Abstract
Early adverse life events (EALs) have been associated with regional thinning of the subgenual cingulate cortex (sgACC), a brain region implicated in the development of disorders of mood and affect, and often comorbid functional pain disorders, such as irritable bowel syndrome (IBS). Regional neuroinflammation related to chronic stress system activation has been suggested as a possible mechanism underlying these neuroplastic changes. However, the interaction of genetic and environmental factors in these changes is poorly understood. The current study aimed to evaluate the interactions of EALs and candidate gene polymorphisms in influencing thickness of the sgACC. 210 female subjects (137 healthy controls; 73 IBS) were genotyped for stress and inflammation-related gene polymorphisms. Genetic variation with EALs, and diagnosis on sgACC thickness was examined, while controlling for race, age, and total brain volume. Compared to HCs, IBS had significantly reduced sgACC thickness (p = 0.03). Regardless of disease group (IBS vs. HC), thinning of the left sgACC was associated with a significant gene-gene environment interaction between the IL-1β genotype, the NR3C1 haplotype, and a history of EALs (p = 0.05). Reduced sgACC thickness in women with the minor IL-1β allele, was associated with EAL total scores regardless of NR3C1 haplotype status (p = 0.02). In subjects homozygous for the major IL-1β allele, reduced sgACC with increasing levels of EALs was seen only with the less common NR3C1 haplotype (p = 0.02). These findings support an interaction between polymorphisms related to stress and inflammation and early adverse life events in modulating a key region of the emotion arousal circuit.
Keywords: Early adverse life events, Glucocorticoids, Proinflammatory cytokines, NF-κB, Cortical thickness, Subgenual anterior cingulate cortex, Functional somatic pain
Introduction
Early adverse life events (EALs) have been implicated in the vulnerability and morbidity related to various psychiatric and chronic medical disorders (McGowan and Szyf 2010; Chu et al. 2013; Drevets et al. 2008; Provencal et al. 2012), including functional pain syndromes such as irritable bowel syndrome (IBS) and fibromyalgia (Bradford et al. 2012; Berman et al. 2012; Labus et al. 2014; Jiang et al. 2013). Converging evidence suggests that by interacting with genetic variance, EALs may have negative effects on the development of brain mechanisms concerned with emotional arousal, stress, and resilience (de Rooij et al. 2012; Ducci et al. 2008; Chen et al. 2011; van der Doelen et al. 2013; Cousijn et al. 2010). Early adverse life events have been implicated in the epigenetic modification of genes related to the stress system, and have been shown to alter the translational expression of genes in peripheral mononuclear blood cells (“conserved transcriptional response to adversity”) (Slavich and Cole 2013) related to the hypothalamus–pituitary–adrenal (HPA) axis (Cottrell and Seckl 2009; Bale et al. 2010). The processes that influence transcription factor binding affinity (e.g. gene polymorphism, methylation, histone modification) can substantially affect a person's propensity to develop certain diseases or endophenotypes (Slavich and Cole 2013), and variations in these processes may explain why only a fraction of individuals with a history of EALs develop adult pathology (Schmidt 2011; Homberg 2012).
Changes in stress sensitivity and functioning of the HPA axis may underlie the association between EALs and risk for disease. For example, animal studies indicate that exposure to EALs results in increased basal corticosterone levels, and significant decreases in the glucocorticoid (GC) receptor total cells in the hilus and granule cell layers (Uys et al. 2006). Epigenetic regulation of the GCR is associated with prolonged decreased levels of GCR mRNA (McGowan et al. 2009). Human studies have shown that adults with a history of EALs have increased cytosine methylation in the promoter region of the GC receptor gene NR3C1 and methylation of this region leads to attenuated cortisol responses to the dexamethasone/corticotropin releasing hormone test (a standardized neuroendocrine challenge test) (Tyrka et al. 2012). These studies suggest that in the face of real or perceived threats (stressors), GC signaling redistributes energy to promote cell survival (Herman et al. 2012). This in turn promotes neurogenesis required to restore normal behavior after stress (Lehmann et al. 2013). In this respect, GC signaling controls stress reactivity through inhibition of the HPA axis (Herman et al. 2012).
A history of EALs is also associated with elevated pro-inflammatory cytokines, which in turn can cause neuroinflammation and increased risk for disease (Dantzer et al. 2008; McAfoose and Baune 2009; Miller et al. 2009; Groer and Morgan 2007; Hartwell et al. 2013). For example, individuals with post-traumatic stress disorder (PTSD) had increased proinflammatory levels and lower anti-inflammatory levels than healthy control subjects (von Kanel et al. 2007). In the presence of stress or with exposure to EALs, GC and cytokines interact to influence cell proliferation. Glucocorticoids can also cause the release of macrophage inhibitory factor, an inflammatory cytokine, from macrophages and T cells (Calandra et al. 1995). These studies, therefore, provided a basis for selecting genes associated with the HPA axis (stress) for further analysis in this study.
Recent research has shown that individuals with exposure to different types of stress (long term, acute, or chronic) have increased vulnerability for neuroplastic changes in brain regions known to precipitate or exacerbate disorders of mood (McEwen 2005) and chronic pain (Rodriguez-Raecke et al. 2013). The stress-related brain changes are related to GC signaling (Solomon et al. 2012), modulating gene transcription by either repressing or facilitating the transcription of target genes (Herman et al. 2005; Anacker et al. 2011). The sgACC and adjacent ventromedial prefrontal cortex (vmPFC) are key regions of an emotion arousal circuit, which play important roles in the modulation of sensory perception and pain modulation (Labus et al. 2013a), memory, autonomic function, and neuroendocrine responses (Radley et al. 2008). Various studies have demonstrated that the sgACC is part of the emotional arousal network, which is involved in the feedback inhibition of the amygdala (Labus et al. 2008; Berman et al. 2012; Jiang et al. 2013; Kilpatrick et al. 2010; Hong et al. 2014b; Pezawas et al. 2005; Stein et al. 2007), and IBS patients show abnormalities in the connectivity of this circuit (Labus et al. 2013b). Morphological reductions in the left sgACC have been reported in patients with disorders of mood and affect (Ansell et al. 2012; Lipsman et al. 2010; Singh et al. 2012) and functional gastrointestinal pain (Jiang et al. 2013). Decreased right and left sgACC volumes have been observed in subjects with PTSD compared to non-PTSD subjects exposed to trauma (Kitayama et al. 2006; Rauch et al. 2003). In postmortem studies of suicide victims, reduced grey matter volume of the bilateral sgACC was attributed to the presence of genes that were associated with a decrease in neuronal and glial cell size, number, and density (Ongur et al. 1998; Gerritsen et al. 2012; McEwen 2005), while deep brain stimulation of the sgACC has shown improved symptoms of refractory depression (Johansen-Berg et al. 2008).
Functional pain syndromes are characterized by widespread somatic symptoms, generalized hypersensitivity to somatic, visceral and auditory stimuli, and a high comorbidity with disorders of mood and affect (Ellingson et al. 2013; Gupta et al. 2014; Labus et al. 2013b; Hong et al. 2014a). Structural and functional brain imaging studies in several of these disorders have shown alterations in sensory and affective brain circuits and regions, including grey matter reductions in the cingulate and specifically in the left sgACC, even in patients without psychiatric comorbidity (Jiang et al. 2013; Labus et al. 2013a, 2014). It remains unclear if the observed structural alterations are a consequence of the experience of chronic pain (somatic or emotional), or are due to genetically/epigenetically determined vulnerability factors associated with stress or EALs, which contribute to chronic somatic and emotional pain.
By studying a large sample of female subjects including a subgroup of IBS subjects without psychiatric comorbidity, we aimed to evaluate interactions between regional thinning of sgACC, self-reported EALs, and several candidate HPA axis gene polymorphisms, which have been implicated in stress and inflammation. Specifically, we aimed to address the following questions—(1) Is a history of EALs associated with a reduction in sgACC thickness? (2) Is there an interaction between EALs and candidate single nucleotide polymorphisms (SNPs) related to stress and inflammation in influencing sgACC structure? (3) Is there a difference in these interactions between healthy control subjects and patients with chronic abdominal pain?
Subjects and methods
Subjects
Five hundred and twenty-seven individuals (304 HCs, 223 IBS) were recruited through the Digestive Disease Clinic at the University of California Los Angeles (UCLA), and advertisements in the local community. The UCLA Medical Institutional Review Board approved all procedures, and all subjects provided informed written consent. Exclusion criteria comprised pregnancy, substance abuse, abdominal surgery, tobacco dependence, and psychiatric illness. From the original sample, a subset of 210 right-handed premenopausal female subjects (137 HCs, 73 IBS) completed structural MRI scans. During a clinical assessment, a gastroenterologist or a nurse practitioner with expertise in functional gastroenterological disorders made a diagnosis of IBS, based on the ROME II or ROME III symptoms at the time of the study (Drossman 2007). Bowel habit was also determined at this time. Subjects were asked to indicate their race in terms of African, Caucasian, Asian, Hawaiian, or American Indian ancestry. Subjects claiming at least partial African ancestry were classified as African. Remaining subjects claiming Caucasian ancestry were classified as Caucasian, and subjects claiming Asian, Hawaiian or American Indian ancestry were classified as Asian.
Behavioral measures
The Early Traumatic Inventory-Self Report (ETI-SR) covers four domains of childhood/early adverse life events that occurred before the age of 18 years—general trauma (31 items), physical (9 items), emotional (7 items), and sexual abuse (15 items) (Bremner et al. 2005). In addition to calculating subscale scores, the number of items receiving a positive response was calculated for each subject, resulting in a total ETI score. Prevalence of ETI was calculated using a cutoff score ≥9 on the ETI total score. This value was determined by conducting a median split on the ETI total score plus one standard deviation to determine the cutoffs (Bremner et al. 2007), a procedure consistent with other studies (Rooks et al. 2012). Comorbid affective and mood disorders were measured using the Hospital Anxiety and Depression Scale (HAD) (Zigmond and Snaith 1983).
Genotyping
Genomic DNA was extracted from saliva samples at the UCLA Biological Samples Processing Core (BSPC). Samples for DNA isolation were collected using the Oragene™ DNA Self-Collection Kit (DNA Genotek Inc., Ottawa, Canada). DNA obtained using this kit is comparable in quality and quantity to DNA extracted from blood (Reynolds et al. 2007). SNPType genotyping was done using the Fluidigm Biomark system (Dube et al. 2008). A sample of DNA (10–60 ng) was pre-amplified using Qiagen Multiple PCR master mix (Qiagen Inc., Valencia CA, USA). Samples were diluted and partitioned uniformly into 765 reaction chambers for each panel, which were then thermocycled under hot conditions at 95uC for 10 min followed by 40 cycles of two-step PCR—15 s at 95uC for denaturing, and 1 min at 60uC for annealing and extension, and then amplification. Signals from all chambers were recorded at the end of each PCR cycle. The assays were designed using Fluidigm's proprietary technology described in detail by Dube et al. (2008). The samples and assays were loaded onto a GT 96*96 Dynamic array and processed as per Fluidigm protocol. The genotyping calls were made using Fluidigm SNP genotyping software. This included counting the number of positive FAM chambers (target gene) and number of positive VIC chambers (RNase P-reference gene) from each panel to calculate the target gene/reference gene ratios. To our knowledge, all participants were genetically unrelated.
MRI
A high resolution structural image was acquired with a magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence with the following parameters— repetition time (TR) = 2,200 and 20 ms, echo time (TE) = 3.26 and 3 ms, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 1.0 × 1.0 × 1.0 mm voxel size. Since studies from different protocols were combined, we applied a general linear model to determine protocol influences on total gray matter volume and controlling for age. Results indicated that the protocols were similar to each other. Some of the female subjects used in this study have been used in other published studies (Jiang et al. 2013; Labus et al. 2011, 2013b, 2014; Hong et al. 2014a; Gupta et al. 2014; Kurth et al. 2012).
Cortical thickness (CT)
The LONI (Laboratory of Neuroimaging) pipeline (http://pipeline.loni.usc.edu/), a graphical workflow environment, was utilized for image preprocessing and CT analysis. For details of the methodological procedure method, see recent investigations of neuroplastic differences between IBS patients and HC (Jiang et al. 2013; Hong et al. 2014b). Briefly, cortical thickness maps estimated in FreeSurfer 4.0 (Fischl and Dale 2000) were registered to International Consortium for Brain Mapping (ICBM) brain surface and then vertex-wise correspondences were established between all cortical surface models. A separate tissue-classification pipeline workflow was employed to obtain total gray matter volume (TGMV), which was used as a covariate in analysis. Data are available on our Pain website (http://painrepository.org/) as part of the Pain and Interoception Network (PAIN) repository. In each hemisphere, sgACC was manually delineated on the 3D ICBM brain atlas by two well-trained technicians with good command of neuroanatomical knowledge. The 3D region of interest masks was mapped back onto the ICBM surface space based on their Euclidean coordinates (Fig. 1).
Fig 1.
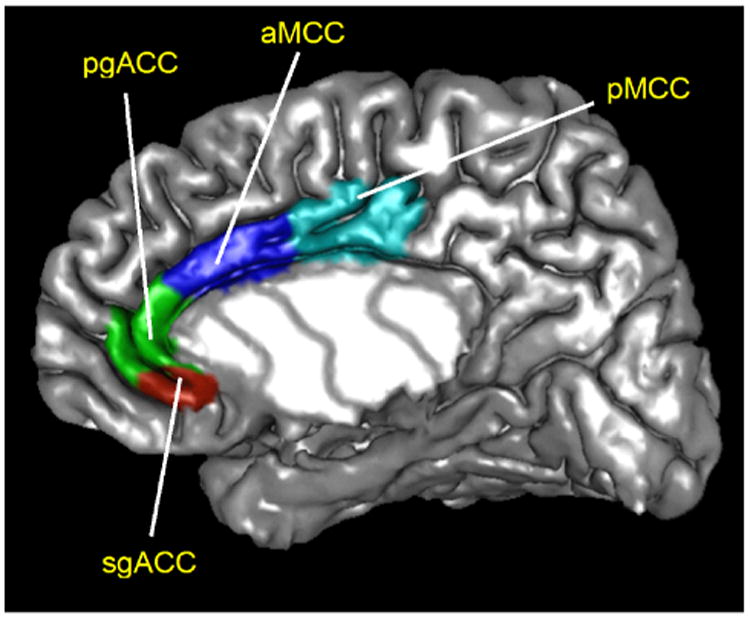
Manual delineation of the subgenual anterior cingulate cortex (sgACC) within the cingulate cortex. sgACC subgenual anterior cingulate cortex, pgACC pregenual anterior cingulate cortex, aMCC anterior mid cingulate cortex, pMCC posterior mid cingulate cortex
Statistical analysis
To study the interactions of EALs with candidate genes that may play a role in observed structural brain changes due to neuroinflammation, we focused on HPA axis genes (Blackwell and Christman 1997). To determine the potential gene predictors of sgACC thickness, a penalized ordinary least squares linear regression analysis controlling for TGMV, race and age was performed using Mendel software, version 13 (Lange et al. 2013; Zhou et al. 2010). This initial analysis was conducted on 11 available HPA-related genotyped SNPs (NR3C1, CRHR1, FKBP5, CRHBP) (see Supplementary Table 1) in our database, which were selected based on a literature search. From these initial 11 SNPs, we only examined SNPs that show a significant association with sgACC volume after false discovery rate (FDR) correction (Benjamini 2010). This approach has been applied in genome-wide studies as a dimension reducing technique (Haghighi et al. 2014). The glucocorticoid receptor (GCR) NR3C1 genes (rs2963155 and rs33389) were the only SNPs that had a significant association with sgACC volumes after adjusting for FDR using a graphic sharpening method (uncorrected p = 0.007, 0.009, respectively q's < 0.05) (Pike 2011). Interleukin-1β (IL-1β) gene (rs16944) was not found to be significant in an additional penalized ordinary least squares linear regression analysis similar to the initial Mendel analysis. However, to test our hypothesis that that stress and inflammation systems interact with EALs to influence sgACC structure, given that the NR3C1 gene is associated with stress-induced activation of NF-κB, a protein complex required for the transcription and production of proinflammatory cytokines, including interleukin-1 (IL-1) (Blackwell and Christman 1997), we focused on the interaction between these NR3C1 SNPs and a SNP in the promotor region of the IL-1β gene (rs16944), which contains a binding site for NF-κB. Hardy–Weinberg equilibrium, pairwise linkage disequilibrium (LD), genotype success rate and minor allele frequency were calculated using Mendel (Lange et al. 2005). The cut off value for divergence from Hardy–Weinberg equilibrium was (p ≥ 0.05). A haplotype analysis performed using Haploview determined that the two NR3C1 SNPs were in linkage disequilibrium, indicating that based on allele frequencies the alleles at two loci were non-randomly significantly associated with each other using genomic proximity, and thus subsequent analyses were performed using the NR3C1 haplotype, given that these tightly linked alleles are likely to be inherited together (Barrett et al. 2005).
Differences in clinical and demographics variables between HC and IBS were examined using the general linear model (GLM), specifying disease group as a factor. To further describe these differences, we calculated Cohen's effect size d in the scale of standard deviation units, and values are interpreted as low (d = 0.20), moderate (d = 0.50), and high (d = 0.80). A p value ≤0.05 was considered statistically significant. Next, differences between HCs and IBS on thickness of the right and left sgACC were tested using the GLM specifying disease group as a factor and age and TGMV as covariates.
Finally, we examined the main and interaction effects of genetic variation, EALs (total ETI score and emotional ETI subscore) and health status on the sgACC cortical thickness while controlling for race, age and TGMV. Specifically, the cortical thickness of the left and right sgACC, were modeled as a function of 3 covariates (age, TGMV, race), 4 main effects (health status, EALs, GCR haplotype, IL-1β SNP), and 11 terms representing the 2, 3, and 4 way interactions of the main effects. ETI scores were normalized using a square root transform. Analyses were performed comparing homozygotes for the most common/major alleles [CA/CA for the NR3C1 haplotype (MC) and GG for the IL-1β SNP (mc)] against heterozygotes and homozygous for the minor alleles/less common alleles [non-CA/CA for the NR3C1 haplotype (LC) and AA or AG for the IL-1β SNP (lc)]. Statistical analyses were performed using SPSS version 19.
Results
Sample description
Mean age, EAL prevalence and levels as measured by the early traumata inventory (ETI), symptoms of anxiety and depression as measured by the Hospital Anxiety and Depression Scale (HAD), and racial distribution are presented in Table 1. No significant differences in age were found [F(1, 208) = 1.37, p = 0.24; Cohen's d = 0.17] between IBS and HCs. Using the Rome II and III criteria, 23 patients were classified as IBS with constipation, 21 with diarrhea, 20 with alternating/mixed bowel habit, and 9 were unspecified.
Table 1. Description of subjects.
| HC (SD) | IBS (SD) | F | p value | Female IBS vs. female HC Effect size (es) Cohen's d | ||
|---|---|---|---|---|---|---|
| N | 137 | 73 | ||||
| Bowel habit | ||||||
| Constipation | 23 | |||||
| Diarrhea | 21 | |||||
| Alternating/mixed | 20 | |||||
| Unspecified | 9 | |||||
| Normal | 137 | 0 | ||||
| Age | M = 28.62 (9.06) | M = 30.18 (9.43) | 1.37 | 0.24 | 0.17 | |
| HAD anxiety | M = 3.25 (2.53) | M = 5.69 (3.62) | 4.21 | 0.04* | 0.83 | |
| HAD depression | M = 1.19 (1.64) | M = 2.51 (2.35) | 21.89 | <0.001** | 0.69 | |
| EAL (ETI) | ||||||
| EAL prevalencea | 16 (12 %) | 16 (22 %) | 3.25 | 0.08 | ||
| EAL total (sqrt) | M = 1.52 (1.07) | M = 1.89 (1.16) | 5.28 | 0.02* | 0.34 | |
| EAL general | M = 1.35 (1.38) | M = 1.95 (1.72) | 1.17 | 0.28 | 0.40 | |
| EAL physical | M = 0.97 (1.28) | M = 1.21 (1.40) | 1.80 | 0.18 | 0.18 | |
| EAL emotional | M = 0.70 (1.30) | M = 1.23 (1.68) | 12.4 | 0.001** | 0.37 | |
| EAL sexual | M = 0.40 (0.98) | M = 0.48 (1.14) | 0.96 | 0.33 | 0.08 | |
| CT left sgACC (mm)b | M = 1.79 (0.39) | M = 1.64 (0.35) | 4.75 | 0.03* | –0.40 | |
| CT right sgACC (mm)b | M = 1.69 (0.34) | M = 1.62 (0.40) | 0.84 | 0.36 | –0.20 | |
|
| ||||||
| HC (SD) | IBS (SD) | χ2 | p value | Female IBS vs. female HC Effect size (es) Cohen's d | ||
|
| ||||||
| Race | ||||||
| African | 21 (15.3 %) | 10 (13.7 %) | ||||
| Asian | 51 (37.2 %) | 18 (24.7%) | 5.17 | 0.16 | ||
| Caucasian | 59 (43.1 %) | 43 (58.9 %) | ||||
| Missing | 6 (4.3 %) | 2 (2.7 %) | ||||
N number of subjects, M mean, SD standard deviation, es effect size (Cohen's d), IBS irritable bowel syndrome, HC healthy controls, Bowel Habit for IBS subjects based on ROME II/III diagnostic criteria, HAD Hospital Anxiety and Depression Scale, EAL early adverse life events, ETI early traumatic inventory (values square root), CT cortical thickness, sgACC subgenual anterior cingulate cortex, mm adjusted in millimeters
p < 0.05,
p < 0.01
EAL total scores ≥9
Controlled for age and total grey matter volume (TGMV)
Prevalence of comorbid affective and mood symptoms
Although mood and affect-related symptoms were within normal ranges, there were significant main effects for symptoms of anxiety [F(1, 208) = 4.21, p = 0.04; d = 0.83] and depression [F(1, 208) = 21.89, p < 0.001; d = 0.69] with higher levels in IBS compared to HCs. When factoring gene polymorphisms (GC NR3C1 haplotype and IL-1β SNP) neither anxiety nor depression impacted sgACC thickness.
Prevalence and severity of early adverse life events
Even though EALs were reported by both groups, the prevalence of EALs (cutoff score ≥9 for the EAL total score) was almost twice as high in IBS (22 %) compared to HCs (12 %) [χ2(1) = 3.25, p = 0.08]. Significant and greater total EAL levels were observed in IBS patients [F(1, 207) = 5.28, p = 0.02; d = 0.34]. However, out of all four EAL subscores (general, physical, sexual, emotional), IBS subjects differed significantly from HCs only on the emotional subscale [F(1, 207) = 12.41, p < 0.001; d = 0.37]. Although there was a trend for higher EAL total mean scores for both African and Caucasian subjects compared to Asian subjects and subjects who did not report their race (missing), no significant EAL score differences were found between the races [F(1, 3) = 1.179, p = 0.35].
Cortical thickness
After controlling for age and TGMV, sgACC thickness was reduced in IBS compared to HCs, with statistically significant differences observed for the left sgACC [F(1, 208) = 4.75, p = 0.03; d = −0.40] (Table 1). In the combined sample (IBS + HCs), a significant negative correlation between left sgACC thickness and the EALs emotional subscale score was observed [r (208) = −0.15, p = 0.03].
Genotyping results
The genotype success rate was ≥95 % for all single nucleotide polymorphisms (SNPs). The minor allele frequencies observed (Table 2) for each SNP in our population were comparable to what has been reported for dbSNP samples (http://www.ncbi.nlm.nih.gov/projects/SNP/). No significant group differences in frequency distribution were detected.
Table 2. Description of single nucleotide polymorphisms.
| SNP | Gene | Chr | Position | Alleles major/minor | MAF IBS | MAF HCs | p value | HWE |
|---|---|---|---|---|---|---|---|---|
| rs33389 | NR3C1 | 5 | 142700499 | C/T | 0.94/0.06 | 0.91/0.09 | 0.230 | 0.630 |
| rs2963155 | NR3C1 | 5 | 142756004 | A/G | 0.81/0.19 | 0.79/0.21 | 0.578 | 0.757 |
| rs16944 | IL-1β | 2 | 113594867 | G/A | 0.53/0.47 | 0.61/0.39 | 0.110 | 0.403 |
|
| ||||||||
| Haplotype | Most common allele combinations | Allele Freq IBS | Allele Freq HCs | p value | HWE | |||
|
| ||||||||
| NR3C1 | CA/TA/CA/TG | 0.81/0.00/0.13/0.06 | 0.79/0.00/0.12/0.09 | 0.686 | 0.910 | |||
SNP single nucleotide polymorphisms, Chr chromosome position, MAF minor allele frequency, HWE Hardy–Weinberg equilibrium, NR3C1 glucocorticoids, IL-1β interleukin-1 beta, IBS irritable bowel syndrome, HC healthy controls
Relationship between genetic variation, EALs, and health status in influencing thickness of the left sgACC
A significant interaction between the NR3C1 haplotype and the IL-1β genotype and EAL total score was demonstrated for the left sgACC [F(1, 208) = 4.05, p = 0.05] (Fig. 2). Individuals homozygous or heterozygous for the minor IL-1β allele combination (lc), demonstrated decreased cortical thickness in the left sgACC with increasing levels of total EALs regardless of NR3C1 haplotype status (MC or LC) (MC/lc: p = 0.02, LC/lc: p = 0.02 respectively). Individuals homozygous for the major IL-1β allele (mc) demonstrated reduced thickness of the left sgACC with increasing total EAL levels only with the lesser common NR3C1 haplotype (LC) (LC/mc: p = 0.02). In contrast, individuals homozygous for the major IL-1β allele (mc) and homozygous for the most common NR3C1 haplotype (MC) demonstrated increased left sgACC thickness with increasing total EAL levels (MC/mc: p = 0.007). No significant main or interaction effects were observed for disease group. Only NR3C1 haplotype (MC), not the IL-1β allele, showed a significant main effect. Individuals who were homozygous for the most common NR3C1 haplotype (MC) had increased left sgACC thickness compared to individuals with the lesser common NR3C1 haplotype (LC) regardless of the level of total EALs [F(1, 208) = 5.58, p = 0.02] (see Fig. 3). No significant results were observed for the right sgACC. Although there was a trend there were no significant results observed for the emotional EAL subscale-gene interactions on either left or right sgACC (p > 0.06).
Fig 2.
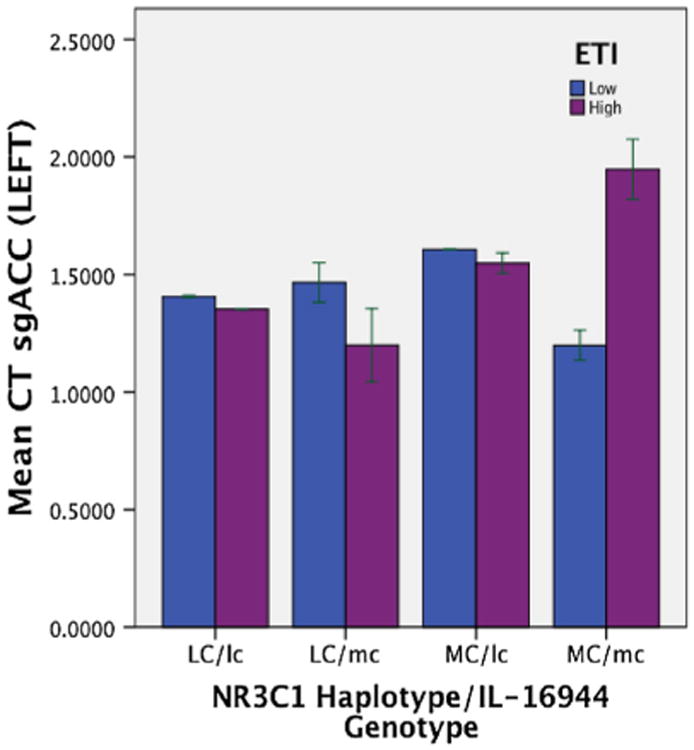
Mean sgACC cortical thickness is shown for four genetic groups with different levels of EALs. NR3C1 glucocorticoids, IL-1β Interleuken-1 Beta, lc homozygous or heterozygous with the minor IL-1β allele (AA or AG), mc homozygous for the major IL-1β allele (GG), LC homozygous/heterozygous with lesser common NR3C1 haplotypes (non-CA/CA), MC homozygous with most common NR3C1 haplotype (CA/CA), CT cortical thickness; sgACC subgenual anterior cingulate cortex, EALs early adverse life events as measured by ETI total score (early traumatic inventory)
Fig 3.
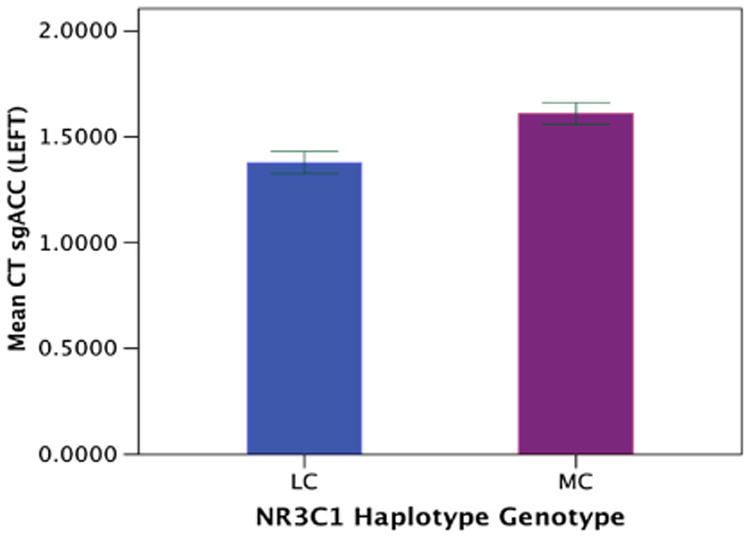
Mean sgACC cortical thickness is shown for NR3C1 genetic groups regardless of EALs. NR3C1 glucocorticoids, LC homozygous/heterozygous with lesser common NR3C1 haplotypes (non-CA/CA), MC homozygous with most common NR3C1 haplotype (CA/CA), CT cortical thickness, sgACC subgenual anterior cingulate cortex
Discussion
This study aimed to test the hypothesized interactions between candidate gene polymorphisms and early adversity in shaping the structure of a brain region (sgACC) involved in the emotional arousal aspects of pain regulation. The key findings are: (1) emotional early adverse life events were present in both HCs and IBS (more common in IBS), and were associated with regional thinning of the sgACC. (2) Thinning of the left sgACC was associated with significant interactions between the IL-1β genotype, the NR3C1 haplotype, and a history of EALs. (3) Regardless of disease or self-reported EAL history, the most common GCR gene NR3C1 haplotype was associated with increased left sgACC thickness, compared to individuals with the lesser common NR3C1 haplotypes.
Differences in EALs between patients with IBS and HC subjects
Even though reported by both groups, the prevalence and intensity of EAL experiences were significantly higher in IBS compared to HC subjects. These findings are consistent with studies that show a greater incidence of EALs in IBS compared to HCs (Bradford et al. 2012; Wu 2012; Talley et al. 1998). Upon examining subscales, IBS patients showed greater scores on the emotional trauma scale and sample items on the ETI emotional subscale include the following, “Often put down or ridiculed,” or “Often told that one is no good” (Bremner et al. 2005). There is an extensive literature demonstrating that a history of early emotional adversity is associated with various anxiety and depression symptoms and comorbidities in the adult (Chitkara et al. 2008; Berman et al. 2012). These comorbidities contribute to the disproportional impairment of health-related quality of life, increased somatic symptoms and psychological distress among IBS (Heitkemper et al. 2011). Alterations in HPA axis responses to a visceral stressor in IBS patients with a history of EALs have been observed (Chang 2011), which have been attributed to epigenetic modulation of the GC signaling system, resulting in reduced GCR mRNA expression and associated reduction in negative feedback inhibition within the HPA axis (Weaver et al. 2001).
Differences in EALs and regional thickness of sgACC between IBS and HC subjects
Early adversity in vulnerable individuals is believed to have long-term effects on brain systems involved in stress responsiveness and emotional arousal (including corticolimbic inhibition) (Gee et al. 2013; Tottenham and Sheridan 2009; McEwen 2008), thereby increasing the risk of developing adult stress-related disorders (Chaloner and Greenwood-Van Meerveld 2013; Pietrek et al. 2013). In the current study, the thickness of the left sgACC was significantly reduced and this reduction was correlated with higher ratings of the emotional subscale of the ETI across both groups. This is consistent with findings from several fMRI studies, which provide evidence for reduced activity and connectivity of the sgACC, a cingulate subregion implicated in modulation of emotional arousal. For example, using rectal distention stimuli, Ringel et al. (2008) reported a trend for reduced activity in the sgACC in IBS and HC subjects with abuse history compared to those subjects without. We recently reported that higher levels of EALs in male and female IBS, but not HC subjects was associated with decreased resting state connectivity of the ACC with the salience network (Gupta et al. 2014).
Epigenetic modulation of GCR gene expression may play an important role in this EAL associated neuroplasticity (Binder et al. 2008). For example, in postmortem brains of suicide victims, a history of EALs was associated with decreased levels of GCR mRNA and increased methylation of the GCR NR3C1 promoter (Szyf et al. 2008), suggesting an interaction between EALs and GCR gene transcription (McGowan et al. 2009). Morphometric changes (e.g. reduction in sgACC thickness) have been observed in depressed subjects (Drevets et al. 2008), and these changes were related to a stress-induced decrease in glial cell size (McEwen and Magarinos 2001; Czeh et al. 2006). Since glial cells provide support to neuronal function (Vazquez-Chona et al. 2011), a loss in glial cells may result in loss of neuronal density as well. Astrocytes are modulated by GCs and by IL-1 in sustaining expression of adhesion molecules and chemokines which in turn attract leukocytes to the central nervous system (CNS) (Rozovsky et al. 1995; Moynagh 2005). More recently, significant structural differences between female IBS patients compared to female HCs have shown significant decrease in sgACC thickness, with positive correlations with IBS symptom severity (Jiang et al. 2013). The fact that the changes were not correlated with the duration of symptoms suggests that the regional GM changes may be a consequence of genetic or epigenetic factors, predating the development of IBS symptoms (Zhang et al. 2011; Blalock et al. 2011).
Relationship between EALs, genetic variation, and health status in influencing thickness of the sgACC
Immune response genes, in particular those related to the innate immune response (including IL-1β) are highly sensitive to social environmental conditions, and stress-related alterations in GC signaling are likely to play a key role in modulating changes in proinflammatory gene expression (Cole 2009; Cole et al. 2009). Such changes have been reported not only in association with ongoing chronic stress (Raison and Miller 2003), but their lifelong persistence has been demonstrated in non-human primates following early adverse life conditions (Cole et al. 2012). While GC receptor activation typically downregulates inflammatory gene expression, chronic psychosocial stress can result in desensitization of GCR proteins and a failure of GCs to regulate GCR response genes, even in the presence of normal or elevated GC levels (Sorrells et al. 2009; Slavich and Cole 2013). As a result of decreased GCR regulation by GCs, NF-kB is derepressed, e.g. NF-kB is no longer inhibited from binding to gene promoters, leading to increased inflammatory activity (Blackwell and Christman 1997).
In the current study, the IL-1β SNP rs16944 by itself did not interact with EALs and diagnosis to affect significant changes in sgACC thickness. However, a significant interaction between this IL-1β SNP, the GCR gene NR3C1 haplotype, and EALs was demonstrated. In individuals with the minor IL-1β allele (lc), sgACC thickness decreased with increasing EALs regardless of NR3C1 haplotype and disease group. Even though gene methylation or transcription was not assessed in this study, one could speculate that the most common and least common NR3C1 haplotypes are associated with reduced GCR mediated NF-kB derepression with increasing EALs. In individuals with the minor IL-1β allele, this derepression could result in regional neuroinflammatory changes leading to cortical thinning in the sgACC. These results are similar to animal studies that show impaired GC regulation of cytokine release leading to increased inflammatory responses and a ‘defensive phenotype’ that increases susceptibility to disease, infection and injury in animals that have experienced EALs (Miller et al. 2009; Avitsur et al. 2006; Zhang et al. 2006). However, in individuals homozygous for the major IL-1β allele combination (mc), only the lesser common NR3C1 haplotypes (LC) was associated with decreased sgACC thickness, while the most common NR3C1 haplotype (MC) was associated with increased sgACC thickness with increasing EAL levels. In summary, women with less common alleles in either the IL-1β or NR3C1 genes appear to be more susceptible to EAL-related reductions in sgACC thickness, while women homozygous for the major IL-1β allele and homozygous for the most common NR3C1 haplotype appear to be protected against such effects. While one study has found that under chronic behavioral stress, dendritic shrinkage in pyramidal cells of the anterior cingulate and paralimbic regions occurs (Radley et al. 2004), another study found increased apical dendritic branching and complexity after administration of GCs (Wellman 2001). This increase in proximal dendritic branching could be a compensatory mechanism in response to distal dendritic atrophy (Wellman 2001), because GCs are not only differentially expressed in various types of cells and tissues (Provencal et al. 2012; Uddin et al. 2010; Gadek-Michalska et al. 2013), but they also may be unable to discriminate between different sites of action (Wellman 2001). However, the exact mechanisms are unknown and future studies will need to determine the molecular mechanism behind these changes. There are also times in the presence of enriched environments (e.g. healthy diet intake or exercise), when GCs can promote cell survival and restoration of normal behavior, and thus protect against grey matter reductions even after experiencing adverse-related stress (Lehmann et al. 2013; Herman et al. 2012). Consistent with this concept are recent reports showing that the most common alleles in GCR NR3C1 SNPs (rs33389 and rs2963155) are protective against developing temporomandibular pain disorders (Smith et al. 2013).
Limitations
The study has several limitations. To increase statistical power, we focused our genotype analyses on SNPs that ranked highest in the Mendel analysis, and did not include other well-characterized SNPs (such as the FKBP5) which have been implicated in modulating the epigenetic effects of early adversity on GCR expression (Binder et al. 2008). Similarly, we focused the analysis on one brain region and only one parameter of grey matter (cortical thickness). As we did not directly assess the molecular consequences of early adversity in terms of DNA methylation and gene transcription, we can only speculate about the mechanisms that mediate the observed correlations between EALs and regional brain structure. We did not measure female sex hormones, and therefore could not address a possible influence of cyclical sex hormone variations on the current findings. However, to minimize menopause-related influences on brain structure (Spencer et al. 2008), we studied predominantly premenopausal women during the follicular phase of their menstrual cycle.
Conclusions and possible pathophysiological implications
Our study found support for the hypothesized interactions between genes related to the immune and stress systems and EALs in shaping the structure of a brain region involved in emotional arousal aspects of pain regulation. As these interactions occurred in both HCs and IBS, the prevalence of EALs may lead to a greater prevalence of such brain changes in both groups. Together with previous reports of similar brain changes in patients with depression, the current finding suggests that EALs are associated with sgACC thinning, but that other factors and brain changes determine the specific symptom expression in different populations. Future studies in larger samples and in samples with clinical levels of psychiatric comorbidities should address the question if observed reductions in this region and other brain regions (including subregions of the insula and cingulate cortices, and hippocampus) are related to similar gene and early environment interaction effects on the expression of central inflammatory mediators.
Supplementary Material
Acknowledgments
This research was supported in part by grants from the National Institute of Health: R01 DK048351 (EAM), P50 DK064539 (EAM), P30 DK041301 (UCLA Cure Center), K01 DK085133 (LAK); Pilot scans were provided by Ahamsom-Lovelace Brain Mapping Center. The authors acknowledge the valuable editorial contributions made to the manuscript by Cathy Liu.
Drs. Mayer, Labus, Bradesi, Chang, and Kilpatrick's work has been funded by the NIH.
Abbreviations
- SgACC
Subgenual anterior cingulate cortex
- VmPFC
Ventromedial prefrontal cortex
- IBS
Irritable bowel syndrome
- HC
Healthy control
- EALs
Early adverse life events
- SNPs
Single nucleotide polymorphisms
- GCR
Glucocorticoid receptor NR3C1
- IL-1β
Proinflammatory cytokines interleuken-1β
- HPA
Hypothalamus–pituitary–adrenal axis
- fMRI
Functional brain imaging
- CT
Cortical thickness
- GM
Gray matter
- GI
Gastroenterological
- ETI
Early traumatic inventory
- HAD
Hospital and Anxiety Depression Scale
- MP-RAGE
Magnetization-prepared rapid acquisition gradient echo
- TR
Repetition time
- TE
Echo time
- NF-κB
Nuclear factor-κB
- TGMV
Total grey matter volume
- LD
Linkage disequilibrium
- GLM
General linear model
- CNS
Central nervous system
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-015-0996-9) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no conflict of interest.
Ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of California Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Contributor Information
Arpana Gupta, Email: aagupta9@gmail.com, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA; David Geffen School of Medicine, University of California Los Angeles (UCLA), Los Angeles, USA; Division of Digestive Diseases, University of California Los Angeles (UCLA), Los Angeles, USA.
Jennifer Labus, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA; David Geffen School of Medicine, University of California Los Angeles (UCLA), Los Angeles, USA; Division of Digestive Diseases, University of California Los Angeles (UCLA), Los Angeles, USA.
Lisa A. Kilpatrick, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA; David Geffen School of Medicine, University of California Los Angeles (UCLA), Los Angeles, USA; Division of Digestive Diseases, University of California Los Angeles (UCLA), Los Angeles, USA
Mariam Bonyadi, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA.
Cody Ashe-McNalley, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA.
Nuwanthi Heendeniya, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA.
Sylvie Bradesi, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA; David Geffen School of Medicine, University of California Los Angeles (UCLA), Los Angeles, USA; Division of Digestive Diseases, University of California Los Angeles (UCLA), Los Angeles, USA.
Lin Chang, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA; David Geffen School of Medicine, University of California Los Angeles (UCLA), Los Angeles, USA; Division of Digestive Diseases, University of California Los Angeles (UCLA), Los Angeles, USA.
Emeran A. Mayer, Email: emayer@ucla.edu, Oppenheimer Center for Neurobiology of Stress, and Pain and Interoception Network (PAIN), University of California Los Angeles (UCLA), CHS 42-210 MC737818, 10833 Le Conte Avenue, Los Angeles, CA 90095-7378, USA; David Geffen School of Medicine, University of California Los Angeles (UCLA), Los Angeles, USA; Division of Digestive Diseases, University of California Los Angeles (UCLA), Los Angeles, USA; Ahmanson-Lovelace Brain Mapping Center, University of California Los Angeles (UCLA), Los Angeles, USA.
References
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrino. 2011;36(3):415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72(1):57–64. doi: 10.1016/J.Biopsych.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20(4):339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–319. doi: 10.1016/J.Biopsych.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamini Y. Discovering the false discovery rate. J R Stat Soc B. 2010;72:405–416. [Google Scholar]
- Berman S, Suyenobu B, Naliboff BD, Bueller J, Stains J, Wong H, Mandelkern M, Fitzgerald L, Ohning G, Gupta A, Labus JS, Tillisch K, Mayer EA. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. NeuroImage. 2012;63(4):1854–1863. doi: 10.1016/J.Neuroimage.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17(1):3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Buechel HM, Popovic J, Geddes JW. Landfield PW (2011) Microarray analyses of laser-captured hippocampus reveal distinct gray and white matter signatures associated with incipient Alzheimer's disease. J Chem Neuroanat. 2011;42(2):118–126. doi: 10.1016/J.Jchemneu.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, Chang L. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol H. 2012;10(4):385–390. doi: 10.1016/J.Cgh.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. The early trauma inventory self report (ETI-SR) Gastroenterology. 2005;128(4):A340. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the early trauma inventory-self report. J Nerv Ment Dis. 2007;195(3):211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Chaloner A, Greenwood-Van Meerveld B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci. 2013;7:13. doi: 10.3389/fnins.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140(3):761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103(3):765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DA, Williams LM, Harris AW, Bryant RA, Gatt JM. Early life trauma predicts self-reported levels of depressive and anxiety symptoms in nonclinical community adults: relative contributions of early life stressor types and adult trauma exposure. J Psychiatr Res. 2013;47(1):23–32. doi: 10.1016/j.jpsychires.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression. Curr Dir Psychol Sci. 2009;18(3):132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Mendoza SP, Capitanio JP. Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosom Med. 2009;71(6):591–597. doi: 10.1097/Psy.0b013e3181aa95a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci USA. 2012;109(50):20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009 doi: 10.3389/Neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, van Wingen G, Fernandez G. Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci USA. 2010;107(21):9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31(8):1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij SR, Costello PM, Veenendaal MVE, Lillycrop KA, Gluckman PD, Hanson MA, Painter RC, Roseboom TJ. Associations between DNA methylation of a glucocorticoid receptor promoter and acute stress responses in a large healthy adult population are largely explained by lifestyle and educational differences. Psychoneuroendocrino. 2012;37(6):782–788. doi: 10.1016/J.Psyneuen.09.010. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA. Introduction. The Rome Foundation and Rome III. Neurogastroent Motil. 2007;19(10):783–786. doi: 10.1111/J.1365-2982.2007.01001.X. [DOI] [PubMed] [Google Scholar]
- Dube S, Qin J, Ramakrishnan R. Mathematical analysis of copy number variation in a DNA sample using digital PCR on a nanofluidic device. PLoS ONE. 2008;3(8):e2876. doi: 10.1371/journal.pone.0002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13(3):334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013 doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek-Michalska A, Spyrka J, Rachwalska P, Tadeusz J, Bugajski J. Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol Rep. 2013;65(5):1163–1175. doi: 10.1016/s1734-1140(13)71474-9. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen L, Tendolkar I, Franke B, Vasquez AA, Kooijman S, Buitelaar J, Fernandez G, Rijpkema M. BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Mol Psychiatry. 2012;17(6):597–603. doi: 10.1038/mp.2011.51. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrino. 2007;32(2):133–139. doi: 10.1016/j.psyneuen.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Gupta A, Kilpatrick L, Labus JS, Tillisch K, Braun A, Hong JY, Ashe-McNalley C, Naliboff B, Mayer EA. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76(6):404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi A, Melka MG, Bernard M, Abrahamowicz M, Leonard GT, Richer L, Perron M, Veillette S, Xu CJ, Greenwood CM, Dias A, El-Sohemy A, Gaudet D, Paus T, Pausova Z. Opioid receptor mu 1 gene, fat intake and obesity in adolescence. Mol Psychiatry. 2014;19(1):63–68. doi: 10.1038/mp.2012.179. [DOI] [PubMed] [Google Scholar]
- Hartwell KJ, Moran-Santa Maria MM, Twal WO, Shaftman S, DeSantis SM, McRae-Clark AL, Brady KT. Association of elevated cytokines with childhood adversity in a sample of healthy adults. J Psychiatr Res. 2013;47(5):604–610. doi: 10.1016/j.jpsychires.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Cain KC, Burr RL, Jun SE, Jarrett ME. Is childhood abuse or neglect associated with symptom reports and physiological measures in women with irritable bowel syndrome? Biol Res Nurs. 2011;13(4):399–408. doi: 10.1177/1099800410393274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45(4):292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR. The stress-coping (mis)match hypothesis for nature × nurture interactions. Brain Res. 2012;1432:114–121. doi: 10.1016/j.brainres.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, Smith SR, Tillisch K, Naliboff B, Mayer EA. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014a;34(43):14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Labus JS, Jiang Z, Ashe-Mcnalley C, Dinov I, Gupta A, Shi Y, Stains J, Heendeniya N, Smith SR, Tillisch K, Mayer EA. Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. PLoS ONE. 2014b;9(1):e84564. doi: 10.1371/journal.pone.0084564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, Ashe-McNalley C, Hong JY, Tillisch K, Toga AW, Mayer EA. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS ONE. 2013;8(9):e73932. doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rush-worth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, Labus JS, Mayer EA, Naliboff BD. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS) Biol Psychol. 2010;84(2):272–278. doi: 10.1016/j.biopsycho.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90(2–3):171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Labus JS, Stains J, Ebrat B, Vianna E, Jiang ZG, Tillisch K, Naliboff BD, Mayer EA. Mild visceral stimuli interfere with attentional processes in IBS but not healthy control subjects. Gastroenterology. 2012;142(5):S553. [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. NeuroImage. 2008;41(3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Ebrat B, Jiang ZG, Stains J, Tillisch K, Naliboff BD, Mayer EA. The effect of cognitive load on interoceptive processing. Gastroenterology. 2011;140(5):S368–S369. [Google Scholar]
- Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, Feier N, Bueller J, Stains J, Smith S, Suyenobu B, Naliboff B, Mayer EA. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013a doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, Feier N, Bueller J, Stains J, Smith S, Suyenobu B, Naliboff B, Mayer EA. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013b;154(10):2088–2099. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Tillisch K, Ebrat B, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155(1):137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Sinsheimer JS, Sobel E. Association testing with mendel. Genet Epidemiol. 2005;29:36–50. doi: 10.1002/gepi.20073. [DOI] [PubMed] [Google Scholar]
- Lange K, Papp JC, Sinsheimer JS, Sripracha R, Zhou H, Sobel EM. Mendel: the Swiss army knife of genetic analysis programs. Bioinformatics. 2013;29(12):1568–1570. doi: 10.1093/bioinformatics/btt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci. 2013;33(7):2961–2972. doi: 10.1523/JNEUROSCI.3878-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, McIntyre RS, Giacobbe P, Torres C, Kennedy SH, Lozano AM. Neurosurgical treatment of bipolar depression: defining treatment resistance and identifying surgical targets. Bipolar Disord. 2010;12(7):691–701. doi: 10.1111/J.1399-5618.2010.00868.X. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiology of disease. 2010;39(1):66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/Nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh PN. The interleukin-1 signalling pathway in astrocytes: a key contributor to inflammation in the brain. J Anat. 2005;207(3):265–269. doi: 10.1111/j.1469-7580.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/Nn1463. [DOI] [PubMed] [Google Scholar]
- Pietrek C, Elbert T, Weierstall R, Muller O, Rockstroh B. Childhood adversities in relation to psychiatric disorders. Psychiatry Res. 2013;206(1):103–110. doi: 10.1016/j.psychres.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Pike N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol. 2011;2(3):278–282. doi: 10.1111/J.2041-210x.2010.00061.X. [DOI] [Google Scholar]
- Provencal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang DS, Bennett AJ, Pierre PJ, Friedman DP, Cote SM, Hallett M, Tremblay RE, Suomi SJ, Szyf M. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci. 2012;32(44):15626–15642. doi: 10.1523/Jneurosci.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28(22):5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. NeuroReport. 2003;14(7):913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Do T, Hongo D, Kuramoto K, Biggs W, Frech C. Comparison of high density genotyping results from saliva and blood samples on Affymetrix GeneChip GenomeWide SNP 6.0 arrays. 2007. [Google Scholar]
- Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, Whitehead WE, Naliboff BD, Berman S, Mayer EA. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134(2):396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Structural brain changes in chronic pain reflect probably neither damage nor atrophy. PLoS ONE. 2013;8(2):e54475. doi: 10.1371/journal.pone.0054475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks C, Veledar E, Goldberg J, Bremner JD, Vaccarino V. Early trauma and inflammation: role of familial factors in a study of twins. Psychosom Med. 2012;74(2):146–152. doi: 10.1097/PSY.0b013e318240a7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Laping NJ, Krohn K, Teter B, O'Callaghan JP, Finch CE. Transcriptional regulation of glial fibrillary acidic protein by corticosterone in rat astrocytes in vitro is influenced by the duration of time in culture and by astrocyte-neuron interactions. Endocrinology. 1995;136(5):2066–2073. doi: 10.1210/endo.136.5.7720656. [DOI] [PubMed] [Google Scholar]
- Schmidt MV. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrino. 2011;36(3):330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Chen MC, Kelley RG, Garrett A, Mitsunaga MM, Bararpour L, Howe M, Reiss AL, Gotlib IH. Volumetric reductions in the subgenual anterior cingulate cortex in adolescents with bipolar I disorder. Bipolar Disord. 2012;14(6):585–596. doi: 10.1111/j.1399-5618.2012.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clin Psychol Sci. 2013;1(3):331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Mir E, Bair E, Slade GD, Dubner R, Fillingim RB, Greenspan JD, Ohrbach R, Knott C, Weir B, Maixner W, Diatchenko L. Genetic variants associated with development of TMD and its intermediate phenotypes: the genetic architecture of TMD in the OPPERA prospective cohort study. J Pain. 2013;14(12):T91–T101. doi: 10.1016/j.jpain.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36(3):736–745. doi: 10.1016/J.Neuroimage.03.022. [DOI] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49(1):46–60. doi: 10.1002/Em.20357. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42:47–53. doi: 10.1136/gut.42.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS ONE. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci USA. 2010;107(20):9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, Muller CJ, Marais L, Harvey BH, Stein DJ, Daniels WM. Early life trauma decreases glucocorticoid receptors in rat dentate gyrus upon adult re-stress: reversal by escitalopram. Neuroscience. 2006;137(2):619–625. doi: 10.1016/j.neuroscience.2005.08.089. [DOI] [PubMed] [Google Scholar]
- van der Doelen RH, Kozicz T, Homberg JR. Adaptive fitness; early life adversity improves adult stress coping in heterozygous serotonin transporter knockout rats. Mol Psychiatry. 2013;18(12):1244–1245. doi: 10.1038/mp.2012.186. [DOI] [PubMed] [Google Scholar]
- Vazquez-Chona FR, Swan A, Ferrell WD, Jiang L, Baehr W, Chien WM, Fero M, Marc RE, Levine EM. Proliferative reactive gliosis is compatible with glial metabolic support and neuronal function. BMC Neurosci. 2011;12:98. doi: 10.1186/1471-2202-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflam-matory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Weaver IC, La Plante P, Weaver S, Parent A, Sharma S, Diorio J, Chapman KE, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Mol Cell Endocrinol. 2001;185(1–2):205–218. doi: 10.1016/s0303-7207(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wu JC. Psychological co-morbidity in functional gastrointestinal disorders: epidemiology, mechanisms and management. J Neurogastroenterol Motil. 2012;18(1):13–18. doi: 10.5056/jnm.2012.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol. 2006;73(1):72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Li LJ, Li ZX, Li WH, Ma N, Hou CL, Zhang ZJ, Zhang ZQ, Wang LF, Duan L, Lu GM. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord. 2011;133(1–2):294–299. doi: 10.1016/J.Jad.03.040. [DOI] [PubMed] [Google Scholar]
- Zhou H, Sehl ME, Sinsheimer JS, Lange K. Association screening of common and rare genetic variants by penalized regression. Bioinformatics. 2010;26(19):2375–2382. doi: 10.1093/bioinformatics/btq448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


