are mostly driven by differences in HIV prevalence among older MSM partners; third, YMSM and in particular Black YMSM having an older Black MSM partner are at highest risk; and fourth, NG and CT, particularly rectal infections, account for a sizeable portion of all HIV infections. These results emphasize the need for HIV prevention efforts targeting all YMSM, holistic HIV and STI testing strategies, and suggest that prevention interventions focusing on transmission between YMSM and older MSM might be highly effective.
Supplementary Material
SDC 1: Crew450 study
SDC 2: Sexual partnership formation and dissolution
SDC 3: Disease Transmission
SDC 4: Population size, age range, and simulated time horizon
SDC 5: Implementation and Validation
SDC 6: Counterfactual scenarios
SDC 7: Addendum to results
SDC 1–7 are combined into one Supplemental Digital Content file, Supplementary Digital Content.pdf
Acknowledgments
Source of Funding: This project was supported by grants from the National Institute on Drug Abuse (Co-PIs: Mustanski and Garofalo; R01DA025548; PI: Mustanski, R01DA025548-02S1; PI: Birkett, R03DA033906, K08DA037825) and the National Institute on Drug Abuse funded Center for Prevention Implementation Methodology (Ce-PIM PI Brown, P30DA027828).
We thank the Chicago Department of Public Health for providing detailed data about HIV diagnoses among 15 to 24 year old YMSM in Chicago from 2009 to 2013. We thank the reviewers for their constructive and helpful comments and ideas. We also thank Gregory L Philips II, Alexander Gutfraind, Richard D’Aquila, Hank Seifert, Uri Wilensky, and Hendricks Brown for helpful comments. We thank Daniel Ryan for his assistance in data analysis.
Footnotes
Earlier version of this study were presented at the IMPACT 2nd Annual Chicago LGBTQ Health & Wellness Conference, Chicago, Nov. 20, 2013, the Mathematics and HIV Workshop: Operations Research and Network Modeling for HIV Treatment and Prevention, Burnaby BC, Canada, March 29–30, 2014, the Ce-PIM Science Advisory Board Meeting, Chicago, May 1–2, 2014, and the INFORMS Annual Meeting 2014, San Francisco, Nov. 9–12, 2014.
Conflicts of Interest
All authors declare no conflicts of interest.
All authors conceived the study idea, contributed original ideas, and edited drafts of the article. E. Beck led the research, writing, and analysis. B. Armbruster supervised analytic methods.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- 1.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS ONE. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. CDC Fact Sheet: Estimates of New HIV Infections in the United States, 2006–2009. Atlanta, GA: 2011. pp. 1–6. [Google Scholar]
- 3.Balaji AB, Bowles KE, Le BC, et al. High HIV incidence and prevalence and associated factors among young MSM, 2008. AIDS. 2013;27(2):269–278. doi: 10.1097/QAD.0b013e32835ad489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan PS, Peterson J, Rosenberg ES, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PLoS ONE. 2014;9(3):e90514. doi: 10.1371/journal.pone.0090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maulsby C, Millett G, Lindsey K, et al. HIV among Black men who have sex with men (MSM) in the United States: a review of the literature. AIDS Behav. 2014;18(1):10–25. doi: 10.1007/s10461-013-0476-2. [DOI] [PubMed] [Google Scholar]
- 6.Mustanski BS, Newcomb ME, Du Bois SN, et al. HIV in young men who have sex with men: a review of epidemiology, risk and protective factors, and interventions. J Sex Res. 2011;48(2–3):218–253. doi: 10.1080/00224499.2011.558645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men--STD Surveillance Network, United States, 2010–2012. CID. 2014;58(11):1564–1570. doi: 10.1093/cid/ciu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537–543. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 9.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Trans Infec. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin F, Prestage GP, Imrie J, et al. Anal sexually transmitted infections and risk of HIV infection in homosexual men. J Acquir Immune Defic Syndr. 2010;53(1):144–149. doi: 10.1097/QAI.0b013e3181b48f33. [DOI] [PubMed] [Google Scholar]
- 11.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 12.Hotton A, Gratzer B. Howard Brown Health Center: STI Annual Report, 2011. Chicago, IL: Howard Brown Center; 2012. [Google Scholar]
- 13.Millett GA, Peterson JL, Wolitski RJ, et al. Greater risk for HIV infection of black men who have sex with men: a critical literature review. Am J Public Health. 2006;96(6):1007–1019. doi: 10.2105/AJPH.2005.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vriend HJ, Lugner AK, Xiridou M, et al. Sexually transmitted infections screening at HIV treatment centers for MSM can be cost-effective. AIDS. 2013;27(14):2281–2290. doi: 10.1097/QAD.0b013e32836281ee. [DOI] [PubMed] [Google Scholar]
- 15.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS. 2007;21(15):2083–2091. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 16.Clerkin EM, Newcomb ME, Mustanski B. Unpacking the racial disparity in HIV rates: the effect of race on risky sexual behavior among Black young men who have sex with men (YMSM) J Behavioral Medicine. 2011;34(4):237–243. doi: 10.1007/s10865-010-9306-4. [DOI] [PubMed] [Google Scholar]
- 17.Berry M, Raymond HF, McFarland W. Same race and older partner selection may explain higher HIV prevalence among black men who have sex with men. AIDS. 2007;21(17):2349–2350. doi: 10.1097/QAD.0b013e3282f12f41. [DOI] [PubMed] [Google Scholar]
- 18.Joseph HA, Marks G, Belcher L, et al. Older partner selection, sexual risk behaviour and unrecognised HIV infection among black and Latino men who have sex with men. Sex Trans Infec. 2011;87(5):442–447. doi: 10.1136/sextrans-2011-050010. [DOI] [PubMed] [Google Scholar]
- 19.Mustanski B, Birkett M, Kuhns LM, et al. The Role of Geographic and Network Factors in Racial Disparities in HIV Among Young Men Who have Sex with Men: An Egocentric Network Study. AIDS Behav. 2014 doi: 10.1007/s10461-014-0955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newcomb ME, Mustanski B. Racial differences in same-race partnering and the effects of sexual partnership characteristics on HIV Risk in MSM: a prospective sexual diary study. J Acquir Immune Defic Syndr. 2013;62(3):329–333. doi: 10.1097/QAI.0b013e31827e5f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond HF, McFarland W. Racial mixing and HIV risk among men who have sex with men. AIDS Behav. 2009;13(4):630–637. doi: 10.1007/s10461-009-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tieu HV, Murrill C, Xu G, et al. Sexual partnering and HIV risk among black men who have sex with men: New York City. J Urban Health. 2010;87(1):113–121. doi: 10.1007/s11524-009-9416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurt CB, Matthews DD, Calabria MS, et al. Sex with older partners is associated with primary HIV infection among men who have sex with men in North Carolina. J Acquir Immune Defic Syndr. 2010;54(2):185–190. doi: 10.1097/QAI.0b013e3181c99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson BT, Redding CA, DiClemente RJ, et al. A network-individual-resource model for HIV prevention. AIDS Behav. 2010;14(Suppl 2):204–221. doi: 10.1007/s10461-010-9803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maglio PP, Sepulveda MJ, Mabry PL. Mainstreaming modeling and simulation to accelerate public health innovation. Am J Public Health. 2014;104(7):1181–1186. doi: 10.2105/AJPH.2014.301873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonabeau E. Agent-based modeling: Methods and techniques for simulating human systems. P Natl Acad Sci USA. 2002;99:7280–7287. doi: 10.1073/pnas.082080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm V, Railsback SF. Individual-based Modeling and Ecology. New Jersey: Princeton University Press; 2005. [Google Scholar]
- 28.Kuhns LM, Kwon S, Ryan DT, et al. Evaluation of Respondent-Driven Sampling in a Study of Urban Young Men Who Have Sex with Men. J Urban Health. 2014 doi: 10.1007/s11524-014-9897-0. Epub: 2014 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mustanski B, Ryan DT, Garofalo R. Associations of sexually transmitted infections with condom problems among young men who have sex with men. Sex Transm Infect. 2014;41:427–432. doi: 10.1097/OLQ.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodreau SM, Carnegie NB, Vittinghoff E, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PLoS ONE. 2012;7(11):e50522. doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krivitsky P, Handock M. A separable model for dynamic networks. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2014;76:29–46. doi: 10.1111/rssb.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vittinghoff E, Douglas J, Judson F, et al. Per-Contact Risk of Human Immunodeficiency Virus Transmission between Male Sexual Partners. Am J Epidemiol. 1999;150:306–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 33.Bissessor M, Tabrizi SN, Fairley CK, et al. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control. J Clinical Microbiology. 2011;49(12):4304–4306. doi: 10.1128/JCM.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Inf Dis. 2008;198(5):687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 35.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weintrob AC, Grandits GA, Agan BK, et al. Virologic response differences between African Americans and European Americans initiating highly active antiretroviral therapy with equal access to care. J Acquir Immune Defic Synd. 2009;52(5):574–580. doi: 10.1097/QAI.0b013e3181b98537. [DOI] [PubMed] [Google Scholar]
- 37.Swindells S, Cobos DG, Lee N, et al. Racial/ethnic differences in CD4 T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS. 2002;16(13):1832–1834. doi: 10.1097/00002030-200209060-00020. [DOI] [PubMed] [Google Scholar]
- 38.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. CID. 2005;41(1):67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 39.Phillips GKL, Garofalo R, Mustanski B. Do recruitment patterns of young men who have sex with men (YMSM) recruited through respondent-driven sampling (RDS) violate assumptions? J Epidemiol Community Health. 2014 doi: 10.1136/jech-2014-204206. Epub: 2014 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Census Bureau. State and County QuickFacts. Chicago (city), Illinois: 2015. [Accessed 04/01, 2015]. Available at: http://quickfacts.census.gov/qfd/states/17/1714000.html. [Google Scholar]
- 41.Chicago Department of Public Health. HIV/STI Surveillance Report, 2013. Chicago, IL: City of Chicago; 2013. [Google Scholar]
- 42.Chicago Department of Public Health. The Heterosexual HIV Epidemic in Chicago: Insights into the Social Determinants of HIV. Chicago, IL: City of Chicago; 2011. [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control. [Accessed 04/01/2015];WISQARS (Web-based Injury Statistics Query and Reporting System) 2015 Available at: http://www.cdc.gov/injury/wisqars/
- 44.Jones RC, Harper-Jemison DM, Clark J, et al. City of Chicago. 2013. Leading Causes of Death in Chicago, 2007–2009. [Google Scholar]
- 45.Morris M, Goodreau S, Moody J. Sexual networks, concurrency, and STD/HIV. In: Holmes K, editor. Sexually transmitted diseases. New York: McGraw-Hill; 2007. pp. 109–126. [Google Scholar]
- 46.Chicago Department of Public Health. HIV Risk and Prevention Behaviors Among Men Who Have Sex With Men, Chicago, 2008 and 2011. Chicago, IL: City of Chicago; Dec, 2012. [Google Scholar]
- 47.Mustanski B, Newcomb ME. Older sexual partners may contribute to racial disparities in HIV among young men who have sex with men. J Adolescent Health. 2013;52(6):666–667. doi: 10.1016/j.jadohealth.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oster AM, Wiegand RE, Sionean C, et al. Understanding disparities in HIV infection between black and white MSM in the United States. AIDS. 2011;25(8):1103–1112. doi: 10.1097/QAD.0b013e3283471efa. [DOI] [PubMed] [Google Scholar]
- 49.Chesson HW, Pinkerton SD. Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. J Acquir Immune Defic Syndr. 2000;24(1):48–56. doi: 10.1097/00126334-200005010-00009. [DOI] [PubMed] [Google Scholar]
- 50.Xiridou M, Vriend HJ, Lugner AK, et al. Modelling the impact of chlamydia screening on the transmission of HIV among men who have sex with men. BMC Infectious Diseases. 2013;13:436. doi: 10.1186/1471-2334-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies BAS, Turner K, Ward H. How robust are the natural history parameters used in chlamydia transmission dynamic models? A systematic review. Theor Biol and Med Model. 2014;11(8) doi: 10.1186/1742-4682-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1: Crew450 study
SDC 2: Sexual partnership formation and dissolution
SDC 3: Disease Transmission
SDC 4: Population size, age range, and simulated time horizon
SDC 5: Implementation and Validation
SDC 6: Counterfactual scenarios
SDC 7: Addendum to results
SDC 1–7 are combined into one Supplemental Digital Content file, Supplementary Digital Content.pdf