Abstract
Background
Biochemical changes induced in red blood cells (RBCs) during storage may impair their function upon transfusion. Transfusion-associated stresses may further amplify storage lesion effects including increased phosphatidylserine (PS)-exposure at the RBC membrane, microparticle (MP)-release and adhesion to endothelial cells (ECs). RBC stress susceptibility in vitro was investigated in relation to storage time and additive solution.
Study design and methods
Leukocyte-reduced whole blood donations (n=18) were paired, mixed and re-split before separating the RBCs for storage in SAGM or AS-1 solutions. Samples were taken after 3, 21 or 35 days. For oxidative stress treatment RBCs were exposed to 0.5mM tert-butylhydroperoxide. Transfusion-associated stress was simulated by overnight culture at 37 °C with plasma containing inflammatory mediators. PS-exposure and MPs were measured by flow cytometry and adhesion to EC was tested under flow conditions. PS specificity of adhesion was tested by blocking with PS-containing lipid vesicles.
Results
Oxidative stress induced significantly higher PS-exposure and adhesion to ECs in RBCs stored for 35 days compared to 3 days (p<0.04). PS-containing vesicles blocked RBC-EC adhesion. After overnight culture with or without plasma, PS-exposure and EC-adhesion were significantly increased (p<0.05). MP numbers increased with longer RBC storage and after RBC culture with plasma. Culture conditions influenced MP numbers from day 35 RBCs. RBCs stored in SAGM had significantly higher PS-exposure after stress treatment than AS-1 RBCs (p<0.02).
Conclusion
Storage for 35 days significantly increased RBC susceptibility to oxidative and in vitro transfusion-associated stresses and was higher for RBCs stored in SAGM compared to AS-1.
Keywords: Red blood cells, storage lesion, oxidative stress, stress susceptibility, transfusion, phosphatidylserine, adhesion, AS-1, SAGM, microparticles
INTRODUCTION
During storage of up to 6 weeks, red blood cells (RBCs) undergo biochemical and physical changes, summarized in the term ‘storage lesion’ that reflect storage-induced stresses.1,2 In addition to storage time, the function of stored RBCs is affected by donor variation and different processing and storage protocols, including leukocyte-reduction and additive solutions.3–5 Upon transfusion, RBCs encounter further stress conditions and their survival and function in the transfusion recipient may be impaired, particularly after longer storage.6,7 Specific stress factors may differ between individual recipients depending on their clinical condition but as a minimum, upon transfusion stored RBCs will be subjected to a change in temperature, osmolality and increased pH as well as potentially mechanical and oxidative stresses, such as cardiac by-pass, trauma and inflammatory environments including sepsis.8,9 While changes to RBCs due to storage-related stresses are increasingly well characterized, much less is known about how stored RBCs are affected by stresses associated with transfusion.
We have previously shown that increased storage time results in increased adhesion of leukoreduced stored RBCs to endothelial cells (ECs) and that adhesion followed a different pattern depending on storage in saline-adenine-glucose-mannitol (SAGM) or AS-1 solutions.10,11 Increased adhesion of RBCs to ECs may reduce blood flow and thereby impair the tissue oxygenation function of RBCs.12 The mechanisms of adhesion of stored RBC to ECs have not been clearly elucidated.
Phosphatidylserine (PS), which in healthy cells is confined to the inner membrane leaflet, translocates to the outer leaflet in stressed and apoptotic cells.13 PS-exposure on the outer RBC membrane is a stress marker and it has been suggested to mediate RBC-EC adhesion and target the RBCs for erythrophagocytic clearance from the circulation.13–15 PS-exposure is inducible in a larger percentage of longer-stored RBC compared to shorter storage time indicating increased stress susceptibility after prolonged storage.15,16 Stored RBCs also contain accumulated RBC-derived microparticles (MPs) that have exposed PS and may perturb blood flow by enhancing coagulation,17,18 vasoconstriction,19 and contribute to immunomodulatory effects.20,21 We found in a previous study that the number of RBC-derived MPs increased more during storage in SAGM compared to AS-1 solution.11 Furthermore, MP-release from stored RBCs subjected to additional stress conditions was found to be increased in longer-stored RBCs.16
SAGM and AS-1 solutions have the same chemical constituents; however AS-1 has increased concentrations of glucose and adenine to provide greater energy sources and increased mannitol to help stabilize the RBC membranes.11 Consequently AS-1 RBC components typically have significantly lower hemolysis during component storage compared to SAGM-RBCs,11 although whether this is of clinical significance is unclear.
The aim of the current study was to investigate (1) whether high PS-exposure mediates increased adhesion of stored RBCs to ECs, (2) whether longer-stored RBCs are more susceptible to increased PS-exposure and MP-release using an in vitro culture model to simulate sepsis and transfusion, and (3) to compare RBC stress susceptibility after storage in SAGM or AS-1 solutions.
MATERIALS AND METHODS
RBC components
This study was approved by the Australian Red Cross Blood Service Human Research Ethics Committee.
Whole blood (WB) was obtained from healthy, blood group A-positive (A+) donors. Twelve WB donations (470 mL) were collected into blood collection packs with citrate-phosphate-dextrose anticoagulant (Fresenius Kabi AG, Bad Homburg, Germany), processed into leukocyte-reduced RBC concentrate and resuspended in SAGM solution (100 mL). For the paired study to compare SAGM and AS-1 solutions, 18 WB donations were collected into packs with an in-line WB leukoreduction filter (WBF3 filter; Pall Medical, Portsmouth, UK). Paired donations were collected less than 2 hours apart. After leukoreduction, paired donations were pooled and then equally divided into the original collection packs. This ‘pool-and-split’ study design was used to limit biological differences arising from inherent inter-donor variability. The RBCs were separated by centrifugation at 5,000 xg for 10 min at room temperature (RT) and processed using a blood separator (Macopress; MacoPharma, Mouvaux, France). One of the pair was resuspended in SAGM (Pall) and the other in AS-1 (Adsol; Baxter, Jiutepec, Mexico). Due to technical problems two of the AS-1 RBCs could not be used resulting in seven AS-1 and nine SAGM RBCs (i.e. 7 pairs).
The RBCs were stored under standard blood banking conditions at 2–6 °C and sampled aseptically at days 3, 21 and 35. Full blood counts were performed using an automated hematology analyzer (CellDyn Ruby, Abbott, Santa Clara, CA). Extracellular pH was measured at 22 °C with a pH meter (PHM210; Radiometer, Lyon, France).
Flow cytometry analysis of RBC PS-exposure and MPs
To detect PS-exposure, 1 × 106 RBCs in phosphate buffered saline (PBS) containing 0.1 % human serum albumin (HSA) (Albumex 20; CSL, Parkville, Victoria, Australia) (PBS/HSA) were incubated with 5 µL fluorescein isothiocyanate (FITC)-labelled lactadherin (Haematologic Technologies, Essex Junction, Vermont) (final volume, 100 µL) for 30 minutes in the dark at RT. After staining, RBCs were washed and resuspended in 300 µL PBS/HSA for flow cytometry (FACS CantoII with Diva software, BD Biosciences, San Jose, CA). Data were analyzed using FlowJo software (Treestar, Ashland, OR).
MPs in RBC supernatants, plasma and culture supernatants were enumerated and characterized as previously described.22 Briefly, for the determination of the cellular source of MPs, the sample was mixed with FITC-labelled anti-CD41 (platelet marker) or phycoerythrin (PE)-labelled anti-CD235a (RBC marker) (BD Biosciences). PS-exposure on MPs was determined using FITC-labelled lactadherin (Haematologic Technologies). Samples were diluted with 0.2 µm-filtered PBS, pH 7.2 to a final volume of 100 µL in an absolute count tube (TruCount tubes, BD BioSciences) before incubation in the dark at RT for 30 minutes. An additional 300 µL of filtered PBS was added to each sample prior to flow cytometry. A gating strategy previously optimized for MP detection was applied22 and the absolute count of MPs was calculated according to the manufacturer’s instructions (TruCount tubes, BD Biosciences).
In vitro stress treatments
a) tert-butylhydroperoxide (tBuOOH) oxidation
RBCs were washed twice and 20 × 106 RBCs/mL in 50 mL PBS were incubated with 0.5 mM tBuOOH (final concentration) for 30 minutes at 37 °C. Titration experiments identified that 0.5 mM tBuOOH was the optimal concentration to induce PS-exposure on 90% of RBCs without causing severe morphological shape changes to the RBCs. The tBuOOH-treated RBCs were washed twice in 30 mL PBS and resuspended in flow perfusion buffer ((Media 199, (Gibco, Grand Island, NY) with 1 % HSA)), or PBS/HSA for flow cytometry analysis.
b) RBC culture to simulate transfusion-associated stress
An in vitro transfusion-associated stress model was designed to simulate in vivo body temperature and septic plasma. To generate inflammatory plasma, WB from healthy donors was incubated with 50 ng/mL lipopolysaccharide (LPS) (E. coli 0111:B4; Sigma Chemical, St. Louis, MO), or without for control, for 24 hours at 37 °C in 5 % CO2-air. After culture, the plasma was collected and stored at –70 °C. The plasma levels of cytokines ((interleukin (IL)-1β, IL-6, IL-10, tumor necrosis factor (TNF)-α, interferon-γ)) and MPs were determined by a cytokine multiplex assay and flow cytometry, respectively. Further details and data are available in the on-line Supplementary Information.
For the in vitro transfusion-associated stress model, RBC samples were diluted to 40 % hematocrit in their own supernatant (generated by centrifugation of a separate aliquot of the same RBC sample at 5,000 xg for 5 minutes at 4 °C). Inflammatory plasma or supernatant were added at a final 1/5 dilution, such that the RBC samples had a 32% final hematocrit. RBCs were incubated overnight at 37 °C in 5 % CO2-air.
RBC adhesion to ECs
RBC adhesion to ECs was analyzed under continuous flow perfusion to simulate in vivo microvascular blood flow using a modification of our previously described method.10,11 Briefly, blood group A+ human umbilical vein ECs (HUVECs) were cultured to confluence on a collagen-coated 6-channel μ-slide IV0.4 (ibidi, Planegg, Germany). The slide was mounted into a perfusion chamber heated to 37 °C, which was positioned on an inverted microscope (Model IX71, Olympus, Japan) and connected to a syringe pump (KDS-270; KD Scientific, Holliston, MA). RBCs (1×108 RBC/mL in M199/1% HSA perfusion buffer) were perfused at a shear stress of 0.3 dyne/cm2 at 37 °C. After 10 minutes continuous perfusion, images were captured by a CCD camera that took 10 images two seconds apart for each of 15 different, randomly selected fields of view. Adherent RBCs were defined as RBCs that remained adherent for at least 16 seconds (corresponded to eight consecutive images). The results were calculated as the mean number of adherent RBCs/mm2.
Lipid vesicle preparation and blocking of RBC-EC adhesion
Phosphatidylcholine (PC) vesicles and PS-containing PC vesicles were prepared using previously described methods.23 Specific details are available in the on-line Supplementary Information. Briefly, large unilamellar vesicles (LUV, 100 nm) were made by passing a suspension of large multilamellar vesicles through a 100 nm polycarbonate filter (Whatman, GE Healthcare, Uppsala, Sweden) using a manual extruder (Avestin, Ottawa, Canada). Vesicles were stored at RT and used within 7 days of preparation.
The optimal concentration of LUV for blocking experiments was determined by the ability of the PS-containing vesicles to inhibit the binding of FITC-lactadherin to tBuOOH-treated RBC, compared to PC vesicles. Specific details are available in the on-line Supplementary Information. Based on these titrations, 1 mM concentration of LUV was selected as the optimal dose to distinguish specific inhibition by PS-PC vesicles from background inhibition by PC-only vesicles.
To test inhibition of RBC-EC adhesion by lipid vesicles, HUVECs were pre-incubated with a 1 mM suspension of LUV for 30 minutes at 37 °C. Lipid vesicles were added at 10 µM final concentration to the tBuOOH-treated RBCs immediately before flow perfusion commenced to ensure the presence of excess LUV during the perfusion experiment.
RBC Glutathione (GSH)
RBC samples were centrifuged at 1,700 xg for 5 minutes at 4 °C. A 50 µL aliquot of the RBC pellet was pipetted into a fresh tube and immediately frozen at −70 °C. The levels of GSH were determined using the GSH/GSSG Ratio Assay Kit (Calbiochem/Merck-Millipore, Darmstadt, Germany) according to the manufacturer’s instructions.
Statistical analysis
Data are shown as mean ± standard error of the mean (SEM). Statistical analysis was performed using software (Prism; GraphPad, La Jolla, CA). Wilcoxon matched pairs signed rank test was used to determine differences between SAGM and AS-1 RBCs. Two-way ANOVA was used to determine differences between treatments groups. Repeated measures ANOVA was used to determine differences across RBC storage duration. Significance was defined as p less than 0.05.
RESULTS
Longer storage increased RBC susceptibility to oxidative stress
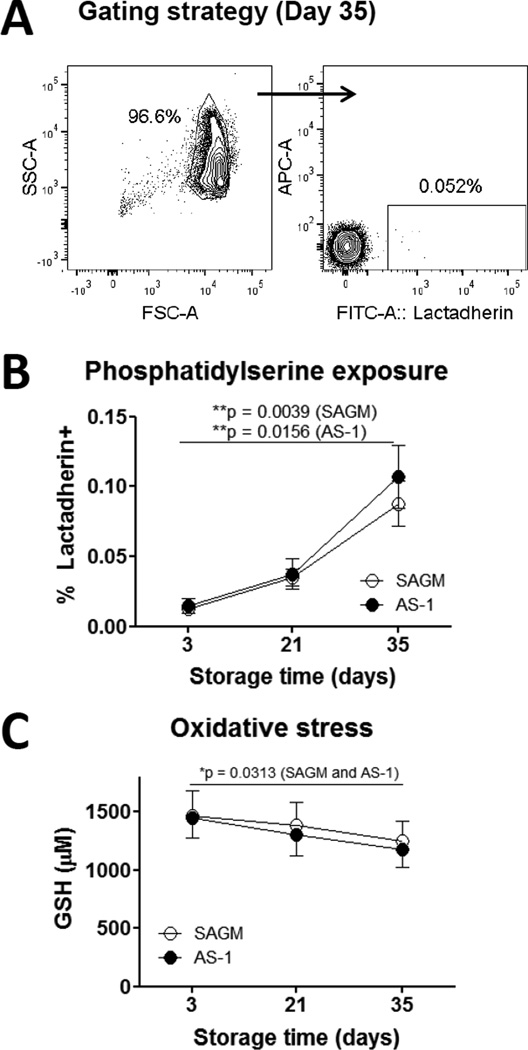
The frequency of lactadherin-binding PS+ RBCs increased significantly during 35 days storage in SAGM or AS-1 solutions (p < 0.016), although the absolute frequency of PS+ RBCs remained very low (less than 0.2 % on day 35) (Fig. 1A, 1B). Over 35 days storage, the concentration of reduced GSH decreased (p = 0.03) (Fig. 1C).
Figure 1. Longer-stored RBCs had increased PS-exposure and decreased GSH.
(A) Flow cytometry plots of day 35 stored RBCs showing the gating strategy and PS-exposure detected by staining with FITC-labelled lactadherin. Log-scale light scatter plot (left) was used to set the RBC gate, which was then displayed on a fluorescence plot (right). 20,000 events were collected. (B) Frequency of PS+ RBCs during 35 days storage of SAGM RBCs (n = 9) and AS-1 RBCs (n = 7). (C) Concentration of RBC reduced glutathione (GSH) in SAGM RBCs (n = 9) and AS-1 RBCs (n = 7) after 3, 21 and 35 days of storage. SAGM RBCs (○), AS-1 RBCs (●) Significance defined as *p < 0.05 and **p < 0.01.
Oxidative stress treatment induced greater adhesion of RBCs to ECs
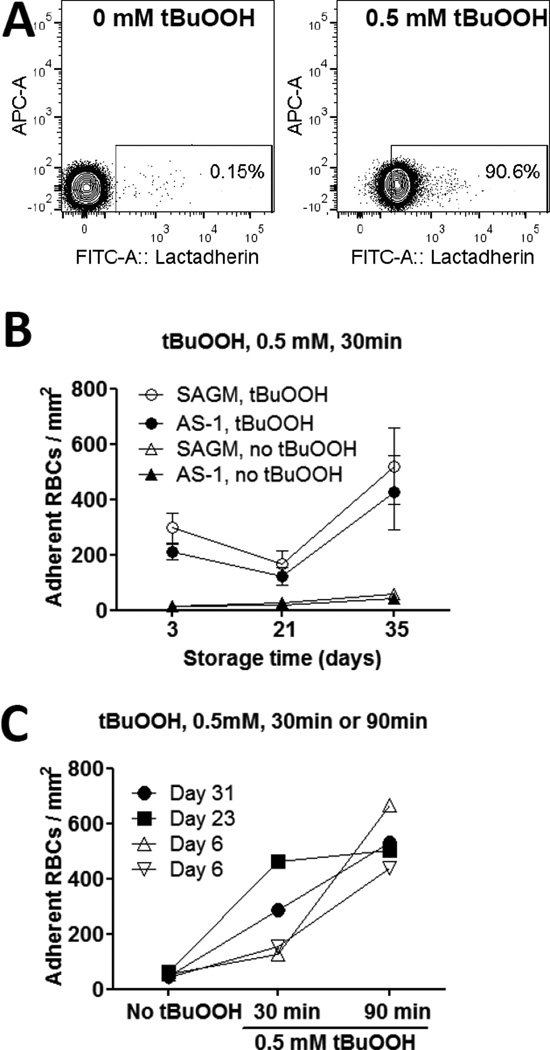
To determine whether oxidative treatment of stored RBCs affected their interaction with ECs, RBCs were treated with 0.5 mM tBuOOH for 30 min to induce PS-exposure in at least 90% of the RBCs (Fig. 2A). RBC-EC adhesion was measured under continuous flow perfusion. Adhesion of tBuOOH-treated RBCs to ECs was significantly higher for RBCs stored for 35 days compared to 21 days in SAGM (p = 0.039) or AS-1 solutions (p = 0.016) (Fig. 2B).
Figure 2. Longer-stored RBCs were more susceptible to oxidative stress.
(A) Flow cytometry plots of tBuOOH-induced PS exposure on day 35 stored RBCs detected by FITC-labelled lactadherin. Control, no tBuOOH (left plot); treated with 0.5 mM tBuOOH for 30 min (right plot). Representative of five independent experiments. (B) RBCs after 3, 21 and 35 days of storage were treated with 0.5 mM tBuOOH for 30 min and then tested for adhesion to ECs under continuous flow perfusion conditions at 37 °C. Adhesion of tBuOOH-treated RBCs to ECs was significantly higher after 35 days of storage compared to 21 days of storage for SAGM RBCs (○) (p = 0.039) and AS-1 RBCs (●) (p = 0.016). Control, no tBuOOH SAGM RBCs (△) AS-1 RBCs (▲) are shown for comparison. Results are for 7 pairs. (C) RBCs at different storage ages from separate donors were treated with or without 0.5 mM tBuOOH for 30 min or 90 min as indicated and then tested for adhesion to ECs under continuous flow perfusion conditions at 37 °C. Day 6 (▽, donor 1); Day 6 (△, donor 2); Day 23 (■); Day 31 RBCs (●).
Extended tBuOOH-treatment for 90 minutes resulted in EC-adhesion of fresher RBCs (day 6) to reach similar levels as seen for longer-stored RBCs (day 23 or 31) treated with tBuOOH for only 30 minutes (Fig. 2C).
Oxidized RBC adhesion to EC was blocked by PS-containing vesicles
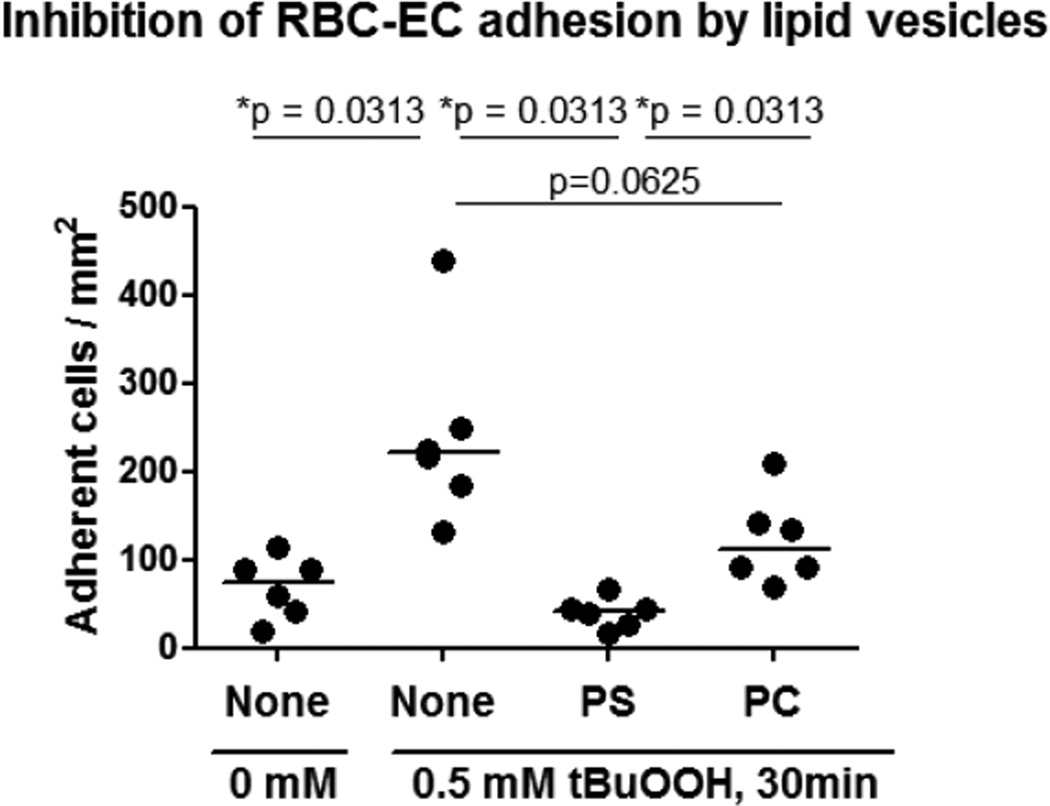
Incubation of ECs with PS-containing vesicles significantly reduced the adhesion of tBuOOH-treated RBCs to ECs compared to untreated ECs (p = 0.03) (Fig. 3). PC-only control vesicles did not significantly block tBuOOH-treated RBC adhesion to ECs (Fig. 3).
Figure 3. PS-containing lipid vesicles blocked adhesion of oxidized RBCs to ECs.
Adhesion of RBCs to ECs was measured under continuous flow perfusion conditions at 37 °C, before and after tBuOOH-treatment of RBCs and with or without pre-incubation of ECs with PS-containing or PC-only (control) lipid vesicles as indicated. Horizontal bars represent the median of six individual SAGM-RBC units tested at median storage time of 34 days (range 23 to 42 days). Significance defined as *p < 0.05.
PS-exposure was higher in longer-stored RBCs after in vitro transfusion-associated stress conditions
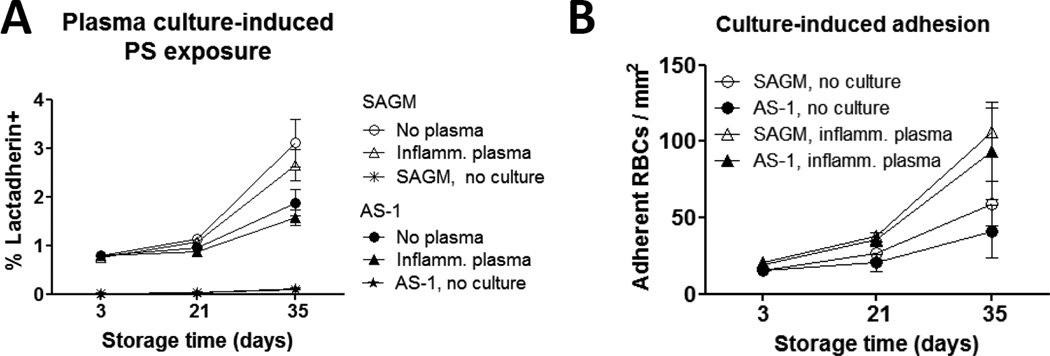
To more closely model physiological and transfusion-associated stress conditions an overnight 37 °C culture assay was used. Details of the cytokine and MP content of the inflammatory plasma and RBC supernatants used in these experiments are available in the on-line Supplementary Information to this article. Culture of stored RBCs in the presence of inflammatory plasma or RBC supernatant resulted in significantly higher PS-exposure compared to uncultured stored RBCs at all storage time-points (p < 0.001) (Fig. 4A). However, there was no significant difference in the level of PS-exposure induced by culture of RBCs in inflammatory plasma compared to their own supernatant. Cultured day 21- and day 35-stored SAGM RBCs had significantly higher frequency of PS+ RBCs compared to their AS-1 counterparts (p = 0.016 for both time-points).
Figure 4. In vitro transfusion-associated stress increased PS-exposure and RBC-EC adhesion.
(A) PS-exposure on stored RBCs before and after overnight culture at 37 °C in the presence of inflammatory plasma or RBC supernatant. PS-exposure was detected by staining with FITC-labelled lactadherin and flow cytometry. Culture induced significantly increased PS-exposure, which increased with longer storage of RBCs. Culture in RBC supernatant, Day 3 to 35, SAGM RBCs (○) (p = 0.004), AS-1 RBCs (●) (p = 0.016). Cultured in inflammatory plasma, Day 3 to 35, SAGM RBCs (▲) (p = 0.004), AS-1 RBCs (●) (p = 0.031). There was no significant difference between culture in inflammatory plasma or RBC supernatant. SAGM RBCs had significantly higher levels of PS-exposure induced by overnight culture compared to AS-1 RBCs at day 21 and day 35 of storage, p = 0.016 for both time-points and culture in RBC supernatant or inflammatory plasma (n = 7 pairs). Controls (‘no culture’, meaning stored samples were not incubated overnight) are also shown, SAGM RBCs (*), AS-1 RBCs (▲). (B) RBC adhesion to ECs before and after overnight culture at 37°C in the presence of plasma. RBC adhesion was measured under continuous flow perfusion conditions. After overnight culture, adhesion of RBCs stored for 35 days was significantly increased compared to RBCs stored for 3 days in SAGM (△) (p = 0.004) or AS-1 (▲) (p = 0.016). Uncultured RBCs had lower adhesion compared to cultured RBCs, but similarly showed increased adhesion with longer storage time; Day 3 to 35, SAGM RBCs (○) (p = 0.014); AS-1 RBCs (●) (strong trend, p = 0.075). After overnight culture, the number of adherent day 35 RBCs was significantly increased compared to no culture for AS-1 RBCs (p = 0.031) and almost significant for SAGM RBCs (p = 0.055). Results are from 9 SAGM RBCs and 7 AS-1 RBCs.
Stress-treated day 35 RBCs adhered more to ECs than shorter stored RBCs
After overnight culture, adhesion of day 35-stored RBCs to ECs under flow perfusion conditions was significantly increased compared to RBCs stored for 3 days (SAGM RBCs, p = 0.004; AS-1RBCs, p = 0.016) (Fig. 4B). The mean number of adherent day 35 RBCs doubled after overnight culture compared to no culture, which was statistically significant for AS-1 RBCs (p = 0.031) and almost significant for SAGM-RBCs (p = 0.055). Similar to the findings for PS-exposure, RBC adhesion to ECs was comparable whether cultured in inflammatory plasma or RBC supernatant (n=3, data not shown). The difference in the level of adhesion of SAGM RBCs compared to AS-1 RBCs was not significant at the storage time-points tested.
Number of MPs increased in longer-stored RBCs after in vitro transfusion-associated stress and depended on the presence of plasma
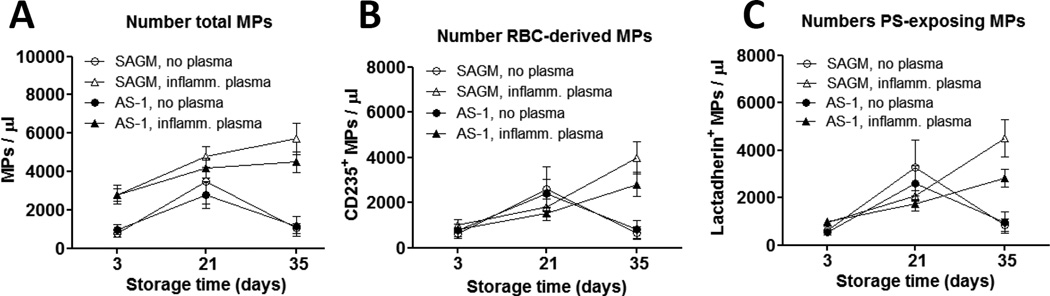
Following culture in the presence of plasma or RBC supernatant, the numbers of MPs released by RBCs stored for 21 days increased compared to day 3 RBCs (Fig. 5). However, a contrasting effect of the presence or absence of plasma was observed following the culture of day 35 RBCs. Whereas day 35 RBCs cultured in plasma released higher numbers of MPs compared to day 21 RBCs, day 35 RBCs cultured in their own supernatant had decreased MP release compared to day 21 RBCs. As a result, MP numbers in cultured day 35 RBC were significantly different depending on the presence or absence of plasma during culture for total MPs (Fig 5A), RBC-derived CD235+ MPs (Fig. 5B) and PS+ MPs (Fig. 5C) (p < 0.05 for all types of MPs for SAGM RBCs and AS-1 RBCs).
Figure 5. Number of MPs after in vitro transfusion-associated stress was influenced by RBC storage duration and presence of plasma.
Stored RBCs were cultured overnight at 37 °C in the presence of inflammatory plasma or RBC supernatant. The numbers of total MPs (A), RBC-derived CD235a+ MPs (B) and lactadherin-binding PS+ MPs (C) released into the culture supernatant was quantified by flow cytometry. The numbers of MPs following culture of RBCs in plasma significantly increased with longer storage duration of SAGM RBCs (▲) (total MPs, p = 0.027; CD235a+ and PS+ MPs, p = 0.008) and AS-1 RBCs (○) (total MPs, not significant; CD235a+ and PS+ MPs, p = 0.016). There was no significant storage effect following culture of SAGM RBCs (○) or AS-1 RBCs in RBC supernatant. The differences in numbers of MPs following culture in the presence of plasma compared to RBC supernatant were significant at day 35 of storage for SAGM RBCs (total MPs, p = 0.004; CD235+ MPs, p = 0.008; PS+ MPs, p = 0.004) and AS-1 RBCs (total MPs, p = 0.031; CD235+ MPs, p = 0.031; PS+ MPs, p = 0.047). The difference in the number of PS+ MPs released by day 35 SAGM RBCs cultured in plasma was significantly higher than their AS-1 counterparts (p = 0.047). Results are from 9 SAGM RBCs and 7 AS-1 RBCs.
This finding was not an artefact caused by MPs present in the plasma because the same amount and batch of plasma was added to day 3, 21 and 35 RBCs, but the MP numbers after culture in presence or absence of plasma were not significantly different on days 3 and 21 (Fig. 5). Furthermore, in the presence of plasma during overnight culture, MP numbers increased progressively with longer storage of RBCs. This resulted in a storage effect with significantly higher numbers of CD235a+ MPs and PS+ MPs released by day 35 RBCs compared to day 3 RBCs (SAGM RBCs, p = 0.008; AS-1 RBCs, p = 0.016) (Fig 5). In contrast, no significant difference in MP numbers was observed between day 3 and day 35 in the absence of plasma, further suggesting that the presence of plasma was required for culture-induced MP release from day 35 RBCs.
The numbers of culture-induced MPs were not significantly different between RBCs stored in SAGM or AS-1 solutions, except for the numbers of PS+ MPs released by day 35 RBCs cultured in plasma, in which SAGM RBCs released significantly higher numbers of PS+ MPs compared to AS-1 RBCs (p = 0.047) (Fig 5C).
DISCUSSION
Based on our previous findings that longer-stored RBCs adhere in greater numbers to ECs and release more MPs,10,11 we extended our in vitro experiments to investigate the effects of physiological conditions that would be encountered by stored RBCs upon transfusion, including changed temperature, pH, exposure to plasma, inflammatory and oxidative stresses. Here we showed that longer-stored RBCs are more susceptible to in vitro transfusion-associated stress conditions than shorter stored RBCs. Our results implicate PS-exposure by stressed stored RBCs as a potentially significant consequence following transfusion.
Storage alone resulted in a significant, although small, increase in the frequency of PS+ RBCs (Fig 1B). In this study, lactadherin was used to detect PS exposure as it is more sensitive than annexin V.22,24 Consistent with the increased PS-exposure by longer-stored RBCs, the intracellular levels of GSH, an important RBC antioxidant, significantly declined during storage (Fig 1C). Other studies have reported reduced de novo synthesis of GSH by RBCs stored for more than 21 days.25,26 Together these results predict that longer-stored RBCs would have increased stress-susceptibility such as oxidative stress.
Treatment of stored RBCs with oxidant tBuOOH, which induced at least 90% of RBCs to expose PS at the outer membrane, was associated with significantly increased adhesion of longer-stored RBCs to ECs (Fig 2B). Shorter-stored RBCs required extended (90 minutes) oxidant treatment to cause a similar frequency of adherence as longer-stored RBCs treated for only 30 minutes (Fig 2C). This confirmed a higher oxidative stress-susceptibility in longer-stored RBCs. Adhesion of oxidized RBCs was blocked by PS-containing lipid vesicles and only partly blocked by control vesicles that lacked PS (Fig 3). Thus, RBC-EC adhesion is at least in part mediated by a PS-specific interaction, which supports the finding by Koshkaryev and collegues who blocked adhesion of stored RBCs to ECs with recombinant annexin V.27 PS has been shown to contribute to RBC-EC adhesion by several mechanisms, including via a PS-receptor that becomes externalised on activated ECs,28,29 via endothelial matrix bound thrombospondin-1 (TSP-1),30 via PS-binding chemokine CXC ligand 16 (CXCL16) which is upregulated on ECs by inflammatory cytokines31 and via the bi-specific molecule lactadherin that links RBCs and ECs by binding PS on RBCs and integrin alphaVbeta3 on ECs.14,32 Whether PS-mediated adhesion of stored RBCs to ECs triggers endothelial-erythrophagocytosis, other phagocytic activity or vascular effects,14,15 remains to be determined.
In vitro culture of RBCs emphasized the higher stress-susceptibility of longer-stored RBCs, which induced higher PS-exposure, adhesion to ECs and MP release (Figs 4 and 5). Interestingly, culture in the presence of inflammatory plasma had similar effects on PS-exposure and EC-adhesion as culture in RBC supernatant. It would seem that culture at physiological temperature was a key factor for the observed effects. Our results match those reported by Burger and colleagues,16 who cultured stored RBCs at 40 % hematocrit in their own supernatant at 37 °C overnight and described increased PS-exposure in longer-stored RBCs as well as increased hemolysis and potassium leakage. Added to this, we showed that EC-adhesion of RBCs at all storage time-points increased after 37 °C culture. This may have been mediated by the increased PS-exposure by cultured stored RBCs, which follows with our tBuOOH experiments and the role for PS in RBC-EC adhesion. The level of adhesion was lower than seen after tBuOOH treatment, which was likely due to the lower frequency of PS+ RBCs in the culture experiments (i.e. 3% for cultured day 35 RBCs compared to 90 % for tBuOOH-treated RBCs). Furthermore, the effect may have been dampened due to PS+ MPs present in the plasma and RBC supernatant, which could have blocked adhesion similarly to our PS-containing vesicles. In a previous study we reported that LPS treatment increased the strength of adhesion of stored RBCs to ECs,33 however we did not test this in the current study.
Despite the seeming lack of additional effect of inflammatory plasma on PS-exposure and adhesion to ECs compared to culture in RBC supernatant, it did impact the number of MPs present after culture of day 35 RBCs (Fig 5). The explanation for the observed reduced number of MPs in cultured day 35 RBCs incubated with RBC supernatant is unclear. It is possible that MPs released by longer-stored RBCs during 37 °C culture may be less resilient and that incubation in the presence of plasma may help to stabilize the MPs. It is known that plasma constituents, such as lipid moieties and GPI-linked proteins, can be readily adsorbed into RBC membranes.34,35 Thus, MPs could be stabilized by incorporating plasma constituents into their membrane, however this will require further investigation. The notion that the biochemical characteristics of MPs is dynamic and changes during storage of RBCs is supported by proteomic studies.36,37 The observed differences in numbers of MPs after 37 °C culture of stored RBCs with or without plasma indicate that plasma should be present during in vitro experiments designed to investigate transfusion-associated stress responses of stored RBCs. The presence of human plasma or serum has been found to be important in other studies of storage effects on RBCs. For example, Burger and colleagues showed that CD47 on longer-stored RBCs undergoes conformational conversion to an ‘eat-me’ signal, but only in the presence of human serum.38
SAGM RBCs were more susceptible to in vitro transfusion-associated stress compared to AS-1 RBCs, as shown by the significantly higher PS-exposure after culture of day 21 and day 35 SAGM RBCs. In conjunction with the results from our previous paired study of RBCs stored in SAGM or AS-1,11 which tested different storage time-points and reported higher adhesion of SAGM RBCs on day 28 and lower at day 42, suggests that dynamic changes accelerate after 21 days of storage. Consistent with our previous report,11 MP formation was significantly higher in SAGM RBCs compared to AS-1 RBCs, and following in vitro transfusion-associated stress treatment, released significantly higher numbers of PS+ MPs. Together with the higher frequency of PS-exposure by stressed SAGM RBCs compared to AS-1 RBCs suggests that transfused SAGM RBCs may have greater propensity to contribute to PS-mediated coagulopathy or to be targeted for rapid clearance from the circulation.7,15
Our in vitro transfusion-associated stress model has limitations. It does not fully represent the transfusion recipient where all types of cells in the blood and tissues contribute to the immune response. The response to LPS by healthy volunteers, as used in this study, is likely to be different from that of critically-ill patients with sepsis.39 Our inflammatory plasma contained a significant amount of IL-10, indicating a marked suppressive immune response to LPS, which may have dampened any pro-inflammatory effects. IL-10 is known to suppress the inflammatory response of ECs.40 Other stress-challenges, such as mechanical or osmotic fragility following RBC culture,41,42 may also have been informative.
In conclusion, longer-stored RBCs have increased susceptibility to in vitro transfusion-associated stress. This was evident by increased PS-exposure, EC-adhesion and MP release. RBCs stored in SAGM may have greater vulnerability to transfusion-associated stress due to increased propensity to expose PS and to release higher numbers of PS+ MPs compared to AS-1 RBCs. For in vitro models of transfusion, warming of stored RBCs to 37 °C and the inclusion of human plasma appear to be important factors that influence the experimental outcome. The clinical significance of our findings is unclear. However, increased PS-exposure could target transfused RBCs for rapid removal from the circulation and dysregulate phagocyte responses and the microcirculation,13,15,43 whilst increased release of MPs loaded with reactive constituents could promote hemostatic and immune disturbances.19,44,45 Such sequelae could have adverse consequences for certain patient groups with significant systemic inflammatory or coagulopathic co-morbidities, such as patients with septic shock or severe hemorrhagic trauma.46 Consideration for tailoring RBC component inventory management such that patients with heightened oxidative stress load are transfused with shorter-stored RBCs could be warranted. Our results support the continued need for research focussed on strategies to further improve the quality of stored RBCs that maximize their resilience to transfusion-associated stresses.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Donor services staff at the Australian Red Cross Blood Service for assistance with collecting whole blood packs and the Processing staff, in particular Peter Agrotis, for assistance with the processing of the whole blood. We also thank the staff at the BMDI Cord Blood Bank Melbourne for providing umbilical cords for EC preparation.
Funding:
This work was funded by NIH Grant 1R01 HL095470-01A1. We acknowledge the Australian governments that fully fund the Australian Red Cross Blood Service for the provision of blood products and services to the Australian community.
Abbreviations
- EC(s)
endothelial cell(s)
- GSH
glutathione
- LUV
large unilamellar vesicles
- MP(s)
microparticle(s)
- PS
phosphatidylserine
- RT
room temperature
- SAGM
saline-adenine-glucose-mannitol
- tBuOOH
tert-butylhydroperoxide
- WB
whole blood
Footnotes
Conflict of interest:
There are no conflicts of interest.
REFERENCES
- 1.D'Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2014 Aug 6; doi: 10.1111/trf.12804. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Hess JR. Red cell storage. J Proteomics. 2010;73:368–373. doi: 10.1016/j.jprot.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 3.van ’t Erve TJ, Wagner BA, Martin SM, Knudson CM, Blendowski R, Keaton M, et al. The heritability of metabolite concentrations in stored human red blood cells. Transfusion. 2014;54:2055–2063. doi: 10.1111/trf.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acker JP, Hansen AL, Kurach JD, Turner TR, Croteau I, Jenkins C. A quality monitoring program for red blood cell components: in vitro quality indicators before and after implementation of semiautomated processing. Transfusion. 2014;54:2534–2543. doi: 10.1111/trf.12679. [DOI] [PubMed] [Google Scholar]
- 5.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for "omics" analyses. Blood transfusion. 2012;10(Suppl 2):s7–s11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 7.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48(7):1478–1485. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen B, Matot I. Aged erythrocytes: a fine wine or sour grapes? Br J Anaesthes. 2013;111(S1):i62–i70. doi: 10.1093/bja/aet405. [DOI] [PubMed] [Google Scholar]
- 9.Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Haematol. 2014;165:3–16. doi: 10.1111/bjh.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anniss AM, Sparrow RL. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion. 2006;46(9):1561–1567. doi: 10.1111/j.1537-2995.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 11.Sparrow RL, Sran A, Healey G, Veale MF, Norris PJ. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: a paired study. Transfusion. 2014;54:560–568. doi: 10.1111/trf.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiu YT, McIntire LV. In vitro studies of erythrocyte-vascular endothelium interactions. Ann Biomed Eng. 2003;31:1299–1313. doi: 10.1114/1.1630320. [DOI] [PubMed] [Google Scholar]
- 13.Lang F, Abed M, Lang E, Föller M. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal. 2014;21:138–153. doi: 10.1089/ars.2013.5747. [DOI] [PubMed] [Google Scholar]
- 14.Fens MH, van Wijk R, Andringa G, van Rooijen KL, Dijstelbloem HM, Rasmussen JT, et al. A role for activated endothelial cells in red blood cell clearance: implications for vasopathology. Haematologica. 2012;97(4):500–508. doi: 10.3324/haematol.2011.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosman GJ. Survival of red blood cells after transfusion: processes and consequences. Front Physiol. 2013;4:376. doi: 10.3389/fphys.2013.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger P, Kostova E, Bloem E, Hilarius-Stokman P, Meijer AB, van den Berg TK, et al. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br J Haematol. 2013;160:377–86. doi: 10.1111/bjh.12133. [DOI] [PubMed] [Google Scholar]
- 17.Rubin O, Delobel J, Prudent M, Lion N, Kohl K, Tucker EI, et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53(8):1744–1754. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Lv L, Liu S, Ma G, Su Y. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox sanguinis. 2013;105(1):11–17. doi: 10.1111/vox.12014. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Zhao W, Christ GJ, Gladwin MT, Kim-Shapiro DB. Nitric oxide scavenging by red cell microparticles. Free Rad Biol Med. 2013;65:1164–1173. doi: 10.1016/j.freeradbiomed.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leuk Biol. 2008;84:1316–1325. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 21.Danesh A, Inglis HC, Jackman RP, Wu S, Deng X, Muench MO, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123:687–696. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sparrow RL, Chan KS. Microparticle content of plasma for transfusion is influenced by the whole blood hold conditions: pre-analytical considerations for proteomic investigations. J Proteomics. 2012;76:211–219. doi: 10.1016/j.jprot.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boland MP, Hatty CR, Separovic F, Hill AF, Tew DJ, Barnham KJ, et al. Anionic phospholipid interactions of the prion protein N terminus are minimally perturbing and not driven solely by the octapeptide repeat domain. J Biol Chem. 2010;285:32282–32292. doi: 10.1074/jbc.M110.123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou J, Fu Y, Zhou J, Li W, Xie R, Cao F, et al. Lactadherin functions as a probe for phosphatidylserine exposure and as an anticoagulant in the study of stored platelets. Vox Sang. 2011;100:187–195. doi: 10.1111/j.1423-0410.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 25.Gevi F, D'Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–180. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long term storage of human erythrocytes on the glutathione synthesis rate and steady-state concentration. Transfusion. 2011;51:1450–1459. doi: 10.1111/j.1537-2995.2010.03026.x. [DOI] [PubMed] [Google Scholar]
- 27.Koshkaryev A, Zelig O, Manny N, Yedgar S, Barshtein G. Rejuvenation treatment of stored red blood cells reverses storage-induced adhesion to vascular endothelial cells. Transfusion. 2009;49:2136–2143. doi: 10.1111/j.1537-2995.2009.02251.x. [DOI] [PubMed] [Google Scholar]
- 28.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 29.Setty BN, Betal SG. Microvascular endothelial cells express a phosphatidylserine receptor: a functionally active receptor for phosphatidylserine-positive erythrocytes. Blood. 2008;111:905–914. doi: 10.1182/blood-2007-07-099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manodori AB, Barabino GA, Lubin BH, Kuypers FA. Adherence of phosphatidylserine-exposing erythrocytes to endothelial matrix thrombospondin. Blood. 2000;95:1293–1300. [PubMed] [Google Scholar]
- 31.Borst O, Abed M, Alesutan I, Towhid ST, Qadri SM, Foller M, et al. Dynamic adhesion of eryptotic erythrocytes to endothelial cells via CXCL16/SR-PSOX. Am J Physiol Cell Physiol. 2012;302:C644–C651. doi: 10.1152/ajpcell.00340.2011. [DOI] [PubMed] [Google Scholar]
- 32.Guchhait P, Dasgupta SK, Le A, Yellapragada S, Lopez JA, Thiagarajan P. Lactadherin mediates sickle cell adhesion to vascular endothelial cells in flowing blood. Haematologica. 2007;92:1266–1267. doi: 10.3324/haematol.11379. [DOI] [PubMed] [Google Scholar]
- 33.Anniss AM, Sparrow RL. Variable adhesion of different red blood cell products to activated vascular endothelium under flow conditions. Am J Hematol. 2007;82:439–445. doi: 10.1002/ajh.20837. [DOI] [PubMed] [Google Scholar]
- 34.Frame T, Carroll T, Korchagina E, Bovin N, Henry S. Synthetic glycolipid modification of red blood cell membranes. Transfusion. 2007 May;47(5):876–882. doi: 10.1111/j.1537-2995.2007.01204.x. [DOI] [PubMed] [Google Scholar]
- 35.Sloand EM, Maciejewski JP, Dunn D, Moss J, Brewer B, Kirby M, Young NS. Correction of the PNH defect by GPI-anchored protein transfer. Blood. 1998;92:4439–4445. [PubMed] [Google Scholar]
- 36.Kriebardis AG, Antonelou MH, Stamolulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–1953. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 37.Bosman GJ, Lasonder E, Luten M, Roerdinkholder-Stoelwinder B, Novotný VM, Bos H, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–835. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 38.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. 2012;119:5512–5521. doi: 10.1182/blood-2011-10-386805. [DOI] [PubMed] [Google Scholar]
- 39.Dorresteijn MJ, Draisma A, van der Hoeven JG, Pickkers P. Lipopolysaccharide-stimulated whole blood cytokine production does not predict the inflammatory response in human endotoxemia. Innate Immunity. 2010;16:248–253. doi: 10.1177/1753425909339923. [DOI] [PubMed] [Google Scholar]
- 40.Huet O, Laemmel E, Fu Y, Dupic L, Aprico A, Andrews KL, et al. Interleukin 10 antioxidant effect decreases leukocytes/endothelial interaction induced by tumor necrosis factor alpha. Shock. 2013;39:83–88. doi: 10.1097/SHK.0b013e318278ae36. [DOI] [PubMed] [Google Scholar]
- 41.Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Daly AR, et al. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99:325–331. doi: 10.1111/j.1423-0410.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 42.Bosman GJ, Cluitmans JC, Groenen YA, Werre JM, Willekens FL, Novotny VM. Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during red blood cell storage. Transfusion. 2011;51(5):1072–1078. doi: 10.1111/j.1537-2995.2010.02929.x. [DOI] [PubMed] [Google Scholar]
- 43.Hod EA, Spitalnik SL. Stored red blood cell transfusions: Iron, inflammation, immunity, and infection. Transfus Clin Biol. 2012;19:84–89. doi: 10.1016/j.tracli.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol. 2014;34:313–320. doi: 10.1161/ATVBAHA.113.302378. [DOI] [PubMed] [Google Scholar]
- 45.Saas P, Angelot F, Bardiaux L, Seilles E, Garnache-Ottou F, Perruche S. Phosphatidylserine-expressing cell by-products in transfusion: A pro-inflammatory or an anti-inflammatory effect? Transfus Clin Biol. 2012;19:90–97. doi: 10.1016/j.tracli.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Sparrow RL. Red blood cell storage duration and trauma. Transfus Med Rev. 2014 doi: 10.1016/j.tmrv.2014.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.