Abstract
Objective
Overexpression of dopamine and cAMP-regulated phosphoprotein, Mr 32000 (DARPP-32), and its truncated isoform (t-DARPP) are associated with gastric tumorigenesis. Herein, we investigated the role of DARPP-32 proteins in regulating angiopoietin 2 (ANGPT2) and promoting tumor angiogenesis.
Design
Quantitative real-time RT-PCR, immunoblotting, luciferase reporter, immunofluorescence, immunohistochemistry, and angiogenesis assays were applied to investigate the regulation of angiogenesis by DARPP-32 proteins.
Results
Overexpression of DARPP-32 significantly increased the mRNA and protein levels of ANGPT2 in gastric cancer cells. The overexpression of DARPP-32 T34A mutant or the N-terminal truncated isoform, t-DARPP, led to similar effects ruling out the T34-dependent regulation of protein phosphatase 1 activity in regulating ANGPT2. DARPP-32 proteins induced a secreted form of ANGPT2, which was detectable in the media, functionally active, and able to induce angiogenesis, measured by the HUVEC tube formation assay. Antibody blocking of the secreted ANGPT2 abrogated its function. To identify the mechanism by which DARPP-32 regulates ANGPT2, we examined the activities of NF-κB and STAT3, known regulators of angiogenesis. The results ruled out NF-κB and showed induction of STAT3 phosphorylation, activation, and nuclear localization. Inhibition or knockdown of STAT3 significantly attenuated the induction of ANGPT2 by DARPP-32 proteins. In vivo xenografts models demonstrated that overexpression of DARPP-32 promotes angiogenesis and tumor growth. Analyses of human gastric cancer tissues showed a strong correlation between DARPP-32 and ANGPT2.
Conclusion
Our novel findings establish the role of DARPP-32-STAT3 axis in regulating ANGPT2 in cancer cells to promote angiogenesis and tumorigenesis.
Keywords: Stomach cancer, tube formation, STAT3, PPP1R1B, angiogenesis
Introduction
Almost one million cases of gastric cancer are diagnosed each year, establishing this disease as the fourth most common cancer and the second leading cause of cancer related deaths worldwide 1, 2. In some regions of the world such as in Asia and Latin America, gastric carcinoma is the most common malignancy, and the incidence of gastric cancer is almost 10-fold higher than the United States 3–5. Because of the lack of early specific symptoms, the diagnosis of gastric cancer is typically delayed in most patients until cancer has invaded the muscularis propria and patients present with advanced stages, becoming at higher risk of poor response to therapy and disease recurrence 6–8.
Formation of tumor blood vessels is a crucial event during cancer formation and metastasis 9. Tumor neovascularization depends on the production of specific angiogenic factors; either by host or tumor cells shifting the angiogenic balance toward a proangiogenic phenotype 10. ANGPT2 is a family member of the human ANGPT-TIE system 11. It is primarily produced by endothelial cells 12 and stored in Weibel-Palade bodies from where it can be rapidly released upon stimulation to act as an autocrine regulator of endothelial cell functions. Recent studies have shown that the overexpression of ANGPT2 correlates with poor prognosis in several cancers 13–16. ANGPT2 plays a key role in tumor initiation 17 and increases the number of tumor vessel sprouts, possibly owing to the decreased pericyte coverage and more unstable vessels 18. ANGPT2 acts as a vessel destabilizing agent that induces permeability and leads to dissociation of cell-cell contacts in cultured endothelial cells 19. The latest reports have suggested that ANGPT2 can be regulated by vascular endothelial growth factor A (VEGFA), insulin-like growth factor 1 (IGF1), hypoxia inducible factors 1 (HIF1), and platelet-derived growth factor B (PDGFB) 11, 12, 20. In addition, tumor necrosis factor-α (TNF-α)–induced activation of NF-κB can up-regulate ANGPT2 expression in HUVEC cells 21. Consistent with the proposed link between inflammation and angiogenesis, activation of the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway has been implicated in tumor angiogenesis in many types of human cancers 22, 23. In fact, the inhibition or knockdown of STAT3 has been shown to suppress angiogenesis in different human cancers 24–26. However, the role of STAT3 in regulating the ANGPT-TIE system in cancer cells has not been explored.
Dopamine and adenosine 3′,5′-cyclic monophosphate-regulated phosphoprotein Mr 32000 (DARPP-32), also known as protein phosphatase 1 regulatory (inhibitor) subunit 1B (PPP1R1B), is abundantly expressed in spiny neurons of the neostriatum 27. We have previously shown that the DARPP-32 gene also encodes alternatively spliced mRNA that generates an additional protein isoform known as truncated DARPP-32 (t-DARPP) 28. Both DARPP-32 and t-DARPP are frequently overexpressed in gastric adenocarcinomas 28, 29. DARPP-32 promotes activation of chemokine (C-X-C motif) receptor 4 (CXCR4) and cancer cell invasion 30. In addition, overexpression of DARPP-32 proteins is associated with a potent anti-apoptotic advantage for gastric cancer and breast cancer cells through a p53-independent mechanism that involves preservation of the mitochondrial membrane potential, activation of phosphoinositide-3-kinase (PI3K)/AKT, and increased B-cell CLL/lymphoma 2 (BCL2) levels 29, 31, 32. Additionally, DARPP-32 proteins promote resistance of cancer cells to gefitinib and trastuzumab by promoting stabilization and interaction of epidermal growth factor receptor (EGFR), erb-b2 receptor tyrosine kinase 2 (ERBB2), and erb-b2 receptor tyrosine kinase 3 (ERBB3) which lead to activation of PI3K-AKT signaling 33–36.
The primary objective of this study was to investigate the role of DARPP-32 proteins in promoting angiogenesis in gastric cancer. We have uncovered that DARPP-32 proteins enhance angiogenesis through regulation of tumor-derived ANGPT2. We have demonstrated that DARPP-32 proteins induce ANGPT2 expression in gastric cancer cells through regulation of STAT3 activity. These novel findings underscore the importance of DARPP-32 proteins in regulating angiogenesis, which is a crucial step in promoting gastric tumorigenesis.
Materials and Methods
Detailed protocols and standard procedures are described in the Supplementary Methods.
Cell culture
Human gastric cancer cell lines were obtained from American Tissue Culture Collection (ATCC, Manassas, VA) and maintained in culture using F12 medium (GIBCO, Carlsbad, CA) (AGS and SNU-16) or Dulbecco’s modified Eagle’s medium (GIBCO) (MKN-28) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and 1% penicillin/streptomycin (GIBCO). Human umbilical vein endothelial cells (HUVEC) were purchased from PromoCell (Heidelberg, Germany), and maintained in culture using Endothelial Cell Growth Medium (Cedarlane, Burlington, NC).
DARPP-32 and t-DARPP overexpression
The expression plasmids for FLAG-DARPP-32 and FLAG-t-DARPP were generated by PCR amplification of the full-length DARPP-32 cDNA. Flag-tagged–DARPP-32 or –t-DARPP were cloned into the adenoviral (pACCMV) shuttle vector, a kind gift from Dr. David Carbone (Ohio State University). The recombinant adenoviruses were generated by co-transfecting HEK-293 cells (ATCC) with the shuttle and (pJM17) backbone adenoviral plasmids (a gift from Dr. Carbone) using the Calcium Phosphate Transfection Kit (Applied Biological Materials Inc., Richmond, BC). Stably transfected AGS cells expressing DARPP-32 or t-DARPP were generated using pcDNA3 plasmids (Invitrogen) as described previously 32, 36.
Western blotting
Western blotting was conducted as previously described36. Detailed antibody information is included in Supplementary Methods.
Quantitative Real-time RT-PCR
63 gastric cancer and 39 normal gastric tissue samples were collected from the National Cancer Institute Cooperative Human Tissue Network (CHTN) and the pathology archives at Vanderbilt University Medical Center (Nashville, TN). All tissue samples were collected, coded, and de-identified in accordance with the Vanderbilt University Institutional Review Board-approved protocols. Quantitative real-time PCR (qRT-PCR) was conducted as previously described 36.
HUVEC tube formation assay
HUVEC tube formation assay in AGS and MKN-28 cells was conducted following the protocol of In Vitro Angiogenesis Assay Tube Formation Kit (Trevigen, Gaithersburg, MD). A detailed protocol is included in Supplementary Methods.
Immunofluorescence and Immunohistochemistry
Immunofluorescence and Immunohistochemistry were performed as previously described 36. Detailed antibody information is included in Supplementary Methods.
Luciferase reporter assay
Luciferase activity was measured using the Dual-Luciferase Reporter Assay kit (Promega, Madison, WI) according to the manufacturer’s instructions. Detailed protocols and plasmid information are described in Supplementary Methods.
In vivo experiments
Five-week-old female NIHS-Lystbg Foxn1nu Btkxid nude mice were purchased from Harlan Laboratories, Inc. (Frederick, MD) and maintained under specific pathogen-free conditions. Tetracycline inducible (Tet-on system) AGS cells stably expressing DARPP-32 or empty vector 32 were injected subcutaneously (2×106 cells per site) into the flanks. Fresh doxycycline (400 μg/ml) was added to the drinking water three times a week starting from the injection day. Tumor volume was calculated by the formula: tumor volume = 1/2 (length×width2). All mice were sacrificed and tumors were collected when the tumor volume of control group reached 400mm3. The Vanderbilt Institutional Animal Care and Use Committee approved all animal work.
Results
Overexpression of DARPP-32 proteins up-regulate ANGPT2 mRNA and protein expression in gastric cancer cells
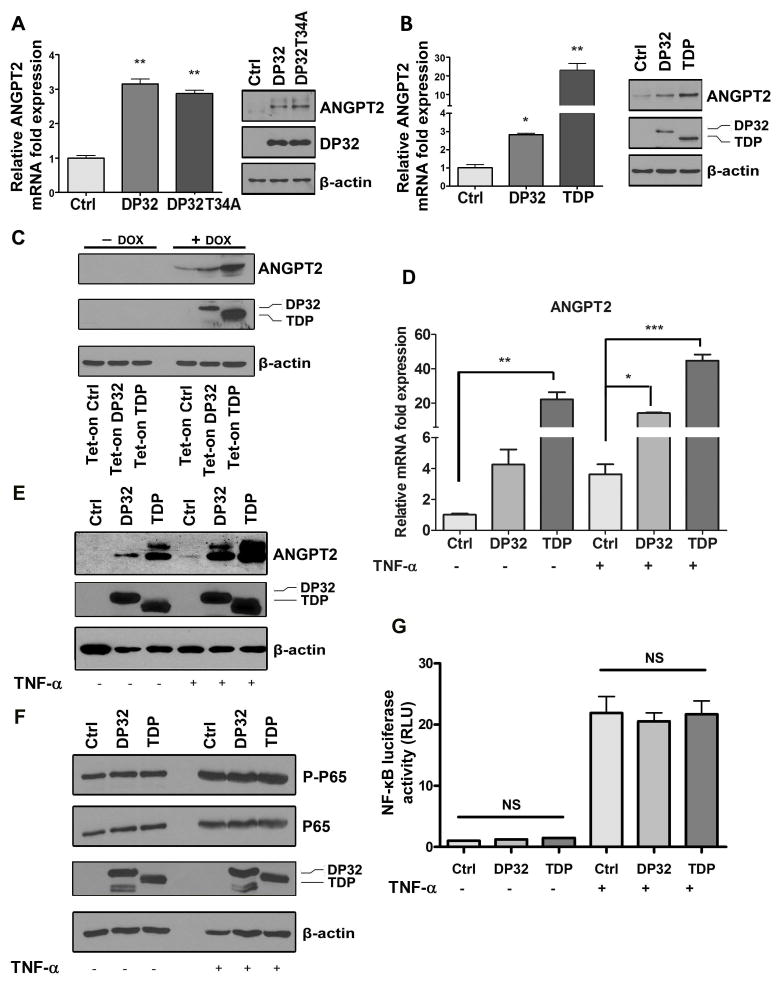
The Western blot analysis and qRT-PCR results demonstrated that DARPP-32 overexpression leads to up-regulation of ANGPT2 at the mRNA and protein levels in AGS and MKN28 gastric cancer cell lines, which express low levels of endogenous DARPP-32 proteins (Figure 1A and B, Supplemental Figure 1A and B). We next examined if the phosphorylation of threonine 34 residue (T34) of DARPP-32 is essential for the induction of ANGPT2. The results indicated that mutant DARPP-32 (T34A) can induce ANGPT2 mRNA and protein levels suggesting that it is not a critical site (Figure 1A, Supplemental Figure 1A). These results were further confirmed using truncated DARPP-32, known as t-DARPP, which lacks the N-terminal domain and the T34 site of DARPP-32 (Figure 1B, Supplemental Figure 1B). These findings ruled out the N-terminal T34-dependent PP1 regulatory function of DARPP-32 37 as a mechanism regulating ANGPT2 in cancer. Using an inducible Tet-on expression system, our data indicated that the induction of AGS-Tet-on-DARPP-32 or AGS-Tet-on-t-DARPP stable cells with doxycycline for 48 hours markedly increased ANGPT2 protein levels (Figure 1C). These results confirmed that the T34 phosphorylation site of DARPP-32 is not necessary for the induction of ANGPT2 and suggested that both DARPP-32 and t-DARPP are potent inducers of ANGPT2.
Figure 1. DARPP-32 proteins up-regulate ANGPT2 expression in gastric cancer cells without activating NF-κB signal pathway.
A–B) The qRT-PCR and Western blot analysis of ANGPT2 mRNA (left panels) and protein (right panels) levels in AGS cells transiently overexpressing DARPP-32 (DP32), DARPP-32T34A mutant (DP32T34A), or t-DARPP (TDP). Error bars indicate standard deviation (SD), * P<0.05, ** P<0.01 compared with control (One way ANOVA). C) Western blot analysis of ANGPT2, DARPP-32, and t-DARPP in AGS/Tet-on cells stably expressing empty vector (Ctrl), DARPP-32 (DP32), and t-DARPP (TDP). D–E) The qRT-PCR and Western blot analysis of ANGPT2 expression in AGS cells transiently overexpressing DARPP-32, t-DARPP, or control with or without TNF-α treatment; * P<0.05, ** P<0.01, *** P<0.001 compared with control (One way ANOVA). F) Western blot analysis showing phospho-P65 (S536) (P-P65), P65, DARPP-32, and t-DARPP in AGS cells transiently overexpressing DARPP-32, t-DARPP, or control with or without TNF-α treatment. G) NF-κB luciferase reporter analysis in AGS cell stably expressing pGL4.32 [luc2P/NF-κB-RE/Hygro] and transiently overexpressing DARPP-32, t-DARPP, or control treated with or without TNF-α; NS, not significant.
DARPP-32 proteins enhance TNF-α–induced ANGPT2 expression through activation of STAT3
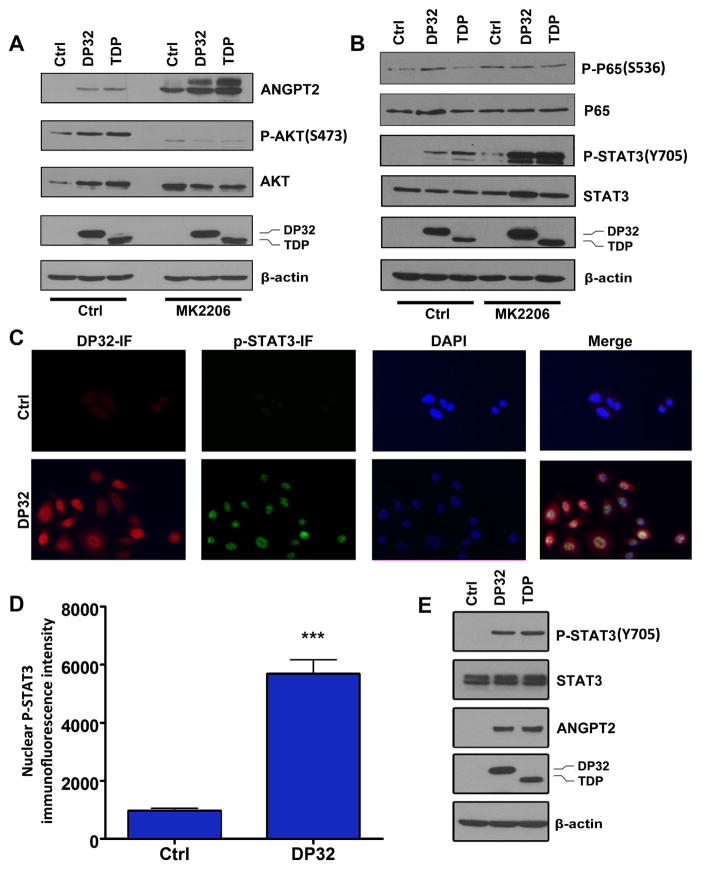
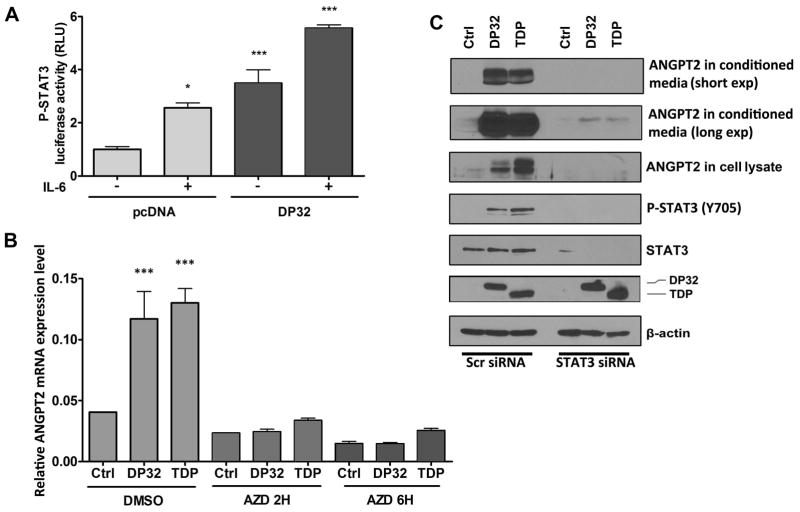
Previous studies have shown that TNF-α can induce ANGPT2 levels in HUVEC 21. Therefore, to investigate whether the induction of ANGPT2 expression by DARPP-32 proteins in cancer cells is mediated by TNF-α, we treated AGS cells transiently expressing DARPP-32, t-DARPP, or empty vector with TNF-α overnight and evaluated ANGPT2 mRNA and protein expression. As expected, our results showed that TNF-α induced ANGPT2 mRNA and protein levels in AGS cells. However, DARPP-32 or t-DARPP induced equal or higher levels of ANGPT2 mRNA and protein than treatment with TNF-α alone (Figure 1D and E). Interestingly, our data indicated that the combination of TNF-α treatment and overexpression of DARPP-32 or t-DARPP induced significantly higher levels of ANGPT2 mRNA and protein than DARPP-32 or t-DARPP overexpression alone (Figure 1D and E). These results suggested that DARPP-32 proteins could synergistically enhance TNF-α–mediated induction of ANGPT2 expression in gastric cancer cells. In an attempt to determine the mechanism by which DARPP-32 proteins induce ANGPT2 expression, we investigated the role of DARPP-32 proteins in activating NF-κB. Western blot data indicated that DARPP-32 or t-DARPP expression has no effect on phosphorylation of NF-κB–p65 (S536) protein in AGS cells with or without TNF-α treatment (Figure 1F). In line with these results, the luciferase reporter assay data indicated no significant difference in NF-κB reporter activity between control and DARPP-32 proteins expressing AGS cells with or without TNF-α treatment (Figure 1G). Collectively, these results demonstrated no activation of the NF-κB pathway by DARPP-32 and t-DARPP in gastric cancer cells, suggesting that induction of ANGPT2 by DARPP-32 proteins involves a different signaling mechanism. We have previously shown that overexpression of DARPP-32 proteins activates the AKT pathway, promoting gastric cancer cell survival and chemotherapeutic drug resistance 32. Accordingly, we investigated if the DARPP-32-AKT axis is involved in the regulation of ANGPT2. Our data indicated that the inhibition of AKT by MK2206 in AGS cells did not abrogate the induction of ANGPT2 expression by DARPP-32 proteins (Figure 2A). Surprisingly, the ANGPT2 expression levels were increased following inhibition of AKT (Figure 2A and Supplemental Figure 2). Based on these results, we concluded that the induction of ANGPT2 by DARPP-32 proteins is independent of the AKT pathway in gastric cancer cells. Confirming our results in Figure 1, DARPP-32 and t-DARPP expression had no effect on NF-κB but surprisingly induced phosphorylation of STAT3 (Y705), which was further enhanced following inhibition of AKT (Figure 2B). The substantial increase in the levels of phospho-STAT3 following AKT inhibition in DARPP-32 proteins expressing cells suggests that the DARPP-32-STAT3 axis may be involved in resistance to AKT inhibitors. The immunofluorescence data confirmed activation of STAT3 by DARPP-32, as indicated by increased nuclear p-STAT3 (Y705) levels in AGS cells (Figure 2C–E). In accordance with this result, the luciferase reporter assay data indicated that DARPP-32 expression significantly induced STAT3 transcriptional activity with and without IL-6 treatment (P<0.01, Figure 3A). To investigate the role of STAT3 in the regulation of ANGPT2 by DARPP-32 proteins, we blocked STAT3 activity with a specific inhibitor (AZD1480) or siRNA knockdown in AGS cells transiently expressing DARPP-32, t-DARPP, or empty vector. The suppression of STAT3 significantly decreased the induction of ANGPT2 mRNA (P<0.05, Figure 3B) and protein (Figure 3C) expression by DARPP-32 proteins in AGS cells. Notably, the decrease in ANGPT2 protein levels was observed in both cell lysate and conditioned media in response to STAT3 knockdown (Figure 3C). Together, these results indicated that DARPP-32 proteins induce ANGPT2 mRNA and protein expression through activation of STAT3.
Figure 2. DARPP-32 proteins up-regulate ANGPT2 expression and phosphorylate STAT3.
A–B) Western blot analysis of ANGTP2, phospho-AKT (S473) (P-AKT), phospho-P65 (S536), phospho-STAT3 (Y705) (P-STAT3) in AGS cells transiently overexpressing control, DARPP-32, or t-DARPP treated with or without AKT inhibitor MK2206 (5 μM, overnight). C) Immunofluorescence for DARPP-32 and phospho-STAT3 (Y705) in AGS cells transiently overexpressing DARPP-32 or control (200X). D) Quantitative data for phospho-STAT3 in panel C, *** P<0.001 (t test). E) Western blots confirming immunofluorescence data shown in panel C.
Figure 3. DARPP-32 proteins-induced up-regulation of ANGPT2 expression is dependent on STAT3 pathway.
A) STAT3 reporter analysis in AGS cells stably expressing DARPP-32 (DP32) or control (pcDNA) with or without IL-6 treatment (200 ng/ml, 4 hours), * P<0.05, *** P<0.001 compared with AGS cells stably expressing pcDNA without IL-6 treatment (One way ANOVA). B) ANGPT2 mRNA level in AGS cells transiently overexpressing DARPP-32 proteins or control with or without STAT3 inhibitor AZD1480 (300 nmol/L, overnight), *** P<0.001 compared with AGS control cells without AZD1480 treatment (One way ANOVA). C) Western blot analysis of ANGPT2 in cell lysates and conditioned media of AGS cells transiently overexpressing DARPP-32 proteins or control, with control or STAT3 siRNA knockdown.
DARPP-32 proteins promote angiogenesis through up-regulation of ANGPT2 expression
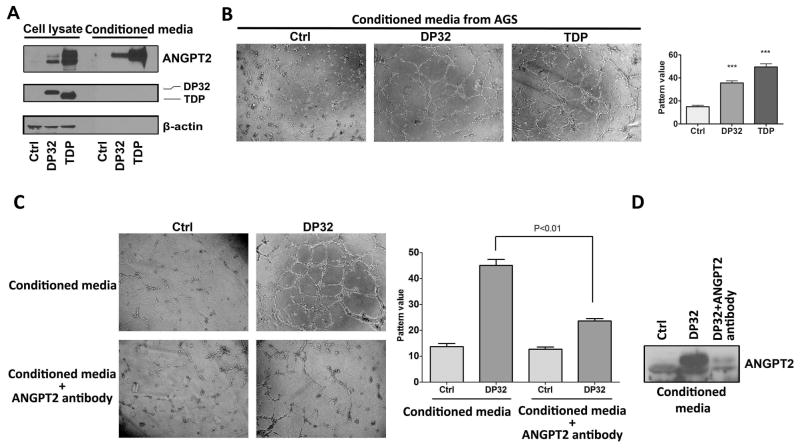
Our data indicated that DARPP-32 proteins induce ANGPT2 expression. The secreted ANGPT2 protein is known to promote angiogenesis; therefore, we investigated the role of DARPP-32 proteins in angiogenesis in gastric cancer. We utilized the HUVEC tube formation in in vitro assay as a measure of angiogenesis. Conditioned media from AGS and MKN-28 cells transiently overexpressing DARPP-32 or t-DARPP contained higher levels of the ANGPT2 protein (Figure 4A and Supplemental Figure 3C), and induced significantly more HUVEC tube formation than control cells (P<0.05, Figure 4B and Supplemental Figure 3A and B). The removal of ANGPT2 from conditioned media of DARPP-32–overexpressing AGS cells using a specific ANGPT2 antibody binding method significantly decreased the HUVEC tube formation (P<0.01, Figure 4C and D). While the conditioned media from AGS wild type cells induced very weak tube formation compared with ANGPT2 or VEGF-α (Supplemental Figure 5A and B). These results clearly indicated that DARPP-32 proteins promote angiogenesis by inducing ANGPT2 expression in gastric cancer cells.
Figure 4. DARPP-32 proteins enhance angiogenesis in gastric cancer in vitro.
A) Western blot data showing ANGPT2 protein levels in cell lysates or conditioned media from AGS cells transiently overexpressing DARPP-32 proteins or control. B) HUVEC tube formation analysis (left panel, 100X) and quantification data (right panel) using conditioned media from AGS cells transiently overexpressing DARPP-32 proteins or control, *** P<0.001 compared with AGS control cells (One way ANOVA). C) HUVEC tube formation analysis (left panel, 100X) and quantification data (right panel, One way ANOVA) using conditioned media from AGS cells transiently overexpressing DARPP-32 or control, with or without ANGPT2 binding antibody. D) Western blot analysis of ANGPT2 in conditioned media from the same cells as panel C.
DARPP-32 proteins regulate tumor growth and angiogenesis in xenograft mouse models
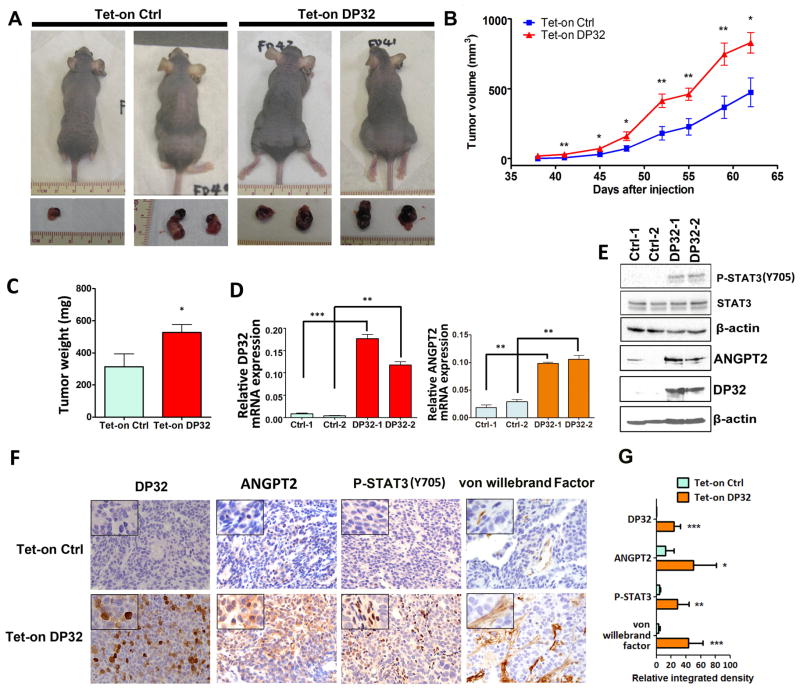
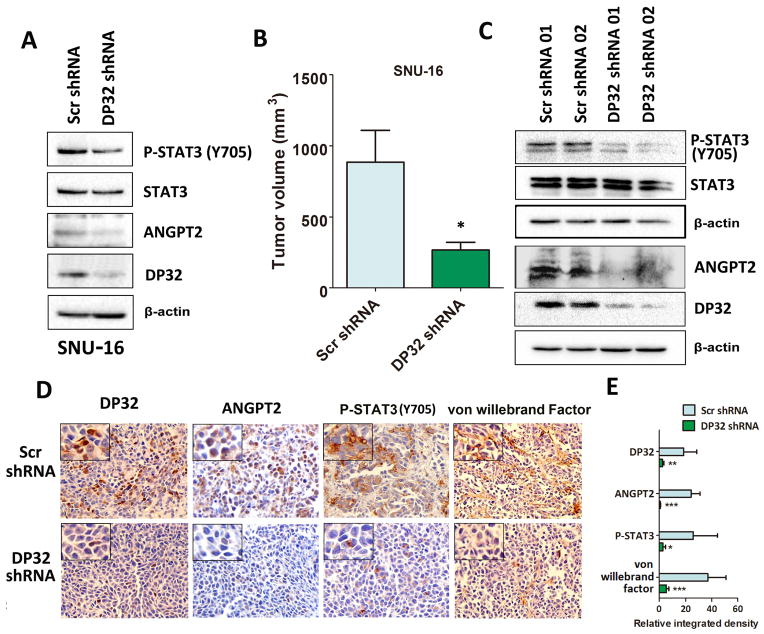
We next investigated the role of DARPP-32 in xenograft gastric tumor growth and angiogenesis in vivo. We utilized the Tet-on inducible AGS cells stably expressing DARPP-32 or empty vector to inject nude mice. Tumors derived from AGS-DARPP-32 cells grew substantially faster than the AGS control tumors (P<0.05, Figure 5A–C). Western blot and qRT-PCR analyses confirmed the higher expression levels of DARPP-32, ANGPT2 and phospho-STAT3 (Y705) in the DARPP-32 xenograft tumors than control tumors (P<0.01, Figure 5D and E). In addition, immunohistochemistry analysis indicated that DARPP-32 expression was associated with high protein levels of ANGPT2, phospho-STAT3 (Y705) and von Willebrand factor, a marker of blood vessels, endothelial cells and indicative of angiogenesis in DARPP-32 xenografts (P<0.001, Figure 5F and G). On the other hand, we examined ANGPT2 expression levels in SNU-16/DARPP-32 shRNA or SNU-16/Scramble shRNA xenografts. As expected, Western blot analysis data indicated that ANGPT2 and phospho-STAT3 (Y705) protein levels were significantly lower in SNU-16 cells stably expressing DARPP-32 shRNA than control cells (Figure 6A). As we previously reported 36, we confirmed that knockdown of DARPP-32 expression decreased xenograft tumor growth compared to control (P<0.05, Figure 6B). Consistent with the in vitro data, knockdown of DARPP-32 expression reduced phospho-STAT3 (Y705) and ANGPT2 protein levels in DARPP-32 shRNA xenograft tumor samples as compared to control samples (Figure 6C). Accordingly, immunohistochemistry data demonstrated that ANGPT2, phospho-STAT3 (Y705) and the von Willebrand factor expression level was significantly lower in DARPP-32 knockdown xenograft tumor samples than control samples (P<0.001, Figure 6D and E). Collectively, our results strongly suggested that DARPP-32 proteins enhance angiogenesis and tumor growth in vivo by up-regulation of ANGPT2 expression.
Figure 5. DARPP-32 expression enhances xenograft gastric tumor growth and angiogenesis in vivo.
A) Representative xenograft tumors of sacrificed mice at end of experiment (day 62). AGS cells stably expressing Tet-on inducible DARPP-32 or control were injected subcutaneously (2×106 cells per site) into nude mice. B) Tumor volume was measured at the indicated times; each data point represents the mean ± standard deviation for 8 xenografts. *P<0.05, **P<0.01 (t test). C) Quantification of tumor weight at the end of experiment. The tumor weight is indicated by mean ± standard deviation, * P<0.05 (t test). D) qRT-PCR analysis of DARPP-32 (left panel) and ANGPT2 (right panel) in xenograft tumor samples, ** P<0.01, P<0.001 (One way ANOVA). E) Western blot analysis of phosphor-STAT3 (Y705), ANGPT2 and DARPP-32 in duplicate xenograft tumor samples. F) Immunohistochemical staining (left panel) and quantification data (right panels) of DARPP-32, ANGPT2, phosphor-STAT3 (Y705) and von Willebrand factor from xenograft tumors (100X), each sample was amplified to show the details. G) Quantification data for panel F, *P<0.05, **P<0.01, *** P<0.001 compared with Tet-on Control cells (t test).
Figure 6. Knockdown of endogenous DARPP-32 expression decreases ANGPT2 protein level, xenograft tumor growth, and angiogenesis.
A) Western blot analysis of DARPP-32, ANGPT2, phospho-STAT3 (Y705) (P-STAT3) proteins in SNU-16 cells stably expressing DARPP-32 shRNA (DP32 shRNA) or scrambled shRNA (Scr shRNA). B) SNU-16 cells stably expressing DARPP-32 shRNA or scrambled shRNA were injected subcutaneously (2×106 cells per site) into nude mice. Tumor volume was measured at the indicated times; each data point represents the mean ± standard deviation for 10 xenografts. C) Western blot analysis of DARPP-32 and ANGPT2 proteins in SNU-16 xenograft tumor samples. D) Immunohistochemical staining of DARPP-32, ANGPT2, phosphor-STAT3 (Y705) and von Willebrand factor in SNU-16 xenograft tumors (100X), each sample was amplified to show the details. E) Quantification data for D), *P<0.05, **P<0.01, ***P<0.001 compared with SNU-16 scrambled shRNA cells (t test).
DARPP-32 proteins and ANGPT2 are frequently co-overexpressed in human gastric cancer samples
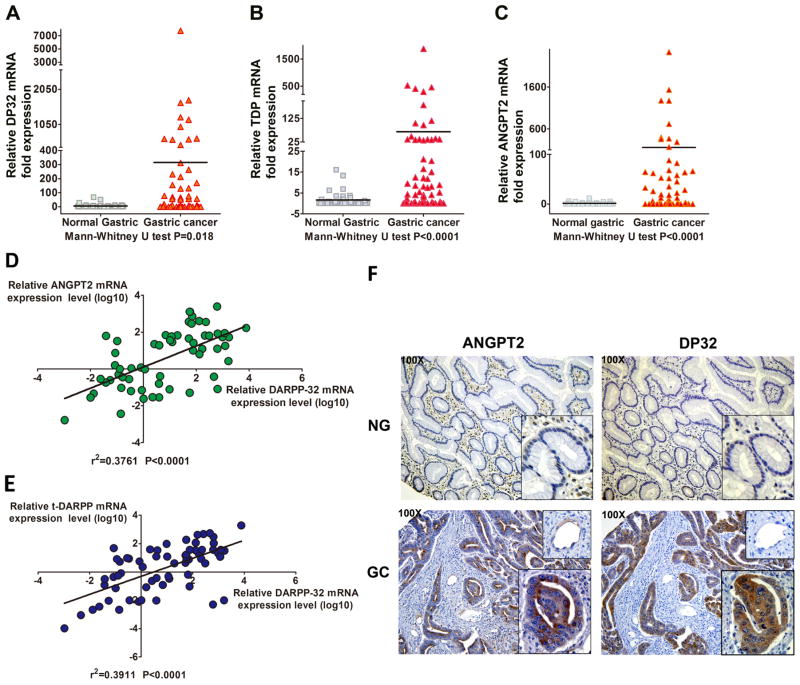
Because of the in vitro data, we investigated the correlation between DARPP-32, t-DARPP and ANGPT2 mRNA expression in de-identified human gastric cancer tissue samples. Our data indicated that DARPP-32, t-DARPP, and ANGPT2 are significantly co-overexpressed in gastric cancer samples (P<0.05, Figure 7A–C). Strong positive correlations were found between ANGPT2 and DARPP-32 (r2=0.4, P<0.0001) or t-DARPP and DARPP-32 (r2=0.4, P<0.0001) mRNA expression levels (Figure 7D and E). These clinical data suggested a possible positive regulation of ANGPT2 expression by DARPP-32 proteins in human gastric cancer.
Figure 7. ANGPT2 and DARPP-32 proteins are up-regulated in human gastric cancer tissue samples.
A–C) qRT-PCR analysis of DARPP-32, t-DARPP, and ANGPT2 mRNA expression in normal gastric and gastric cancer tissue samples. Horizontal line indicated mean. Significance was evaluated by Mann-Whitney U test. D–E) Correlation between ANGPT2 and DARPP-32 (r2=0.4, P<0.0001) or t-DARPP and DARPP-32 (r2=0.4, P<0.0001) mRNA levels in gastric cancer tissue samples (relative mRNA expression levels were converted to log10 values, linear regression). F) Immunohistochemical staining of ANGPT2 and DARPP-32 in normal and gastric cancer tissue samples (100X). Glandula and blood vessel tissues in the normal and gastric cancer samples were amplified to show the details of ANGPT2 and DARPP-32 expression.
Cytosolic co-overexpression of DARPP-32 and ANGPT2 proteins in human gastric epithelial tumor cells
To investigate whether DARPP-32 regulates ANGPT2 through a paracrine or an autocrine mechanism with regard to the cellular origin of ANGPT2, we examined ANGPT2 and DARPP-32 expression level in human normal and gastric cancer tissue samples by immunohistochemistry. Our results demonstrated that normal gastric glandular tissue expressed low (or undetectable) levels of DARPP-32 and ANGPT2 proteins, whereas gastric cancer epithelial cells showed high levels of DARPP-32 and ANGPT2 predominantly in the cytoplasm (Figure 7F). Meanwhile, blood vessel cells in gastric cancer tissues expressed low levels of endogenous ANGPT2 but not DARPP-32. As expected, more blood vessels, indicative of increased angiogenesis, were observed in cancer tissues overexpressing DARPP-32 than normal samples (Figure 7F). These data clearly demonstrated that DARPP-32 regulates epithelial tumor-derived ANGPT2 through an autocrine mechanism in gastric cancer.
Discussion
DARPP-32 proteins are frequently overexpressed and amplified in gastric cancer 28, 29, 38, promoting cancer cell growth, invasion, and survival 29, 30, 36. Angiogenesis is an important biological activity that promotes tumor growth and metastasis 39. ANGPT2 has been recently reported to play a key role in angiogenesis in different types of cancer 11, 40.
In this study, we investigated the expression and regulation of ANGPT2 in gastric cancer. Our results demonstrate that DARPP-32 proteins promote angiogenesis through the regulation of ANGPT2 expression and secretion from gastric cancer cells. The T34, located in the N-terminal domain of DARPP-32, plays a critical role in regulating PP1 activity in the brain 41, 42. Our findings suggest that mutations of the T34 in DARPP-32 as well as t-DARPP, lacking the N-terminal domain, are able to induce ANGPT2. These findings indicate that PP1 is not a critical factor in regulating ANGPT2 in gastric cancer. On the other hand, our results demonstrate that DARPP-32 regulates ANGPT2 by activating STAT3. This is supported by several lines of evidence: 1) We showed that DARPP-32 proteins up-regulated ANGPT2 mRNA and protein levels in different gastric cancer cell models; 2) Knockdown of DARPP-32 expression decreased the ANGPT2 protein level in the SNU-16 gastric cancer cell model; 3) DARPP-32 proteins-induced ANGPT2 expression promoted angiogenesis as indicated by enhanced tube formation of HUVECs in vitro, and increased xenograft tumor growth and vascularization in vivo; 4) Knockdown of DARPP-32 expression down-regulated ANGPT2, decreasing xenograft tumor growth and angiogenesis in vivo; 5) Using STAT3 luciferase reporter and immunofluorescence analysis, we found that DARPP-32 proteins activate STAT3, which was essential for the up-regulation of ANGPT2; 6) Clinical data from human primary gastric tumors indicated that DARPP-32 and ANGPT2 were frequently co-overexpressed and strongly correlated in gastric cancer.
Several cytokines such as hypoxia-inducible factor 1 (HIF-1) and VEGF can regulate ANGPT2 expression in different physiological and pathological conditions, establishing a link between inflammation and cancer 43. Previous studies indicated that TNF-α induces ANGPT2 expression in HUVECs, human lymphatic, or blood vascular endothelial cells 21, 44. Therefore, we postulated that ANGPT2 regulation by TNF-α might be mediated by a possible DARPP-32–NF-κB axis in gastric cancer cells. Our data showed that DARPP-32 proteins enhanced ANGPT2 expression independent of NF-κB. Interestingly, our data indicated that DARPP-32 proteins, in combination with TNF-α, synergistically induced higher ANGPT2 levels than DARPP-32 proteins alone in gastric cancer cells. We have previously shown that the DARPP-32 proteins play an important role in the activation of PI3K/AKT pathway in upper gastrointestinal cancer cells 32, 35. In this study, we examined the potential implication of AKT pathway in the regulation of ANGPT2 by DARPP-32 proteins. Surprisingly, we found that pharmacologic inhibition of AKT enhanced, not suppressing, DARPP-32–induced ANGPT2 expression. Therefore, our results suggested that the regulation of ANGPT2 by DARPP-32 proteins was not mediated by NF-κB or AKT signaling pathways.
STAT3 regulates several critical functions such as proliferation and angiogenesis in human normal and malignant tissues 45–47. Herein, we demonstrated that transient overexpression of DARPP-32 proteins induced strong phosphorylation and nuclear localization of STAT3 (Y705) in gastric cancer cell lines. Conversely, knockdown of DARPP-32 decreased the phospho-STAT3 (Y705) protein level in gastric cancer cells. Importantly, the induction of ANGPT2 by DARPP-32 proteins was sharply decreased by the specific STAT3 inhibitor (AZD1480) or siRNA knockdown in AGS cells. These results clearly demonstrated that the induction of ANGPT2 by DARPP-32 proteins is mediated by the activation of STAT3.
ANGPT2 is a protein secreted by endothelial cells under normal physiological conditions 12. We investigated the role of secreted DARPP-32–induced ANGPT2 protein in angiogenesis using the HUVEC tube formation assay. Our data showed that conditioned media from DARPP-32 proteins overexpressing cells promoted tube formation in HUVECs. We confirmed that this induction of tube formation was dependent on ANGPT2, as the removal of ANGPT2 from the conditioned media abrogated the promotion of tube formation by DARPP-32. Our in vivo xenograft mouse model also demonstrated the pro-angiogenic function of DARPP-32. These results clearly indicated that DARPP-32 enhances angiogenesis through the regulation of ANGPT2 in gastric cancer. VEGF-α is an important cytokine that induces angiogenesis in cancer 48, 49. We found that gastric cancer cells express endogenous levels of VEGF-α that are not modulated by DARPP-32. While our data demonstrate that DARPP-32 proteins induce angiogenesis, mainly through the regulation of ANGPT2, we can not rule out the contribution of other existing factors such as VEGF-α in this process.
Our in vivo animal data demonstrated that DARPP-32 was overexpressed in xenograft tumors and this was accompanied with an increase in ANGPT2 expression, blood vessels, and tumor growth; validating the data from the in vitro cell models. Our in vitro data also indicated that the pro-angiogenic function of DARPP-32 was dependent on the induction of ANGPT2 expression. In contrast, the knockdown of DARPP-32 in xenograft tumors significantly decreased ANGPT2 expression levels, angiogenesis, and tumor growth. ANGPT2 plays an important role in blood vessel formation, especially tumor initiation 17. In fact, a higher level of ANGPT2 was correlated with poor prognosis in breast cancer 13, hepatocellular carcinoma 14 and leukemia 50. Notably, our data indicated higher levels of ANGPT2 expression in primary human gastric tumors and a strong correlation between ANGPT2 and DARPP-32. We could not confirm the correlation with clinical outcome due to our small sample size; analysis of additional clinical samples could establish this pattern. Our findings demonstrated that DARPP-32 and ANGPT2 were highly expressed in the cytoplasm in gastric cancer cells but only ANGPT2 was weakly expressed in tumor-associated endothelial cells in human gastric tumor samples. This is in line with the results from Moon and his colleagues’ study, showing the expression of ANGPT2 in hepatocellular cancer cells 51. Interestingly, tumor stromal tissue displayed even lower expression level of ANGPT2 than normal gastric connective tissue. These results suggested that DARPP-32-induced ANGPT2 in gastric cancer may function as a promoter of angiogenesis through a paracrine mode of action. Further studies will be required to investigate the contribution of endogenous ANGPT2 in gastric cancer epithelial cells to angiogenesis through regulation of blood vessel endothelial cells. Collectively, our data clearly indicated that the DARPP-32-STAT3 axis enhances gastric cancer angiogenesis through the up-regulation of ANGPT2.
In conclusion, our findings demonstrate, for the first time, to our knowledge, that DARPP-32 proteins play a major role in promoting angiogenesis by regulating the expression and secretion of ANGPT2 in gastric cancer cells. The DARPP-32-dependent angiogenesis provides a new paradigm in gastric tumorigenesis which could have an impact on its treatment.
Supplementary Material
What is already known about this subject?
Angiopoietin 2 plays a key role in angiogenesis.
DARPP-32 proteins overexpression was frequently observed along the stages of gastric carcinogenesis.
DARPP-32 proteins promote chemotherapeutic drug resistance and survival of cancer cells.
DARPP-32 promotes invasion of gastric cancer cells.
What are the new findings?
This study demonstrates that DARPP-32 proteins up-regulate ANGPT2 mRNA and protein expression levels in gastric cancer cells.
DARPP-32 proteins regulate ANGPT2 expression and secretion through activation of STAT3 in gastric cancer cells.
ANGPT2 is induced in human gastric tumor epithelial cells, not in tumor associated vascular endothelial cells, by DARPP-32.
This study strongly suggests that the DARPP-32-STAT3-ANGPT2 axis regulates angiogenesis in gastric cancer.
How might it impact on clinical practice in the foreseeable future?
Understand the mechanisms of how DARPP-32 proteins enhance angiogenesis in gastric cancer.
DARPP-32-STAT3-ANGPT2 axis provides a new paradigm in gastric carcinogenesis.
The future development of potential inhibitors against DARPP-32 and ANGPT2 may offer a therapeutic window in gastric cancer.
Acknowledgments
This study was supported by grants from the National Institute of Health; R01CA93999 (WER), Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- DARPP-32
dopamine and cAMP-regulated phosphoprotein, Mr 32000
- t-DARPP
truncated isoform of DARPP-32
- ANGPT2
angiopoietin 2
- VEGFA
vascular endothelial growth factor A
- IGF1
insulin-like growth factor 1
- HIF1
hypoxia inducible factors 1
- PDGFB
platelet-derived growth factor B
- TNF-α
tumor necrosis factor-α
- JAK2
Janus kinase 2
- CXCR4
(C-X-C motif) receptor 4
- PI3K
phosphoinositide-3-kinase
- BCL2
B-cell CLL/lymphoma 2
- EGFR
epidermal growth factor receptor
- ERBB2
erb-b2 receptor tyrosine kinase 2
- ERBB3
erb-b2 receptor tyrosine kinase 3
Footnotes
Conflict of interest: The authors declare no conflict of interest.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or Vanderbilt University.
Author contributions:
Zheng Chen: experimental design and acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis.
Shoumin Zhu: analysis and interpretation of in vitro data; statistical analysis; technical support.
Mohammed Soutto: experimental trouble shooting and assisted in interpretation of data.
Jun Hong: technical and experimental support.
DunFa Peng: assisted in the in vitro experiments.
Abbes Belkhiri: analysis and interpretation of data; experimental troubleshooting; drafting of the manuscript; critical revision of the manuscript.
Zekuan Xu: technical and experimental support.
Wael El-Rifai: study concept and design; study supervision; study support; critical revision of the manuscript for important intellectual content.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clinical microbiology reviews. 2010;23:713–39. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. International journal of cancer Journal international du cancer. 2009;125:666–73. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 4.Davis PA, Sano T. The difference in gastric cancer between Japan, USA and Europe: what are the facts? what are the suggestions? Critical reviews in oncology/hematology. 2001;40:77–94. doi: 10.1016/s1040-8428(00)00131-1. [DOI] [PubMed] [Google Scholar]
- 5.Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, Morgan D. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer causes & control : CCC. 2013;24:249–56. doi: 10.1007/s10552-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P. Is gastric cancer preventable? Gut. 2004;53:1217–9. doi: 10.1136/gut.2004.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crew KD, Neugut AI. Epidemiology of gastric cancer. World journal of gastroenterology : WJG. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishiyama M, Eguchi H. Pharmacokinetics and pharmacogenomics in gastric cancer chemotherapy. Advanced drug delivery reviews. 2009;61:402–7. doi: 10.1016/j.addr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Reinmuth N, Parikh AA, Ahmad SA, Liu W, Stoeltzing O, Fan F, Takeda A, Akagi M, Ellis LM. Biology of angiogenesis in tumors of the gastrointestinal tract. Microscopy research and technique. 2003;60:199–207. doi: 10.1002/jemt.10258. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nature reviews Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 12.Hu B, Cheng SY. Angiopoietin-2: development of inhibitors for cancer therapy. Current oncology reports. 2009;11:111–6. doi: 10.1007/s11912-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sfiligoi C, de Luca A, Cascone I, Sorbello V, Fuso L, Ponzone R, Biglia N, Audero E, Arisio R, Bussolino F, Sismondi P, De Bortoli M. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. International journal of cancer Journal international du cancer. 2003;103:466–74. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 14.Mitsuhashi N, Shimizu H, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F, Yoshidome H, Kato A, Nukui Y, Miyazaki M. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37:1105–13. doi: 10.1053/jhep.2003.50204. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa M, Yamamoto H, Nagano H, Miyake Y, Sugita Y, Hata T, Kim BN, Ngan CY, Damdinsuren B, Ikenaga M, Ikeda M, Ohue M, Nakamori S, Sekimoto M, Sakon M, Matsuura N, Monden M. Hepatic expression of ANG2 RNA in metastatic colorectal cancer. Hepatology. 2004;39:528–39. doi: 10.1002/hep.20048. [DOI] [PubMed] [Google Scholar]
- 16.Lind AJ, Wikstrom P, Granfors T, Egevad L, Stattin P, Bergh A. Angiopoietin 2 expression is related to histological grade, vascular density, metastases, and outcome in prostate cancer. The Prostate. 2005;62:394–9. doi: 10.1002/pros.20163. [DOI] [PubMed] [Google Scholar]
- 17.Nasarre P, Thomas M, Kruse K, Helfrich I, Wolter V, Deppermann C, Schadendorf D, Thurston G, Fiedler U, Augustin HG. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer research. 2009;69:1324–33. doi: 10.1158/0008-5472.CAN-08-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashizume H, Falcon BL, Kuroda T, Baluk P, Coxon A, Yu D, Bready JV, Oliner JD, McDonald DM. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer research. 2010;70:2213–23. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. Journal of cell science. 2005;118:771–80. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 20.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, Johnson RS, Imityaz HZ, Simon MC, Fredlund E, Greten FR, Rius J, Lewis CE. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–59. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim I, Kim JH, Ryu YS, Liu M, Koh GY. Tumor necrosis factor-alpha upregulates angiopoietin-2 in human umbilical vein endothelial cells. Biochemical and biophysical research communications. 2000;269:361–5. doi: 10.1006/bbrc.2000.2296. [DOI] [PubMed] [Google Scholar]
- 22.Ji Y, Wang Z, Li Z, Li K, Le X, Zhang T. Angiotensin II induces angiogenic factors production partly via AT1/JAK2/STAT3/SOCS3 signaling pathway in MHCC97H cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;29:863–74. doi: 10.1159/000171034. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Gao FH, Wang JY, Liu F, Yuan HH, Zhang WY, Jiang B. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung cancer. 2011;73:366–74. doi: 10.1016/j.lungcan.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Bid HK, Oswald D, Li C, London CA, Lin J, Houghton PJ. Anti-angiogenic activity of a small molecule STAT3 inhibitor LLL12. PLoS One. 2012;7:e35513. doi: 10.1371/journal.pone.0035513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Jiang T, Zhu L, Liu J, Cao J, Huang KJ, Qiu ZJ. STAT3-targeting RNA interference inhibits pancreatic cancer angiogenesis in vitro and in vivo. International journal of oncology. 2011;38:1637–44. doi: 10.3892/ijo.2011.1000. [DOI] [PubMed] [Google Scholar]
- 26.Qian WF, Guan WX, Gao Y, Tan JF, Qiao ZM, Huang H, Xia CL. Inhibition of STAT3 by RNA interference suppresses angiogenesis in colorectal carcinoma. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica … [et al.] 2011;44:1222–30. doi: 10.1590/s0100-879x2011007500143. [DOI] [PubMed] [Google Scholar]
- 27.Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- 28.El-Rifai W, Smith MF, Jr, Li G, Beckler A, Carl VS, Montgomery E, Knuutila S, Moskaluk CA, Frierson HF, Jr, Powell SM. Gastric cancers overexpress DARPP-32 and a novel isoform, t-DARPP. Cancer research. 2002;62:4061–4. [PubMed] [Google Scholar]
- 29.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer research. 2005;65:6583–92. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 30.Zhu S, Hong J, Tripathi MK, Sehdev V, Belkhiri A, El-Rifai W. Regulation of CXCR4-mediated invasion by DARPP-32 in gastric cancer cells. Molecular cancer research : MCR. 2013;11:86–94. doi: 10.1158/1541-7786.MCR-12-0243-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vangamudi B, Peng DF, Cai Q, El-Rifai W, Zheng W, Belkhiri A. t-DARPP regulates phosphatidylinositol-3-kinase-dependent cell growth in breast cancer. Molecular cancer. 2010;9:240. doi: 10.1186/1476-4598-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belkhiri A, Dar AA, Zaika A, Kelley M, El-Rifai W. t-Darpp promotes cancer cell survival by up-regulation of Bcl2 through Akt-dependent mechanism. Cancer research. 2008;68:395–403. doi: 10.1158/0008-5472.CAN-07-1580. [DOI] [PubMed] [Google Scholar]
- 33.Muto J, Shirabe K, Sugimachi K, Maehara Y. A review of angiogenesis in hepatocellular carcinoma. Hepatology research : the official journal of the Japan Society of Hepatology. 2014 doi: 10.1111/hepr.12310. [DOI] [PubMed] [Google Scholar]
- 34.Belkhiri A, Dar AA, Peng DF, Razvi MH, Rinehart C, Arteaga CL, El-Rifai W. Expression of t-DARPP mediates trastuzumab resistance in breast cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4564–71. doi: 10.1158/1078-0432.CCR-08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong J, Katsha A, Lu P, Shyr Y, Belkhiri A, El-Rifai W. Regulation of ERBB2 receptor by t-DARPP mediates trastuzumab resistance in human esophageal adenocarcinoma. Cancer research. 2012;72:4504–14. doi: 10.1158/0008-5472.CAN-12-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu S, Belkhiri A, El-Rifai W. DARPP-32 increases interactions between epidermal growth factor receptor and ERBB3 to promote tumor resistance to gefitinib. Gastroenterology. 2011;141:1738–48. e1–2. doi: 10.1053/j.gastro.2011.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HB, Horiuchi A, Watanabe T, Shih SR, Tsay HJ, Li HC, Greengard P, Nairn AC. Characterization of the inhibition of protein phosphatase-1 by DARPP-32 and inhibitor-2. The Journal of biological chemistry. 1999;274:7870–8. doi: 10.1074/jbc.274.12.7870. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee K, Peng D, Brifkani Z, Belkhiri A, Pera M, Koyama T, Koehler EA, Revetta FL, Washington MK, El-Rifai W. Dopamine and cAMP regulated phosphoprotein MW 32 kDa is overexpressed in early stages of gastric tumorigenesis. Surgery. 2010;148:354–63. doi: 10.1016/j.surg.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 40.Morrissey C, True LD, Roudier MP, Coleman IM, Hawley S, Nelson PS, Coleman R, Wang YC, Corey E, Lange PH, Higano CS, Vessella RL. Differential expression of angiogenesis associated genes in prostate cancer bone, liver and lymph node metastases. Clinical & experimental metastasis. 2008;25:377–88. doi: 10.1007/s10585-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 41.Sim AT. The regulation and function of protein phosphatases in the brain. Molecular neurobiology. 1991;5:229–46. doi: 10.1007/BF02935548. [DOI] [PubMed] [Google Scholar]
- 42.Slobodyansky E, Aoki Y, Gaznabi AK, Aviles DH, Fildes RD, Jose PA. Dopamine and protein phosphatase activity in renal proximal tubules. The American journal of physiology. 1995;268:F279–84. doi: 10.1152/ajprenal.1995.268.2.F279. [DOI] [PubMed] [Google Scholar]
- 43.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. The Journal of biological chemistry. 1999;274:15732–9. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- 44.Yan ZX, Jiang ZH, Liu NF. Angiopoietin-2 promotes inflammatory lymphangiogenesis and its effect can be blocked by the specific inhibitor L1-10. American journal of physiology Heart and circulatory physiology. 2012;302:H215–23. doi: 10.1152/ajpheart.00895.2011. [DOI] [PubMed] [Google Scholar]
- 45.Rhee YH, Jeong SJ, Lee HJ, Koh W, Jung JH, Kim SH, Sung-Hoon K. Inhibition of STAT3 signaling and induction of SHP1 mediate antiangiogenic and antitumor activities of ergosterol peroxide in U266 multiple myeloma cells. BMC cancer. 2012;12:28. doi: 10.1186/1471-2407-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokogami K, Yamashita S, Takeshima H. Hypoxia-induced decreases in SOCS3 increase STAT3 activation and upregulate VEGF gene expression. Brain tumor pathology. 2012 doi: 10.1007/s10014-012-0122-0. [DOI] [PubMed] [Google Scholar]
- 47.Shin J, Lee HJ, Jung DB, Jung JH, Lee EO, Lee SG, Shim BS, Choi SH, Ko SG, Ahn KS, Jeong SJ, Kim SH. Suppression of STAT3 and HIF-1 alpha mediates anti-angiogenic activity of betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS One. 2011;6:e21492. doi: 10.1371/journal.pone.0021492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, Frasca D, Koker M, Konig K, Meder L, Rauh D, Buettner R, Wolf J, Brekken RA, Neumaier B, Christofori G, Thomas RK, Ullrich RT. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. The Journal of clinical investigation. 2013 doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasahira T, Kurihara M, Bhawal UK, Ueda N, Shimomoto T, Yamamoto K, Kirita T, Kuniyasu H. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. British journal of cancer. 2012;107:700–6. doi: 10.1038/bjc.2012.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maffei R, Martinelli S, Santachiara R, Rossi D, Guarnotta C, Sozzi E, Zucchetto A, Rigolin GM, Fiorcari S, Castelli I, Fontana M, Coluccio V, Leonardi G, Zucchini P, Tripodo C, Cuneo A, Gattei V, Del Poeta G, Forconi F, Gaidano G, Torelli G, Marasca R. Angiopoietin-2 plasma dosage predicts time to first treatment and overall survival in chronic lymphocytic leukemia. Blood. 2010;116:584–92. doi: 10.1182/blood-2009-11-252494. [DOI] [PubMed] [Google Scholar]
- 51.Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16:552–7. doi: 10.1097/01.MP.0000071841.17900.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.