Abstract
Maternal hypertension is common during pregnancy, and multiple studies have reported on an association between maternal hypertension and congenital heart defects (CHDs) in offspring; however, there is variability in the quality of these studies. A systematic review and meta-analysis was conducted on the associations between untreated and treated maternal hypertension and the risk of CHDs, evaluating CHDs overall as well as specific CHD subtypes. A systematic search of peer-reviewed articles published before August 2013 identified 16 studies evaluating the associations between untreated and treated maternal hypertension and CHDs. Summary relative risk (RR) estimates were calculated using fixed-effects models and random-effects models. Significant associations were observed between maternal hypertension and overall CHDs, for both treated [RR 2.0; 95 % confidence interval (CI) 1.5, 2.7] and untreated (RR 1.4; 95 % CI 1.2, 1.7) hypertension, as well as for overall hypertension regardless of treatment status (RR 1.8; 95 % CI 1.5, 2.2). The magnitude of effect was similar for the majority of CHD sub-types evaluated. The effects were also similar among women with hypertension who used one of multiple specific hypertension medications. There was no evidence of publication bias, and our results were robust to several factors considered in sensitivity analyses (e.g., source of exposure data, adjustment for potential confounders, and study design). Maternal hypertension was associated with CHDs. By understanding the specific mechanisms involved, appropriate strategies may be developed to reduce this risk, in order to prevent CHDs.
Keywords: Hypertension, Pregnancy, Congenital heart defects, Meta-analysis
Introduction
Congenital heart defects (CHDs) are among the most common birth defects and are present in about 6–12 per 1000 live births [6, 19, 21]. CHDs are also the most common cause of mortality among all infant deaths related to birth defects [40]. Many of the affected individuals who survive will experience lifelong morbidity and/or will require serious medical treatments.
Despite their high prevalence and clinical significance, the etiology of CHDs remains unknown for most affected individuals [7, 20]. Several maternal characteristics and conditions, such as maternal obesity and diabetes, are suspected CHD risk factors. One of the medical conditions during pregnancy that has been evaluated as a risk factor for CHDs is maternal hypertension, based on the possibility that maternal hypertension could result in changes in blood flow to the uterus during pregnancy [2, 8, 9, 29, 33, 34]. Approximately 2–10 % of pregnant women have hypertension during pregnancy, including both pregestational (onset before pregnancy) and gestational (onset during pregnancy) hypertension [23].
There are several reports of a higher risk of CHDs in off-spring among women with hypertension who are treated with antihypertensive medications, as well among those who are untreated; however, not all of the results from previous studies have been consistent, and these results have not been collectively compared. Further, there is variation in the quality of these studies (e.g., self-reported exposure status vs. medical records, case–control vs. cohort studies, and adjustment for important potential confounders such as body mass index).
To determine whether all recent published epidemiologic studies, in combination, support an association between maternal hypertension and the risk of CHDs in offspring, we conducted the first systematic review and meta-analysis of this association. The goals of this study were to estimate the summary relative risks (RRs) for this association and to examine the evidence of heterogeneity across studies and publication bias.
Materials and Methods
Systematic Review
This systematic review and meta-analysis was conducted in accordance with preferred reporting of items for systematic review and meta-analysis (PRISMA) statement guidelines [30]. A systematic search of peer-reviewed journals was conducted to identify prospective studies assessing untreated and treated hypertension during pregnancy and the risk of CHD in offspring. We searched the US National Library of Medicine MEDLINE database for the published articles in English from 1978 to August 2013 using Ovid and PubMed. Our search terms included “hypertension,” “pregnancy induced hypertension,” “antihypertensive agents,” “angiotensin-converting enzyme inhibitors,” “antihypertensive drugs,” “antihypertensive medication,” “beta-blocker,” “Angiotensin converting enzyme (ACE) inhibitor,” “calcium channel blocker,” “pharmaceutical preparations,” “pregnancy-high-risk,” “maternal drug use,” “pregnancy trimester, first,” “pregnancy trimester, second,” “pregnancy trimester, third,” “maternal exposure,” “infant, newborn,” “maternal,” “abnormalities-drug induced,” “pregnancy complications,” “cardiovascular,” “cardiovascular abnormalities,” “heart defects,” “congenital,” “congenital heart defects,” “coronary vessel anomalies,” “myocardial bridging,”, “heart septal defects,” “aortopulmonary septal defect,” “truncus arteriosus, persistent,” “endocardial cushion defects,” “heart septal defects,” “atrial,” “heart septal defects, ventricular,” “epidemiological studies,” “cohort studies,” “cross sectional studies,” “case–control studies,” “incidence,” and “prevalence.” A review of article bibliographies was carried out to select additional relevant articles. Further, a Scopus search was conducted to identify other articles that cited each article of interest.
Study Selection
The following eligibility criteria for selecting articles was used: (1) studies published in English, (2) original epidemiological studies (case–control, cohort, or cross-sectional studies), (3) studies that examined the association between maternal hypertension or hypertensive medication and CHDs overall or specific CHD subtypes (e.g., atrioventricular septal defects) in infants, and (4) articles that reported risk estimates (i.e., RRs or odds ratios) and 95 % CIs or had raw data that enabled us to calculate the risk estimates. In the event of multiple publications using the same data, we included the study that provided the most comprehensive information (e.g., longest time periods of study or most CHD cases included for analysis).
Data Extraction
Two investigators (A.R., A.J.A.) independently screened the title and abstract of each article to determine whether it met the eligibility criteria. For any study which one or both screeners deemed potentially eligible from the title/abstract screen, the full text of the article was independently reviewed and eligibility was determined (A.R., A.J.A.). When eligibility determination was discrepant between the screeners, resolution was reached through discussion.
Information on important aspects of each study was extracted from each included article. These data included authors, period of study, publication year, study location, data collection method, study design, sample size (including case and control counts), and source of exposure (self-report vs. medical records). We abstracted the reported effect estimates and 95 % CIs or calculated them using the available counts/raw data if they were missing. For simplicity, we reported all estimates of effect as RRs, assuming that odds ratios were valid estimates of the RR. We abstracted estimates for the association with CHDs overall and CHD subtypes when available using the adjusted risk estimate when present. We abstracted the effect of treated and untreated hypertension separately when able (e.g., Caton et al. reported both [8]). For each effect, we abstracted the adjustment variables and timing of hypertension onset (pregestational, gestational, or type unspecified). When separate effects were reported based on the timing of hypertension onset (e.g., gestational hypertension, hypertension type unspecified), we abstracted the effect for hypertension type unspecified or pregestational when type unspecified was not available. For each effect, we also abstracted the medication treatment status (untreated, treated, or unspecified). For the effects of treated hypertension, we further abstracted hypertension medication type (any medication, ACE inhibitors, beta-blockers, calcium channel blockers), and when effects of hypertension medications were reported for more than one time period (e.g., first trimester as well as third trimester), the effect for the first trimester was used (e.g., [26]).
Each study's potential for bias was independently assessed by two investigators (A.R., A.J.A.), using the Newcastle–Ottawa Quality Assessment Scale for observational studies [38]. Any disagreement in score was resolved through discussion.
Statistical Analysis
For the main analyses, we calculated summary effect estimates separately for untreated and treated hypertension. Because the majority (∼93 %) of women with hypertension do not take hypertension medications during early pregnancy [33], effects for studies that did not report on medication treatment status were analyzed with untreated hypertension. For these analyses, we conducted separate comparisons for CHDs overall and specific CHD subtypes (when two or more studies reported associations for a given CHD subtype). If a study reported on specific CHD sub-types but not CHDs overall, it was not included in the analyses of CHDs overall. Studies that reported on the effects of medications were analyzed with treated hyper-tension. We first conducted analyses based on hypertension medications overall (not including effects based on specific medications). We also conducted three additional separate comparisons based on ACE inhibitors, beta-blockers, and calcium channel blockers, respectively. There were not enough studies of treated hypertension and specific CHD subtypes to evaluate specific CHD subtypes.
Because the effects of treated and untreated hypertension were fairly similar (see “Results”), we also conducted a post hoc analysis for CHDs overall based on overall hypertension exposure, which incorporated estimates both from studies that assessed treated and untreated hypertension. Only one estimate of the effect of overall hypertension was considered in each study for this analysis. Thus, we used the effect estimate from each study based on the following priority: (1) untreated hypertension and CHDs overall; (2) treated hypertension (hypertension medications overall) and CHDs overall; (3) treated hypertension (specific hypertension medication used) and CHDs overall (i.e., some papers only evaluated one specific hypertension medication); and (4) untreated hypertension and specific CHD subtypes (i.e., one paper looked at a specific subtype and did not evaluate CHDs overall) [39].
We repeated this analysis in order to conduct several sensitivity analyses. To evaluate whether individual studies were driving the combined estimate, we iteratively removed each study one at a time and estimated the combined effect based on all other studies. To evaluate differences in adjustment for confounders, we identified and conducted a sensitivity analysis restricted to studies that had the greatest degree of control for potential confounders. These studies were defined based on adjusting for at least four of the following important potential confounders (based on the literature): maternal age, race/ethnicity, parity, body mass index, smoking, and diabetes (either adjusting for diabetes or excluding women with diabetes). We also conducted sensitivity analyses by the study type (case–control vs. cohort) and the type of data collection (self-reported vs. medical records). A sensitivity analysis was also performed among studies with a total score of >6 on the Newcastle–Ottawa Quality Assessment Scale (i.e., studies with a relatively lower suspected potential for bias).
For all analyses, we initially tested for heterogeneity across studies using Cochran's Q test. We computed summary RR estimates and 95 % CIs using fixed-effects models, based on inverse variance weighing to compute summary RR estimates, or used the DerSimonian and Laird method [15] to compute estimates based on random-effects models. Specifically, when there was evidence of heterogeneity across studies (p < 0.05), we estimated the effect using the random-effects model, which provides a more appropriate summary effect estimate between heterogeneous study-specific estimates. Otherwise, when evidence of heterogeneity was not observed from the Q test, we estimated the effect using the fixed-effects analysis. All analyses were computed using Stata 13.0 (Stata Corp, College Station, TX, USA). As the Stata “meta” command requires values for standard errors (SEs) and none of the studies reported SEs, we calculated the SEs using the following formula:
For each analysis, forest plots were generated to visualize the study-specific RR estimates and a summary RR estimate, and we used boxes of varying size to represent the relative weight of an individual study toward the computation of the summary RR estimate. We evaluated the potential for publication bias using Egger's test (p < 0.05) and by visual examination of the symmetry in funnel plots (Stata “metabias” and “metafunnel” commands).
Results
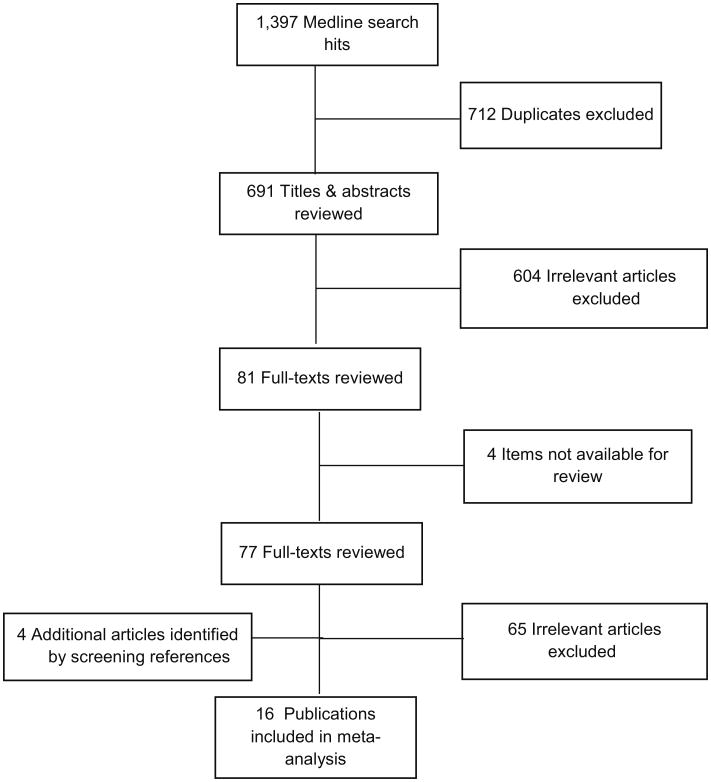
We identified 16 articles published between 1990 and 2013 for the meta-analysis (Fig. 1), using our inclusion criteria. The characteristics of the included studies are listed in Table 1. There were five studies conducted in the USA, nine in Europe, and two in Canada. Among the selected studies, there were nine case–control studies, six cohort studies, and one cross-sectional study.
Fig. 1. Flowchart of study selection.
Table 1. Characteristics of publications included in the meta-analysis.
| Author(s) | Publication year | Study location | Study period | Number of cases/controlsa | Treatment status | Exposure source | Adjustment variablesb |
|---|---|---|---|---|---|---|---|
| Case–control studies | |||||||
| Tikkanen and Honein [37] | 1990 | Finland | 1982–1983 | 408/756 | Untreated | Self-reported | A, B, C. D, E, F, G, and I |
| Ferencz et al. [18] | 1993 | USA | 1981–1989 | 3377/3572 | Untreated and treated | Self-reported | A, E, G, H, J, K, and I |
| Sorensen et al. [36] | 2001 | Hungary | 1980–1996 | 4467/38,151 | Treated | Self-reported | A, B, J, and M |
| Kallen and Ollauson [22] | 2003 | Sweden | 1995–2001 | 5015/577,730 | Treated | Self-reported | A, E, O, and M |
| Caton et al. [8] | 2009 | USA | 1997–2003 | 5021/4796 | Untreated and treated | Self-reported | A, B, H, M, U, V, and W |
| Nakhai-Pour [32] | 2010 | Canada | 2009–2010 | 1441/54,878 | Treated | Medical records | No |
| Banhidy et al. [4] | 2011 | Hungary | 1980–1996 | 4480/22,843 | Untreated | Medical records | A, G, J, M, and X |
| Zen et al. [41] | 2011 | Brazil | 2005–2007 | 250/303 | Untreated and treated | Self-report | No |
| Vereczkey et al. [39]c | 2013 | Hungary | 1980–1996 | 77/38,151 | Untreated | Self-reported | A and M |
| Cohort studies | |||||||
| Cooper et al. [12] | 2006 | USA | 1985–2000 | 305/29,507 | Treated | Medical records | A, B, H, P, Q and R |
| Schoendorfer et al. [35]. | 2008 | Europe | 1986–2003 | 7/1098 | Treated | Self-reported | No |
| Malm et al. [28] | 2008 | Finland | 1996–2001 | <11/348,989 | Treated | Medical records | A, G, J, and M |
| Lennestal et al. [25] | 2009 | Sweden | 1995–2006 | 12,660/1,030,703 | Treated | Self-reported | A, M, R, S, T and U |
| Davis et al. [14] | 2011 | USA | 1996–2000 | 506/49,836 | Treated | Medical records | No |
| Li et al. [26] | 2011 | USA | 1995–2008 | 7700/465,754 | Untreated and treated | Medical records | A, H, M, V and U |
| Liu et al. [27] | 2013 | Canada | 2002–2010 | 2377/2,278,838 | Untreated | Medical records | A, B, D, I, L, M, N, S, U, V, and Y |
Number of subjects with/without heart defects are listed when the study was not a case–control study
Adjustment variables: A maternal age, B maternal illness, C maternal ultrasound examination, D hypertension prior to index pregnancy, E maternal smoking, F maternal deodorant use, G maternal occupation, H maternal race/ethnicity, I alcohol consumption, J therapeutic drugs, K life style exposure, L region of birth, M parity, N multiple gestational pregnancy, O years of involuntary childlessness, P rural residence, Q income quartile, R maternal year of birth, S tobacco use, T previous miscarriage, U maternal body mass index, V gestational diabetes/preexisting diabetes, W use of fertility medication/procedure, X use of folic acid during pregnancy, Y infant sex
This paper only evaluated atrioventricular canal defects
All studies reported the mother's hypertension status during the first trimester of pregnancy, which is the critical exposure time period for the development of CHDs. There were eight studies that examined the effect of untreated hypertension and twelve studies that evaluated treated hypertension (four studies reported estimates for both treated and untreated). While the majority of studies provided effect estimates adjusted for a range of covariates, four studies reported either unadjusted effect estimates or raw data which was used to calculate the unadjusted effect estimates (Table 1).
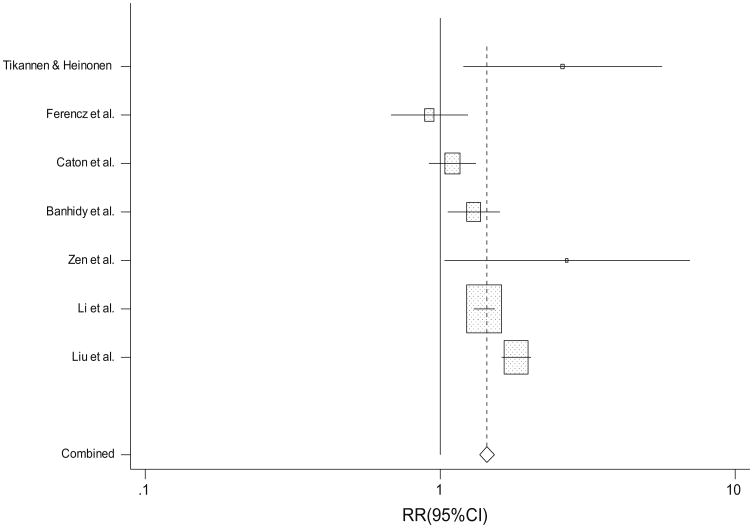
Seven studies evaluated the association between untreated maternal hypertension during pregnancy and CHDs overall (Table 2; Fig. 2) (one additional study evaluated a CHD subtype but not CHDs overall [39]). Untreated maternal hypertension was significantly associated with CHDs overall (random-effect RR 1.4; 95 % CI 1.2, 1.7; heterogeneity p < 0.001). The magnitude of the effect estimate was also positive for the association between untreated maternal hypertension and each of seven CHD subtypes (range of RRs 1.1–2.0). Among these, statistically significant associations (p < 0.05) were present with conotruncal defects, atrioventricular septal defects, and ventricular septal defects (range of RRs 1.3–1.7), and there was no evidence of heterogeneity across studies for any of these effects.
Table 2. Summary of relative risks (RRs) for the association between untreated maternal hypertensiona and congenital heart defects.
| Cardiac defects | No. of studies | Summary RR (95 % CI) | Heterogeneity p value | Egger's test p valueb |
|---|---|---|---|---|
| Any congenital heart defect | 7 | 1.38 (1.15, 1.67) | <0.001c | 0.939 |
| Conotruncal defects | 2 | 1.30 (1.04, 1.62) | 0.255 | – |
| Atrioventricular septal defects | 3 | 1.65 (1.10, 2.49) | 0.190 | 0.717 |
| Left ventricular outflow tract obstruction | 2 | 1.13 (0.86, 1.47) | 0.631 | – |
| Right ventricular outflow tract obstruction | 2 | 1.20 (0.94, 1.53) | 0.021 | – |
| Ventricular septal defects | 2 | 1.32 (1.08, 1.61) | 0.255 | – |
| Atrial septal defects | 2 | 2.01 (0.85, 4.74) | <0.001c | – |
| Heterotaxy/situs inversus | 2 | 1.17 (0.68, 2.01) | 0.086 | – |
Studies that assessed hypertension medications but not maternal hypertension were not included
At least three studies are required for performance of Eggers test
When evidence of heterogeneity was observed, the effect from the random-effects model was reported
Fig. 2. Study-specific and summary RRs and 95 % CIs from the meta-analysis of untreated maternal hypertension and congenital heart defects.
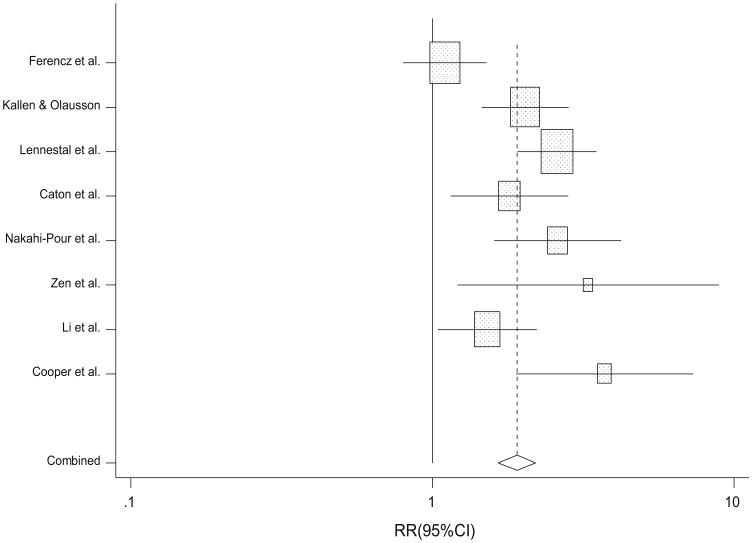
A total of eight studies evaluated the association between treated maternal hypertension (hypertension medications overall) and CHDs overall (Table 3; Fig. 3). A significant association between treated maternal hypertension and CHDs overall was observed (random-effect RR 2.0; 95 % CI 1.5, 2.7; heterogeneity p = 0.001). The magnitude of effect was positive for the association between maternal hypertension treated with each of three specific types of hypertension medications (ACE inhibitors, beta-blockers, and calcium channel blockers; range of RRs 1.2–2.1). However, the association was only statistically significant for beta-blockers (random-effects RR 2.1; 95 % CI 1.6, 2.7; heterogeneity p = 0.04).
Table 3. Summary of relative risks (RRs) for the association between treated maternal hypertensiona and congenital heart defects.
| Exposure | No. of studies | Summary RR (95 % CI) | Heterogeneity p value | Egger's test p value |
|---|---|---|---|---|
| Hypertension medications overall | 8 | 2.03 (1.54, 2.68) | 0.001 | 0.710 |
| ACE inhibitor | 4 | 2.12 (0.76, 5.93) | <0.001b | 0.556 |
| Beta-blockers | 3 | 2.10 (1.64, 2.70) | 0.037b | 0.461 |
| Calcium channel blockers | 3 | 1.16 (0.86, 1.55) | 0.347 | 0.232 |
Use of hypertension medication
When evidence of heterogeneity was observed, the effect from the random-effects model was reported
Fig. 3. Study-specific and summary RRs and 95 % CIs from the meta-analysis of treated maternal hypertension and congenital heart defects.
Based on the results of the Egger's test (Tables 2, 3), there was no evidence of publication bias observed for any of the analyses we conducted. We also constructed funnel plots (data not shown) for the analyses of untreated/treated hypertension and CHDs overall, and these also did not suggest evidence of publication bias.
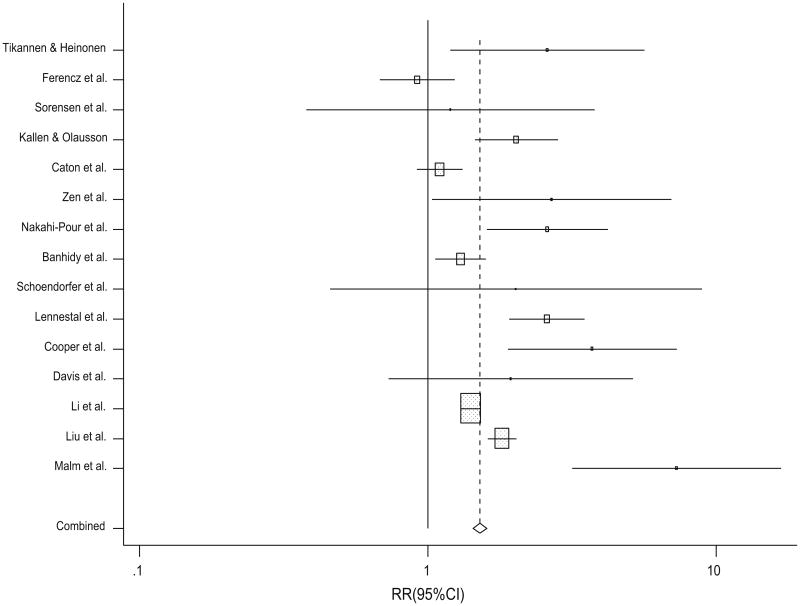
For the effects of untreated and treated hypertension and CHDs overall, the direction of the effect estimates was positive for all but one study (Figs. 2, 3). Because the results were similar between untreated and treated maternal hypertension and CHDs overall and CHD subtypes (range of RRs 1.1–2.1), we conducted post hoc analyses for CHDs overall, based on overall hypertension exposure (Fig. 4). This analysis incorporated estimates both from studies that assessed treated and untreated hypertension. The combined estimate for this association was similar to the main analyses (random-effects RR 1.8; 95 % CI 1.5, 2.2).
Fig. 4. Study-specific and summary RRs and 95 % CIs from the meta-analysis of overall maternal hypertension and congenital heart defects.
We repeated this analysis, and after eliminating each individual study one at a time and analyzing all other studies, the range of the combined estimates was similar (RR 1.7–1.9). To evaluate potential confounding, we repeated the analysis of overall hypertension exposure among the five studies which had the greatest degree of control for potential confounders [8, 22, 24, 26, 27] (i.e., adjusted for at least four of the following: maternal age, race/ethnicity, parity, body mass index, diabetes, smoking), and these results were also similar to the main results (RR 1.7). We also repeated this analysis separately among case– control studies and cohort studies, and results were similar to the main results (data not shown). Further, we also repeated this analysis separately among studies that determined exposure status based on medical records versus self-report, and results were also similar to the main results (data not shown). After assigning a Newcastle–Ottawa Quality Assessment Scale score to each study (range 5–9), we repeated this analysis among studies with a total score of >6, and results were also similar to the main results (data not shown).
Discussion
We found that maternal hypertension during pregnancy is associated with CHDs in offspring. Although we included studies that varied widely in terms of their case definition, control selection, exposure assessment, sample size, study design, adjustment for confounders, time period, and geography, the majority of results from individual studies are consistent. We found positive associations between both treated and untreated hypertension, as well as overall hypertension (regardless of treatment status). Further, we found similar associations for many of the CHD subtypes and specific hypertension medications evaluated, and the magnitudes of effects for all comparisons were in the positive direction. The consistency of the observed effects across these analyses and in our sensitivity analyses supports an association between maternal hypertension and CHDs. This association is supported by the fact that individual estimates for 14/15 studies included in our analysis of overall hypertension exposure and combined CHDs were positive (Fig. 4). The results from our sensitivity analyses further suggest that our main results were robust to inclusion of studies and were not driven by a single study, and were also not due to differences in adjustment for potential confounders, study design, or exposure record source (self-report vs. medical records).
Although the majority of women with hypertension during pregnancy do not use hypertension medications, it is difficult to separate the effect of hypertension versus hypertension medications in epidemiologic studies. In our analyses, we observed an association between untreated maternal hypertension and CHDs, which suggests that the association between hypertension and CHDs is not simply due to teratogenic effects of medication alone. However, the magnitude of effect for the association between treated hypertension and CHDs (RR 2.0) was larger than that for untreated hypertension (RR 1.4), which might suggest that hypertension medications lead to an additional increase in risk. Alternatively, it is possible that the women on anti-hypertensive medications were also the women with the most severe underlying hypertension and that this trend partially represents a dose–response relationship for the underlying hypertension. It is also likely that a small proportion of women included in our analysis of untreated hypertension did actually use hypertension medications. Furthermore, it is possible that there are underlying risk factors for both hypertension and CHDs that overlap (e.g., genes with pleiotropic effects), and research efforts need to focus on elucidating genetic factors that affect hypertension and CHD risk.
The American Congress of OBGYN Task Force on Hypertension suggests against antihypertensive medications use for women with mild-to-moderate chronic hypertension during pregnancy [34], and treatment of gestational hypertension is also usually not recommended [31]. Angiotensin-converting enzyme (ACE) inhibitors are known to be associated with adverse pregnancy outcomes, such as preterm birth, fetal growth restriction, and small for gestational age, and are recommended against during pregnancy [16]. Beta-blockers are among the most common antihypertensive medications used during pregnancy [16, 34]; however, the safety of their use during pregnancy is controversial [1]. Calcium channel blockers are also commonly recommended during pregnancy and are generally considered to have low risks to the fetus [1, 31].
In our analyses, women who took ACE inhibitors or beta-blockers specifically had about twice the risk of having a child with a CHD compared to women without hypertension, although the association with ACE inhibitors was not statistically significant. The magnitude of the nonsignificant association with calcium channel blockers was smaller. It may be that certain medications might be preferable to the others in terms of CHD risk, though we were unable to show definitive differences. Further, we were unable to assess the effects of hypertension control or to analyze blood pressure as a continuous variable, and further research in these areas would be informative.
Hypertension in pregnancy has been associated with adverse birth outcomes including fetal growth retardation and preterm delivery, as well as certain birth defects, including hypospadias [3, 5, 17]. The mechanisms by which hypertension or hypertension medications may increase risk of CHDs have not been fully delineated. It has been proposed that both maternal hypertension and hypertensive medications might cause uteroplacental insufficiency, decreasing blood flow to the uterus during pregnancy, thus lowering fetal blood pressure [2, 8, 9, 29, 33, 34]. Fetal intracardiac blood flow alterations and cell death have been proposed as two important mechanisms for abnormal heart development in the fetus [10, 11]. Chronic hypertension specifically has been reported to be associated with threatened abortion and placental disorders, and there may be shared pathways/mechanisms involved in these outcomes and risk of heart defects in the offspring (e.g., alterations in placental blood flow) [13]. Further, beta-blockers and calcium channel blockers can cross the placenta and may result in hypoglycemia and seizures in the fetus [14].
Our study had several strengths, including the large sample analyzed (nearly five million total subjects analyzed). We conducted separate analyses to independently evaluate the effect of treated versus untreated hypertension. Additionally, due to presumed heterogeneous etiologies, many CHD subtypes were analyzed separately, and therefore, we were able to estimate the range of risks for these subtypes. We also estimated the range of risks for hypertensive medication subtypes on overall CHDs. Further, we conducted sensitivity analyses, which suggested that our results were not influenced by differences in study design (case–control vs. cohort) or source of exposure assessment (medical records vs. self-report).
Our study also had certain limitations, many of which are common among meta-analyses, including the quality of the individual studies included. For example, our analysis was limited to studies that were published in English and the studies that we evaluated varied in terms of adjustment variables, study design, hypertension and cardiac pheno-type definitions, time, and geography. However, these limitations are frequent among meta-analyses, and we did not find any evidence of publication bias. Further, our sensitivity analyses did not indicate that our results were due to several differences between studies (i.e., exposure data source, adjustment for potential confounders, study design). There are several areas in which future studies could further our understanding of hypertension risk. There were insufficient data to assess the effects of pregestational versus chronic hypertension or to evaluate a dose–response relationship between maternal hypertension medication and CHDs. There were also only a limited available number of studies that have evaluated specific CHD sub-types; however, fairly similar effects were observed across the specific CHD subtypes analyzed.
Our analyses suggest that the risk of CHDs in offspring was approximately 80 % higher among women with hypertension compared to those without hypertension. The risk among women with treated hypertension specifically may be slightly higher, though perhaps less so among women who use calcium channel blockers. Given that hypertension is a relatively common exposure among mothers of reproductive age, it may account for a substantial proportion of CHD risk. Our findings suggest that future work should focus on better understanding the specific mechanisms involved and then developing and implementing strategies to reduce risk and thereby prevent CHDs. For example, the relationship between maternal hypertension and specific CHD subtypes should be further delineated. Further research is needed to better inform individual clinical management as well as public health planning. For example, it is unclear whether the observed CHD risk could be reduced by using intervention strategies focused on controlling blood pressure. Ultimately, this future work may lead to prevention approaches, in order to decrease CHD risk.
Acknowledgments
This work was supported by a Grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5P01HD070454). We thank Helena Vonville, MLS, MPH for assistance with the literature search.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Al Khaja KA, Sequeira RP, Alkhaja AK, Damanhori AH. Drug treatment of hypertension in pregnancy: a critical review of adult guideline recommendations. J Hypertens. 2014;32(3):454–463. doi: 10.1097/HJH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 2.Alwan S, Polifka JE, Friedman JM. Angiotensin II receptor antagonist treatment during pregnancy. Birth Defects Res A. 2005;73(2):123–130. doi: 10.1002/bdra.20102. [DOI] [PubMed] [Google Scholar]
- 3.Ananth CV, Peedicayil A, Savitz DA. Effect of hypertensive diseases in pregnancy on birthweight, gestational duration, and small-for-gestational-age births. Epidemiology. 1995;6(4):391–395. doi: 10.1097/00001648-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Banhidy F, Acs N, Puho EH, Czeizel AE. Chronic hypertension with related drug treatment of pregnant women and congenital abnormalities in their offspring: a population-based study. Hypertens Res. 2011;34(2):257–263. doi: 10.1038/hr.2010.227. [DOI] [PubMed] [Google Scholar]
- 5.Bateman BT, Huybrechts KF, Fischer MA, Seely EW, Ecker JL, Oberg AS, Franklin JM, Mogun H, Hernandez-Diaz S. Chronic hypertension in pregnancy and the risk of congenital malformations: a cohort study. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107(3):E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 7.Botto LD, Lin AE, Riehle-Colarusso T, Malik S National Birth Defects Prevention Study. Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A. 2007;79(10):714–727. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 8.Caton AR, Bell EM, Druschel CM, Werler MM, Lin AE, Browne ML, McNutt LA, Romitti PA, Mitchell AA, Olney RS, Correa A National Birth Defects Prevention Study. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009;54(1):63–70. doi: 10.1161/HYPERTENSIONAHA.109.129098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. Postmarketing surveillance for angiotensin-converting enzyme inhibitor use during the first trimester of pregnancy—United States, Canada and Isreal, 1987–1995. MMWR Morb Mortal Wkly Rep. 1997;46:2. [PubMed] [Google Scholar]
- 10.Clark EB. Mechanisms in the pathogenesis of congenital cardiac malformations. In: Pierpont ME, Moller JH, editors. The genetics of cardiovascular disease. Martinus Nijhoff Publishing; Boston, MA: 1986. p. 8. [Google Scholar]
- 11.Clark EB. Pathogenetic mechanisms of congenital cardiovascular malformations revisited. Semin Perinatol. 1996;20(6):465–472. doi: 10.1016/s0146-0005(96)80062-0. [DOI] [PubMed] [Google Scholar]
- 12.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 13.Czeizel AE, Banhidy F. Chronic hypertension in pregnancy. Curr Opin Obstet Gynecol. 2011;23(2):76–81. doi: 10.1097/GCO.0b013e328342b7a9. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL, Eastman D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Dublin S, Platt R. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiol Drug Saf. 2011;20(2):138–145. doi: 10.1002/pds.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Excellence NIfHaC. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. [Accessed June 9, 2014];NICE Guidelines. 2010 [Google Scholar]
- 17.Fang J, Madhavan S, Alderman MH. The influence of maternal hypertension on low birth weight: differences among ethnic populations. Ethn Dis. 1999;9(3):369–376. [PubMed] [Google Scholar]
- 18.Ferencz C, Rubin J, Loffredo C, Magee C. Epidemiology of Congenital Heart Disease: the Baltimore Washington Infant Study 1981–89. Futura Publishing Company; Mount Kisco: 1993. [Google Scholar]
- 19.Hoffman JI. Congenital heart disease: incidence and inheritance. Pediatr Clin North Am. 1990;37(1):25–43. doi: 10.1016/s0031-3955(16)36830-4. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL American Heart Association Council on Cardiovascular Disease in the Young. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KC, Rouleau J. Temporal trends in Canadian birth defects birth prevalences, 1979–1993. Can J Public Health. 1997;88(3):169–176. doi: 10.1007/BF03403882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol. 2003;17(3):255–261. doi: 10.1016/s0890-6238(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 23.Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy. 2007;26(1):67–76. doi: 10.1080/10641950601147945. [DOI] [PubMed] [Google Scholar]
- 24.Lennestal R, Otterblad Olausson P, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65(6):615–625. doi: 10.1007/s00228-009-0620-0. [DOI] [PubMed] [Google Scholar]
- 25.Lennestal R, Olausson P, Kallen B. Maternal use of anti-hypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in infants. Eur J Clin Phamacol. 2009;65:10. doi: 10.1007/s00228-009-0620-0. [DOI] [PubMed] [Google Scholar]
- 26.Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011;343:d5931. doi: 10.1136/bmj.d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Joseph KS, Lisonkova S, Rouleau J, Van den Hof M, Sauve R, Kramer MS. Association between maternal chronic conditions and congenital heart defects: a population-based cohort study. Circulation. 2013;128(6):583–589. doi: 10.1161/CIRCULATIONAHA.112.001054. [DOI] [PubMed] [Google Scholar]
- 28.Malm H, Artama M, Gissler M, Klaukka T, Merilainen J, Nylander O, Padlan M, Palva E, Ritvannen A, Tyrkko K. First trimester use of ACE-inhibitors and risk of major malformations. Reprod Toxicol. 2008;05:5. [Google Scholar]
- 29.Mastrobattista JM. Angiotensin converting enzyme inhibitors in pregnancy. Semin Perinatol. 1997;21(2):124–134. doi: 10.1016/s0146-0005(97)80055-9. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Moussa HN, Arian SE, Sibai BM. Management of hyper-tensive disorders in pregnancy. Women's Health. 2014;10(4):385–404. doi: 10.2217/whe.14.32. [DOI] [PubMed] [Google Scholar]
- 32.Nakhai-Pour HR, Rey E, Berard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Res B. 2010;89(2):147–154. doi: 10.1002/bdrb.20238. [DOI] [PubMed] [Google Scholar]
- 33.Roberts CL, Bell JC, Ford JB, Hadfield RM, Algert CS, Morris JM. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy. 2008;27(3):285–297. doi: 10.1080/10641950701826695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts JMAP, Bakris G, et al. Hypertension in pregnancy report of the American College of Obstetricians and Gynecologists' Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):9. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 35.Schoendorfer C, Hannemann D, Meister R, Eléfant E, Maarschalkerweerd B, Arnon J, Vial T, Pinilla E, Clementi M, Gnansia E, Santis M, Malm H, Dolivo A, Schaefer C. The safety of calcium channel blockers during pregnancy: a prospective, multicenter, observational study. Reprod Toxicol. 2008;26:24. doi: 10.1016/j.reprotox.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen HT, Czeizel AE, Rockenbauer M, Steffensen FH, Olsen J. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand. 2001;80(5):397–401. [PubMed] [Google Scholar]
- 37.Tikkanen J, Heinonen OP. Risk factors for cardiovascular malformations in Finland. Eur J Epidemiol. 1990;6(4):348–356. doi: 10.1007/BF00151707. [DOI] [PubMed] [Google Scholar]
- 38.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed Mar 24, 2015];2013 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 39.Vereczkey A, Kosa Z, Csaky-Szunyogh M, Gerencser B, Czeizel AE. Birth outcomes of cases with conotruncal defects of heart—a population-based case–control study. J Maternal-Fetal Neonatal Med. 2014 doi: 10.3109/14767058.2014.918598. [DOI] [PubMed] [Google Scholar]
- 40.Yang Q, Chen H, Correa A, Devine O, Mathews TJ, Honein MA. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A. 2006;76(10):706–713. doi: 10.1002/bdra.20308. [DOI] [PubMed] [Google Scholar]
- 41.Zen TD, Rosa RF, Zen PR, Trevisan P, da Silva AP, Ricachinevsky CP, Paskulin GA. Gestational and family risk factors for carriers of congenital heart defects in southern Brazil. Pediatr Int. 2011;53(4):551–557. doi: 10.1111/j.1442-200X.2011.03341.x. [DOI] [PubMed] [Google Scholar]