Abstract
The processes associated with transition to castration independent prostate cancer growth are not well understood. Cellular senescence is a stable cell cycle arrest that occurs in response to sublethal stress. It is often overcome in malignant transformation to confer a survival advantage. CCAAT/Enhancer Binding Protein (C/EBP) β function is frequently deregulated in human malignancies and interestingly, androgen dependent prostate cancer cells express primarily the LIP isoform. We found that C/EBPβ expression is negatively regulated by androgen receptor activity and that treatment of androgen dependent cell lines with anti-androgens increases C/EBPβ mRNA and protein levels. Accordingly, we also find that C/EBPβ levels are significantly elevated in primary prostate cancer samples from castration resistant compared with therapy naive patients. Chromatin immunoprecipitation demonstrated enhanced binding of the androgen receptor to the proximal promoter of the CEBPB gene in the presence of dihydroxytestosterone. Upon androgen deprivation, induction of C/EBPβ is facilitated by active transcription as evident by increased histone 3 acetylation at the C/EBPβ promoter. Also, the androgen agonist R1881 suppresses the activity of a CEBPB promoter reporter. Loss of C/EBPβ expression prevents growth arrest following androgen deprivation or anti-androgen challenge. Accordingly, suppression of C/EBPβ under low androgen conditions results in reduced expression of senescence-associated secretory genes, significantly decreased number of cells displaying heterochromatin foci, and increased numbers of Ki67 positive cells. Ectopic expression of C/EBPβ caused pronounced morphological changes, reduced PC cell growth, and increased the number of senescent LNCaP cells. Lastly, we found that senescence contributes to prostate cancer cell survival under androgen deprivation, and C/EBPβ deficient cells were significantly more susceptible to killing by cytotoxic chemotherapy following androgen deprivation. Our data demonstrate that up-regulation of C/EBPβ is critical for complete maintenance of androgen deprivation induced senescence and that targeting C/EBPβ expression may synergize with anti-androgen or chemotherapy in eradicating prostate cancer.
Keywords: C/EBPβ, androgen dependent prostate cancer, senescence
Introduction
Prostate cancer (PC) is the most prevalent malignancy in adult men in the United States.1 Although early detection and treatment of localized disease is often curative, PC remains a leading cause of cancer death. Anti-androgen therapy is the most effective approach in patients with advanced disease and induces significant responses in almost all patients.2 However, androgen deprivation achieved by pharmacologic or surgical castration results in only limited apoptosis of tumor cells 3, 4 and accordingly only partial tumor regression. Indeed after a period of disease control most patients develop castration resistant growth and PC progression, which is responsible for the majority of the morbidity and mortality associated with this disease.2 Identifying mechanisms that engender castration resistance is crucial for the design of future therapeutic strategies. Progress has been made understanding the mechanisms associated with eventual emergence of castration resistant prostate cancer (CRPC). However, less is known about the early adaptation associated with androgen deprivation.5-7
Members of the CCAAT/enhancer binding protein (C/EBP) family of transcription factors are characterized by a conserved C-terminus, which contains both a DNA-binding basic region (BR) and leucine-zipper (LZ), collectively referred to as the bZIP domain. C/EBPβ is a widely expressed transcription factor that promotes proliferation or terminal differentiation and growth arrest in several different cell types.8 These opposing functions seem to be regulated by the expression of different C/EBPβ translational isoforms from three in frame start codons within an intron-less mRNA.9, 10 The two high molecular weight C/EBPβ isoforms, termed liver-enriched activating proteins (LAP and LAP*), contain N-terminal transactivation domains (TAD), whereas the liver-enriched inhibitory protein (LIP) lacks these TADs. LIP can dominantly inhibit LAPs and other C/EBP members via heterodimerization or by recruiting transcriptional repressors.11 C/EBPβ activity affects several facets of PC disease progression. C/EBPβ regulates the expression of steroidogenic genes including StAR and cytochrome p450 aromatase,12, 13 and its activity is modulated in response to dihydrotestosterone, estrogen and progesterone.14-17 It has also been suggested that C/EBPβ can act as a co-repressor of the androgen receptor (AR) in PC.18, 19 Although C/EBPβ is not detected in healthy prostate, luminal epithelial cells up-regulate C/EBPβ in the case of proliferative-inflammatory atrophy, a precursor to PC, 20 and C/EBPβ participates in the regulation of metastatic genes and PC cell survival.21, 22 However, the contribution of C/EBPβ to the emergence of castration resistant growth has not been previously investigated.
Cellular senescence is a stable cell cycle arrest that occurs in response to a variety of intrinsic and extrinsic sublethal stress stimuli.23, 24 Accumulating data point to an important role of senescence in cancer progression.23, 24 Recently, several groups demonstrated that in response to androgen deprivation PC cells undergo senescence, and that the acquisition of senescence is associated with emergence of castrate resistant growth.5-7 In other lineages C/EBPβ and its downstream target genes are critical for the induction and maintenance of oncogene induced senescence, associated with over-expression of activated Ras or BRAF.8, 25, 26 C/EBPβ can directly bind to target gene promoters and enhancers to induce senescence-associate factors IL-6 and IL-8, but can also suppress E2F-1 target genes and induce growth arrest dependent on E2F:pRb.8
We now demonstrate that upon androgen deprivation C/EBPβ is rapidly up-regulated in androgen dependent PC cells and that AR binds to and suppresses the C/EBPβ proximal promoter. Increased expression of C/EBPβ under these conditions is necessary for acquisition of the senescent phenotype. Accordingly, preventing C/EBPβ up-regulation increases the susceptibility of PC cells to apoptosis induced by chemotherapy.
Results
CRPC is associated with increased CEBPB
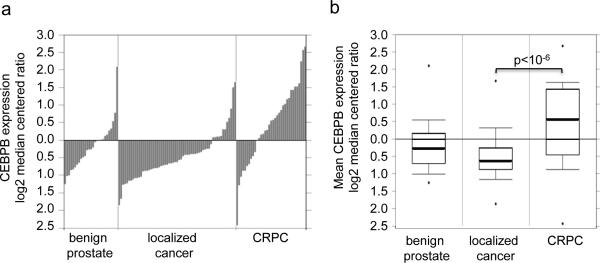
To determine whether C/EBPβ levels correlate with human prostate cancer progression we interrogated the Oncomine database.27 In the Grasso et al data set 28 that included gene expression patterns from 28 benign prostate tissues, 59 localized PC, and 35 CRPC samples CEBPB expression was significantly (p<1.9 × 10−6) elevated in CRPC compared with localized disease (Figures 1a, 1b).
Figure 1.
C/EBPβ expression increases in castration resistant prostate cancer. Individual patient (a) and mean (b) CEBPB expression as log2 median centered ratio for benign prostate, localized prostate cancer, and castration resistant prostate cancer (CRPC).
Inhibition of AR induces CEBPB transcription
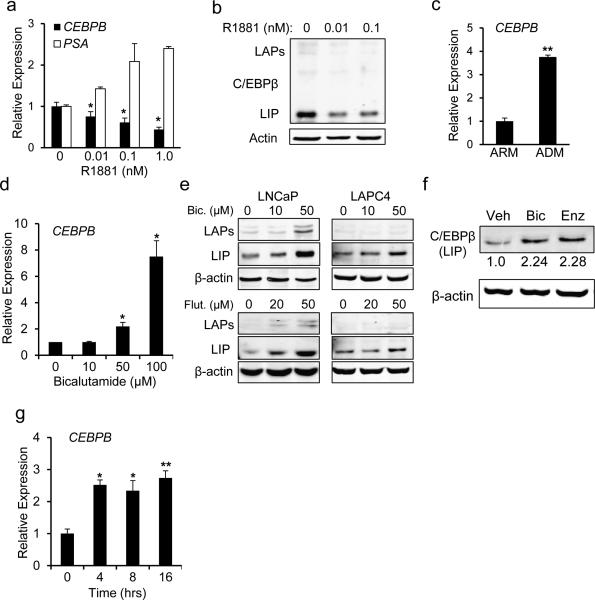
Treatment of LNCaP cells with the synthetic AR agonist R1881 for 24 hrs results in a dose-dependent 2.5-fold decrease in CEBPB mRNA and protein expression (Figures 2a, 2b), and as expected, prostate specific antigen (PSA) transcript levels increased under these conditions. Conversely, culturing LNCaP cells in androgen depleted media (ADM) for 7 days resulted in a significant 3.8-fold increase in C/EBPβ expression (Figure 2c). Pharmacologic inhibition of the AR using bicalutamide resulted in a dose dependent rise in CEBPB transcript abundance, achieving a 7.5-fold increase at the highest dose tested (Figure 2d). Accordingly, we detected increased protein levels of C/EBPβ in both LNCaP and LAPC4 cells treated with bicalutamide, or flutamide (Figure 2e). Since bicalutamide, or flutamide may have an AR agonist effect we also tested the effect of enzalutamide, which does not have agonistic effects. Similar to bicalutamide, incubation with 20 μM enzalutamide resulted in increased C/EBPβ levels (Figure 2f). CEBPB RNA levels were rapidly up-regulated within 4 hrs of exposure of LNCaP cells to bicalutamide (Figure 2g).
Figure 2.
C/EBPβ expression is regulated by AR activity in prostate cancer cell lines. LNCaP cells were cultured in the indicated concentrations of R1881 for 24 hrs and RNA (a) or protein (b) were analyzed for the expression of the indicated gene products. (c) LNCaP cells were cultured in androgen replete (ARM) or androgen depleted (ADM) media for 9 days and CEBPB RNA levels analyzed. Mean and SD from 3 independent experiments are shown. (d) CEBPB transcripts levels in LNCaP cells cultured with the indicated concentration of bicalutamide were measured in 3 independent experiments using qRT-PCR. (e) LNCaP or LAPC4 cells were cultured with the indicated doses of bicalutamide (Bic), flutamide (Flut), or (f) enzalutamide (enz) for 24 hours and the cell lysates were subjected to Western blotting. Representative gels with relative band intensity values are shown. (g) LNCaP cells were exposed to bicalutamide at 50 μM and C/EBPβ expression was assessed at the indicated time points. The average normalized CEBPB transcripts levels from 3 independent experiments are shown. *-p<0.01, **- p<0.001
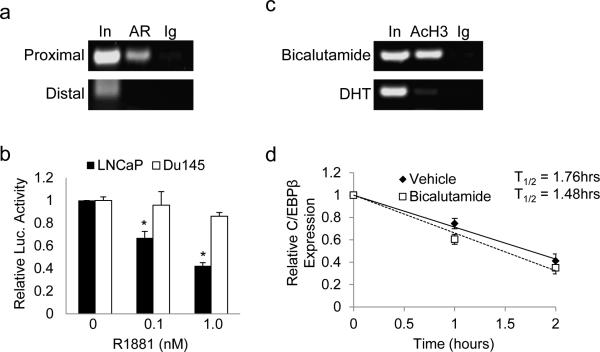
To assess binding of AR to the CEBPB promoter, LNCaP cells were cultured in full media and subjected to chromatin immunoprecipitation (ChIP) analysis. Precipitated DNA was amplified using primers spanning the proximal (-131 to -242 bp) or distal (-2098 to -1983 bp) regions of the human CEBPB promoter. We observed AR binding to the proximal but not the distal region (Figure 3a). Next, CEBPB-luc, containing proximal promoter region (−888 to +64) linked to a luciferase reporter, was co-transfected into LNCaP or DU145 PC cells with CMV-β-galactosidase as internal control. Reproducibly, luciferase activity significantly decreased by 2.5-fold in LNCaP cells cultured with 1 nM R1881 for 24 hrs compared with vehicle control (Figure 3b). This effect on CEBPB promoter activation was mediated by the AR as R1881 did not reduce luciferase activity in similarly transfected DU145 cells which lack AR. Treatment of LNCaP cells with bicalutamide for 4 hours induced acetylation of histone H3 bound to the CEBPB proximal promoter, whereas culture with dihydrotestosterone (DHT) suppressed this mark of active transcription (Figure 3c). Importantly, we did not observe significant changes in the half-life of CEBPB RNA in response to bicalutamide, indicating that the stability of CEBPB transcripts was unaffected (Figure 3d). Collectively, these results show that the AR suppresses transcription of CEBPB.
Figure 3.
AR binds and represses the CEBPB gene. (a) LNCaP cells were subjected to ChIP analysis with antibodies against androgen receptor (AR) or normal rabbit IgG (Ig). PCR was used to amplify DNA fragments centered at -187 bp (proximal) or an upstream fragment centered at -2040 bp (distal) of the CEBPB gene. (b) LNCaP or DU145 cells were transiently transfected with CEBPB-Luc and cultured with vehicle or R1881 at the indicated dose. Fold activation of the reporter relative to vehicle treated cells was determined after normalization to β-galactosidase activity. The average from 3 independent experiments is presented (c) LNCaP cells were cultured in the presence of bicalutamide (50 μM) or dihydroxytestosterone (DHT) (10 nM) for 4 hrs and subjected to ChIP using antibodies against acetylated histone 3 (AcH3) or normal rabbit IgG (Ig). A representative gel of PCR amplified proximal promoter DNA fragment is shown. (d) LNCaP cells were cultured in the presence of vehicle or bicalutamide (50 μM) and after 4 hrs actinomycin D (5 μg/ml) was added to the media. Total cellular RNA was harvested at the indicated time points, reverse transcribed to cDNA and relative CEBPB transcript levels were measured using qRT-PCR. The slope of a linear best-fit line for each group was determined to calculate the CEBPB RNA half-life, based on three repetitions.
Ectopic expression of C/EBPβ suppresses LNCaP cell growth
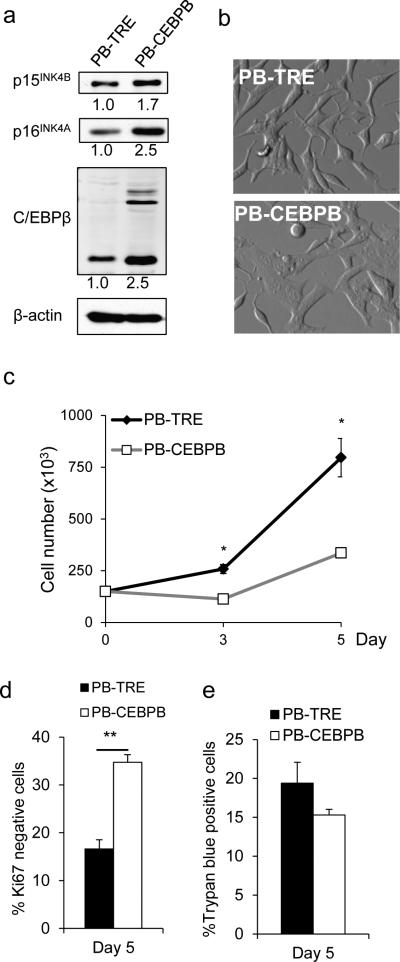
Because C/EBPβ expression was inversely regulated by AR activity, an essential signal for PC cell growth, we next evaluated whether ectopic expression induces growth arrest similar to androgen deprivation. C/EBPβ was inducibly expressed in LNCaP cells using the Tet-regulated transposon-based piggyBac vector 29 and cells with stable integration of the transgene or an empty vector (TRE) control were selected by puromycin (2 mg/ml). Treatment of LNCaP PBCEBPB cells with doxycycline for 5 days induced an increase in the expression of C/EBPβ 2.5-fold (Figure 4a), similar to the increase seen in LNCaP cells cultured in enzalutamide. C/EBPβ forced expression was associated with increased levels of the cell cycle inhibitors p16INK4A and p15INK4B and a flattened morphology (Figure 4a, 4b). Next, we tested the effect of C/EBPβ overexpression on LNCaP cell proliferation. Equal numbers of LNCaP PB-CEBPB or PB-TRE control cells were seeded in doxycycline containing media and enumerated after 3 and 5 days. Ectopic C/EBPβ expression resulted in a significant 2.3-fold decreased rate of proliferation (figure 4c) and a significantly increased number of Ki67 negative cells 5 days after doxycycline treatment (figure 4d) without increased cell death (figure 4e). These data demonstrate that elevated levels of C/EBPβ are sufficient to suppress the growth of androgen-sensitive PC cells.
Figure 4.
Ectopic expression of C/EBPβ suppresses LNCaP cell growth. (a) LNCaP PB-CEBPB and PB-TRE control cells were cultured for 5 days in doxycycline 0.5 μg/ml. Cell lysates were obtained and a representative Western blot analysis for C/EBPβ, p15INK4B and p16INK4A is shown. Numbers below the blots indicate the relative band density. (b) Phase-contrast images of LNCaP PB-TRE and PB-CEBPB cells after 5 days in culture with 0.5 μg/ml doxycycline. (c) 1.5E5 LNCaP PB-CEBPB or PB-TRE cells were seeded in media containing 0.5 μg/ml doxycycline. Cells were enumerated on day 3 and 5. (d) Cells were cultured under similar conditions and the Ki67 expression was analyzed after five days of doxycycline treatment. (e) The number of dead cells on day 5 was assessed by trypan blue exclusion. All graphs represent the average of three experiments, error bars: SEM; * p<0.01; ** p<0.02.
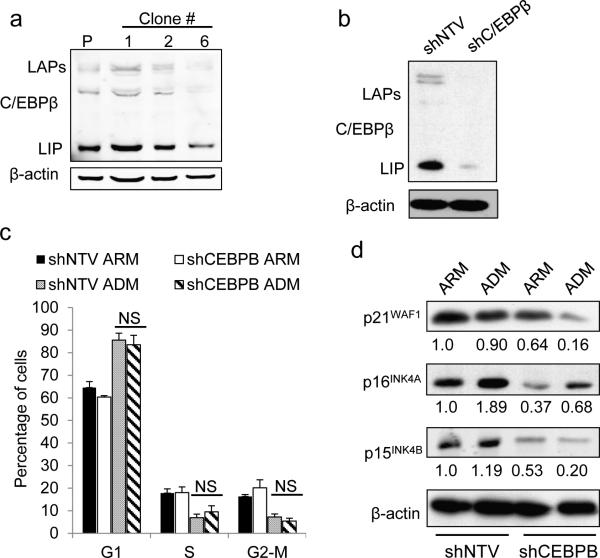
C/EBPβ is required for complete maintenance of androgen deprivation induced growth arrest Persistence of PC cells under androgen-deprived conditions is an initial step towards development of castration resistant growth. Upon androgen deprivation LNCaP cells undergo cell cycle arrest and enter a senescent state.5-7 Importantly, androgen deprivation-induced senescence is only partly reversible and cells continue to display senescence markers and poor proliferation after re-exposure to androgen.6 Because C/EBPβ upregulation was associated with androgen deprivation and its expression sufficient to suppress LNCaP proliferation, we next targeted C/EBPβ to test its function in androgen-deprivation-induced growth arrest. We utilized two independent methods: inducible shRNA and transcription activator-like effector nucleases (TALENs).30, 31 We designed a pair of TALENs to target the CEBPB gene, transfected LNCaP cells, and screened individual clones for C/EBPβ expression. Subclone 6, was identified as having CEBPB knockdown (presumably due to incomplete targeting of all alleles in the polyploid LNCaP cells) (Figure 5a), and used in our subsequent experiments. A complete stable deletion of all CEBPB alleles could not be achieved, suggesting C/EBPβ plays a critical role for cell survival. As control we employed subclone 1 in which C/EBPβ expression is similar to that observed in parental cells. In addition, C/EBPβ expression was effectively knocked down in LNCaP cells utilizing a doxycycline inducible shRNA against CEBPB (shCEBPB) compared with a non-targeting vector control (shNTV) (Figure 5b). We used flow cytometry to investigate the effect of C/EBPβ depletion on PC cell cycle distribution (Figure 5c). For these studies we employed cells expressing the inducible shRNA as stable deletion of C/EBPβ via TALEN required adaptation to low C/EBPβ levels. Cells expressing shCEBPB or shNTV both went into G1 cell cycle arrest when cultured in androgen depleted media (ADM) (Figure 5c).
Figure 5.
C/EBPβ is required for high expression levels of cell cycle inhibitors upon AR inhibition. (a) Total cellular proteins extracted from parental (P) LNCaP cells or individual clones transfected with TALEN-C/EBPβ were subjected to Western blotting using C/EBPβ or actin antibodies. (b) LNCaP cells harboring an inducible shRNA against C/EBPβ (shC/EBPβ) or non-targeting vector (NTV) were cultured with doxycycline (200 ng/ml) for 48 hrs and protein extracts were subjected to immunoblotting with the indicated antibodies. (c) LNCaP cells harboring shCEBPB or non-targeting vector (shNTV) cultured with doxycycline (200 ng/ml) in androgen replete (ARM) or androgen depleted media (ADM) for 7 days, were stained with propidium iodide and DNA content was analyzed by flow cytometry. Mean distribution of cells in G1, S, or G2/M from three experiments. (d) LNCaP cells expressing shNTV or shCEBPB were cultured in ARM or ADM for 7 days and the expression of the indicated cell cycle regulators was assessed by Western blotting.
The growth arrest induced by androgen deprivation is associated with changes in cell cycle inhibitors including p16INK4A, p15INK4B and p21WAF1. Because C/EBPβ has been shown to regulate the expression of these genes, we next evaluated their expression in LNCaP cells following androgen deprivation. The levels of p16INK4A, p15INK4B and p21WAF1 proteins were diminished in C/EBPβ deficient cells compared with shNTV cultured in ARM or ADM (Figure 5d). In contrast to p15INK4B and p21WAF1, p16INK4A protein levels mildly increased in shCEBPB cells following culture in ADM, compared to culture in ARM. However, p16INK4A levels in shCEBPB cells are markedly lower compared to shNTV control cells cultured in either ARM or ADM (Figure 5d).
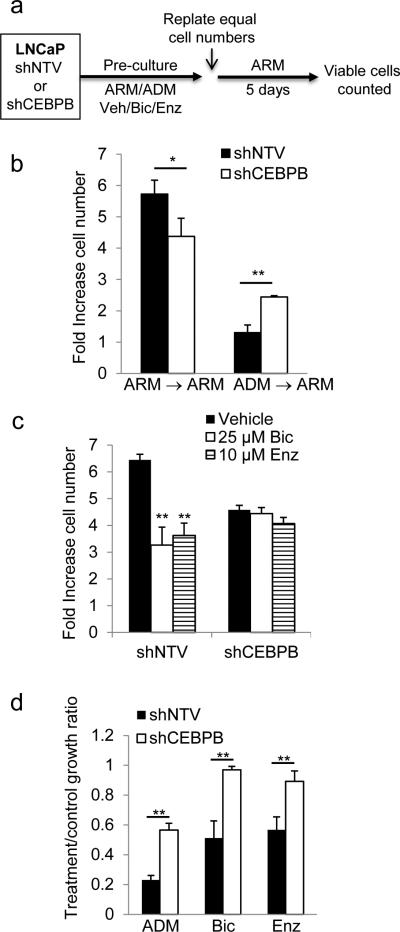
To evaluate whether C/EBPβ is required to maintain growth arrest of LNCaP cells challenged by androgen deprivation, we pre-cultured LNCaP cells harboring shNTV or shC/EBPβ in ADM or androgen-replete media (ARM) with doxycycline for 7 days, re-seeded equal number of cells in ARM and enumerated viable cells 5 days later (Figure 6a). As expected from the observed inhibition of G1 to S cell cycle progression (Figure 5c), culture in ADM for 7 days resulted in diminished cell accumulation (not shown). Although in ARM the rate of proliferation of shCEBPB cells was modestly lower than shNTV cells, C/EBPβ knockdown was associated with a better, although incomplete, recovery of proliferation after pre-culture in ADM (Figure 6b).
Figure 6.
C/EBPβ is required for complete maintenance of growth arrest induced by AR inhibition in LNCaP cells. (a) LNCaP cells expressing shCEBPB or shNTV were pre-cultured in doxycycline under the indicated conditions or in ARM control media, 1.5E5 cells from each pre-culture condition were replated and enumerated after 5 days, as depicted in the diagram. (b) After 7 days pre-culture in ARM or ADM equal number of shCEBPB or shNTV LNCaP cells were re-plated in ARM in a 6-well dish and counted 5 days later. The average fold-increase in cell number is shown. (c) Similarly, cells were pre-cultured for 4 days with bicalutamide (Bic), enzalutamide (Enz), or vehicle control and doxycycline, re-plated in ARM and enumerated after 5 days as described above. (d) The relative growth ratio of cells pre-cultured in ADM, bicalutamide (Bic), or enzalutamide (Enz), over cells cultured in ARM control conditions. Bar graphs represent the mean of at least three experiments ±SEM; * p<0.05; ** p<0.005.
Charcoal stripped FBS lacks androgen and multiple other growth factors. To define the specific effect of androgen-depletion, LNCaP cells harboring shNTV or shCEBPB were pre-cultured with doxycycline and bicalutamide, enzalutimide, or vehicle for 4 days, and subsequently re-plated at equal numbers in ARM without inhibitors (Figure 6a). In contrast to shNTV cells which demonstrated 2-fold decreased recovery, CEBPB depleted cells completely recovered their proliferation once released from AR-inhibition (Figure 6c). To better reflect recovery and adjust for the different proliferation rate in ARM of shCEBP compared to shNTV cells, we also plotted these data as a ratio of growth of cells pre-cultured in ADM, bicalutamide, or enzalutimide to cells pre-cultured in ARM (Figure 6d). This normalization highlights the recovery of proliferation of shCEBPB compared to shNTV cells in each experiment, indicating that C/EBPβ is required for maintenance of complete growth arrest induced by androgen deprivation and its suppression alleviates the phenotype, at least in part.
C/EBPβ elevation induces senescence
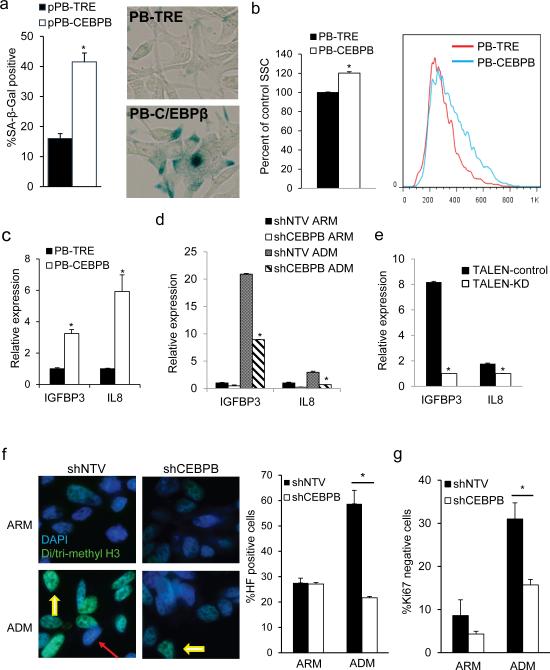
Because C/EBPβ seemed to play a role in maintaining growth arrest following androgen deprivation, we next evaluated whether over-expression of C/EBPβ was sufficient to induce senescence in LNCaP cells. Senescent cells are characterized by an increase in cell volume, granularity and lysosomal mass indicated by senescence associated β-galactosidase (SA-β-gal) activity.32 Compared to control PB-TRE, PB-CEBPB cells had a significantly increase in the number SA-β-gal positive cells and the level of cell granularity, as assessed by side scatter, (Figures 7a and 7b). Expression of several secreted gene products is elevated in senescent cells and is referred to as the senescence associated secretory phenotype (SASP).24 Release of these secreted factors promotes paracrine growth arrest, and C/EBPβ was shown to be central to induction of SASP genes.25, 33, 34 Accordingly, overexpression of C/EBPβ led to a significant increase in the transcript levels of two SASP-associated genes, IL8 and IGFBP3 (Figure 7c). Similarly, we observed increased IGFBP3 and IL8 levels upon androgen deprivation, which was abrogated by either CEBPB shRNA or TALEN targeting (Figures 7d and 7e). Another feature of senescent cells is accumulation of tightly packed heterochromatin foci (HF) characterized by increased di- or trimethylated histone 3 on lysine 9 (H3K9me2, H3K9me3).35 When cultured in ADM, LNCaP-shNTV cells display a 2-fold increase in the number of HF positive cells. In contrast, we did not observe an increase in HF in shCEBPB cells cultured under similar conditions (Figure 7f). Senescent cells exit cell cycle, and stain negative for Ki67. A similar proportion of shNTV or shCEBPB cells expressed Ki67 in ARM. However, the proportion of Ki67-negative cells after one week of culture in ADM was 2-fold lower in cells lacking C/EBPβ (Figure 7g). Together, these data indicate that in PC cells C/EBPβ is necessary for induction of senescence upon androgen-deprivation.
Figure 7.
C/EBPβ is required for androgen deprivation induced senescence. (a) The percent of SA-β-gal-positive PB-TRE or PB-CEBPB cells was quantified after 5 days of culture in 0.5 μg/ml doxycycline. An average from 3 experiments (left) and representative images are shown (right). (b) Quantification of cell granularity by the median side scatter value of G1 PB-TRE and PBCEBPB cells 5 days after seeding in doxycycline. A representative histogram is shown on the right. (c) IL8 and IGFBP3 expression were quantified by qRT-PCR in PB-TRE and PB-CEBPB cells cultured under the above conditions. Conversely, total cellular RNA was extracted from shNTV or shCEBPB (d), or TALEN-control or TALEN-KD (e) LNCaP cells after 7 days in culture in ARM or ADM and transcript levels of IL8 and IGFBP3 were assessed by qRTPCR. (f) Expression of an shRNA against CEBPB or non-targeting vector (shNTV) control were induced by doxycycline in LNCaP cells cultured in androgen replete (ARM) or depleted media (ADM). After 7 days cells were stained for senescence associated heterochromatin foci (HF) (yellow arrows). Red arrow points to HF negative cell. Representative photomicrographs are shown (left, 600X magnification). The average and standard error of three independent counts is shown (right panel). *p<0.001. (g) Ki67 was quantified using flow cytometry in shNTV or shCEBPB LNCaP cells cultured in ARM or ADM for 7 days and the average proportion of Ki67 negative cells from 3 experiments is presented. *p<0.01.
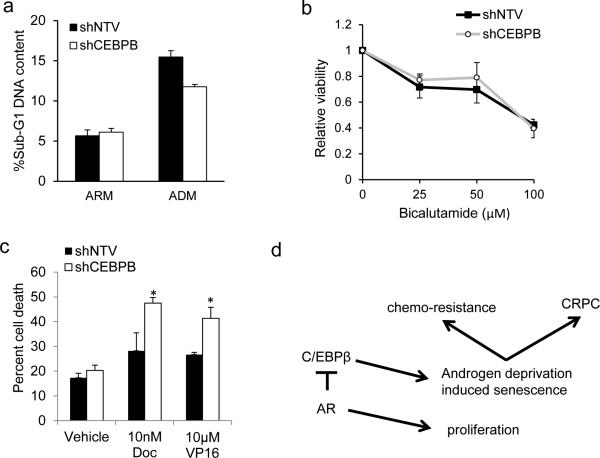
Cellular senescence engenders a pro-survival phenotype. Given rapid induction of C/EBPβ in the absence of AR signaling and its role in directing senescence, we evaluated whether targeting C/EBPβ synergizes with anti-androgen agents or chemotherapy in killing PC cells. Culture of LNCaP shNTV and shCEBPB in ADM (Figure 8a) or exposing them to bicalutamide (Figure 8b) increased the number of dead cells relative to ARM cultures. However, there was no difference in cell viability between shNTV and shCEBPB cells (Figure 8a and 8b). However, after pre-culture in ADM, treatment with docetaxel or etoposide induced a significant 68% or 55% increase in cell death, respectively, in LNCaP cells harboring shCEBPB compared with shNTV (Figure 8c). Together, these data demonstrate that C/EBPβ promotes a pro-survival, drug-resistant phenotype during androgen deprivation.
Figure 8.
C/EBPβ protects from chemotherapy following androgen deprivation. (a) LNCaP cells were cultured for one week in ARM or ADM and cell death was evaluated flow cytometric analysis of sub-G1 DNA content. (b) LNCaP shNTV or shCEBPB lines cultured in ADM were exposed to bicalutamide at the indicated dose for 48 hrs and relative cell viability was evaluated by the WST-1 assay. The average cell viability from 3 independent experiments is shown. (c) LNCaP lines harboring shNTV or shCEBPB were cultured for 7 days in androgen depleted media (ADM), re-plated in androgen replete media (ARM), and treated with vehicle control, 10 nM docetaxel (Doc), or 10 μM etoposide (VP16) for 48 hours. Cell survival was assessed by trypan blue exclusion and the average percentage of dead cells from 3 independent experiments is shown. (d) Model of the regulation of C/EBPβ expression by AR. Upon AR inhibition C/EBPβ expression will increase to promote senescence, chemoresistence, and emergence of CRPC.
Discussion
Accumulating evidence points to a strong connection between senescence and tumor progression.23, 24, 36 Oncogene induced senescence promotes the eventual emergence of subpopulations of aggressive, malignant cells and thus may be considered a tumor promoting state. In PC, androgen deprivation induced senescence promotes the development of tumor progression and resistance to apoptosis,5 fostering the emergence of cancer initiating cells.37 Increased numbers of senescent cells have been observed in tissue sections from tumors in patients that had been treated with neoadjuvant androgen deprivation therapy.7 Also, cellular senescence induced by androgen deprivation dramatically increases reactive oxygen species and DNA double-strand breaks, and leads to the outgrowth of hormone-refractory populations in cultured LNCaP cells.5 CRPC emerges as a result of multiple adaptations, including AR gene amplification, abnormal AR activation, or enhanced steroidogenesis.2 These changes are acquired and propagated as a result of selective pressure exerted on PC cells by an androgen poor milieu while cells are protected by the androgen deprivation-induced senescence state. Previous reports have suggested that androgen deprivation leads to a senescent state in both LNCaP and LAPC4 cells, but the mechanism by which these cells become senescent was not well described.5-7 Here, we demonstrate that PC cells respond to androgen withdrawal by up-regulating CEBPB transcription, that loss of C/EBPβ lead to a reduction in the number of senescent cells following androgen deprivation, and that ectopic expression of C/EBPβ induces the expression of senescent markers indicating that C/EBPβ plays a central role in cellular senescence induced by androgen deprivation, and that impeding the senescent response via inhibition of C/EBPβ expression keeps PC cells susceptible to chemotherapy, validating C/EBPβ as a therapeutic target in androgen dependent PC.
Probing the Oncomine database revealed elevated expression of CEBPB in human CRPC samples. As CRPC is often characterized by active AR signaling, this finding may seemingly be at odd with our in vitro data showing that AR activity suppresses C/EBPβ expression. Several potential explanations may account for elevated C/EBPβ levels despite active AR signaling in CRPC. It has been demonstrated that there is substantial divergence in AR gene targets when comparing castrate-resistant to androgen sensitive cells.38 Further, androgen deprivation or castration-resistance is associated with decreased AR occupancy on repressive DNA elements39 and the expression of many AR-repressed genes increases in castrate-resistant cells.39 Finally, although castrate-resistant cells often exhibit increased levels of AR, AR signaling relative to androgen sensitive prostate cancer cells may not increase because of diminished ligand levels. Therefore, C/EBPβ de-repression may persist in cells that have developed castration-resistance.
Our data demonstrate that inhibition of AR leads to rapid upregulation of CEBPB RNA, loss of AR interaction with the CEBPB promoter, and increased promoter H3K acetylation. To our knowledge, this is the first demonstration that CEBPB is a direct AR transcriptional repressive target. This observation is consistent with previous reports showing that AR can inhibit gene expression through interaction with transcriptional co-repressors at proximal promoter regions.40, 41,42 Derepression of CEBPB occurs within 4 hours of exposure to anti-androgens. Conversely, treatment with AR agonist R1881 results in diminished expression of C/EBPβ and suppression of the activity of a CEBPB promoter luciferase reporter. Accordingly, exposure to DHT leads to a decrease in activating AcH3 histone marks on the promoter. These findings indicate that AR suppresses CEBPB expression directly through regulation of the promoter. Examination of the CEBPB promoter sequence did not identify an androgen response element (ARE), suggesting indirect binding of AR. In other contexts, AR directly interacts with Sp1 to regulate gene expression in the absence of an ARE making Sp1 a potential mediator of AR regulation of CEBPB.43, 44
Treatment of LNCaP cells with anti-androgens or culture in hormone-depleted media leads to G1 arrest and cellular senescence. PC cells require AR signaling for transition from G1 to S and accordingly we did not observe continued proliferation of cells that had been cultured in hormone depleted media or with anti-androgens regardless of CEBPB knockdown. Androgen deprivation induced senescence had a profound long-lived effect on PC cell proliferation in the presence of normal C/EBPβ levels even after reintroduction of androgens, as previously observed.6 We found that after androgen deprivation, C/EBPβ deficiency allowed LNCaP cells to resume proliferation when re-seeded in ARM. Thus, C/EBPβ plays an important role in the complete maintenance of senescent growth arrest induced by androgen deprivation.
Senescent cells develop unique secretory paracrine activities, conferring a pro-malignant microenvironment by secreting an array of cytokines and proinflammatory mediators.24, 45, 46 Our results raise the possibility that C/EBPβ also promotes the expression of senescence-associated secretory genes such as IL-8 and IGFBP3 and the cell cycle inhibitors p21WAF1 and p15INK4B. IL-8 signaling has been shown to promote cell survival, angiogenesis and senescence in pre-clinical models of PC.8, 47, 48 It activates the PI3K-AKT-mTOR pathway, which is critical for cell survival during androgen deprivation.49 IGFBP3 is strongly upregulated following androgen deprivation and promotes tumor growth in a mouse PC model.50, 51 In PC patients the percentage of cells positive for p15INK4B was shown to increase with tumor grade.52-54 Expression of p21WAF1 correlates with a worsened prognosis both before and after androgen deprivation therapy, and in vitro studies of p21WAF1 have shown that it suppresses apoptotic response to chemotherapy.55-57 These findings are consistent with our results showing that C/EBPβ deficient cells, with decreased p21WAF1 and p15INK4B levels, were more sensitive to chemotherapy post-androgen withdrawal. Increased p16INK4A expression is not essential to androgen induced senescence,5 and we see suppression of p16INK4A in C/EBPβ deficient LNCaP cells. Overall, C/EBPβ promotes PC senescence and thereby potentially chemo-resistance and progression to castration-resistant growth through multiple transcriptional targets during androgen deprivation therapy (Figure 8d).
Our delineation of CEBPB upregulation in human hormone-refractory PC further support the concept that C/EBPβ-dependent induction of senescence during androgen blockade promotes castration-resistant progression by providing the opportunity to respond the selective pressure of anti-androgen therapy. Importantly, these data indicate the potential utility of targeting C/EBPβ in combination with androgen deprivation for novel PC therapy.
Materials and Methods
Cell lines and plasmids
LNCaP cells were maintained in RPMI media without phenol red with 10% heat inactivated fetal bovine serum (HI-FBS) (Hyclone) supplemented with penicilin/streptomycin. LAPC4 cells were maintained in Iscove's modified Dulbecco's media (IMDM) supplemented with 15% HI-FBS, 1 nM DHT, and penicillin/streptomycin. DU145 cells were maintained in RPMI with 10% HI-FBS, and 293T cells were cultured in Dulbecco modified Eagle medium (DMEM) with 10% HI-FBS. Cells were grown in a humidified incubator maintained at 37°C with 5% CO2. Cells were split 1:4 and were used until passage 40. Cells transduced with pTRIPZ-shRNA or transfected with pPB-TRE-Puro were maintained in tetracycline-screened FBS (Hyclone). For androgen deprivation cells were cultured in phenol red-free media supplemented with 10% charcoal-stripped-FBS (csFBS) (Hyclone). Androgen receptor was blocked using enazlutamide (Selleckchem Houston, TX USA) or bicalutamide (Sigma-Aldrich St. Louis, MO USA).
pTRIPZ-shRNA (Open Biosystems) lentiviral vectors were generated as described 58 and stably transduced cells were selected with puromycin (2 μg/ml) after 48 hrs. Expression of shRNA was induced by treating cells with 200 ng/ml doxycycline, replaced every 48 hrs and confirmed by fluorescence microscopy detection of RFP.
The pPB-TRE-Puro plasmid (kindly provided by Jolene Ooi and Pentao Liu) contains a multiple cloning site downstream of a TRE element, and a CAG promoter upstream of rtTA, IRES, and apuromycin resistance gene. The mouse Cebpb ORF including the 3’UTR (1-1400) was ligated as BamHI/NotI fragment into the BglII/NotI digested pPB-TRE-Puro plasmid 29 to generate the pPB-CEBPB which was confirmed by sequencing. Stable PB-TRE and PB-CEBPB cell lines were generated by transfecting equal parts pCMV-hyperpiggybase and piggybac vectors by lipofection.
Western Blotting
Protein samples from whole cell lysates and nuclear extracts were prepared and subjected to Western blotting as previously described.59 After blocking with Odyssey blocking buffer (LI-COR Bioscience Lincoln, NE USA) membranes were incubated overnight with the following primary antibodies: p15INK4B (M-20), C/EBPβ (C-19), AR (N-20) (Santa Cruz Biotechnology), β-actin (AC15) (Sigma-Aldrich). For target protein detection, membranes were incubated with secondary antibodies, goat anti-mouse Alexafluor 670 (Life Technologies, Carlsbad, CA) or goat anti-rabbit antiserum (LI-COR) and imaged on a Li-Cor Odyssey Fc infrared imaging system.
Quantitative real-time PCR
Total RNA was isolated from cells and first strand cDNA was synthesized as previously described.58 β-actin transcript was used as a reference to normalize samples and relative expression was calculated as described.58 Each sample was assayed in triplicates and each experiment was repeated at least three times. Oligonucleotides were custom ordered from Sigma-Aldrich, and their sequences are presented in Table 1.
Table 1.
Primers used for ChIP and RNA analysis.
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| ChIP | ||
| CEBPB proximal | GGCCGCCCTTATAAATAACC | TATTAGTGAGGGGGCTGGTG |
| CEBPB upstream | ATAATGGTGGCTGGCGATAG | CCTTCCTCACTGCAAAATGG |
| Real-time PCR | ||
| TMPRSS2 | AATCCCCATCCGGGACAGT | AGGAGTCGCACTCTATCCCA |
| PSA | GCAGCATTGAACCAGAGGAG | AGAACTGGGGAGGCTTGAGT |
| ACTB | GACCTGGCTGGCCGGGACCT | GGCCATCTCTTGCTCGAAGT |
| GAPDH | CCACCCATGGCAAATTCC | GATGGGATTTCCATTGATGACA |
| CEBPB | AAACTCTCTGCTTCTCCCTCTGC | CTGACAGTTACACGTGGGTTGC |
| IL8 | TCTGGCAACCCTAGTCTGCT | GCTTCCACATGTCCTCACAA |
| BDNF | GCGTGTGTGACAGTATTAGT | CTGGGTAGTTCGGCACTGGG |
| CGA | GCGGTGGAAGAGCCATCAT | TCTGTGGCTTCACCACTTTTCTC |
| IGFBP3 | GCCAGCGCTACAAAGTTGAC | ATGTGTACACCCCTGGGACT |
| P16 INK4A | CCGAATAGTTACGGTCGGAGG | CACCAGCGTGTCCAGGAAG |
| P15 INK4B | GAGGCGCGCGATCCAG | CACCAGCGTGTCCAGGAAG |
| P21 CIP1/WAF1 | ACTCTCAGGGTCGAAAACGG | GATGTAGAGCGGGCCTTTGA |
Luciferase reporter assays
Using PCR, a DNA fragment from -888 to +64 base pairs (bp) relative to the initiation of transcription (Ensembl database) of the human CEBPB promoter was cloned as a BamHI/MluI fragment into BglII/MluI digested pGL3-luciferase reporter vector (Promega, Medison,WI USA) to generate CEBPB-Luc that was confirmed by Sanger sequencing. LNCaP or DU145 cells were seeded in 6-well plates and transfected using Lipofectamine 2000. Each well was cotransfected with 1.4 μg of the reporter plasmid and 10 ng of CMV-β-galactosidase as an internal control. Twenty four hours after transfection cells were treated with vehicle (0.01% ethanol) or the androgen agonist R1881 (Sigma-Aldrich) and after additional 24 hrs lysed (Reporter Lysis Buffer, Promega) and assayed for luciferase and β-galactosidase activity. Fold activation relative to the vehicle treated cells after correction for β-galactosidase activity was determined. Lysates from non-transfected cells were used as baseline.
TALEN construction and CEBPB gene editing
TALEN DNA constructs targeting the human CEBPB ORF were constructed using the Golden Gate Talen assembly kit (Addgene. Cambridge, MA USA).30 Targeting sequences were designed using the Cornell University TAL Effector Nucleotide Targeter 2.0 web based software. Golden Gate assembly of the repeat-variable di-residue sequence was performed according to the manufacturer's instructions and the completed TALEN pairs were ligated into the pTAL3 vector. The complete TALEN ORF including the repeat-variable and FokI domains was excised using XhoI and ApaI restriction endonuceases and ligated into the pcDNA3.1(+) vector. LNCaP cells cotransfected with TALEN expression vectors targeting CEBPB were seeded in 96 well dishes, and individual clones were screened for C/EBPβ expression by Western blotting.
DNA content analysis and Flow cytometry
Cell cycle analysis by DNA content was performed as previously described.60 Ki67 expressing cells were identified by flow cytometry using APC-anti-Ki67 antibody (BioLegend, San Diego, CA). Flow cytometry analysis was performed using a BD FACSCalibur machine (BD Biosciences), Sub-cellular debris and dead cells were gated out and singlet discrimination was performed by gating on FL2-A and FL2-W channels and data were interpreted using FloJo Cytometric Analytical software (TreeStar).
Chromatin immunoprecipitation (ChIP)
5E6 LNCaP cells were used in each ChIP reaction as previously described,58 using antibodies against C/EBPβ, AR, rabbit IgG (Santa Cruz Biotechnology), or acetylated histone H3 (06–599) (Millipore, Billerica, MA USA). DNA fragments corresponding to the promoters of interest were detected by PCR using the primers presented in Table 1.
Cell viability and proliferation assays
Viability was determined using the WST-1 assay (Roche). Briefly, cells were seeded into 96 well plates, allowed to adhere for 48 hrs and then treated for an additional 48 hours. WST-1 reagent (Roche) was directly added to the wells and after incubation absorbance was read at 450 nm using a BioRad Microplate Reader Model 680. The 670 nm reference absorbance and readings from blank wells containing only cell culture media and DMSO (vehicle) were subtracted from experimental wells. Relative viability was determined by dividing absorbance readings from vehicle treated wells. For cell proliferation, LNCaP cells were grown for 7 days in growth or hormone-depleted media. Cells were trypsinized, stained with Trypan blue dye and viable cells were enumerated using a hemocytometer. 1.5E5 cells per well were seeded into 6-well plates in ARM. After 5 days cells were similarly enumerated.
SA-β-Gal Chromogenic Assay
SA-β-Gal positive cells were stained using the chromogenic assay as described.61 Five random fields of view were imaged on a Leica E600 microscope by brightfield microscopy at 200x magnification. Positive cells were identified as those containing blue precipitate throughout the cytoplasm.
Immunofluorescent staining and SAHF-quantification
LNCaP cells were seeded onto Poly-D-lysine coated glass coverslips and following treatment were fixed in 4% paraformaldehyde. Cells were washed and incubated in permeabilization buffer (TBS, 2% BSA, 0.5% Triton-X 100, 0.1% sodium azide). After blocking, cells were incubated with anti-di/trimethyl H3K9 (1:250, Cell Signaling Technology Danvers, MA USA), washed and incubated with goat anti-mouse-Alexafluor 488 conjugated secondary antibody (Life Technologies). Cells were washed and mounted on glass slides for analysis by fluorescence microscopy. SAHF were imaged on a Leica E800 fluorescence microscope with a CCD camera and imaged at 630X magnification with an oil immersion objective. Fluorescent micrographs of SAHF from 6-7 random fields of view were quantified using ImageJ (National Institutes of Health Bethesda, MD USA). Intensely stained nuclei with multiple fluorescent foci that colocalized with DAPI staining were counted as positive cells containing SAHF.
Statistical analysis
Statistical comparison of two groups of samples was conducted using the Student's t-test. Comparisons of multiple groups of samples was performed using the analysis of variance followed by multiple comparisons with the Student's t-test and the Holm-Bonferonni Correction (α/(n-k+1), where n = number of comparisons and k = rank of p-value).
Acknowledgments
This research was supported by grants from the St. Baldrick's Foundation (to I.P-P.), the Mitchell Foundation (to I.P-P.), the Walsh Prostate Cancer Research Fund (to I.P-P. and A.D.F.), the Samuel Waxman Cancer Research Foundation (to A.D.F.), Department of Defense grant PC131609 (to D.J.B.), National Institutes of Health T32 CA60441 (to T.B), and the Giant Food Children's Cancer Research Fund and P30 CA006973.
Footnotes
Authorship Contributions:
D.J.B. designed and performed the research, analyzed data and wrote the manuscript; J.Z. and T.B. performed the research; S.R.D. designed the research and analyzed data; A.D.F. designed the research, analyzed data and wrote the manuscript; I.P-P. designed and performed the research, analyzed data and wrote the manuscript.
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures. 2013 2013, pp http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- 2.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. The New England journal of medicine. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Isaacs JT. Activation of programmed (apoptotic) cell death for the treatment of prostate cancer. Advances in pharmacology. 1996;35:281–306. doi: 10.1016/s1054-3589(08)60278-1. [DOI] [PubMed] [Google Scholar]
- 4.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Burton DG, Giribaldi MG, Munoz A, Halvorsen K, Patel A, Jorda M, et al. Androgen deprivation-induced senescence promotes outgrowth of androgen-refractory prostate cancer cells. PLoS One. 2013;8:e68003. doi: 10.1371/journal.pone.0068003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewald JA, Desotelle JA, Church DR, Yang B, Huang W, Laurila TA, et al. Androgen deprivation induces senescence characteristics in prostate cancer cells in vitro and in vivo. Prostate. 2013;73:337–345. doi: 10.1002/pros.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pernicova Z, Slabakova E, Kharaishvili G, Bouchal J, Kral M, Kunicka Z, et al. Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia. 2011;13:526–536. doi: 10.1593/neo.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebastian T, Malik R, Thomas S, Sage J, Johnson PF. C/EBPbeta cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 2005;24:3301–3312. doi: 10.1038/sj.emboj.7600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calkhoven CF, Muller C, Leutz A. Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev. 2000;14:1920–1932. [PMC free article] [PubMed] [Google Scholar]
- 10.Timchenko NA, Welm AL, Lu X, Timchenko LT. CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBPbeta mRNA and regulates translation of C/EBPbeta isoforms. Nucleic Acids Res. 1999;27:4517–4525. doi: 10.1093/nar/27.22.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanabe A, Kumahara C, Osada S, Nishihara T, Imagawa M. Gene expression of CCAAT/enhancer-binding protein delta mediated by autoregulation is repressed by related gene family proteins. Biol Pharm Bull. 2000;23:1424–1429. doi: 10.1248/bpb.23.1424. [DOI] [PubMed] [Google Scholar]
- 12.Christenson LK, Johnson PF, McAllister JM, Strauss JF., 3rd CCAAT/enhancer-binding proteins regulate expression of the human steroidogenic acute regulatory protein (StAR) gene. J Biol Chem. 1999;274:26591–26598. doi: 10.1074/jbc.274.37.26591. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE. Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001;61:2328–2334. [PubMed] [Google Scholar]
- 14.Bagchi MK, Mantena SR, Kannan A, Bagchi IC. Control of uterine cell proliferation and differentiation by C/EBPbeta: functional implications for establishment of early pregnancy. Cell cycle. 2006;5:922–925. doi: 10.4161/cc.5.9.2712. [DOI] [PubMed] [Google Scholar]
- 15.Boruk M, Savory JG, Hache RJ. AF-2-dependent potentiation of CCAAT enhancer binding protein beta-mediated transcriptional activation by glucocorticoid receptor. Mol Endocrinol. 1998;12:1749–1763. doi: 10.1210/mend.12.11.0191. [DOI] [PubMed] [Google Scholar]
- 16.Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, et al. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci U S A. 2006;103:1870–1875. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Gonit M, Salazar MD, Shatnawi A, Shemshedini L, Trumbly R, et al. C/EBPalpha redirects androgen receptor signaling through a unique bimodal interaction. Oncogene. 2010;29:723–738. doi: 10.1038/onc.2009.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia L, Berman BP, Jariwala U, Yan X, Cogan JP, Walters A, et al. Genomic androgen receptor-occupied regions with different functions, defined by histone acetylation, coregulators and transcriptional capacity. PLoS One. 2008;3:e3645. doi: 10.1371/journal.pone.0003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Bergh A, Damber JE. Increased expression of CCAAT/enhancer-binding protein beta in proliferative inflammatory atrophy of the prostate: relation with the expression of COX-2, the androgen receptor, and presence of focal chronic inflammation. Prostate. 2007;67:1238–1246. doi: 10.1002/pros.20595. [DOI] [PubMed] [Google Scholar]
- 21.Kim MH, Fields J. Translationally regulated C/EBP beta isoform expression upregulates metastatic genes in hormone-independent prostate cancer cells. Prostate. 2008;68:1362–1371. doi: 10.1002/pros.20801. [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, Minton AZ, Agrawal V. C/EBPbeta regulates metastatic gene expression and confers TNF-alpha resistance to prostate cancer cells. Prostate. 2009;69:1435–1447. doi: 10.1002/pros.20993. [DOI] [PubMed] [Google Scholar]
- 23.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nature reviews Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual review of pathology. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huggins CJ, Malik R, Lee S, Salotti J, Thomas S, Martin N, et al. C/EBPgamma suppresses senescence and inflammatory gene expression by heterodimerizing with C/EBPbeta. Mol Cell Biol. 2013;33:3242–3258. doi: 10.1128/MCB.01674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusa K, Zhou L, Li MA, Bradley A, Craig NL. A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci U S A. 2011;108:1531–1536. doi: 10.1073/pnas.1008322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, 2nd, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. Journal of cell science. 2000;113(Pt 20):3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 33.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 34.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cahu J, Bustany S, Sola B. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells. Cell death & disease. 2012;3:e446. doi: 10.1038/cddis.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, Lamb AD, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell. 2013;23:35–47. doi: 10.1016/j.ccr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457–471. doi: 10.1016/j.ccr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 41.Svensson C, Ceder J, Iglesias-Gato D, Chuan YC, Pang ST, Bjartell A, et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson C, Ceder J, Iglesias-Gato D, Chuan YC, Pang ST, Bjartell A, et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res. 2014;42:999–1015. doi: 10.1093/nar/gkt921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu S, Jenster G, Epner DE. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol Endocrinol. 2000;14:753–760. doi: 10.1210/mend.14.5.0461. [DOI] [PubMed] [Google Scholar]
- 44.Yuan H, Gong A, Young CY. Involvement of transcription factor Sp1 in quercetin-mediated inhibitory effect on the androgen receptor in human prostate cancer cells. Carcinogenesis. 2005;26:793–801. doi: 10.1093/carcin/bgi021. [DOI] [PubMed] [Google Scholar]
- 45.Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 46.Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98:245–249. doi: 10.1038/sj.bjc.6604087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manna S, Singha B, Phyo SA, Gatla HR, Chang TP, Sanacora S, et al. Proteasome inhibition by bortezomib increases IL-8 expression in androgen-independent prostate cancer cells: the role of IKKalpha. J Immunol. 2013;191:2837–2846. doi: 10.4049/jimmunol.1300895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seaton A, Scullin P, Maxwell PJ, Wilson C, Pettigrew J, Gallagher R, et al. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis. 2008;29:1148–1156. doi: 10.1093/carcin/bgn109. [DOI] [PubMed] [Google Scholar]
- 49.MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, et al. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- 50.Kawabata R, Oie S, Takahashi M, Kanayama H, Oka T, Itoh K. Up-regulation of insulin-like growth factor-binding protein 3 by 5-fluorouracil (5-FU) leads to the potent anti-proliferative effect of androgen deprivation therapy combined with 5-FU in human prostate cancer cell lines. International journal of oncology. 2011;38:1489–1500. doi: 10.3892/ijo.2011.991. [DOI] [PubMed] [Google Scholar]
- 51.Mehta HH, Gao Q, Galet C, Paharkova V, Wan J, Said J, et al. IGFBP-3 is a metastasis suppression gene in prostate cancer. Cancer Res. 2011;71:5154–5163. doi: 10.1158/0008-5472.CAN-10-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, et al. Overexpression of the cell cycle inhibitor p16INK4A in high-grade prostatic intraepithelial neoplasia predicts early relapse in prostate cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:544–550. [PubMed] [Google Scholar]
- 53.Lee CT, Capodieci P, Osman I, Fazzari M, Ferrara J, Scher HI, et al. Overexpression of the cyclin-dependent kinase inhibitor p16 is associated with tumor recurrence in human prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5:977–983. [PubMed] [Google Scholar]
- 54.Zhang Z, Rosen DG, Yao JL, Huang J, Liu J. Expression of p14ARF, p15INK4b, p16INK4a, and DCR2 increases during prostate cancer progression. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19:1339–1343. doi: 10.1038/modpathol.3800655. [DOI] [PubMed] [Google Scholar]
- 55.Baretton GB, Klenk U, Diebold J, Schmeller N, Lohrs U. Proliferation-and apoptosis-associated factors in advanced prostatic carcinomas before and after androgen deprivation therapy: prognostic significance of p21/WAF1/CIP1 expression. Br J Cancer. 1999;80:546–555. doi: 10.1038/sj.bjc.6690390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez LA, Yang J, Vazquez ES, Rodriguez-Vargas Mdel C, Olive M, Hsieh JT, et al. p21 modulates threshold of apoptosis induced by DNA-damage and growth factor withdrawal in prostate cancer cells. Carcinogenesis. 2002;23:1289–1296. doi: 10.1093/carcin/23.8.1289. [DOI] [PubMed] [Google Scholar]
- 57.Steinman RA, Johnson DE. p21WAF1 prevents down-modulation of the apoptotic inhibitor protein c-IAP1 and inhibits leukemic apoptosis. Molecular medicine. 2000;6:736–749. [PMC free article] [PubMed] [Google Scholar]
- 58.Paz-Priel I, Houng S, Dooher J, Friedman AD. C/EBPalpha and C/EBPalpha oncoproteins regulate nfkb1 and displace histone deacetylases from NF-kappaB p50 homodimers to induce NF-kappaB target genes. Blood. 2011;117:4085–4094. doi: 10.1182/blood-2010-07-294470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paz-Priel I, Cai DH, Wang D, Kowalski J, Blackford A, Liu H, et al. CCAAT/enhancer binding protein alpha (C/EBPalpha) and C/EBPalpha myeloid oncoproteins induce bcl-2 via interaction of their basic regions with nuclear factor-kappaB p50. Mol Cancer Res. 2005;3:585–596. doi: 10.1158/1541-7786.MCR-05-0111. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Scott E, Sawyers CL, Friedman AD. C/EBPalpha bypasses granulocyte colony-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood. 1999;94:560–571. [PubMed] [Google Scholar]
- 61.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nature protocols. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]