Abstract
Objective
To study the cross-sectional association between physical activity measured with an accelerometer, structural knee abnormalities and cartilage T2-values assessed with 3T MRI.
Methods
We included 274 subjects from the Osteoarthritis Initiative cohort without definite radiographic osteoarthritis (KL 0 and 1) and at most mild pain, stiffness and functional limitation in the study knee (WOMAC 0–1), which had not limited their activity due to knee pain. Physical activity was measured over seven days with an ActiGraph GT1M accelerometer. Subjects were categorized by quartile of physical activity based on the average daily minutes of moderate/vigorous activity (mv-PA). MR images of the right knee (at 48-months visit) were assessed for structural abnormalities using a modified WORMS score and for T2-relaxation times derived from segmented cartilage of 4 femorotibial regions and the patella. WORMS-grades and T2-measurements were compared between activity quartiles using a linear regression model. Covariates included age, sex, BMI, knee injury, family history of knee replacement, knee symptoms, hip and ankle pain and daily wear time of the accelerometer.
Results
Higher mv-PA was associated with increased severity (p=0.0087) and number of lesions of the medial meniscus (p=0.0089) and severity of bone marrow edema lesions (p=0.0053). No association between cartilage lesions and mv-PA was found. T2-values of cartilage (loss, damage, abnormalities) tended to be greater in the higher quartiles of mv-PA, but the differences were non-significant.
Conclusion
In knees without radiographic osteoarthritis in subjects with no or mild knee pain, higher physical activity levels were associated with increases in meniscal and BMEP lesions.
Keywords: Physical activity, Accelerometer, Osteoarthritis, knee, MRI, T2-relaxation time
Introduction
Since morphological cartilage degeneration in osteoarthritis (OA) is progressive and irreversible, the identification of modifiable risk factors is essential to delay the development of the disease. Physical activity (PA) of certain types and intensity may increase the risk of developing knee OA, but the evidence for this is conflicting. Occupations with repetitive knee bending(1,2) and elite runners or soccer players(3,4) have an increased risk of knee osteoarthritis, though the latter may be due to sport-related injuries. Some studies have suggested that moderate levels of PA in the general population are associated with an increased risk of radiographic knee OA or knee replacement,(5,6) but other studies have not found this association.(7,8)
While most studies of PA and knee OA have assessed disease with radiographs, recent studies(9–11) have used Magnetic Resonance Imaging (MRI). MRI can assess structural abnormalities of the knee joint and can provide information on the biochemical composition of the cartilage matrix by quantifying water content, collagen integrity and proteoglycan content using T2 and T1rho relaxation time measurements.(12,13) Some MRI studies suggest that moderate and vigorous PA may have beneficial effects on cartilage volume(14) and composition.(15) In contrast, in an analyses of subjects without knee pain from the Osteoarthritis Initiative (OAI) we have found an increased prevalence of lesions seen on MRI in cartilage, meniscus and subchondral bone and increased joint effusion(16,17) as well as higher mean tibiofemoral T2-relaxation times(18) in most active indiviuals, suggesting that higher PA levels may be associated with increased joint tissue degradation.
All of these studies assessed PA by questionnaires, which are prone to a reporting bias leading to an over-estimation of PA.(19) In the only study to our knowledge that has used objective measures of PA, Dore et al(20) examined longitudinal changes in MR imaging findings and found no difference in new cartilage lesions in subjects who performed ≥10,000 steps/day by pedometer compared to those with fewer steps. However, the more active subjects had an increased incidence of bone marrow lesions and worsening of meniscal damage, especially in those with pre-existing knee structural abnormalities.
Because PA is a modifiable behavior, is recommended in treatment guidelines for the non-pharmacological management of knee pain,(21) may lessen disability,(22) and might be beneficial for cartilage protection,(23,24) it is important to gain a better understanding of the relationship of objectively measured PA and knee health using MRI. To do so, we used the OAI clinical data, radiographs, and 3 Tesla knee MRIs with a T2-mapping sequence,(25) and data from an OAI ancillary study of PA assessed objectively with accelerometers(26,27) to evaluate the cross-sectional association of PA with knee structural abnormalities and cartilage T2-relaxation times in knees without radiographic OA in subjects with no, or at most mild, knee symptoms and who had not limited or reduced their physical activities due to knee pain.
Materials and Methods
Subjects
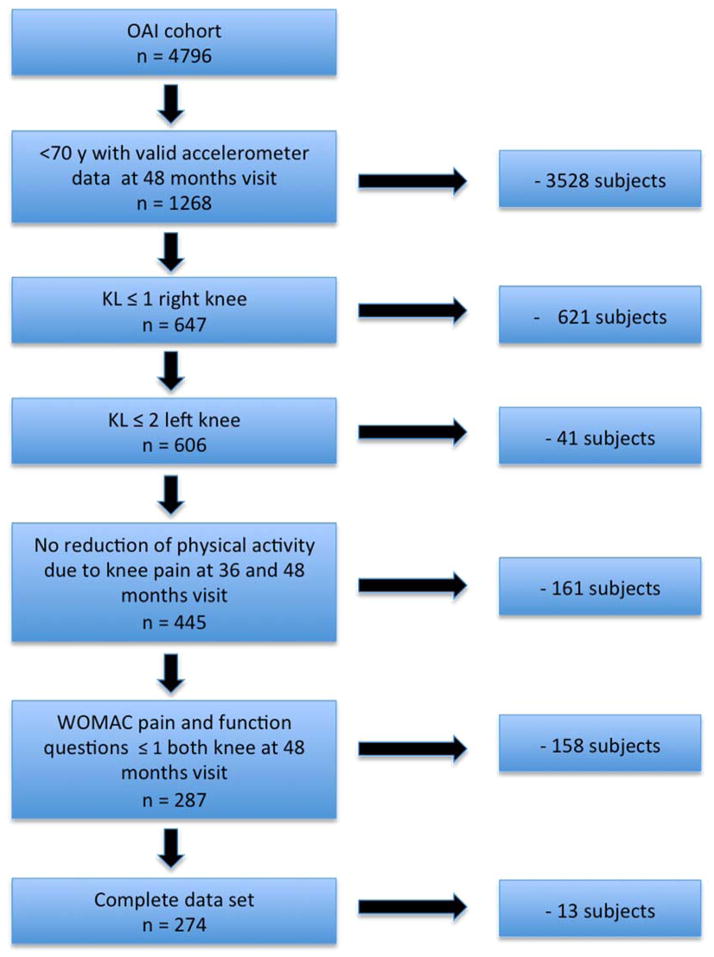
Data are from the OAI, a longitudinal, observational multicenter study of the natural evolution of knee OA in 4796 subjects sponsored by the National Institutes of Health. Details of the study protocol are available at www.oai.ucsf.edu. The study was compliant with the Helsinki Declaration. All subjects included in this study provided informed consent. The study protocol, amendments, and informed consent documentation were reviewed and approved by the local institutional review boards. Participants recruited at five clinical sites either had symptomatic knee OA (progression subcohort) or were at increased risk of developing it due to risk factors (incidence subcohort); a small number of subjects without knee pain, radiographic OA or risk factors were also enrolled (control subcohort).(25,28) An ancillary study of PA assessed by accelerometer began at the 48-month follow-up visit.(26,27) We used imaging and clinical data of this visit for the analysis. A subset of 274 subjects was selected for our study using the following inclusion criteria at the 48-months visit: age <70, a Kellgren-Lawrence (KL) grade of ≤1 in the right knee and ≤2 in the left knee. In addition each question of the three Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)(29) subscales for pain (5 questions), stiffness (2 questions) and disability (17 questions) had to be answered with a score of ≤1 (mild) for all questions in both knees. Only participants who reported that they had not limited or reduced their activities due to knee pain in the past 30 days at both the month 36 and month 48 visits were included. Subjects with rheumatoid arthritis, MRI contraindications and incomplete data were excluded from the OAI (Figure 1). Of the 274 included subjects, 272 had risk factors for knee OA and two were from the control subcohort without risk factors.
Figure 1.
Patient selection according to the inclusion criteria.
Imaging
Radiographs
Knee radiographs were acquired at 48 months using the fixed flexion protocol(30) and read for Kellgren-Lawrence grade as previously described(31).
MR Imaging protocol
MR images of the right knee were obtained using four identical 3.0 Tesla scanners (Trio, Siemens) and quadrature transmit-receive coils (USA Instruments, Aurora, Oh, USA) at one of four sites (Ohio State University, Columbus, OH; University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; and Memorial Hospital of Rhode Island, Pawtucket, RI). Details of the acquisition protocol have been published(32) and included the following sequences: 1) coronal proton density-weighted fast spin-echo (FSE); 2) sagittal 3-D dual echo in the steady state (DESS) with selective water excitation; 3) sagittal intermediate-weighted FSE with fat suppression; and 4) sagittal T2-weighted multi-echo spin-echo (SE).
Whole-Organ Magnetic Resonance Imaging Score (WORMS) grading
MR images were evaluated for presence and grade of cartilage, meniscal and bone marrow edema pattern (BMEP) lesions using WORMS(33), modified as previously described(17). Two radiologists (MK with 11 and LN with 8 years of experience) who were masked to the PA data analyzed all 274 MRI studies separately, and in case of diverging findings consensus readings were performed with a third radiologist (TML with 22 years of experience). Meniscus lesions were graded 0–4 in each of 6 regions (medial/lateral and anterior/body/posterior). Cartilage grades (0–6) and BMEP grades (0–3) were also scored in 6 regions (patella, trochlea, medial/lateral femur, and medial/lateral tibia). BMEP was defined as areas of poorly marginated increases in T2 signal intensity in the fat suppressed imaging sequences. For each type of lesion (meniscus, cartilage, BMEP), a maximum score per knee (WORMS max) was defined as the highest score in any of the regions. We also calculated a lesion score for each knee by summing the scores over all regions (WORMS sum).
Quantitative T2-relaxation time measurements
We used the sagittal 2-D multi-echo SE images of the right knee for segmentation and quantification of T2-relaxation time using an in-house developed spline-based, semiautomated software segmentation algorithm in MATLAB (MathworksInc, El Segundo, CA).(34,35) Segmentation of the cartilage was performed on the first echo sequence to maximize signal-to-noise ratio. All segmentation was performed by three individuals (MK, WL and HA) in the following compartments: patella, medial/lateral femoral condyle, medial/lateral tibia. The trochlea was not segmented because of interfering flow artifacts from the popliteal artery.
Inter-reader reproducibility errors of this technique were minimal as reported in a prior study.(36)
Physical activity levels
PA was assessed in a consecutive sample of 2127 consenting participants at the 48-month visit with a uniaxial accelerometer (ActiGraph GT1M, ActiGraph, Pensacola, FL, USA) that measures vertical acceleration and deceleration and has been validated in several studies, showing a high correlation with metabolic equivalent and total energy expenditure as well as with the ground reaction force as measured with a force plate.(26,37) The reliability and accuracy of ActiGraph accelerometers have also been demonstrated in subjects with OA.(38–40)
Participants were instructed to wear the accelerometer on a belt on the natural waistline for seven consecutive days.(26) PA was recorded through a weighted activity count, where the weights are proportional to the magnitude of measured acceleration. A valid day of monitoring required ≥10 wear hours in a 24-hour period. Non-wear periods were defined as ≥90 minutes of no activity counts.(27) At least four valid days of monitoring were required to provide a reliable estimate of PA.(26) Valid estimates were available for 1927 (91%) activity substudy participants.
For each of our 274 participants the average daily time (minutes) spent with moderate to vigorous physical activity (mv-PA), based on a threshold of >2020 counts per minute, was calculated from the sum of daily total minutes of mv-PA divided by the number of valid days. We than stratified the subjects according to their activity levels (average daily minutes of mv-PA) into quartiles with the following ranges: 1. (=lowest) quartile: 0.6–10.2 min., 2. quartile: 10.6–22.4 min., 3. quartile: 22.8–38.9 min., 4. (=highest) quartile 39.6–133.9 min., (Table 1).
Table 1.
Subject characteristics at 48 months visit
| mv-PA quartiles of average (daily minutes of moderate to vigurous activity)
|
||||||
|---|---|---|---|---|---|---|
| Quartiles (lowest to highest mvPA) | All subjects | 1. quartile | 2. quartile | 3. quartile | 4. quartile | P-value |
| Range of mvPA (min) | 0.6 – 133.9 | 0.6 – 10.2 | 10.6 – 22.4 | 22.8 – 38.9 | 39.6 – 133.9 | |
| Number of subjects | n = 274 | n = 68 | n = 69 | n = 69 | n = 68 | |
| Gender* | <0.001† | |||||
| Male | 134 (48.9) | 28 (41.2) | 23 (33.3) | 35 (50.7) | 48 (70.6) | |
| Female | 140 (51.1) | 40 (58.8) | 46 (66.7) | 34 (49.3) | 20 (29.4) | |
| Cohort* | 0.691 | |||||
| Normal | 2 (0.7) | 0 (0) | 1 (1.5) | 1 (1.5) | 0 (0) | |
| Incidence | 253 (92.3) | 64 (94.1) | 64 (92.8) | 61 (88.4) | 64 (94.1) | |
| Progression | 19 (6.9) | 4 (5.9) | 4 (5.8) | 7 (10.3) | 4 (5.9) | |
| KL grade‡ | 81 (29.6) | 20 (29.4) | 22 (31.9) | 17 (25.0) | 22 (32.6) | 0.744 |
| Risk factors* | ||||||
| History of knee injury | 57 (21.1) | 11 (16.2) | 12 (17.4) | 17 (25.0) | 17 (25.1) | 0.352 |
| Knee injury since baseline | 9 (3.3) | 1 (1.5) | 3 (4.35) | 2 (2.9) | 3 (4.4) | 0.740 |
| History of knee surgery | 13 (4.7) | 1 (1.5) | 4 (5.8) | 3 (4.4) | 5 (7.5) | 0.409 |
| Family history of knee replacement | 47 (17.2) | 8 (11.8) | 10 (14.7) | 7 (10.3) | 22 (32.6) | 0.002† |
| Heberden’s nodes | 67 (24.7) | 13 (19.1) | 19 (27.5) | 20 (29) | 15 (22.4) | 0.560 |
| Ipsilateral knee symptoms (WOMAC)** | ||||||
| Knee pain | 0.4 (0.8) | 0.5 (0.8) | 0.4 (0.9) | 0.4 (0.7) | 0.4 (1.1) | 0.937 |
| Knee stiffness | 0.4 (0.7) | 0.3 (0.6) | 0.4 (0.7) | 0.5 (0.8) | 0.5 (0.8) | 0.353 |
| Functional limitation | 0.7 (1.7) | 0.5 (1.3) | 0.8 (2.0) | 0.6 (1.7) | 0.7 (1.6) | 0.827 |
| Contralateral knee symptoms (WOMAC)** | ||||||
| Knee pain | 0.3 (0.7) | 0.3 (0.7) | 0.4 (0.9) | 0.3 (0.6) | 0.3 (0.6) | 0.698 |
| Knee stiffness | 0.4 (0.7) | 0.4 (0.7) | 0.4 (0.7) | 0.4 (0.8) | 0.4 (0.7) | 0.923 |
| Disability | 0.6 (1.6) | 0.6 (1.5) | 0.8 (2.2) | 0.5 (1.1) | 0.7 (1.4) | 0.787 |
| Bilateral hip pain§ | 113 (41,2) | 28 (41.2) | 25 (36.2) | 30 (43.5) | 30 (44.1) | 0.779 |
| Bilateral ankle pain§ | 13 (4.7) | 4 (5.9) | 5 (7.3) | 3 (4.4) | 1 (1.5) | 0.426 |
| Age^ | 59.1 ± 5.5 | 62.1 ± 5.5 | 58.3 ± 5.1 | 58.2 ± 6.0 | 57.8 ± 5.4 | < 0.001† |
| BMI^ | 26.8 ± 4.2 | 27.5 ± 4.4 | 27.1 ± 5.0 | 26.6 ± 3.9 | 25.8 ± 3.1 | 0.094 |
Values are number (%),
Number (%) of KL 1,
Average and StdDev,
Values are mean (± standard deviation),
Number (%) of subjets, who reported pain on either side at least two times since baseline,
Statistically significant (P < 0.05).
KL grade = Kellgren Lawrence grade. WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index. BMI = body mass index.
Statistical analysis
Statistical analysis was performed using JMP version 11 (SAS Institute, Cary, NC, USA) and STATA version 12 software (StataCorp LP, College Station, TX, USA). In addition to descriptive statistics, chi-squared tests were used to assess the differences in nominal variables such as gender, cohort and OA risk factors between the PA groups. For continuous variables such as age and BMI, differences between PA groups were determined using Kruskal-Wallis test. Multivariate linear regression analysis was used to assess the associations of the predictor mv-PA quartiles with the outcome variables T2-relaxation time and WORMS scores (max score and sum score of menisci, cartilage and BMEP). For each quartile the adjusted mean of the outcome variable (+/−SEM) is reported. P-values represent significance of trends between the strata. For this test the strata were treated as continuous scales in the model. Because of the relatively large number of comparisons made, we set the level of significance to p<0.01 (Table 2).
Table 2.
T2 relaxation time and WORMS grades by quartile of average daily minutes of moderate to vigorous physical activity
| Average daily minutes of moderate to vigourous activity
|
|||||
|---|---|---|---|---|---|
| 1. quartile (lowest) | 2. quartile | 3. quartile | 4. quartile (highest) | p | |
| n = 68 | n = 69 | n = 69 | n = 68 | ||
| Meniscus | |||||
| WORMS max | 1.43 (0.24) | 1.60 (0.22) | 1.78 (0.23) | 1.98 (0.22) | 0.0087 |
| WORMS sum | 2.04 (0.53) | 2.70 (0.49) | 2.85 (0.51) | 3.31 (0.49) | 0.0089 |
| Sum. of lesions Medial Meniscus | 1.19 (0.43) | 1.52 (0.41) | 1.76 (0.42) | 2.42 (0.40) | 0.0017 |
| Sum. of lesions Lateral Meniscus | 0.84 (0.29) | 1.16 (0.28) | 1.09 (0.28) | 0.89 (0.27) | 0.9266 |
| Cartilage | |||||
| WORMS max | 3.10 (0.39) | 2.94 (0.32) | 3.08 (0.32) | 3.43 (0.31) | 0.2477 |
| WORMS sum | 5.9 (0.73) | 5.7 (0.68) | 5.5 (0.71) | 6.4 (0.67) | 0.5813 |
| BMEP | |||||
| WORMS max | 1.1 (0.20) | 1.0 (0.19) | 1.3 (0.19) | 1.5 (0.19) | 0.0053 |
| WORMS sum | 1.6 (0.34) | 1.4 (0.32) | 2.1 (0.33) | 2.1 (0.31) | 0.0498 |
| T2 (ms) | |||||
| LFC | 35.8 (0.44) | 36.6 (0.42) | 36.4 (0.42) | 36.5 (0.39) | 0.1292 |
| LT | 29.5 (0.48) | 30.3 (0.44) | 30.2 (0.46) | 30.3 (0.43) | 0.131 |
| MFC | 38.1 (0.54) | 38.5 (0.51) | 38.9 (0.51) | 38.8 (0.48) | 0.102 |
| MT | 30.7 (0.43) | 31.7 (0.41) | 31.4 (0.42) | 31.8 (0.39) | 0.027 |
| P | 34.4 (0.6) | 35.0 (0.61) | 34.3 (0.62) | 35.1 (0.59) | 0.495 |
| Average of all regions | 33.8 (0.38) | 34.7 (0.35) | 34.4 (0.35) | 34.6 (0.32) | 0.054 |
Least square means (Std error), adjusted for age, sex, BMI, family history of knee replacement, history of knee surgery or injury, WOMAC pain, stiffness and disability (bilateral) hip and ankle pain (bilateral), average daily wear time of accelerometer. WORMS max = highest score in any subcompartment. WORMS sum score = sum of individual subcompartments. Bold numbers are statistically significant (p < 0.01), italic numbers are trends (p < 0.05). BMEP = bone marrow edema pattern. LFC = lateral femoral condyle, LT = lateral tibia, MFC = medial femoral condyle, MT = medial tibia, P = patella
Covariates included in all analyses were age, sex, BMI, family history of knee joint replacement, history of knee surgery, history of knee injury including injuries since the enrollment into the OAI study, WOMAC knee pain, WOMAC stiffness and disability scores for left and right knees. We also included chronic hip pain and ankle pain to control for possible pain induced alterations of the gait pattern that could have led to non physiological loading of the right knee joint. Pain was defined ‘chronic’ if it was reported at least 2 times at the follow-up visits since baseline. We also adjusted for the mean daily wear time of the accelerometer (range 11–20 hours/day).
In addition, we analyzed the prevalence of structural knee lesions in relation to PA. For this purpose WORMS lesions were dichotomized counting lesions as present with WORMS grades >1 for cartilage and >0 for meniscus and BMEP. Significance of trends of prevalence and odds ratios was calculated using a multivariate logistic regression with the same covariates as described above.
Results
SubjectCharacteristics
Subject characteristics including OA risk factors are presented in Table 1. Not surprisingly, participants in the highest quartile of mv-PA were more often male (p<0.001) and were younger (p<0.001) and had by trend a lower mean BMI (p=0.094) than those in the lower activity levels. They also had a higher frequency of family history of knee replacement (p=0.002)
Level of Physical Activity and Meniscus, Cartilage and BMEP Lesions
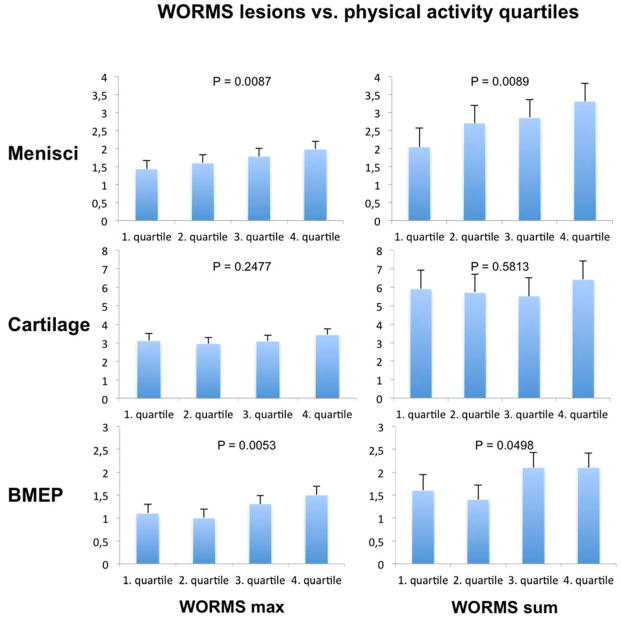
Overall more morphological knee abnormalities were found with increasing levels of average daily minutes of mv-PA (Table 2, Figure 2). Across all quartiles the subjects showed a significant gradual increase of the severity (WORMS maximum score) of meniscus lesions (p=0.0087) and severity of BMEP lesions (p=0.0053) and a significant increase in the sum of WORMS lesions in the menisci (p=0.0089) with increasing mv-PA. The association between the sum of meniscus lesions and PA quartiles was present only in the medial meniscus (p=0.0017). There was also a non-significant trend for an increasing sum of BMEP lesions (p=0.0498) by mv-PA quartile. There was no significant association between the severity or sum of cartilage lesions and quartiles of mv-PA. There were no differences by medial vs. lateral compartments in the association of mv-PA with BMEP and cartilage lesions (data not shown). To exclude that the significant increase of meniscus lesions and BMEP with mv-PA was actually triggered by outliers of the 4th mv-PA quartile that showed the widest range of mv-PA minutes per day (39.6–133,9 min per day) we further stratified the highest quartile (n = 68) in sub quartiles (n = 17). But when analyzing the highest sub-quartile (n = 17 range = 70–133.9 min/day) we did not find a further increase of the sum of meniscus lesions (mean 3 compared to 3.3 of the entire 4th quartile) or sum of BMEP (mean 1.3 compared to 1.5 of the entire 4th quartile).
Figure 2.
Least square means and standard error of the maximum score (WORMS max) and sum of WORMS lesions (WORMS sum) of menisci, cartilage and BMLs. Means are adjusted for age, sex, BMI, family history of knee replacement, history of knee surgery or injury, WOMAC pain, stiffness and disability (bilateral) hip and ankle pain (bilateral), average daily wear time of accelerometer. BMEP = bone marrow edema pattern.
While the prevalence of all types of structural lesions was greatest in the highest mv-PA quartile, these findings were not significant. There was a non-significant trend for an increasing prevalence of medial meniscus lesions by mv-PA (p=0.031) (Table 3).
Table 3.
Prevalence of dichotomized WORMS findings of any grade by average daily minutes of moderate to vigorous physical activity
| Average daily minutes of moderate to vigurous activity
|
p | |||||
|---|---|---|---|---|---|---|
| 1. quartile (lowest) | 2. quartile | 3. quartile | 4. quartile (highest) | OR (95% CI) | ||
| n = 68 | n = 69 | n = 69 | n = 68 | |||
| WORMS Meniscus lesions | 38 (55.9) | 37 (53.6) | 41 (59.4) | 47 (69.1) | 2.01 (0.85–4.83) | 0.098 |
| WORMS medial Meniscus lesions | 29 (42.7) | 26 (37.7) | 29 (42.0) | 41 (60.3) | 2.45 (1.09–5.64) | 0.031 |
| WORMS lateral Meniscus lesions | 21 (30.9) | 23 (33.3) | 28 (40.6) | 24 (35 3) | 1.19 (0.51–2.81) | 0.584 |
| WORMS cartilage lesions > grade 1 | 56 (82.4) | 58 (86.9) | 60 (86.9) | 65 (95.6) | 1.68 (0.65–4.49) | 0.275 |
| WORMS BMEP | 46 (67.7) | 41 (59.4) | 46 (66.7) | 49 (72.1) | 1.90 (0.81–4.53) | 0.071 |
Number of subjects with lesions (%), significance of trend (p<0.01) tested with logistic regression, covariates included age, sex, BMI, family history of knee replacement, history of knee surgery or injury, WOMAC pain, stiffness and disability (bilateral) hip and ankle pain (bilateral), average daily wear time of accelerometer. BMEP = bone marrow edema pattern.
Level of physical activity and cartilage T2-relaxation time
Overall T2-values of the medial femoral condyle, the medial and lateral tibia, the patella and the average joint cartilage tended to be greater in the higher quartiles of mv-PA, but the differences were relatively small and non-significant. A non-significant trend was found for T2 in the medial tibia (p=0.027) (Table 2).
Discussion
The results of this cross-sectional study show that middle-aged individuals without radiographic knee OA and with no or only mild symptoms, who had higher mv-PA levels as measured by accelerometry had increased severity of meniscal lesions, predominantly in the medial meniscus, and more severe BMEP lesions. We did not find an association between the severity or prevalence of focal cartilage lesions and mv-PA. Moreover, no significant association between T2-relaxation time values in the cartilage indicative of cartilage matrix degradation and mv-PA was found.
The majority of studies investigating the role of occupational and community physical activity levels regarding the risk of the development of knee OA in middle aged and older adults have used self-report measures of physical activity and radiographic findings in the knee or total joint replacement. Findings from these studies have been conflicting.(1,2,4–7,42) Recently published longitudinal analyses of persons without knee OA at baseline from three large cohorts did not find a clear association between higher levels of general physical activity and the risk of developing knee OA.(8,43) Felson et al. performed analyses that combined data from the OAI and Multicenter Osteoarthritis cohorts, both of which used the Physical Activity Study in the Elderly (PASE) questionnaire, and found no association between higher levels of self-reported activity and the risk of developing symptomatic knee OA or joint space narrowing over 30 to 48 months.(8) A study in the Johnston County cohort(43) used the Minnesota Leisure Time Activity Questionnaire and concluded that meeting Health and Human Services (HHS) physical activity guideline of ≥ 150 minutes per week of moderate or greater-equivalent activity was not associated with the incidence of radiographic or symptomatic knee OA. It should be noted that those participants who met the guidelines had a significantly increased risk of developing new joint space narrowing (JSN) (adjusted hazard ratio: 1.42; 95% CI: 1.10, 1.82) and participants in the highest activity level (>= 300 min/week of moderate or strenuous equivalent activity) had a nearly significantly increased risk of incident radiographic knee OA (adjusted hazard ratio: 1.62; 95% CI: 0.97, 2.68) compared to the inactive group.
The insensitivity of radiographic findings to early OA and their inability to detect abnormalities in specific joint tissues as well as the reporting bias induced by the self-report of physical activity may all contribute to these conflicting results. However, there are also disparate findings from studies using MRI to assess OA. In a study of healthy middle-aged individuals, self-report of vigorous physical activity was associated with greater tibial cartilage volume and fewer subchondral bone marrow lesions, suggesting a beneficial effect of greater activity on cartilage health.(14) In contrast, our group previously reported that OAI subjects who were more active, in this instance assessed with the PASE questionnaire, had an increased prevalence of cartilage, meniscus, and ligamentous abnormalities, BMEP and joint effusion on knee MRI as compared to less active subjects.(16,17)
To our knowledge there has been only one other study that examined the relationship between objectively measured physical activity levels and MRI lesions in the knee,(20) which had results that are consistent with ours. Dore et al(20) used a pedometer to identify persons averaging at least 10,000 steps a day, a threshold that has been promoted as a practical goal for achieving recommended physical activity levels,(44) and found in a longitudinal analysis that these individuals had an increased risk of worsening meniscus damage and incident BMEP lesions. Consistent with our results they did not find an increased risk of cartilage damage. It is noteworthy that these results were obtained using different objective methods for assessing physical activity. While pedometers are restricted to the measurement of step counts during walking or running, accelerometers provide a more general measure of PA that can be stratified into intensity levels and may include all types of PA that meet a certain intensity threshold. Classification of activity levels based on pedometers versus accelerometers can be widely divergent.(45)
Higher T2-values are associated with risk factors for OA, including meniscal damage and mal-alignment(10,11,16) and predict progression of cartilage morphological lesions.(46) Thus, assuming that increased PA constitutes a risk factor for cartilage degeneration, higher cartilage T2-values with increasing PA could be expected. However, in the present study we did not find a significant association between higher objectively assessed physical activity and T2-values. These results are somewhat in contrast to our prior analyses of OAI subjects that found individuals in the highest physical activity group based on the PASE questionnaire had higher T2-values in several knee cartilage regions in cross-sectional analyses compared to those in the middle activity level or compared to all lower activity levels.(16,18) This discrepancy is, however, not unexpected when comparing PASE data and data derived from accelerometers. While subjective questionnaires count frequencies of specific types of PA, accelerometers objectively measure intensity, frequency and volume of general PA.
Viewing our findings in a pathophysiological context, it appears likely that the weakest structure according to sustained mechanical load during mv-PA is the medial meniscus while cartilage seems to be relatively resilient towards increased PA. The trend for higher T2-values in the cartilage of the medial tibia indicates a stress associated early cartilage degradation and is consistent with the presence of more severe lesions of the medial meniscus(11). In osteoarthritis, BMEP lesions are associated with increasing severity of cartilage lesions(47). In this context it is interesting that BMEP lesions were more severe in the most active subjects although the severity of cartilage lesions was comparable among the PA strata. One explanation may be that cartilage with preexisting lesions, which were very common at all activity levels in the study knees, is insufficient in absorbing mechanical load, thus transmitting axial forces more directly to the subchondral bone. As a consequence increased loading may lead to a higher mechanical stress of the subchondral bone resulting in more severe BMEPs.
Our findings that increasing levels of physical activity are associated with increases in the severity of meniscus and subchondral bone lesions, however, have to be interpreted with caution. Most importantly, it remains uncertain whether these pre-radiographic MRI findings confer an increased risk of eventually developing clinically manifest knee OA. While there is clear evidence that total and to a lesser extend partial meniscectomy leads to a significantly higher risk in developing OA, there is still a scarcity of data about the role of prevalent meniscal lesions for the development of OA. A study in the MOST cohort comparing 121 cases who developed radiographic OA after 30 months with 294 controls found a 8 to 10 fold increased risk for the development of knee OA associated with the presence of meniscal tears, suggesting that these lesions are an important structural risk factor for the development of OA(48). Also the presence of BEMP lesions was predictive for local structural degeneration of the knee joint in a study of Felson et al, who reported a 6 fold increased risk for the progression of radiographic OA(49) and for cartilage loss in MRI as reported by Hunter et al.(50). Nevertheless it is possible that greater levels of physical activity have both, beneficial and detrimental effects on the knee joint health; whether one or the other predominates in the long run can only be determined from longitudinal studies with clinically relevant endpoints. Only a small proportion of people in OAI and other studies using objective measures actually meet current recommendations for physical activity(43,44) and public health efforts encouraging greater achievement of these goals are under way. Although our findings indicate that higher PA may be considered as a risk factor for knee OA, it is possible that this does not necessarily account for all subjects meeting current PA guidelines (41). Physical activity at the level recommended by these guidelines is by most standards relatively modest and has been linked to a variety of important health benefits; potential effects of activity at these levels on knee joint health need to be balanced against the known benefits. For this reason we wanted to exclude that the significant increase of meniscus lesions and BMEP with mv-PA was actually triggered by outliers that performed mv-PA more than 70 minutes per day, however, now further increase of lesions in this high performing subgroup was found.
Our study has several key strengths. This study is one of only two studies that have evaluated the association between PA and OA with objectively measured physical activity. We used knee MRI, which is sensitive to early abnormalities of OA. We selected subjects with risk factors for the development of OA but without radiographic OA and no or only mild knee symptoms. Moreover we tried to avoid a potential bias by excluding persons with knee pain who might have adapted their physical activity as a result of pain. Therefore, the characteristics of the study cohort are uniquely optimized to detect early degenerative changes in response to PA. However, the finding of asymptomatic subjects with meniscus lesions and BMEPs may also indicate that these subjects were actually tolerating their lesions well and it finally remains unclear if the PA in these patients has to be counted only as risk factor or also a beneficial factor that helps the patients to cope with their knee pain. Moreover this selection may not allow to generalize our results with regard to healthy individuals without risk factors or to subjects who already have knee OA or severe knee pain thus explaining some of the discrepancy of our results compared to other studies as described above. Therefore these results need to be confirmed in a broader cohort including all stages of OA.
There are additional limitations of our study: Firstly, we made a large number of separate comparisons and some of the associations may represent chance findings, for this reason we set the level of significance to p<0.01. Secondly it is very likely that the associations between PA and OA we found were not caused by current activity but rather by activity undertaken in the past and at younger ages in those who are still more active. However, measuring physical activity with an accelerometer can only cover a limited time period and may not allow extrapolating activity levels to longer intervals. Thirdly while accelerometer data measures intensity and duration of PA, it does not identify specific exercise or occupational activities that are more likely to result in joint tissues damage. Finally our study was cross-sectional precluding any inference about causation. Further longitudinal studies are needed to investigate if higher PA leads to an acceleration of knee joint degeneration and which certain types of exercise are more likely to cause joint damage with higher PA levels.
In summary, we found that in knees of subjects with no to mild OA symptoms and without radiographic OA, high levels of physical activity as measured by accelerometer were associated with an increased severity of meniscal and BMEP lesions while cartilage showed no increase of damage in response to PA. The implications of these findings for long-term health of the knee in active individuals remain to be determined.
Bullet points.
This is the first study comparing structural and quantitative MR measures of knee joint degeneration between subjects with different levels of physical activity objectively measured with an accelerometer.
Higher levels of moderate to vigorous physical activity are associated with an increased severity of lesions of the medial meniscus and with more severe bone marrow edema.
Quantitative and structural changes of the cartilage were not associated with levels of moderate to vigorous physical activity
Acknowledgments
Financial support
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. M.K. has received grants from the Gottfried and Julia Bangerter-Rhyner-Foundation.
References
- 1.Felson DT, Hannan MT, Naimark A, Berkeley J, Gordon G, Wilson PW, Anderson J. Occupational physical demands, knee bending, and knee osteoarthritis: results from the Framingham Study. J Rheumatol. 1991;18(10):1587–92. [PubMed] [Google Scholar]
- 2.Coggon D, Croft P, Kellingray S, Barrett D, McLaren M, Cooper C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43(7):1443–9. doi: 10.1002/1529-0131(200007)43:7<1443::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Kujala UM, Kettunen J, Paananen H, Aalto T, Battié MC, Impivaara O, Videman T, Sarna S. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38(4):539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 4.Spector TD, Harris PA, Hart DJ, Cicuttini FM, Nandra D, Etherington J, Wolman RL, Doyle DV. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996;39(6):988–95. doi: 10.1002/art.1780390616. [DOI] [PubMed] [Google Scholar]
- 5.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106(2):151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Simpson JA, Wluka AE, Teichtahl AJ, English DR, Giles GG, Graves S, Cicuttini FM. Is physical activity a risk factor for primary knee or hip replacement due to osteoarthritis? A prospective cohort study. J Rheumatol. 2011;38(2):350–7. doi: 10.3899/jrheum.091138. [DOI] [PubMed] [Google Scholar]
- 7.Manninen P, Riihimaki H, Heliovaara M, Suomalainen O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxford) 2001;40(4):432–7. doi: 10.1093/rheumatology/40.4.432. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, Niu J, Yang T, Torner J, Lewis CE, Aliabadi P, Sack B, Sharma L, Guermazi A, Goggins J, Nevitt MC MOST and OAI investigators. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis Cartilage. 2013;21(6):789–95. doi: 10.1016/j.joca.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, Majumdar S. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29(3):324–34. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, Link TM. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. Eur Radiol. 2009;19(1):132–43. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich KM, Shepard T, de Oliveira VS, Wang L, Babb JS, Schweitzer M, Regatte R. T2 measurements of cartilage in osteoarthritis patients with meniscal tears. AJR Am J Roentgenol. 2009;193(5):W411–5. doi: 10.2214/AJR.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Benjamin Ma C, Link TM, Castillo D-D, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y, Mizuta H. T1ρ and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35(1):147–55. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 14.Racunica TL, Teichtahl AJ, Wang Y, Wluka AE, English DR, Giles GG, O’Sullivan R, Cicuttini FM. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis Rheum. 2007;57(7):1261–8. doi: 10.1002/art.22990. [DOI] [PubMed] [Google Scholar]
- 15.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52(11):3507–14. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 16.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509–20. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776–86. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, McCulloch C, Lynch JA, Link TM. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 2011;63(8):2248–56. doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 20.Doré DA, Winzenberg TM, Ding C, Otahal P, Pelletier J-P, Martel-Pelletier J, Cicuttini FM, Jones G. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. 2013;72(7):1170–5. doi: 10.1136/annrheumdis-2012-201691. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes L, Hagen KB, Bijlsma JWJ, Andreassen O, Christensen P, Conaghan PG, Doherty M, Geenen R, Hammond A, Kjeken I, Lohmander LS, Lund H, Mallen CD, Nava T, Oliver S, Pavelka K, Pitsillidou I, da Silva JA, de la Torre J, Zanoli G, Vliet Vlieland TPM European League Against Rheumatism (EULAR) EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–35. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 22.Dunlop DD, Semanik P, Song J, Sharma L, Nevitt M, Jackson R, Mysiw J, Chang RW Osteoarthritis Initiative Investigators. Moving to maintain function in knee osteoarthritis: evidence from the osteoarthritis initiative. Arch Phys Med Rehabil. 2010;91(5):714–21. doi: 10.1016/j.apmr.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones G, Ding C, Glisson M, Hynes K, Ma D, Cicuttini F. Knee articular cartilage development in children: a longitudinal study of the effect of sex, growth, body composition, and physical activity. Pediatr Res. 2003;54(2):230–6. doi: 10.1203/01.PDR.0000072781.93856.E6. [DOI] [PubMed] [Google Scholar]
- 24.Urquhart DM, Tobing JFL, Hanna FS, Berry P, Wluka AE, Ding C, Cicuttini FM. What is the effect of physical activity on the knee joint? A systematic review. Med Sci Sports Exerc. 2011;43(3):432–42. doi: 10.1249/MSS.0b013e3181ef5bf8. [DOI] [PubMed] [Google Scholar]
- 25.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30(4):783–97. vii. doi: 10.1016/j.rdc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Dunlop DD, Song J, Semanik PA, Chang RW, Sharma L, Bathon JM, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Mysiw WJ, Nevitt MC, Hootman JM. Objective physical activity measurement in the osteoarthritis initiative: Are guidelines being met? Arthritis Rheum. 2011;63(11):3372–82. doi: 10.1002/art.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J, Semanik P, Sharma L, Chang RW, Hochberg MC, Mysiw WJ, Bathon JM, Eaton CB, Jackson R, Kwoh CK, Nevitt M, Dunlop DD. Assessing physical activity in persons with knee osteoarthritis using accelerometers: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2010;62(12):1724–32. doi: 10.1002/acr.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging--the osteoarthritis initiative. Nat Rev Rheumatol. 2012;8(10):622–30. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 30.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, Yu W, Genant HK. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 31.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70(11):1884–6. doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Carballido-Gamio J, Bauer JS, Stahl R, Lee K-Y, Krause S, Link TM, Majumdar S. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12(2):120–35. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carballido-Gamio J, Majumdar S. Atlas-based knee cartilage assessment. Magn Reson Med. 2011;66(2):574–83. doi: 10.1002/mrm.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, Majumdar S, Link TM. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(8):984–9. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowlands AV, Stiles VH. Accelerometer counts and raw acceleration output in relation to mechanical loading. J Biomech United States. 2012;45(3):448–54. doi: 10.1016/j.jbiomech.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Brage S, Wedderkopp N, Franks PW, Andersen LB, Froberg K. Reexamination of validity and reliability of the CSA monitor in walking and running. Med Sci Sports Exerc. 2003;35(8):1447–54. doi: 10.1249/01.MSS.0000079078.62035.EC. [DOI] [PubMed] [Google Scholar]
- 39.Welk GJ, Schaben JA, Morrow JR. Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36(9):1637–45. [PubMed] [Google Scholar]
- 40.Farr JN, Going SB, Lohman TG, Rankin L, Kasle S, Cornett M, Cussler E. Physical activity levels in patients with early knee osteoarthritis measured by accelerometry. Arthritis Rheum. 2008;59(9):1229–36. doi: 10.1002/art.24007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, DC: Department of Health and Human Services; 2008. [Google Scholar]
- 42.Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis Rheum. 2007;57(1):6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 43.Barbour KE, Hootman JM, Helmick CG, Murphy LB, Theis KA, Schwartz TA, Kalsbeek WD, Renner JB, Jordan JM. Meeting physical activity guidelines and the risk of incident knee osteoarthritis: a population-based prospective cohort study. Arthritis Care Res (Hoboken) 2014;66(1):139–46. doi: 10.1002/acr.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, Nieman DC, Swain DP American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 45.White DK, Tudor-Locke C, Felson DT, Gross KD, Niu J, Nevitt M, Lewis CE, Torner J, Neogi T. Walking to meet physical activity guidelines in knee osteoarthritis: is 10,000 steps enough? Arch Phys Med Rehabil. 2013;94(4):711–7. doi: 10.1016/j.apmr.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T1ρ and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(1):69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kijowski R, Stanton P, Fine J, De Smet A. Subchondral bone marrow edema in patients with degeneration of the articular cartilage of the knee joint. Radiology. 2006;238(3):943–9. doi: 10.1148/radiol.2382050122. [DOI] [PubMed] [Google Scholar]
- 48.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, Torner J, Nevitt MC, Sack B, Felson DT. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60(3):831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, Li W, Hill C, Gale D. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, Guermazi A, Genant H, Gale D, Felson DT. Increase in bone marrow lesions associated with cartilage loss: A longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis & Rheumatism. 2006;54(5):1529–1535. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]