Abstract
Plume dispersion modeling systems are often used in assessing human exposures to chemical hazards for epidemiologic study. We modeled the 2005 Graniteville, South Carolina, 54,915 kg railcar chlorine release using both the Areal Locations of Hazardous Atmospheres (ALOHA) and Hazard Prediction and Assessment Capability (HPAC) plume modeling systems. We estimated the release rate by an engineering analysis combining semi-quantitative observations and fundamental physical principles. The use of regional meteorological conditions was validated by comparing concentration estimates generated by two source-location weather data sets. The HPAC model estimated a chlorine plume with 20 ppm outdoor concentrations up to 7 km downwind and 0.25 km upwind/downgrade. A comparative analysis of our two models showed that HPAC was the best candidate for use as a model system on which epidemiologic studies could be based after further model validation. Further validation studies are needed before individual exposure estimates can be reliable and the chlorine plume more definitively modeled.
1. Introduction
Chlorine gas (Cl2) is used widely in science, industry and warfare. Today, chlorine is among the top ten industrial chemicals produced in the United States and is used in many major manufacturing areas such as production of automobiles and pharmaceuticals (Jones et al, 2010). The Association of American Railroads (AAR) noted in 2007 that railroads typically transport over 100,000 tank car loads of toxic inhalation hazard (TIH) chemicals annually, such as chlorine and anhydrous ammonia (AAR, 2007). Many of these transportation routes pass through or in close proximity to population centers and other areas in which an accidental release could have detrimental impact on human health. The elemental form of chlorine is highly reactive and, therefore, does not exist naturally in high concentrations. The majority of human exposures occur only occupationally, purposely or accidently (ATSDR, 2010). Indeed, there have been numerous incidents involving chlorine within the past decade such as the intentional releases in Iraq (2007) and Syria (2011, 2014), and accidental releases from railcars in Macedona, Texas (2004), Graniteville, South Carolina (2005) and Tacoma, Washington (2007). The integral nature of chlorine in manufacturing industries and its ease of use and effectiveness as a terrorist agent supports the notion that releases will continue to occur (Jones et al, 2010; CDC, 2005). Accordingly, there is a continuing need to comprehensively assess the toxicological, ecological and socioeconomic effects of these events for purposes of both planning and response.
Currently, large inhalation events involving toxic irritant gases are assessed using a combination of onsite monitoring and comparing injuries to known health effects. The methodology and instrumentation to detect atmospheric concentrations of toxic gases such as chlorine has existed for many years. Yet, the use of continuous monitoring apparatus in settings such as the occupational workplace represents significant investment and preplanned placement, luxuries that are nearly impossible to attain during emergencies due to their unpredictable nature. The initial focus during response to a TIH incident is always the protection of life and property. Therefore, environmental testing is usually only conducted after the initial release has dissipated and it is safe to deploy monitoring equipment.
Many epidemiologic studies concerning the residual effects of chlorine exposure are limited by the lack of prior knowledge of the respiratory physiology of the exposed, small sample sizes, and, perhaps most importantly (and most relevant to this paper), the lack of well-characterized exposure data such as duration, extent and concentration. It is in bridging this gap where the modern science of atmospheric detection and plume modeling is highly valuable (Farago et al, 2005; BASC, 2003).
These algorithmic simulations of pollutant dispersion are important in managing environmental quality and protecting human health. There is considerable variability in the predictive accuracy and robustness of currently available dispersion modeling systems. All models require two basic inputs: source term (physical descriptors of the release itself) and meteorology. It is commonly believed that meteorology (such as atmospheric stability, wind speed and ambient conditions) have some of the most profound implications on dispersion (Hanna et. al, 1982). As an example, a highly stable/less turbulent atmosphere, such as night, will likely result in diminished dispersion. Topography also greatly influences dispersion; however, many mainstream software packages, particularly those targeted towards the first responder and optimized for rapid usability, do not take into account terrain influences during dispersion. Our study focuses on different models with varying sophistication.
The use of air pollution modeling technologies in epidemiologic studies of irritant gas exposure events is an emerging area of research. A review of exposure assessment techniques by Zou and colleagues noted that modeling systems are becoming primary tools in environmental epidemiology studies as robustness increases (Zou et al, 2009). Historically, particularly for incidents involving dense chlorine gas, dispersion models have either over-predicted or under-predicted the exposure limits forcing emergency decisions to be highly conservative. Applications to determine individual exposure estimates for etiologic studies have been largely unsuccessful. Increasing processing power and incorporation of algorithms addressing randomness, variability and the effects of physical and chemical reactions, particularly for dense gases such as chlorine, have yielded increasingly robust model systems. Unlike earlier modeling software, these newer systems are often able to account for dry deposition, ambient reactions and dynamic weather conditions, such as anti-cyclonic phenomena, but with varying accuracy. Simulations of high concentration events emphasizing the collection of plume migration and eco-toxicological data via field experiments have been performed in studies such as the Joint Urban 2003 (Oklahoma City, Oklahoma) and Jack Rabbit (Dugway Proving Ground, Utah) (NOAA FRD, 2013;). Such field trials provide markers against which existing models can be validated (Hanna et al, 2008a). These experiments have produced valuable contributions, and further validation against known exposure thresholds and other markers collected in the aftermath of an incident are useful to ensure these computerized models generate accurate and precise point estimates of personal exposure, which are particularly useful for subsequent epidemiologic study. The purpose here is to describe the first step in developing a validated chlorine plume dispersion model that can be used to adequately estimate exposure in subsequent etiologic epidemiologic studies.
2. Methods
2.1 Description of the exposure event
On January 6th, 2005 at 2:39 AM eastern standard time a freight train traveling through Graniteville, South Carolina at a speed of 76 km/hr was inadvertently diverted off the main line onto an industrial spur where it collided with a parked train. An incorrectly aligned switch was later determined by the National Transportation Safety Board (NTSB) as the culprit. Both locomotives and several railcars were derailed, including three railcars containing approximately 81,646 kg of pressurized liquid chlorine (Cl2) each (NTSB, 2005a). While all three railcars were damaged, the integrity of one of these cars was compromised, resulting in the release of approximately 55,000 kg of chlorine gas into the surrounding community (NTSB, 2005a; NTSB 2005b). Emergency personnel, including hundreds of volunteer firefighters from local and mutual aid departments along with state and federal hazardous materials and investigative assets, responded to Graniteville during the emergency period.
Graniteville is a textile mill town situated within a small river valley. The 2000 census population, most relevant to this paper, was 7,112 (U.S Census, 2000). The collision and release occurred on the industrial track of Avondale Mills, which was a major manufacturer of textiles and employer at the time. The chlorine release resulted in nine fatalities, over 550 people sought medical care in regional hospitals due to respiratory distress, and the evacuation of nearly 5,400 people living and working nearby during the 3-week clean-up period (NTSB, 2005a).
The 2005 Graniteville, South Carolina accidental release of chlorine following a railroad accident is a prime example of the devastating public health impacts that an incident of this magnitude can have on a community. The large-scale exposure to chlorine gas resulted in widespread human and animal fatality and morbidity. Current research projects studying the health implications of this exposure could be strongly advanced by using a validated model to estimate individual dosage. Here, as a first step in producing a validated model, we have modeled the Graniteville accidental release using two atmospheric dispersion models. The selection of these models is explained further in section 2.4.
2.2 Estimation of the Source Term
An essential first step in implementing such a model is to estimate the contaminant release rate and duration. Here, “release rate” is considered to be the flux of Cl2 through the plane defined as the open gash in the side of the tank car. Initially, the amount of Cl2 released as a function of time after the accident was estimated using an engineering analysis that combined semi-quantitative observations and fundamental principles. Our results were then compared with the findings of other investigators. Both this study and the comparison studies focused on the period of the highest Cl2 emission rate, beginning immediately after the accident occurred.
The rate of Cl2 loss from the tank car was a function of several constants, as well as time-varying, factors. The constants included: the location and orientation of the tank car after coming to rest, the location of the tear on the car, and the size and shape of the tear. The most fundamental time-varying factors were the amount of liquid Cl2 remaining in the tank and the temperature of the Cl2- knowing that these two allows determination of the fluids’ physical properties including vapor pressure, density, viscosity and heat capacity of both liquid-phase and gas-phase Cl2..
Information concerning the orientation of the car and of the size and shape of the tear in its side were obtained from measurements, pictures (NTSB, 2005a and 2005b) and interviews with emergency response personnel. These documents contain pictures and descriptions of the leaking tank car and the jagged, roughly vertical tear in the side of the car, and estimate of the amount of chlorine in the car at several times, and its temperature immediately after the accident. While many of these documents are publicly available, we were able to obtain the maximal amount of documentation using a Freedom of Information Act (FOIA) request. Taking into account the volume of Cl2 in the tank, the tank dimensions, and the angle of repose of the car after the accident, the upper end of the rupture was approximately 1.55 m below the initial vapor/liquid phase.
2.2.1 Background
The progression of events that characterize release of a compressed gas, such as Cl2, stored at a temperature less than or equal to its saturation temperature was described by Britter et al. (2011). Release from a rupture initially well below the liquid/gas interface and well above the bottom of the tank can take place in three consecutive stages. Stage 1 begins with the release of liquid Cl2. Inside the tank near the interface, Cl2 starts to boil and a two-phase, foamy layer consisting of bubbles of vapor/aerosol in the liquid forms. This two-phase layer swells, deepens, and descends. Stage 2 begins as the two-phase layer nears the rupture from above, and the release through the rupture becomes two-phase as well. The exact behavior as this material approaches and flows through the rupture is not very well understood as acknowledged by Britter et al. (2011), but some sophisticated models of this phenomenon have been developed such as the w-model (Leung J.C. 1986; Leung J.C. 1990) and the Homogeneous Equilibrium model (HEM) (Richardson S.M. et al. 2006).
Stage 3 occurs if release continues long enough to completely deplete the two-phase layer such that the release from the tank becomes all vapor. The mass flow rate decreases from Stage 1 to Stage 3 (Britter et al. 2011). Note that the transitions from stage to stage are often not distinct. Further complicating this is the formation of an aerosol phase.
For escape of a saturated liquid through a rupture in a container with a wall thickness equal to or less than 0.1 m, the flow approximates that of flow through a sharp-edged orifice. The flow is said to be “non-equilibrium” because the time required for the liquid to pass through the rupture is too short to allow equilibration to the new pressure condition until it is well outside the vessel; this is often referred to as “choked” flow. Here, the thickness of the tank wall at the site of the tear measured from 0.020 m (range= 0.000034 m) (NTSB, 2005b), indicating that thermodynamic equilibrium was not achieved upstream of the rupture during the initial phase of release.
Thus, the earliest release from the tank should have been liquid-phase Cl2. After release (outside the tank), this liquid jet would have abruptly approached an equilibrium condition at atmospheric pressure, causing “flashing” flow. This complex phenomenon results from the combined effects of rapid evaporation of Cl2, warming of the Cl2 jet due to the entrainment of ambient air, and cooling of the jet and the surrounding air through uptake of latent heat of evaporation. Condensed and frozen water was noted on the outside of the tank car near the rupture in photographs.
2.2.2 Estimation Methods
The methods used here to estimate the rate of Cl2 emission and the cumulative loss from the tank car were chosen to be appropriate for the ultimate goals of this research: to provide estimates of Cl2 exposure in Graniteville for participants in health effects studies, not to develop generally applicable approaches for determining pollutant emission, dispersion and transport. This analysis began by calculating the depth of liquid Cl2 in the car using a tilt angle of 10° reported by a SC Department of Health and Environmental Control (DHEC) responder, which agreed with the approximate angle given as 5-10° reported by the NTSB (2005a), the approximate orientation of the car shown in numerous pictures, and the volume of Cl2 initially present. Based upon NTSB (2005a) pictures, the tear on the side of car was a jagged gash very nearly perpendicular to the ground and 4.88 meters from the higher end of the car (the “A” end). The depth of liquid Cl2 was calculated as a function of the liquid-filled tank volume using a web-based program supplied by LMNO Engineering (2009). Then, to simplify computation further, a cubic equation was derived by regression (R2=0.9989) for directly computing the volume of Cl2 in the tank from its depth and vice versa. The next step was to compute the combined pressure resulting from the average head pressure of liquid along the tear and the vapor pressure in the enclosed space above the liquid. Using an equation for flow through a submerged opening (Perry and Green, 1984), we estimated that the time required for the liquid Cl2 level to reach the top of the vertical tear, using the approximation that the two-phase portion of the fluid does not have a significant effect on the mass release rate.
At the beginning of this stage one, the pressure in the Cl2 gas equaled the vapor pressure of chlorine at the tank temperature, estimated to be −3.3 degrees Celsius (26 F) (NTSB, 2005a), corresponding to a vapor pressure of 317 kPa (46 psia) (Occidental Chemical Corporation, 2000). An overview of the source term information we have used as inputs is shown in Table 1.
Table 1.
Modeling Inputs for Graniteville, SC Chlorine Release
| Release Parameters | |
|---|---|
| Location | Graniteville, SC, USA |
| Latitude: 33° 33′ 43.02″ N | |
| Longitude: 81° 48′ 29.99″ W | |
| Date and time | January 6, 2005 at 02:39 AM EST |
| Upstream pressure in tank (psig) | 46 |
| Temperature in tank (°C) | − 3.3 |
| Release height (m) | 1.0 |
| Area of opening (cm2) | 294 |
|
| |
| Weather/Environment (RAWS Data) | |
|
| |
| Weather conditions | Partly cloudy |
| Atmospheric stability | E |
| Wind speed/direction (m/s) | 1 – 3 SSW |
| Surface roughness (mm) | 100 |
|
| |
| Source Emissions | |
|
| |
| Discharge rate (kg/s) | 523 |
| Duration (s) | 105 |
| Total release (liquid + vapor) (kg) | 54,915 |
2.3 Weather Validation Methods
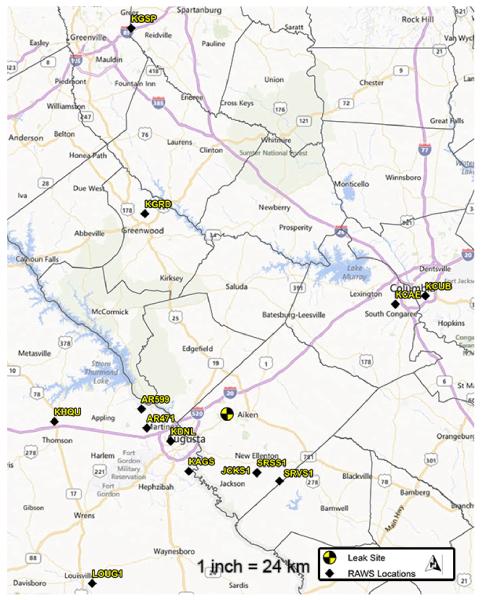
At the time of the incident, there was no on-site meteorological station in Graniteville to provide the most accurate weather data for input into the plume modeling software. Conventionally, in lieu of onsite observations, data from the National Weather Service (NWS) National Climatic Data Center (NCDC) is often used for planning, response and investigative modeling. NWS stations are generally located at airports or areas that serve as population centers. Alternatively, the U.S. Forest Service (USFS) operates Remote Automated Weather Stations (RAWS) which are generally self-sufficient stations located in national forests and similar areas for monitoring forest fires. Information from these mobile stations is transmitted electronically (often via satellite) every hour to the National Interagency Fire Center in Boise, Idaho. We have used archived meteorological surface observations recorded at 13 different RAWS sites collected during the night (and time of incident) on January 6th, 2005 (Figure 1).
Figure 1.
Locations of 13 RAWS sites utilized in weather validation study. Map was developed using ArcGIS ArcMap Version 10.0, Esri, Redlands, CA).
A portable weather station was setup at the incident site late in the morning of January 6th and remained there throughout the duration of cleanup activities. For purposes of validation, we compared archived observational data from the 13 different RAWS sites for five nights beginning on January 7th and ending on January 12th (January 10th was excluded due to missing data) with data from this portable site. We modeled the incident in HPAC using both the RAWS and portable weather station data (micro-environmental) collected on the aforementioned nights. Concentration and surface dosage were calculated at 1,024 points across a three by three mile grid with identical source terms and correlation, mean bias and root mean square error between the two models were calculated using IBM SPSS Statistics (Version 20, IBM, Armonk, NY). The goal of this validation was to support the notion that RAWS data collected during the night of January 6TH provided an accurate representation of the incident site micro-environment in Graniteville. It has been previously reported than an inversion layer may have been present during the morning of the accident which exaggerated the spread of chlorine gas (Buckley et. al, 2012). This inversion would have exasperated the spread of the gas over the surrounding terrain.
2.4 Description of the Models and Usage
The Graniteville incident was modeled in two un-classified versions of standard software packages using the above mentioned source term and meteorological data. First, we used the commonly available Areal Locations of Hazardous Atmospheres (ALOHA) model, which was jointly developed by the National Oceanic and Atmospheric Administration (NOAA) and U.S. Environmental Protection Agency (EPA). Second, we used the Hazard Prediction and Assessment Capability (HPAC, 5.2 Service Pack, DTRA, Ft. Belvoir, VA), developed by the Defense Threat Reduction Agency (DTRA), to incorporate increasingly accurate topographical and meteorological data in an effort to improve the predictive accuracy of our model before proceeding to validation studies. There were several reasons why we selected ALOHA and HPAC instead of other available plume modeling software. Due to its widespread distribution and ease of use, ALOHA may be the most common model in general use by initial responders, such as local fire departments. HPAC has been widely used in studies regarding both the Graniteville and other chlorine releases, and was also utilized by authorized emergency management entities during the response (Hannah et. al, 2008a, 2008b; Buckley et. al, 2007, 2012). We have avoided use of proprietary software because these models may not be in widespread use among those with critical public health and safety decision-making responsibility.
An updated and localized 30 km topographical file of the Graniteville area using the ArcMap geographical information system software (ArcMAP 10, ESRI, Redlands, CA) was utilized with HPAC while simulating the release. No terrain input was used with ALOHA because the standard (and most commonly available) version of the software assumes flat topography and does not accommodate the incorporation of diverse terrain (NOAA, 2013). All models estimated the outdoor atmospheric concentrations of Cl2.
The ALOHA system, part of the Computer-Aided Management of Emergency Operations (CAMEO) suite, is likely the most widely used atmospheric dispersion modeling program at the local level. We used ALOHA version 5.4.3 to model the Graniteville release. The software uses the Dense Gas Dispersion Model (DEGADIS) system to model Gaussian puff and plumes including dispersion of dense gas such as chlorine (NOAA, 1992). Outputs including threat zones can be displayed using the intrinsic CAMEO plotter (MARPLOT) or exported to ArcMap via extensions. The intrinsic CAMEO mapping software, MARPLOT, was used to illustrate the ALOHA output as 60-min Acute Exposure Guideline Level (AEGL) isopleths. Confidence lines were, also, shown on the map.
The HPAC system has several advantages over ALOHA, including the consideration of deposition and depleting effects such as terrain-induced gravity slumping. HPAC consists of several different modules that work together to determine source term, incorporate weather and topographical data, and calculate theoretical migration to develop a dispersion model. Although originally designed for use by the military, the HPAC system is now available to the authorized municipal and research community members and serves as a valuable tool for predicting the effects of hazardous materials releases into the atmosphere and its subsequent impact on a proximal population. The HPAC system uses a second-order closure transport/dispersion model, SCIPUFF, which is a Langrangian model utilizing multi-dimensional Gaussian distributions (DTRA, 2005). In association with a wind field model (SWIFT), the system can describe diffusion processes while allowing variances in concentration fields for purposes of measuring uncertainty. The transport/dispersion and wind field components of HPAC have been validated in the laboratory as well as field trials for short and long-range dispersion over various types of terrain and urban environments (Hanna et al, 2008; Warner et al, 2006; Warner et al, 2008).
3. Results
3.1 Source Term Calculation Results
Cl2 emission began immediately after the accident and continued at a high rate: 523 kg/s. The vapor pressure of Cl2 trapped above the liquid was the dominant force pushing the liquid out of the tank car. We calculated that the liquid level would have reached the top of the tear opening with release of 54,915 kg in about 105 seconds with gas alone slowly escaping afterwards. This represents a loss of nearly 2/3 of the original Cl2 in the tank car, in relatively good agreement with estimates based on the Cl2 remaining in the car at 4:00 PM on the day of the accident from SC DHEC and Norfolk Southern cleanup contractors (NTSB 2005c). The nearest National Weather Service monitoring station in Augusta, Georgia reported an initial ambient temperature of 55° F. Nevertheless, the condensation and ice on the surface of the tank shown in pictures taken on the morning of January 6, 2005 was to be expected given the high rate of Cl2 evaporation and resulting cooling due to the uptake of latent heat.
3.2 Comparison of RAWS and micro-environmental data
For the initial 60 minutes after the accident, cumulative surface dosage results from the HPAC models using RAWS surface observation data were similar to results using observations from a single portable meteorological station positioned near the leaking tank car. Concentration and surface dosage data from the RAWS HPAC plume dispersion model without measured micro-environmental weather data was significantly correlated with the model that included measured micro-environmental data (Table 2). There was overall good agreement between the two models with the exception being as we neared the 60-min averages for certain days. Yet, after calculating an overall strong correlation between the RAWS data and portable weather station, and, therefore, making the assumption that these data were representative of the microenvironment during the incident itself, we are confident that our usage of the RAWS data is suitable for our modeling purposes.
Table 2. Comparison between HPAC models with RAWS and Portable Station Data.
| January 7 | January 8 | January 9 | January 11 | January 12 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| R2 | Mean Bias |
RMSE | R2 | Mean Bias |
RMSE | R2 | Mean Bias |
RMSE | R2 | Mean Bias |
RMSE | R2 | Mean Bias |
RMSE | |
|
Surface Dosage:
| |||||||||||||||
| 60 min | 0.990 | 1.995 | 1.412 | 0.987 | 2.021 | 1.422 | 0.960 | 8.088 | 2.844 | 0.995 | 0.473 | 0.688 | 0.966 | 4.990 | 2.234 |
|
| |||||||||||||||
|
Concentration:
| |||||||||||||||
| 5 min | 0.997 | .000 | .000 | 0.971 | .000 | .001 | 0.936 | .000 | .001 | 0.991 | .000 | .001 | 0.979 | .000 | .001 |
| 15 min | 0.974 | .000 | .001 | 0.965 | .000 | .001 | 0.924 | .000 | .002 | 0.987 | .000 | .000 | 0.891 | .000 | .002 |
| 30 min | 0.975 | .000 | .001 | 0.973 | .000 | .000 | 0.893 | .000 | .001 | 0.991 | .000 | .000 | 0.957 | .000 | .001 |
| 45 min | 0.946 | .000 | .001 | 0.888 | .000 | .000 | 0.624 | .000 | .001 | 0.983 | .000 | .000 | 0.879 | .000 | .000 |
| 60 min | −0.136 | .000 | 7.71 × 10−7 |
0.677 | .000 | .000 | 0.440 | .000 | .000 | 0.977 | .000 | .000 | 0.284 | .000 | .000 |
Note: Statistical analyses shown above include correlation (R2), mean bias and root mean square deviation (RMSE).
3.3 ALOHA and HPAC Model Results
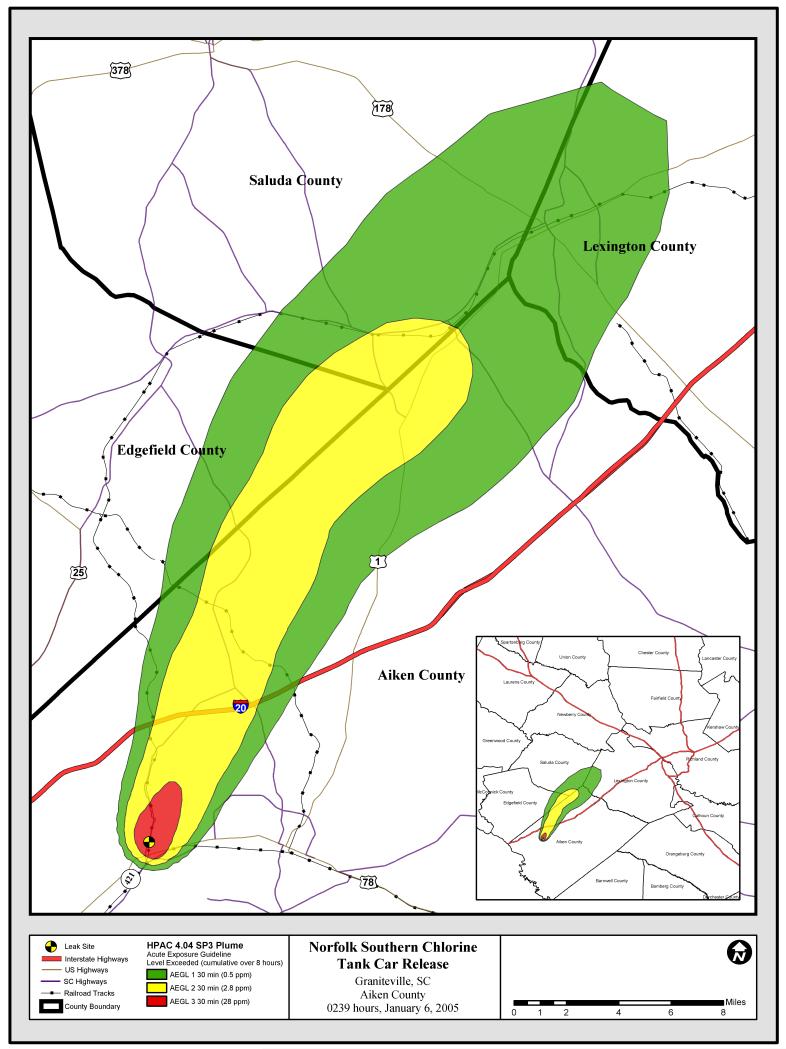
The ALOHA predicted 60-min average concentrations (Figure 2), as shown in Table 3, ranged from 156,000 ppm at a receptor 0.1 km downwind (x) to 10 ppm at a receptor 10 km downwind. We report these values in conservative 60-min averages as this is the standard in ALOHA. Unlike HPAC, the ALOHA system does not report values past 10 km downwind; however, manual calculation of very approximate concentrations is possible using the relevant mathematical modeling equations.
Figure 2.
ALOHA Model of Graniteville, SC chlorine release. Estimated 60-min AEGL isopleths and confidence lines are shown up to a maximum distance of 10 km downwind. AEGL-3 (red), 2 (yellow) and 1 (green) correspond to 20, 2 and 0.5 ppm, respectively.
Table 3. HPAC and ALOHA Model Results for Graniteville, SC Chlorine Release.
| HPAC (30-min) |
HPAC (60-min) |
ALOHA (60-min) |
|
|---|---|---|---|
|
Downwind distance
(km) |
ppm | ppm | ppm |
|
| |||
| 0.1 | 14,900 | 11,642 | 156,000 |
| 0.2 | 6,868 | 5,860 | 81,100 |
| 0.5 | 837 | 553 | 17,200 |
| 1.0 | 89 | 51 | 3,830 |
| 2.0 | 47 | 36 | 672 |
| 5.0 | 0 | 13 | 58.3 |
| 10.0 | 0 | 0.32 | 10 |
| 25.0 | 0 | 0 | N/A |
|
| |||
|
Downwind distance to
concentration |
km | km | km |
|
| |||
| 2,000 ppm | 0.45 (0.22 upwind) |
0.4 (0.18 upwind) |
1.31 - |
| 400 ppm | 0.69 (0.39 upwind) |
0.6 (0.28 upwind) |
2.43 - |
| 20 ppm | 2.6 (0.66 upwind) |
2.6 (0.5 upwind) |
7.57 - |
|
| |||
|
Maximum width to
concentration |
km | km | km |
|
| |||
| 2,000 ppm | 0.54 | 0.4 | - |
| 400 ppm | 0.8 | 0.7 | - |
| 20 ppm | 1.44 | 1.32 | - |
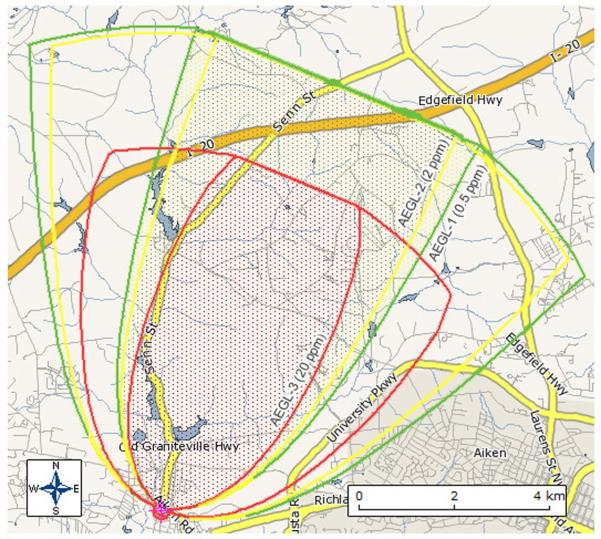
The HPAC output is shown as 30-min AEGL isopleths on an ArcMap GIS layer of the Graniteville incident area (Figure 3). On Table 3, we reported averaged centerline maximum concentrations in both 30-min and 60-min averages. For 30-min averages, these values ranged from 14,900 ppm at 0.1 km downwind to 0 ppm at 25 km downwind. 60-min averages ranged from 11,642 ppm at 0.1 km downwind to 0 ppm at 25.0 km downwind. These maximum 30-min and 60-min concentrations are also shown versus downwind distance graphically (Figure 4). Upwind dispersion up to 0.7 km due to gravitational slumping and maximum width to specific concentrations (2,000, 400, and 20 ppm) were reported for the HPAC model. Although it is possible to show variance in times shorter than 30 minutes, we opted to model 30 and 60-min outputs for comparative purposes to the more output-restricted ALOHA model.
Figure 3.
HPAC Model of Graniteville, SC chlorine release. Estimated 30-min AEGL isopleths are shown. AEGL-3 (red), 2 (yellow) and 1 (green) correspond to 28, 2.8 and 0.5 ppm, respectively.
Figure 4.
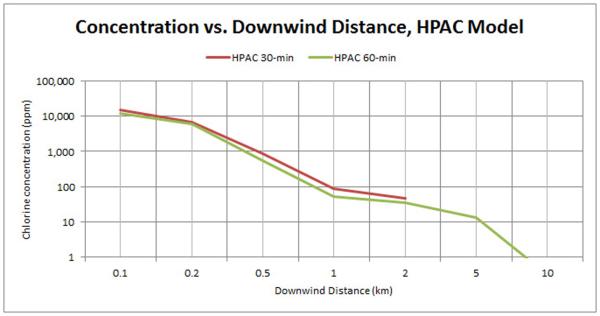
Concentration vs Downwind Distance for HPAC mode. Both 30-min and 60-min curves are shown for the maximum estimated concentration of chlorine at specific centerline distances downwind.
Discussion
Our purpose here has been to identify an atmospheric dispersion modeling system that is suitable for epidemiologic application to etiologic study of toxic inhalation hazard releases. ALOHA lacked the precision required to use it within epidemiological studies. Because it is designed to be used by a wide range of emergency management personnel, ALOHA has limited functionality compared to more “robust” systems such as HPAC. The ALOHA program is intended for use during the immediate response phase of a release and, therefore, sacrifices accuracy for increased speed and ease of use (NOAA, 2011). We opted to compare HPAC with ALOHA, as opposed to other dispersion modeling systems, because ALOHA may be the most widely known and utilized gas dispersion model. Our purpose here is to compare two “tiers” of modeling systems with the aforementioned input parameters, and to identify HPAC as the model of choice to continue our study with. However, a wide variety of models, many of which have been previously compared by Hanna and others, are in use throughout public applications, industry and academia.
The behavior of a dense gas is highly influenced by the geography surrounding the release site, and the failure to account for this terrain leads to concentration estimates which are skewed from reality. Indeed, flat terrain models have been shown to overestimate the range of hazard by nearly a factor of five and direction of migration by up to 90 degrees (McBride et al, 2001). Further, most models like ALOHA which are available freely in the public domain do not consider major removal mechanisms, such as deposition and depletion. These factors are particularly important for a dense gas like chlorine which tends to hug the ground. Furthermore, the flat terrain models tend to incorporate linear removal which is not particularly representative of reality. As a consequence, near-fatal exposures are often approximated beyond distances at which human health impacts are reported. The ALOHA output is suitable for its primary intention, that is, the immediate modeling of an incident by emergency responders for purposes of response. Yet, it is unsuitable as a model for us to base future epidemiologic study on due the relative “fixed” nature of the system as input type and variability is concerned and highly conservative estimated concentration outputs. For this, HPAC is far more suitable because of its ability to incorporate mesoscale wind variations with more advanced algorithms among other improvements. It is for these reasons that we have chosen to proceed with the HPAC system model as a basis for our future epidemiologic studies.
Although HPAC represents a significant advantage over models such as ALOHA, other robust options exist. As an example, the National Atmospheric Release Advisory Center (NARAC) at the Lawrence Livermore National Laboratory (LLNL) in California provides national support to emergency managers for purposes of planning and real-time assessment of chemical, radiological, biological and other similar events. NARAC utilizes the ADAPT/LODI system, which consists of a robust meteorological data assimilation component (ADAPT) and dispersion modeling component (LODI) which considers turbulence, chemical reactions, wet and dry ground deposition, settling due to density and plume rise (NARAC, 2013). Coupled with extensive databases on the properties of agents such as chlorine, accurate topographical data and real-time meteorological observations, NARAC is a critical resource for emergency planning and response. However, unlike HPAC, the advanced methodologies of the NARAC group are available by request only and cannot be performed by unauthorized independent agencies and organizations for research purposes. A 2001 study by LLNL researchers concluded that HPAC and NARAC predictions for a series of simulated scenarios had overall favorable agreement (Warner et al. 2001). We have chosen to proceed with HPAC because it is the most accurate modeling system available to authorized academic researchers. Further, DTRA provides Interagency Modeling & Atmospheric Assessment Center (IMAAC) support through its Technical Reachback unit.
A few papers have been published estimating the Graniteville release rates for purposes different from those of this study. Buckley et al. (2007) described the results of analyses performed by the Savannah River National Laboratory (SRNL) provided in support of the Graniteville emergency response efforts, as well as some more detailed atmospheric transport calculations including HPAC model results in the first 2-3 hours after the accident. They assumed that >62,000 kg of Cl2 was released instantaneously as vapor and aerosol phases. A more comprehensive follow-up analysis on the Graniteville Cl2 release by SRNL covering the same 3-hour time period has also been published (Buckley et al. 2012); it included the estimated emission rate, meteorology, dispersion, as well as the fate and the effects of the chlorine released. In their research, the tear was represented by a 0.8 m long by 0.08 m wide opening extending “just above the mid-point of the tank.” This does agree with our findings. The emission rate was determined using ALOHA code, and the Industrial Transportation (ITRANS) component of the HPAC program. This resulted in a total estimated emission of 59,000 kg, most occurring in the first minute after the accident with the total discharge requiring 5 minutes. They pointed out that ITRANS will not accept a detailed specification of the damage to the tank. The time required for discharge was adjusted using SCIPUFF to achieve better agreement with a short release duration implied by Hanna et al (2008) and Britter (2010). Thus, Buckley et al (2012) concluded that vapor and aerosol release, 59% of the total, was essentially complete after the 3 minutes, while the remaining 41%, a subcooled liquid release, was assumed to have pooled on the ground and then evaporated.
Hanna et al (2008) compared three dense gas dispersion models for several chlorine railcar accidents, including the Graniteville accident. For Graniteville, the duration of source emissions was determined using the PHAST and TRACE models along with a thermodynamic analysis, and the vertical slash in the side of the rail car was represented by a 4-5 inch hole. Based upon the Cl2 release of rate of 1,565 kg/s, they estimated that a two-phase release of liquid and vapor lasted for 34 s, for a discharge of 53,210 kg; the vapor phase release was estimated to continue at the rate of 0.2 kg/s for 3,600 s for an additional discharge of 720 kg. One major reason our results are different is because the Hanna paper, for purposes of comparison between SCIPUFF and other models, assumed flat terrain and utilize a slightly different source term. This was primarily due to the inability of some of the models being compared to incorporate topography (such as ALOHA). The variability between our model and that aforementioned is a prime example of the impact that migration of a heavy gas through complex terrain has on predicted upwind concentrations.
This study also demonstrates the limits of using dispersion modeling for guiding emergency response to accidental releases of compressed, acutely toxic dense gases. Here, 2/3 of a railroad tank car of chlorine was emitted in about 105 s. This is insufficient time to prevent exposure and, in many cases, to prevent death and disabling injury, even when a community is extremely well-prepared. The approach employed here can be of significant value for post-event evaluation, or for response in scenarios with a slower release or for less acutely toxic contaminants. Many modeling systems are incapable of producing exposure estimates in time intervals less than 30 or 60 minutes, or incorporating estimate variability. For purposes of epidemiology, this is highly problematic for the reason mentioned above: many rapid relief incidents occur in minutes. The ability of HPAC to output estimates in short intervals – minutes – is another advantage over ALOHA for our purposes.
Conclusion & Future Directions
Out of our two selected models studied, HPAC is likely the best candidate for use as a model system on which future human epidemiologic studies could be based. HPAC was selected over ALOHA because the system accounts for dynamic plume rise and to a greater degree the dense gas effects, time and space-dependent boundary layers, and mesoscale wind variations over complex terrain. Although the focus of our model development is entirely emergency response, the HPAC modeling system does represent significant improvement over the common ALOHA system. This increased predictive accuracy can have profound impacts during response to hazardous chemical releases. It is recommendable for jurisdictions without access or training on software such as HPAC to fortify mutual aid collaborations with agencies who utilize higher modeling systems.
Yet, there is intrinsic bias in any simulation and performance can be improved (or confirmed) by field studies. Further verification of our model is necessary before epidemiologic application. In other words, to ensure predictive accuracy, a model must be validated using observed indicators of exposure. The next step in improving plume model accuracy is to compare the model to exposure indicator data collected in the aftermath of the incident. Eventually, the resulting validated plume dispersion model could be used to estimate personal exposures for etiologic environmental epidemiology studies. The resulting models presented herein provide preliminary estimates only of outdoor exposure concentrations and should not be interpreted to represent the exact chlorine plume model after the Graniteville exposure event. Further validation studies are needed before individual exposure estimates can be reliably made and the chlorine plume more definitively modeled.
ACKNOWLEDGEMENTS
We acknowledge Ronald G. Meris, Chief of the Reachback Analysis Branch of the Defense Threat Reduction Agency (DTRA), for his scientific review and helpful commentary on this manuscript.
REFERENCES
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Chlorine. 2010 Retrieved January 15, 2013, from http://www.atsdr.cdc.gov/toxprofiles/tp172.pdf. [PubMed]
- Association of American Railroads HazMat Transport by Rail. 2007 Retrieved January 15,2013, from http://www.aar.org.
- Binder RC. Flow Measurement. In: Streeter VL, editor. Handbook for Fluid Dynamics. McGraw-Hill Book Co; New York, NY: 1961. Chap. 14. [Google Scholar]
- Board on Atmospheric Sciences and Climate (BASC) Tracking and Predicting the Atmospheric Dispersion of Hazardous Material Releases: Implications for Homeland Security. The National Academies Press; Washington D.C: 2003. [Google Scholar]
- Britter R, Jeffrey W, Leung J, Hanna S. Toxic Industrial Chemical (TIC) Source Emissions Modeling for Pressurized Liquified Gases. Atmospheric Environment. 2011;45:1–25. [Google Scholar]
- Buckley RL, Hunter CH, Addis RP, Parker MJ. Modeling Dispersion from Toxic Gas Released after a Train Collision in Graniteville, SC. Journal of the Air & Waste Management Association. 2007;57:268–278. doi: 10.1080/10473289.2007.10465329. [DOI] [PubMed] [Google Scholar]
- Buckley Robert L., Hunter Charles H., Werth David W., Whiteside Morgana T., Chen Kuo-Fu., Mazzola Carl A. A case study of chlorine transport and fate following a large accidental release. Atmospheric Environment. 2012;62:184–198. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Chlorine. 2005 Retrieved January 15, 2013, http://www.bt.cdc.gov/agent/chlorine/
- Cave D, Fadam A. Iraqi Militants Use Chlorine in 3 Bombings. The New York Times. 2007 Feb 21; Retrieved January 15, 2013, from http://www.nytimes.com.
- Defense Threat Reduction Agency (DTRA) Hazard Prediction & Assessment Capability 4.04 Manual. Fort Belvoir, VA: 2005. [Google Scholar]
- Farago I, Georgiev K, Havasi A. Proceedings of the NATO Advanced Research Workshop Advances in Air Pollution Modeling for Environmental Security: Vol. 54. NATO Science Series. Springer Journals; Borovetz, Bulgaria: 2005. Advances in Air Pollution Modeling for Environmental Security; p. 406. [Google Scholar]
- Google Inc Google Earth [Software] 2013 Available from http://www.google.com/earth. [Google Scholar]
- Hanna S, Briggs G, Hosker R. Handbook on Atmospheric Diffusion. USA: 1982. DOE/TIC-11223. [Google Scholar]
- Hanna S, Chang J, White J, Bowers J. Evaluation of the Hazard Prediction and Assessment Capability (HPAC) Model with the Oklahoma City Joint Urban 2003 (JU2003) Tracer Observations. Air Pollution Modeling and Its Application XIX. 2008a:63–71. doi:10.1007/978-1-4020-8453-9_7. [Google Scholar]
- Hanna S, Dharmavaram S, Zhang J, Sykes I, Witlox H, Khajehnajafi S, Koslan K. Comparison of Six Widely-Used Dense Gas Dispersion Models for Three Recent Chlorine Railcar Accidents. Process Safety Progress. 2008b;27(3):248–259. doi:10.1002/prs.10257. [Google Scholar]
- The Implementation of the Chemical Facility Anti-Terrorism Standard and the Road Ahead: statement before the Subcommittee on Transportation Security and Infrastructure Protection, of the House Committee on Homeland Security; 110th Cong.; 2007; statement for the record of the American Association of Railroads. [Google Scholar]
- LMNO . Inclined Cylinder Volume Calculation. LMNO Engineering, Research, and Software, Ltd.; [Accessed on April 18, 2009]. www.lmnoeng.com/Volume/InclinedCyl.htm. [Google Scholar]
- Jones R, Wills B, Kang C. Chlorine Gas: An Evolving Hazardous Material Threat and Unconventional Weapon. Western Journal of Emergency Medicine. 2010;11(2):151–156. [PMC free article] [PubMed] [Google Scholar]
- Murdock JW. Mechanics of Fluids. In: Avallone EA, Baumeister T, Sadegh A, Marks LS, editors. Mark’s Standard Handbook for Mechanical Engineers. 11th Ed. McGraw-Hill Professional; New York, NY: 2006. Chap 3.3. [Google Scholar]
- Mcbride MA, Reeves AB, Vanderheyden MD, Lea CJ, Zhou XX. Use of Advanced Techniques to Model the Dispersion of Chlorine in Complex Terrain. Process Safety and Environmental Protection. 2001;79(2):89–102. doi:10.1305/09575820151095175. [Google Scholar]
- National Atmospheric Release Advisory Center (NARAC) NARAC. 2011 Retrieved January 15, 2013, from https://narac.llnl.gov/
- National Oceanic and Atmospheric Administration (NOAA) ALOHA 5.0 Theoretical Description. NOAA Hazardous Materials Response and Assessment Division; Seattle, Washington: 1992. [Google Scholar]
- NOAA Air Resources Laboratory Field Research Division (FRD) Joint Urban 2003 Tracer Field Tests. 2013 Retrieved January 15, 2013 from http://www.noaa.inel.gov/projects/ju03/ju03.htm.
- NOAA Office of Response and Restoration ALOHA. 2013 Retrieved January 15, 2013, from http://response.restoration.noaa.gov/oil-and-chemical-spills/chemical-spills/response-tools/aloha.html.
- NTSB Collision of Norfolk Southern Freight Train 192 With Standing Norfolk Southern Local Train P22 With Subsequent Hazardous Materials Release at Graniteville, South Carolina. 2005a Railroad Accident Report NTSB/RAR-05/04. PB2005-916304. Notation 7710A. Adopted November 29, 2005. January 6, 2005. [Google Scholar]
- NTSB . Materials Laboratory Draft Factual Report. National Transportation Safety Board. Material Laboratory Division; Washington, DC: 2005b. Report No. 05-071. September 8, 2005. [Google Scholar]
- NTSB . Hazardous Materials Group Factual Timeline. Graniteville, SC: 2005c. Appendix N. DCA-05-MR-008. [Google Scholar]
- Occidental Chemical Corporation . OxyChem Chlorine Handbook. Occidental Chemical Corporation; Dallas, TX: 2000. [Google Scholar]
- Warner S, Heagy JF, Platt N, Larson D, Sugiyama G, Nasstrom JS, Foster KT, Bradley S, Bieberbach G. Evaluation of Transport and Dispersion Models: A Controlled Comparison of HPAC and NARAC Predictions. Lawrence Livermore National Laboratory; Livermore, CA: 2001. Retrieved January 14, 2013, from https://e-reports-ext.llnl.gov/pdf/240464.pdf. [Google Scholar]
- Warner S, Platt N, Heagy JF, Jordan JE, Bieberbach G. Comparisons of Transport and Dispersion Model Predictions of the Mock Urban Setting Test Field Experiment. Journal of Applied Meteorology and Climatology. 2006;45:1414–1428. [Google Scholar]
- Warner S, Platt N, Urban JT, Heagy JF. Comparisons of Transport and Dispersion Model Predictions of the Joint Urban 2003 Field Experiment. Journal of Applied Meteorology and Climatology. 2008;47:1910–1928. [Google Scholar]
- Zou B, Wilson J, Zhan B, Yongnian Z. Air pollution exposure assessment methods utilized in epidemiological studies. Journal of Environmental Monitoring. 2009;(11):475–490. doi: 10.1039/b813889c. [DOI] [PubMed] [Google Scholar]
- Murdock JW. Mechanics of Fluids. In: Avallone EA, Baumeister T, Sadegh A, Marks LS, editors. Mark’s Standard Handbook for Mechanical Engineers. 11th Ed. McGraw-Hill Professional; New York, NY: 2006. Chap 3.3. [Google Scholar]
- Perry RH, Green DW. Perry’s Chemical Engineers’ Handbook. 6th Ed. McGraw-Hill Book Co; New York, NY: 1984. [Google Scholar]