Abstract
Traumatic brain injury (TBI) is acquired from an external force, which can inflict devastating effects to the brain vasculature and neighboring neuronal cells. Disruption of vasculature is a primary effect that can lead to a host of secondary injury cascades. The primary effects of TBI are rapidly occurring while secondary effects can be activated at later time points and may be more amenable to targeting. Primary effects of TBI include diffuse axonal shearing, changes in blood brain barrier (BBB) permeability, and brain contusions. These mechanical events, especially changes to the BBB, can induce calcium perturbations within brain cells producing secondary effects, which include cellular stress, inflammation, and apoptosis. These secondary effects can be potentially targeted to preserve the tissue surviving the initial impact of TBI. In the past, TBI research had focused on neurons without any regard for glial cells and the cerebrovasculature. Now a greater emphasis is being placed on the vasculature and the neurovascular unit following TBI. A paradigm shift in the importance of the vascular response to injury has opened new avenues of drug treatment strategies for TBI. However, a connection between the vascular response to TBI and the development of chronic disease has yet to be elucidated. Long-term cognitive deficits are common amongst those sustaining severe or multiple mild TBIs. Understanding the mechanisms of cellular responses following TBI is important to prevent the development of neuropsychiatric symptoms. With appropriate intervention following TBI, the vascular network can perhaps be maintained and the cellular repair process possibly improved to aid in the recovery of cellular homeostasis.
Introduction
Traumatic brain injury (TBI) represents an enormous societal burden both with regard to prevalence/incidence and economic cost (initial treatment and long-term care due to high morbidity), largely irrespective of initial injury mechanism. In fact, TBI is the leading cause of trauma-related morbidity and mortality in developed countries, with over 55 million people affected internationally (24). Interestingly, the mechanism of TBI sustained is often related to the patient’s age with younger patients more likely suffering TBI as the result of motor vehicle accidents, sports, or battlefield exposure to blast waves whereas the elderly population is generally afflicted by falls (neurogenic or cardiogenic in origin). TBI is also distributed bimodally with peak incidences between 15–24 years, and after 75 years of age. Notably, after age 65, patients have increased mortality and worse functional outcome following TBI (169). The most prominent cause of TBIs is motor vehicle collisions (38). Concussive injuries are also high amongst professional athletes and the active military due to the high-risk for a neurotraumatic event to occur on the job (59, 143). These sub-populations should therefore be the focus for future translational studies.
TBI is unique in that it is always acquired from an external force. One difficulty when treating TBI patients is that we never know when, or how, a TBI will occur. Therefore, the contribution of genetic predisposition and comorbidities to overall post-injury outcomes are hard to ascertain. A valuable scale, called the Glasgow Coma Scale (GCS), is used to assess verbal, motor, and eye-opening responses in order to classify TBI severity (80). The score is based on a 15-point scale. Mild injury correlates with a score of 13+, moderate with a score 9–12, and severe with a score of <8. Its clinical utility is mostly to help determine patient status in relation to intracerebral pressure (16). As such, the clinician can use the score to determine the need for appropriate treatment or management. The values obtained from the GCS help define the severity of TBI and allows clinicians to address the injury more appropriately (52). In addition, patients can display cognitive, emotional, and sensory impairments following mild TBIs and even exhibit physical impairments following more severe forms of TBI. Research now suggests that visible signs, or symptoms, of neurological dysfunction from sports-related TBI may not develop for an extended period of time (15).
Imaging modalities have recently been used to detect some of the subtle injury changes associated with TBI. Microdialysis in combination with nuclear magnetic resonance imaging was used to determine that TBI patients have increased anaerobic metabolism dependent on the pentose phosphate pathway (73). Diffusion tensor imaging has been used with mixed success in detecting white track lesions following concussion (163). Functional magnetic resonance imaging (fMRI) has been used to tease out differences in Salience Network functioning following TBI indicative of failed cognitive control (76). In the clinic, Czosynka’s pressure reactivity index can be used to establish a dynamic target for cerebral perfusion pressure (42). Another important consideration is monitoring of cerebral blood flow to prevent the occurrence of ischemia following TBI. Positron emission tomography, perfusion-weighted MRI, and the perfusion computed tomography scan may be used for this endeavor (148).
Two general forms of TBI exist: closed head injury and penetrating head injury (134). TBI is grouped into three levels of severity: mild, moderate and severe (135). Further subclassification of injury is based on symptoms. Subconcussive injury can have no initial symptoms, concussive injury (acute) presents with cognitive, emotional, and sleep symptoms, while juvenile head trauma syndrome presents with a lucid interval and unconsciousness. Post-concussive syndrome involves concussive symptoms lasting greater than 3 months, and chronic traumatic encephalopathy involves a several year symptom free stage followed by onset of neuropsychiatric symptoms (110). Mild TBIs are common and can go undetected with conventional screening tools, although balance tests and cognitive measurements have been used with varied success rates (61, 152). Typically, mild TBI leaves the subject conscious with mild symptoms; however, rodent students have shown that secondary effects often lead to chronic deficits in cognition, memory, and behavior (60, 112). Molecular mechanisms of the secondary effects of mild and moderate TBI are being investigated due to the increased awareness of how these effects contribute to chronic neuropsychiatric symptoms (84). Severe TBI is often life-threatening and requires immediate care. Fewer basic scientists study severe TBI because its effects are more likely irreversible in both the short-term and the long-term.
We have limited knowledge of what brain regions are most affected after a TBI, due to the fact the injury can either be focal or diffuse depending on the type and severity. As such, clinicians struggle with identifying which brain regions have been affected after injury. The brain’s response to TBI is more often globally distributed with a vast number of dilated perivascular spaces (71). Pathologic changes are scattered throughout the brain as well and are highly dependent on neuroinflammation as evident from gliosis (168). With the many different forms of TBI, the diagnosis and treatment plans must be adaptable and situational specific.
To date, the primary focus of TBI research has been on neurons with little emphasis on glial cells or the cerebrovasculature. Indeed, for this review, we identified 1030 published reports that assess neurons, and only 326 reports that assessed other brain cell types, including 46 reports on brain microvessels. The current skewing of research in TBI literature toward neurons is understandable, given the underlying goal of protecting neurons and preserving brain function. A neurocentric approach however has not resulted in successful translation of therapeutics in TBI (78, 102), or other models of neural injury (85). It is clear other approaches are needed to be successful. Microvessel disruption plays a substantial role in primary, secondary and chronic effects of TBI, and understanding the response of the vascular system on brain trauma is critical to our ability to effectively treat brain trauma. As such, it is clear other approaches are needed and this review deals with the response of all brain cell types to trauma and focuses on the potential role of microvessel damage to neuronal loss and dysfunction.
Forms of Traumatic Brain Injury
In human subjects, there are a number of causes of TBI and include motor vehicle collisions, falls, sport-associated injuries, blast exposure, and shaken baby syndrome in infants (Table 2)(189). Besides characterizing the injury based on a specific inciting event, the injury can be described as open or closed head injury, focal or global injury, and impact or blast in origin. Blast injury can be subdivided further into primary, secondary, tertiary, or quaternary injury, a level of detail beyond the scope of this review but discussed in detail elsewhere in the literature (33, 185). The different forms of neurotrauma can further be classified based on clinical features through the GCS assessment, pathological features through advanced imaging assessment, or a combination of the two.
Table 2.
Forms of Traumatic Brain Injury
| TYPE | DESCRIPTION | ANIMAL MODEL | REFERENCES |
|---|---|---|---|
| Blast Traumatic Brain Injury | Blast energy generated from an explosive device contacts the skull and causes acceleration/deceleration injury | Blast model with short driving section for clinical accuracy | Alford et al., 2011, Arun et al., 2013, Cho et al., 2013, DeWitt et al., 2009, Turner et al., 2013 |
| Closed Head Impact | Blunt object strikes the skull without fracturing the bone | Impact-acceleration model | Li et al., 2013, Skandsen et al., 2010, Turner et al., 2012 |
| Concussion and subconcussion | A temporary state of shock due to a violent blow to the head | Controlled closed-head impact with helmet | Angoa-Perez et al., 2014, Bailes et al., 2013, Bailes et al., 2014, Petraglia et al., 2014 |
| Contusion | An area of injured brain where blood capillaries have been ruptured | Fluid Percussion model | Brodhun et al., 2001, Greer et al., 2013, Truettner et al., 2007 |
| Falls | Rapid impact between ground or object with skull due to effects of gravity | Weight drop TBI model | Albert-Weissenberger and Siren, 2010, Asl et al., 2013 |
| Penetrating injury | Fragment from shrapnel or blunt object that fractures skull and causes protrusion into brain parenchyma | Controlled cortical impact with craniectomy | Begum et al., 2014, Clark et al., 1997 |
| Shaken baby syndrome | Abusive head trauma due to rapid acceleration and deceleration of skull when shaken | Controlled cortical impact with intact skull | Dileonardi et al., 2009, Foster et al., 2014, Larner et al., 2004 |
| Spinal Cord Injury | Injury to any part of the spinal cord that leads to inflammation and secondary effects on the CNS | Spinal impact model | Faden et al., 1989, Roth et al., 2014, Xiong et al., 2013 |
While numerous causes of neurotrauma exist, TBI is uniformly associated with both primary and secondary injury mechanisms. The primary injury is induced instantaneously by the application of external forces to the skull and associated brain tissue, depending on the form of TBI. Impact TBI induces focal brain contusions (170), while blast TBI globally shears axons (140) and damages brain microvessels (2) (see Fig 1, Adapted from (175)). The secondary injury follows the primary injury temporally and rodent studies have shown it is associated with induction of signaling cascades that alter metabolic, cellular and molecular events, ultimately leading to alteration in cellular function and/or death (145). This period of secondary injury can last from minutes to years and is mediated by biochemical processes that may be targeted by and amenable to therapeutic development (141); whereas primary injury can only be prevented (through safety devices, preventative measures, reduced exposure, etc).
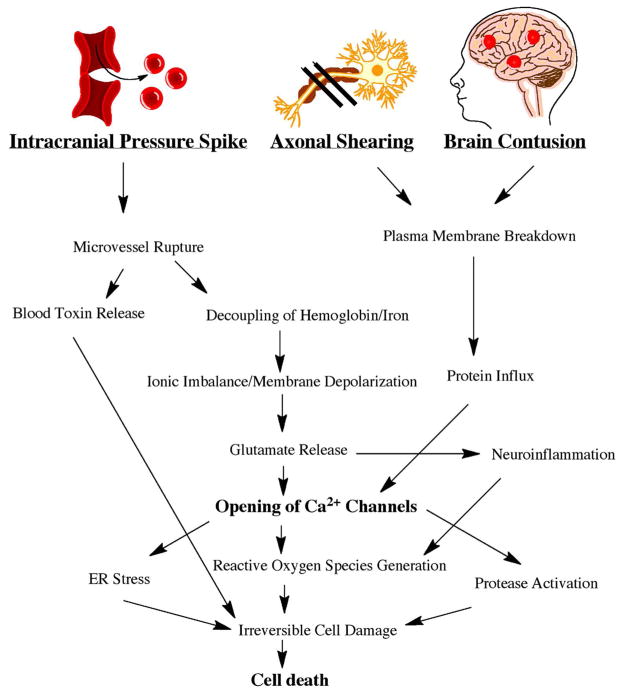
Figure 1.
Pathophysiology of Neuronal Cell Death Following Traumatic Brain Injury. An intracranial pressure spike, axonal shearing, and brain contusion contribute to secondary mechanisms that lead to an increase in Ca2+ channel opening. The generation of reactive oxygen species can ultimately contribute to cell death.
To improve understanding of TBI pathophysiology, as well as develop potential therapeutic agents, an array of TBI models have been created and utilized. Some of the most commonly utilized models include the fluid percussion models; variations of the impact acceleration or weight drop models, the controlled cortical impact models, and variations of the shock tube blast models. These models and others such as penetrating injury models are reviewed in depth elsewhere (5, 9, 182, 189). Importantly, TBI represents a highly heterogeneous condition due to the various mechanisms involved and range in forces that are applied/sustained. As such, it is unlikely that one model is adequate for all TBI research and consequently a multi-faceted approach is likely required. This is reflected in the approach taken by oversight and advisory groups in which consortiums have been formed to evaluate proposed therapeutics across a range of models in search of identifying not only promising therapeutic candidates but also the most appropriate scenarios in which to clinically test agents (83).
Importantly, the diversity of pathophysiological processes involved in neurotrauma has been increasingly recognized with recent studies investigating the role of non-neuronal cell types, such as astrocytes and microglia in neurotrauma, as well as the role of the neurovascular unit and associated blood-brain barrier (BBB). Emerging evidence with in vivo models has implicated these cell types and the BBB in outcomes following neurotrauma, with a particular emphasis on both microvascular and macroscopic components of vascular disruption (75, 96, 111, 114, 137, 186). This is particularly relevant clinically as macroscopic bleeds, such as epidural and subdural hematomas, may be managed neurosurgically, and microvascular/microscopic bleeds have been implicated in neurodegeneration associated with neurotrauma (111, 180).
Traumatic Brain Injury Effects on Neurons
Following TBI there are immediate primary effects and sub-acute secondary effects. Primary effects to the brain from TBI cause damage to neurons, glia, and the vasculature (Table 3). Secondary effects to the brain from TBI include: inflammatory responses (18), cellular stress (50), and apoptotic cascades (31). The physical forces of TBI can shear axons (140), break down plasmalemma (36), and rupture brain microvessels (10). When an axon is torn, or a cell membrane is broken from the external forces of TBI, the neurons can rapidly depolarize and activate voltage gated Ca2+ channels; thereby, increasing intracellular Ca2+ (58). Appropriate modeling of the primary effects of TBI will enable researchers to effectively study therapeutic options for the secondary effects.
Table 3.
Neurotoxic Events in Traumatic Brain Injury
| Primary | Acute Phase Physiology | References |
|---|---|---|
| Intracranial Pressure Spike Hemorrhage | Vascular pressure spike bursts brain microvessels; Brain hemorrhage releases blood-borne toxins and iron into parenchyma | Arun et al., 2012, Abdul-Muneer et al., 2014 |
| Axonal Shearing | External forces physically tear axons; impairs axonal transport and synaptic function | Raghupathi et al., 2004 DiLeonardi et al., 2009 |
| Vasospasm Ischemia | Blood flow disrupted via uneven constriction and dilation in brain vasculature | Izzy et al., 2014 Foster et al., 2014 |
| Secondary | Sub-Acute Phase Physiology | |
| Reactive Oxygen Species Oxidative Stress |
Mitochondria Ca2+ imbalance, mixed with energy failure and NADPH oxidation, triggers oxidative stress and neuronal cell death | Deniaud et al., 2008 Cho et al., 2013 |
| Neuroinflammation | Cytokine release from glial cells promotes cytotoxic effects through macrophage differentiation | Chodobski et al., 2011 Roth et al., 2014 |
| Blood-Brain-Barrier Disruption | Release of Blood toxins into brain parenchyma promoting inflammation and neuronal death | Abdul-Muneer et al., 2013 Alves 2014 |
| Endoplasmic Reticulum Stress | Overabundance of unfolded proteins and intracellular Ca2+ triggers the unfolded protein response | Truttner et al., 2007, Rubovitch et al., 2011, Larner et al., 2004 |
| Glutamate Excitotoxicity | Reduced glutamate uptake activates NMDA receptors | Bullock et al., 1998 Katayama et al., 1990 |
| Protease Activation | Intracellular Ca2+ binds to calmodulin forming calpain kinase; calpain activation cleaves p35 into toxic p25 | Ringger et al., 2004 Huh et al., 2006 |
| Apoptosis | Intrinsic pathway activation both in mitochondria and Endoplasmic reticulum; Ca2+ dependent processes | Clark et al., 2000 Nakagawa et al., 2000 |
| Chronic | Chronic Phase Physiology | |
| Tau Phosphorylation | Tau kinase/phosphatase imbalance leads to hyperphosphorylation of tau | Goldstein et al., 2012 Begum et al., 2014 |
| Amyloid Beta | Increased amyloid precursor protein promotes the toxic oligomerization of amyloid beta | Smith et al., 2003 O’Connor et al., 2008 |
| Neurodegeneration Cognitive deficits | Tau hyperphosphorylation forms neurofibrillary tangles near perivascular regions; promoting neurodegenerative disease and cognitive decline | Mckee et al., 2009 Bailes et al., 2014 |
Axonal injury has emerged as one of the most important pathological features of TBI. The rotational and acceleration/deceleration components of blast-induced TBI, commonly tears axons apart leading to a robust gliosis response and axonal degeneration (34, 56, 95). Axonal swelling and bulb formation are common morphological hallmarks observed following TBI and contribute to decreased action potential firing (44, 45). Axonal injury is a result of TBI and is evident in the white matter areas of the brain (94), and is the initial pathology in neurodegenerative diseases (3). Injury to axons is present in all severities of TBI and may represent a key hallmark of TBI for modeling purposes (77). Therefore, defining the neurodegenerative mechanisms induced by axonal injury would allow us to better model the injury and identify specific targets for neuroprotection.
The mechanical forces of brain trauma can also rupture microvessels (2). Specifically, the traumatic force causes an intracranial pressure spike which causes cerebral microvessels to burst (10). When an external force damages brain microvessels they can release cytotoxic levels of iron into the brain parenchyma (98, 125). Iron promotes Ca2+-dependent mechanisms, which can stimulate cell survival, or trigger cell death depending on severity and duration of iron exposure (119, 120). Ca2+-dependent mechanisms observed following TBI include the unfolded protein response (50, 146), proteasomal degradation (157), autophagy (20), apoptosis (32, 121), and neurodegeneration (155). TBI-induced intracellular Ca2+ increase also prompts reactive oxygen species (ROS) accumulation (30), triggers neuroinflammatory cascades (172), and can influence excitatory amino acids release (164). These secondary effects could potentially be targeted for therapy and warrant further investigation.
Vascular Effects of Traumatic Brain Injury
The external forces of a TBI induce vascular damage with both the initial insult and the ensuing secondary effects (149). It has been proposed that secondary effects can be therapeutically treated because they are driven by pathogenic alterations to signal transduction mechanisms. The vascular effects of TBI include vasospasms, hemorrhage, hypoxia inflammation and BBB disruption. Determining how TBI leads to cell death will provide a better understanding of the secondary effects and provide better therapeutic options. Targets could include BBB restoration after injury, or even using the injury to advantageously deliver drugs during a time window where the BBB is more permeable. This approach will allow pharmacological investigation of mechanisms using drugs that do not easily cross the BBB.
Vasospasms, Subarachnoid Hemorrhage, Edema and Ischemic Hypoxia
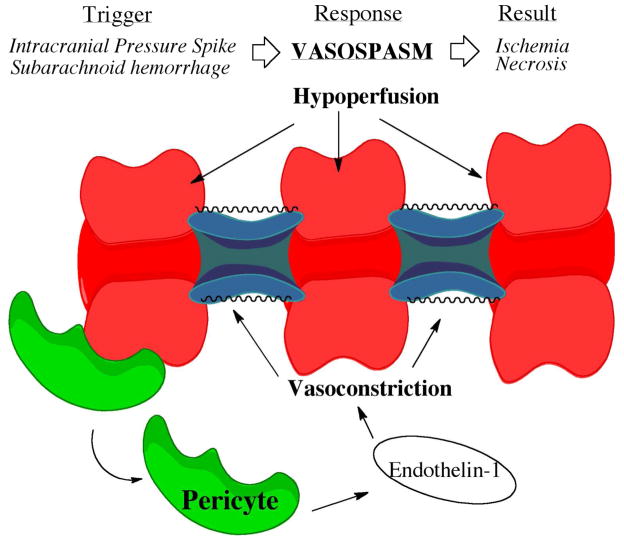
Cerebral vasospasm can be a serious outcome of TBI due to the resulting cerebral hypoperfusion (72). TBI can trigger molecular mechanisms that rapidly mediate vascular tone (See Fig. 2) (29). One such mechanism is the release of a potent vasoconstrictor, endothelin-1, from damaged pericytes (46, 131). Parenchymal contusions and fever increase the risk for vasospasm (162). Up to 68% of individuals with TBI experience short duration vasospasms (87), which are especially common in soldiers exposed to blast TBI (6). Vasospasms resulting from TBI can lead to ischemic episodes, energy depletion and cell necrosis; in other words, an inability for brain cells to compensate for increased metabolic functional overload (97). Vasospasms are frequently associated with recurrent intracranial hypertension and subarachnoid hemorrhage (142, 181). Blast TBI, in particular, can cause cerebral vasospasms that last for more than 30 days and are associated with altered mental status (103). Severe vasospasm must be adequately treated to avoid permanent neurologic deficits or ischemic stroke (17). CT angiography is the diagnostic gold standard for vasospasm (156). Endovascular treatment and vasodilator therapy have both proven to be successful treatment approaches (11, 81).
Figure 2.
Depiction of a Vasospasm Resulting from Traumatic Brain Injury. Vasospasm can be triggered by subarachnoid hemorrhage or blood pressure spikes. Pericytes near vessels release endothelin-1, which triggers vasoconstriction.
Mild TBIs typically exhibit subdural hemorrhaging, while more severe TBIs can display traumatic subarachnoid hemorrhage—increasing the likelihood of diffuse axonal injury, edema, and skull fracture (109). CT imaging is the gold standard for diagnosis of subarachnoid hemorrhage severity (108). The recent establishment of the clinical course score has allowed for the improved monitoring of subarachnoid hemorrhage over time (23), and follow-up CT scans are only needed if new bleeding is a concern (150). TBI has the highest average years of potential life lost for all neurological disorders partly due to traumatic subarachnoid hemorrhage (147). Subarachnoid hemorrhage can lead to increased mortality, extended hospital stays, and central diabetes insipidus (67). Most importantly a likely complication of subarachnoid hemorrhage is cerebral vasospasm (51). A common treatment option for hemorrhagic change that infiltrates the ventricles is a ventriculostomy followed by ventriculoperitoneal shunt placement (28). In severe cases of TBI with subarachnoid hemorrhage where herniation is anticipated, decompressive craniectomy can be performed (90). Fortunately, most intracerebral hemorrhages are minor and will resolve on their own with time (93).
Brain edema following TBI can cause serious complications by limiting brain oxygen delivery and increasing intracranial pressure (124). The pressure increase is more common in children experiencing TBI than adults (48) Hounsfield unit values from brain CT mapped across an intracranial area can be used to accurately predict brain edema following TBI in children (82). A new imaging modality, shear wave elastography, has also been tested in rodents and gives an accurate representation of edema (184). Ulinastatin, a serine protease inhibitor, is currently being investigated for reduction of edema in rodents following TBI (35). Decompressive craniectomies are common in clinical practice (124). Decompressive craniectomies can prevent the intracranial pressure rise and potential herniation associated with edema (22). Decompressive cranectomies are not without risk and a recent report showed 83% of patients developed hygromas and 50% of patients developed aseptic bone resorption (133). Continued work is necessary to find viable alternatives for the treatment of edema.
A primary complication of ischemic hypoxia following TBI is hemispheric hypodensity (53). Ischemic hypoxia can cause the toxic release of hypoxia-inducible factor 1-alpha (159), leading to inflammatory cascades (139). Outcomes from the inflammatory cascade include axonal injury and central myelin damage (178). Point in time monitoring revealed that ~10% of TBI patients experience some ischemic hypoxia (128). Ischemic hypoxia following TBI is associated with poorer functional independence measures (37). Applying techniques for brain oxygen optimization in clinical care has produced the most successful treatment results (105). Continuous neurophysiologic recordings are imperative for appropriate patient management (8). In extreme circumstances, red blood cell transfusion may be required (86). Experimental treatment with mild hypothermia has produced profound preclinical neuroprotection in a variety of TBI models (54, 113) and initial human clinical trials (92) have proved promising.
Blood-Brain-Barrier Disruption: Neuroinflammation and Reactive Oxygen Species Formation
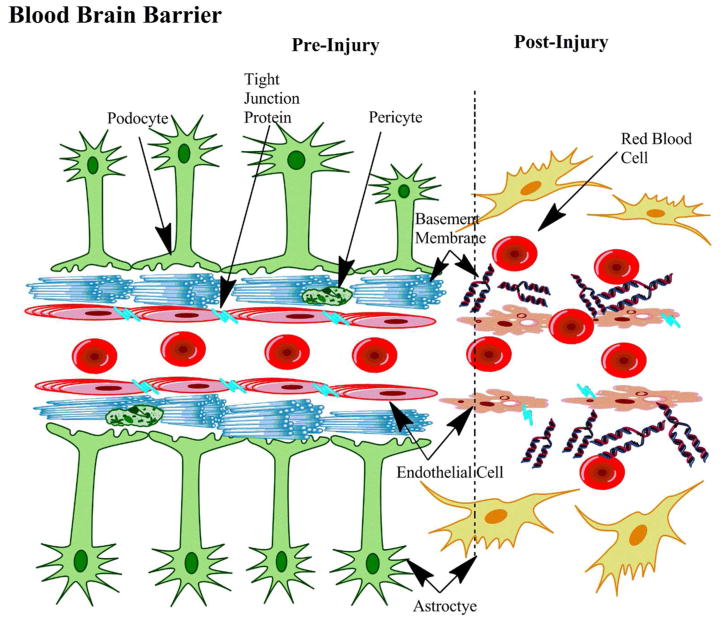
The neurovascular unit can become damaged with TBI. The neurovascular unit controls blood flow to the brain, nutrient delivery, and maintenance of BBB integrity (62). The BBB is an anatomical structure that plays a key role in normal brain physiological regulation. The BBB is composed in part of astrocyte podocytes, a basement membrane, pericytes, and the endothelium connected with tight junction proteins. (14). With regard to TBI, little is known about the role of BBB disruption in disease pathology (7).
Neurovascular inflammation can lead to the formation of ROS (1). Neuronal injury can occur when ROS exceeds the brain’s defense and clearance mechanisms (4). ROS are generated from mitochondrial damage as well as the nicotinamide adenine dinucleotide phosphate-oxidase system at the plasma membrane (188). ROS can eventually contribute to disruption of the BBB, edema, and neuroinflammation (138). ROS contribute to downregulation of tight junction proteins at the microvessel interface (2). In addition, formation of ROS ultimately triggers glial cell activation producing additive injury to the brain parenchyma (149). Oxidative stress stimulates the release of inflammatory factors: interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta (1). ROS also disrupt the vasculatures ability to self-regulate and constrict (43). Ultimately, ROS depression of cerebral metabolism can have long-term consequences manifesting in chronic neurodegeneration and behavioral abnormalities (173). Proton therapy through use of hydrogen infused saline can limit the damage to endothelial cells by preventing BBB disruption and the formation of ROS (74). Another means of limiting oxidative stress is maintenance of mitochondrial homeostasis (130). Uncoupling the mitochondrial oxygen cascade early following TBI has proved beneficial in limiting ROS formation and calcium overload (129). Cerebral microdialysis has been proposed as a diagnostic test for detecting the concentration of free radicals within the brain (65).
BBB disruption is common amongst TBI patients. Approximately 44% of patients who experience severe TBI have BBB breakdown (66). In addition to being effected by ROS, BBB breakdown itself can lead to enhanced neuroinflammation and the formation of ROS (See Fig. 3) (1). Damage to the BBB also leads to the release of neurotoxic proteins into the brain parenchyma (63, 64). Prolonged inflammation can eventually contribute to myelin loss (55). Omega-3 supplementation in rodents can limit BBB breakdown by inhibiting matrix metalloproteinase 9 activity (153). Targeting estrogen receptors may also be a viable therapeutic target due to decreased BBB permeability following TBI in female rats (12).
Figure 3.
The Effects of Traumatic Brain Injury on the Blood-Brain-Barrier. TBI can cause disruptions of tight junction proteins connecting endothelial cells. Astrocytes can undergo astrogliosis and the basement membrane can become disrupted. Ultimately these changes increase the likelihood of red blood cell extravasations.
Tight junctions between endothelial cells maintain vascular integrity, but are often sheared following TBI (183). Blast TBI, in particular, causes a rapid blood pressure spike leading to disruption of the tight junction proteins at the BBB (186). Alternatively, astrogliosis can also lead to BBB modulation over time (100). Astrocytes support endothelial cell function at the neurovascular unit and direct control of permeability (115). Astrocytes and neurons help support the basement membrane surrounding the endothelial cells (161). Podocytes from astrocytes contain vascular endothelial growth factor, which can be released when the BBB is damaged (154). The biomarker, pituitary adenylate cyclase activating polypeptide, offers promising diagnostic potential for determining BBB disruption (25). Currently, digital imaging quantification is the primary method of diagnosis (21).
Secondary Effects of Traumatic Brain Injury
Shortly after TBI, biochemical mechanisms assist in repairing cells that survive the primary insults of TBI. On the other hand, those same mechanisms can cause injured cells to die in order to preserve energy for other cells undergoing the energy-dependent repair process. The brain plays a game of checks and balances (using energy as currency) to determine which neuronal cells are worth saving after TBI. Programmed cell death, or apoptosis, is a necessary mechanism following neuronal injury and may be beneficial to the CNS. However, the resulting effects from an apoptotic event can cause further damage within the brain through the process of neuroinflammation (1).
Even after mild TBI neuronal apoptosis is evident around perivascular regions, indicative of cerebral vascular injury (2). Brain damage from TBI was reported to be associated with decreased mitochondrial membrane potential and increased release of cytochrome C in rodents—both indicative of cellular apoptosis (179). Cleaved-caspase-3 and caspase-3 enzyme activity was reportedly increased in TBI animals versus control (32). Models of TBI have also shown upregulation of caspase gene expression (88). These results indicate that caspase activity contributes to brain tissue loss, and that caspase inhibition may prove to be an effective treatment strategy for TBI.
The secondary effects of TBI can be attenuated with therapeutic interventions given at the right time following neurotrauma. The series of molecular, neurochemical, cellular, and pathophysiological mechanisms resulting from TBI can be targeted with drug therapy designed to manipulate the signaling mechanisms of various cellular and subcellular processes. The secondary mechanisms of TBI include: neuroinflammation, oxidative stress, endoplasmic reticulum (ER) stress, glutamate excitotoxicity, calpain processing, apoptosis and tau hyperphosphorylation (see Fig. 4). The timing and duration of each event is dependent on the type and severity of the TBI in question; therefore, each treatment strategy could be individualized to the specific type of TBI. The aforementioned secondary mechanisms are described in more detail below.
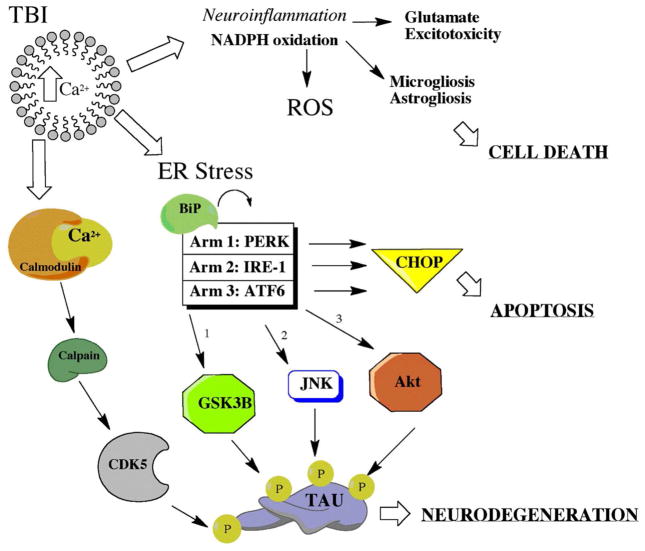
Figure 4.
Complex Interplay of Secondary Mechanisms Following Traumatic Brain Injury. Secondary mechanisms of injury have multiple known effects. A few included are cellular necrosis, apoptosis, neurodegeneration, and tauopathy. Timing of activation and pathway connections are still being teased out with preclinical models of TBI.
Endoplasmic Reticulum Stress, Excitotoxicity and Calpain Processing
TBI occurs over a very short duration, and is often mild in nature, compared to other neural injuries. TBI often goes undetected and chronic deficits often take years to develop (126, 127). TBI harms neurons by perturbing Ca2+ homeostasis in an energy-independent process. The damage done by TBI makes membranes more permeable to Ca2+ and even to extracellular proteins (10). What is known is that neural injury, in particular TBI, causes massive neuronal depolarization and a resulting large influx of Ca2+. Cells which withstand the immediate physical injury from TBI, will have disrupted intracellular Ca2+ levels and oxidative stress (104). As described previously, oxidative stress can induce neuroinflammation, which is known to cause neuronal death and is associated with chronic disease pathology (27).
ER stress has been implicated in a variety of TBI models (19, 50, 151). ER stress then triggers the unfolded protein response (UPR) as an endogenous means of cellular repair. TBI-induced Ca2+ perturbations cause proteins to unfold, and when the ER becomes overwhelmed and struggles to re-fold the unfolded proteins, the UPR ensues (89). In the short-term, the UPR can promote cell survival through three separate mechanisms: (1) attenuation of global protein translation, (2) upregulation of stress response genes, and (3) degradation of unfolded proteins (118). Apoptosis and neurodegeneration are the end-game consequence if ER stress and the UPR are prolonged (40, 117). The UPR has only recently been addressed by TBI investigators because of its new found link to neurodegeneration (116). The timing and duration of the UPR and how exactly it develops into a neurodegenerative phenotype warrants further investigation.
Another secondary mechanism of TBI also involving calcium disruption is glutamate signaling and the phenomenon known as excitotoxicity. TBI triggers a massive depolarization which promotes excessive glutamate release (79). This disrupted regulation in glutamate signaling plays a role in the pathophysiology of TBI through the initiation of secondary injury cascades (26). High extracellular glutamate promotes high levels of intracellular Ca2+ following TBI (174). Secondary injury cascades initiated by glutamate receptors and the disruption of Ca2+ homeostasis will activate calcium-dependent proteases, disrupt energy-dependent processes, and produce oxidative stress (49, 187). Using drug therapy to regulate glutamate release may help to attenuate the secondary effects of TBI exposure.
Loss of microvascular integrity and BBB compromise are often related to brain injuries involving Ca2+ perturbations. In brain trauma models acute and sustained activation of the calpain family of proteases has been implicated in neuronal death and TBI (99). Calpains are acutely activated following TBI within the injured hippocampus and frontal cortex (144). Calpain activation has been observed in degenerating dendrites and atrophic neurons after TBI, providing evidence that calpain activation may participate in neuronal loss after neurotrauma (69). Activation of Ca2+-dependent proteases, such as calpain, is a predicted outcome of membrane depolarization and loss of Ca2+ homeostasis. Its regulation of cytoskeletal dynamics contributes to plasticity (57) and is consistent with injury deficits in axonal architecture and disruption of synaptic plasticity (39). Animals exposed to TBI can exhibit damaged axonal hillocks, suggesting detrimental changes to their synaptic function (13). Moreover, calpain-dependent processes in TBI models have been suggested to attenuate overall electrophysiological responses (132) and invoke more neuronal death (158).
Chronic Effects of Traumatic Brain Injury
Alteration of cerebral blood flow has been linked to delayed neuronal death in the contusion penumbra (47). Hemorrhagic shock causes immediate reduction in mean arterial pressure with continued apoptosis over time due to perpetual lack of perfusion (41). At extended time points TBI continues to cause disruption in the autoregulation of cerebral vasculature (137). A primary mechanism is temporal changes in V1a vasopressin receptors (171). Vasopressin receptor stimulation contributes to cerebral edema that leads to increased intracellular uptake of H2O from the blood (107). The disruption in vascular supply can contribute to perivascular nerve damage (177). In addition, systemic hypotension can contribute to chronic neurodegeneration (101). Persistent activation of calpain proteases is another mechanism by which persistent hypotension leads to neuronal pathology (123). Calpains cleave the contractile components of vascular smooth muscles inhibiting their ability to contract (122).
Not surprisingly, cerebral hypotension has been associated with neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (91). A genetic mutation in Neurogenic locus notch homolog protein 3 increases the risk for small vessel disease following TBI and has been linked as well to Alzheimer’s disease (106). Interestingly, microvasular defects may be linked to Alzheimer’s disease due to the increased incidence of neurological disease in TBI patients (41). Preventative treatment approaches are limited, but a few experimental techniques appear promising. Closely regulated hypothermia therapy can prevent chronic microvessel changes and contustion expansion (190), while compressing the internal jugular vein has also been shown to mitigate axonal injury and vascular disruption (167, 176). Because TBI cannot be predicted a post-injury therapy that mitigates the detrimental chronic effects is therefore needed.
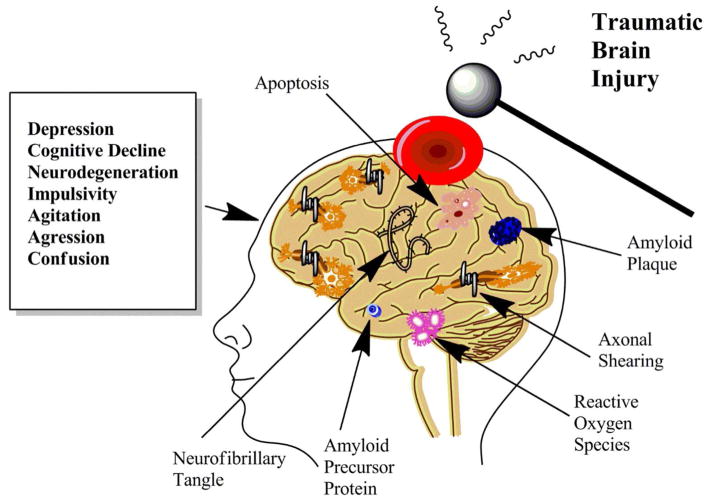
Tau protein hyperphosphorylation and amyloid beta oligomers are well-known markers of neurodegenerative disease. TBI has been shown to hyperphosphorylate tau protein (56), and produce amyloid beta oligomers (166), through mechanisms that are not completely understood. These neurodegenerative factors are activated immediately following TBI and can advance profoundly over time (see Fig. 5). The cellular repair mechanisms associated with these factors are more drawn out, and may contribute to neurodegenerative disease progression and brain matter loss (68, 160). Cortical tissue loss and white matter atrophy resulting from TBI are associated with cognitive deficits (70). Cognitive deficits in rodents have been observed at chronic time points following TBI (136), indicating neurodegenerative disease progression in rodent models of TBI. How the primary effects of TBI manifest into chronic disease states is an area of ongoing investigation. The brains of professional athletes and military veterans are currently being examined with new imaging modalities to discover underlying causes behind neuropsychiatric symptoms such as post-traumatic stress disorder, chronic traumatic encephalopathy, and Alzheimer’s disease (165). Moving forward, TBI research needs to emphasize vascular effects, glial responses and resulting neurodegeneration.
Figure 5.
The Chronic Effects of Traumatic Brain Injury. Some individuals experiencing TBI are more susceptible to chronic effects than others. Environmental and genetic factors play a role. Pathologic changes that may develop include neurofibrillary tangles, axonal shearing, and amyloid plaques to name a few. Neuropsychiatric symptoms may also develop such as depression, impulsivity, cognitive decline, and confusion. This area of chronic deficits following TBI is a topic of growing importance receiving renewed research focus and funding.
Future Directions
Primary and secondary mechanisms of TBI are undergoing continued investigation. What remain to be determined are the tertiary mechanisms that mark the transition from acute injury to chronic neurodegeneration. Studying the broader role of cerebrovascular changes and how these changes interact with neurons and glia must be carefully considered in future investigations. Chronic mechanisms might very well deal with the vasoregulation of cerebral vessels and how microvessels respond to inflammatory markers. In addition, the theory of ‘inflamm-aging’ is likely to take precedence, because aged neurons and glia respond differently to injury-induced stimuli. How these aged cells interact with concurrently aged and previously injured vascular system has not been fully elucidated. Chronic disease states are subject to the effects of both age-related comorbidities and aging itself.
Future studies must incorporate a broader approach utilizing more representative models. Animal models of TBI have been developed, but therapeutics have not yet translated successfully to the clinic. Therefore, an increasing need exists for the development of more clinically relevant TBI models, and the use of advanced MRI and PET imaging to map the dynamics of brain injury responses. This will enable us to devise more effective therapeutic interventions for the amenable secondary effects. Further studies utilizing BBB compromise following TBI could be advantageous for pharmaceutical researchers attempting to alter brain mechanisms. In addition, the balance between protein phosphatases vs. kinases will need to be heavily investigated. Marking the transition to amyloidopathy and/or tauopathy will provide important insight into effective treatment windows. More importantly, understanding chronic mechanisms of injury will allow a more tailored and individualized approach to patient care.
Conclusion
TBI has profound and measurable effects on cerebral vasculature. Due to the variable expression of TBI in the hospital setting, as characterized by the GCS and advanced imaging, individual treatment approaches are a necessity. Understanding complication of TBI such as subarachnoid hemorrhage, vasospasm, and ischemia are necessary in appropriate patient management. Mechanism of injury such as acceleration/deceleration, axonal damage, and BBB disruption are also important to keep in mind. The most promising targets include secondary injury cascades such as oxidative stress, neuroinflammation, and ER stress. Future approaches may target chronic mechanisms involved in the development of neurodegenerative disease. Better pre-clinical models are necessary to map more complex systems such as glutamate toxicity and calpain mediated cell death. Moving forward, treatment of secondary mechanisms affecting vascular dynamics is critical both acutely and chronically. Consideration of age and comorbidities remains a constant factor in any form of neuronal injury. Neurodegeneration and cellular apoptosis are common following TBI, but improved management acutely can prevent the common sequelae of symptoms. In order for successful therapeutic options to translate to the clinic, TBI research needs to move away from the common neurocentric approach and change focus towards vascular effects, glial responses and neurodegeneration.
Table 1.
Abbreviations
| TBI | Traumatic Brain Injury |
| GCS | Glasgow Coma Scale |
| BBB | Blood Brain Barrier |
| ROS | Reactive Oxygen Species |
| NMDA | N-methyl-D-aspartate |
| ER | Endoplasmic Reticulum |
| UPR | Unfolded Protein Response |
| CT | Computerized Tomography |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| CNS | Central Nervous System |
Acknowledgments
This review was supported in part by NIH Grants P01 AG022550, P01 AG027956, P20 GM109098, and U54GM104942 (J.W.S.)
References
- 1.Abdul-Muneer PM, Chandra N, Haorah J. Interactions of Oxidative Stress and Neurovascular Inflammation in the Pathogenesis of Traumatic Brain Injury. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free radical biology & medicine. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adalbert R, Nogradi A, Babetto E, Janeckova L, Walker SA, Kerschensteiner M, Misgeld T, Coleman MP. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain : a journal of neurology. 2009;132:402–416. doi: 10.1093/brain/awn312. [DOI] [PubMed] [Google Scholar]
- 4.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 5.Albert-Weissenberger C, Siren AL. Experimental traumatic brain injury. Experimental & translational stroke medicine. 2010;2:16. doi: 10.1186/2040-7378-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alford PW, Dabiri BE, Goss JA, Hemphill MA, Brigham MD, Parker KK. Blast-induced phenotypic switching in cerebral vasospasm. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12705–12710. doi: 10.1073/pnas.1105860108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves JL. Blood-brain barrier and traumatic brain injury. Journal of neuroscience research. 2014;92:141–147. doi: 10.1002/jnr.23300. [DOI] [PubMed] [Google Scholar]
- 8.Amantini A, Carrai R, Lori S, Peris A, Amadori A, Pinto F, Grippo A. Neurophysiological monitoring in adult and pediatric intensive care. Minerva anestesiologica. 2012;78:1067–1075. [PubMed] [Google Scholar]
- 9.Angoa-Perez M, Kane MJ, Briggs DI, Herrera-Mundo N, Viano DC, Kuhn DM. Animal models of sports-related head injury: bridging the gap between pre-clinical research and clinical reality. Journal of neurochemistry. 2014;129:916–931. doi: 10.1111/jnc.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arun P, Abu-Taleb R, Oguntayo S, Tanaka M, Wang Y, Valiyaveettil M, Long JB, Zhang Y, Nambiar MP. Distinct patterns of expression of traumatic brain injury biomarkers after blast exposure: role of compromised cell membrane integrity. Neuroscience letters. 2013;552:87–91. doi: 10.1016/j.neulet.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Asakuno K, Ishida A. Intraarterial vasodilator therapy immediately rescued pure cortical deafness due to bilateral cerebral vasospasm. Surgical neurology international. 2014;5:61. doi: 10.4103/2152-7806.132031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asl SZ, Khaksari M, Khachki AS, Shahrokhi N, Nourizade S. Contribution of estrogen receptors alpha and beta in the brain response to traumatic brain injury. Journal of neurosurgery. 2013;119:353–361. doi: 10.3171/2013.4.JNS121636. [DOI] [PubMed] [Google Scholar]
- 13.Baalman KL, Cotton RJ, Rasband SN, Rasband MN. Blast wave exposure impairs memory and decreases axon initial segment length. Journal of neurotrauma. 2013;30:741–751. doi: 10.1089/neu.2012.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badaut J, Bix GJ. Vascular neural network phenotypic transformation after traumatic injury: potential role in long-term sequelae. Translational stroke research. 2014;5:394–406. doi: 10.1007/s12975-013-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. Journal of neurosurgery. 2013;119:1235–1245. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- 16.Balestreri M, Czosnyka M, Chatfield DA, Steiner LA, Schmidt EA, Smielewski P, Matta B, Pickard JD. Predictive value of Glasgow Coma Scale after brain trauma: change in trend over the past ten years. Journal of neurology, neurosurgery, and psychiatry. 2004;75:161–162. [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer AM, Rasmussen PA. Treatment of intracranial vasospasm following subarachnoid hemorrhage. Frontiers in neurology. 2014;5:72. doi: 10.3389/fneur.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begum G, Harvey L, Dixon CE, Sun D. ER stress and effects of DHA as an ER stress inhibitor. Translational stroke research. 2013;4:635–642. doi: 10.1007/s12975-013-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begum G, Yan HQ, Li L, Singh A, Dixon CE, Sun D. Docosahexaenoic Acid Reduces ER Stress and Abnormal Protein Accumulation and Improves Neuronal Function Following Traumatic Brain Injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:3743–3755. doi: 10.1523/JNEUROSCI.2872-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS biology. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–579. doi: 10.1161/HYPERTENSIONAHA.113.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bor-Seng-Shu E, Figueiredo EG, Fonoff ET, Fujimoto Y, Panerai RB, Teixeira MJ. Decompressive craniectomy and head injury: brain morphometry, ICP, cerebral hemodynamics, cerebral microvascular reactivity, and neurochemistry. Neurosurgical review. 2013;36:361–370. doi: 10.1007/s10143-013-0453-2. [DOI] [PubMed] [Google Scholar]
- 23.Brandner S, Kellermann I, Hore N, Bozhkov Y, Buchfelder M. Clinical Course Score (CCS): A New Clinical Score to Evaluate Efficacy of Neurotrauma Treatment in Traumatic Brain Injury and Subarachnoid Hemorrhage. Journal of neurosurgical anesthesiology. 2014 doi: 10.1097/ANA.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 24.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44 (Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 25.Bukovics P, Czeiter E, Amrein K, Kovacs N, Pal J, Tamas A, Bagoly T, Helyes Z, Buki A, Reglodi D. Changes of PACAP level in cerebrospinal fluid and plasma of patients with severe traumatic brain injury. Peptides. 2014 doi: 10.1016/j.peptides.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, Marmarou A, Young HF. Factors affecting excitatory amino acid release following severe human head injury. Journal of neurosurgery. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- 27.Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalouhi N, Whiting A, Anderson EC, Witte S, Zanaty M, Tjoumakaris S, Gonzalez LF, Hasan D, Starke RM, Hann S, Ghobrial GM, Rosenwasser R, Jabbour P. Comparison of techniques for ventriculoperitoneal shunting in 523 patients with subarachnoid hemorrhage. Journal of neurosurgery. 2014:1–4. doi: 10.3171/2014.6.JNS132638. [DOI] [PubMed] [Google Scholar]
- 29.Chen B, Mutschler M, Yuan Y, Neugebauer E, Huang Q, Maegele M. Superimposed traumatic brain injury modulates vasomotor responses in third-order vessels after hemorrhagic shock. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013;21:77. doi: 10.1186/1757-7241-21-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho HJ, Sajja VS, Vandevord PJ, Lee YW. Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience. 2013;253:9–20. doi: 10.1016/j.neuroscience.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Clark RS, Chen J, Watkins SC, Kochanek PM, Chen M, Stetler RA, Loeffert JE, Graham SH. Apoptosis-suppressor gene bcl-2 expression after traumatic brain injury in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:9172–9182. doi: 10.1523/JNEUROSCI.17-23-09172.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL, Graham SH. Caspase-3 mediated neuronal death after traumatic brain injury in rats. Journal of neurochemistry. 2000;74:740–753. doi: 10.1046/j.1471-4159.2000.740740.x. [DOI] [PubMed] [Google Scholar]
- 33.Covey DC, Born CT. Blast injuries: mechanics and wounding patterns. Journal of surgical orthopaedic advances. 2010;19:8–12. [PubMed] [Google Scholar]
- 34.Creed JA, DiLeonardi AM, Fox DP, Tessler AR, Raghupathi R. Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. Journal of neurotrauma. 2011;28:547–563. doi: 10.1089/neu.2010.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui T, Zhu G. Ulinastatin Attenuates Brain Edema After Traumatic Brain Injury in Rats. Cell biochemistry and biophysics. 2014 doi: 10.1007/s12013-014-0239-3. [DOI] [PubMed] [Google Scholar]
- 36.Cullen DK, Vernekar VN, LaPlaca MC. Trauma-induced plasmalemma disruptions in three-dimensional neural cultures are dependent on strain modality and rate. Journal of neurotrauma. 2011;28:2219–2233. doi: 10.1089/neu.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cullen NK, Crescini C, Bayley MT. Rehabilitation outcomes after anoxic brain injury: a case-controlled comparison with traumatic brain injury. PM & R : the journal of injury, function, and rehabilitation. 2009;1:1069–1076. doi: 10.1016/j.pmrj.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Dagher JH, Habra N, Lamoureux J, De Guise E, Feyz M. Global outcome in acute phase of treatment following moderate-to-severe traumatic brain injury from motor vehicle collisions vs assaults. Brain injury : [BI] 2010;24:1389–1398. doi: 10.3109/02699052.2010.523042. [DOI] [PubMed] [Google Scholar]
- 39.Dapul HR, Park J, Zhang J, Lee C, DanEshmand A, Lok J, Ayata C, Gray T, Scalzo A, Qiu J, Lo EH, Whalen MJ. Concussive injury before or after controlled cortical impact exacerbates histopathology and functional outcome in a mixed traumatic brain injury model in mice. Journal of neurotrauma. 2013;30:382–391. doi: 10.1089/neu.2012.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 41.Dennis AM, Haselkorn ML, Vagni VA, Garman RH, Janesko-Feldman K, Bayir H, Clark RS, Jenkins LW, Dixon CE, Kochanek PM. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. Journal of neurotrauma. 2009;26:889–899. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Depreitere B, Guiza F, Van den Berghe G, Schuhmann MU, Maier G, Piper I, Meyfroidt G. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. Journal of neurosurgery. 2014;120:1451–1457. doi: 10.3171/2014.3.JNS131500. [DOI] [PubMed] [Google Scholar]
- 43.DeWitt DS, Prough DS. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. Journal of neurotrauma. 2009;26:877–887. doi: 10.1089/neu.2007.0439. [DOI] [PubMed] [Google Scholar]
- 44.Dileonardi AM, Huh JW, Raghupathi R. Differential effects of FK506 on structural and functional axonal deficits after diffuse brain injury in the immature rat. Journal of neuropathology and experimental neurology. 2012;71:959–972. doi: 10.1097/NEN.0b013e31826f5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiLeonardi AM, Huh JW, Raghupathi R. Impaired axonal transport and neurofilament compaction occur in separate populations of injured axons following diffuse brain injury in the immature rat. Brain research. 2009;1263:174–182. doi: 10.1016/j.brainres.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dore-Duffy P, Wang S, Mehedi A, Katyshev V, Cleary K, Tapper A, Reynolds C, Ding Y, Zhan P, Rafols J, Kreipke CW. Pericyte-mediated vasoconstriction underlies TBI-induced hypoperfusion. Neurological research. 2011;33:176–186. doi: 10.1179/016164111X12881719352372. [DOI] [PubMed] [Google Scholar]
- 47.Engel DC, Mies G, Terpolilli NA, Trabold R, Loch A, De Zeeuw CI, Weber JT, Maas AI, Plesnila N. Changes of cerebral blood flow during the secondary expansion of a cortical contusion assessed by 14C-iodoantipyrine autoradiography in mice using a non-invasive protocol. Journal of neurotrauma. 2008;25:739–753. doi: 10.1089/neu.2007.0480. [DOI] [PubMed] [Google Scholar]
- 48.Ewing-Cobbs L, Prasad M, Kramer L, Louis PT, Baumgartner J, Fletcher JM, Alpert B. Acute neuroradiologic findings in young children with inflicted or noninflicted traumatic brain injury. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2000;16:25–33. doi: 10.1007/s003810050006. discussion 34. [DOI] [PubMed] [Google Scholar]
- 49.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 50.Farook JM, Shields J, Tawfik A, Markand S, Sen T, Smith SB, Brann D, Dhandapani KM, Sen N. GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell death & disease. 2013;4:e754. doi: 10.1038/cddis.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faust K, Horn P, Schneider UC, Vajkoczy P. Blood pressure changes after aneurysmal subarachnoid hemorrhage and their relationship to cerebral vasospasm and clinical outcome. Clinical neurology and neurosurgery. 2014;125C:36–40. doi: 10.1016/j.clineuro.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 52.Foreman BP, Caesar RR, Parks J, Madden C, Gentilello LM, Shafi S, Carlile MC, Harper CR, Diaz-Arrastia RR. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. The Journal of trauma. 2007;62:946–950. doi: 10.1097/01.ta.0000229796.14717.3a. [DOI] [PubMed] [Google Scholar]
- 53.Foster KA, Recker MJ, Lee PS, Bell MJ, Tyler-Kabara EC. Factors Associated with Hemispheric Hypodensity after Subdural Hematoma following Abusive Head Trauma in Children. Journal of neurotrauma. 2014 doi: 10.1089/neu.2014.3372. [DOI] [PMC free article] [PubMed]
- 54.Fujita M, Wei EP, Povlishock JT. Effects of hypothermia on cerebral autoregulatory vascular responses in two rodent models of traumatic brain injury. Journal of neurotrauma. 2012;29:1491–1498. doi: 10.1089/neu.2011.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glushakova OY, Johnson D, Hayes RL. Delayed Increases in Microvascular Pathology after Experimental Traumatic Brain Injury Are Associated with Prolonged Inflammation, Blood-Brain Barrier Disruption, and Progressive White Matter Damage. Journal of neurotrauma. 2014 doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Science translational medicine. 2012;4:134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greer JE, Hanell A, McGinn MJ, Povlishock JT. Mild traumatic brain injury in the mouse induces axotomy primarily within the axon initial segment. Acta neuropathologica. 2013;126:59–74. doi: 10.1007/s00401-013-1119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurkoff G, Shahlaie K, Lyeth B, Berman R. Voltage-gated calcium channel antagonists and traumatic brain injury. Pharmaceuticals. 2013;6:788–812. doi: 10.3390/ph6070788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. Jama. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 60.Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. Journal of neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 61.Harmon KG, Drezner J, Gammons M, Guskiewicz K, Halstead M, Herring S, Kutcher J, Pana A, Putukian M, Roberts W American Medical Society for Sports M. American Medical Society for Sports Medicine position statement: concussion in sport. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2013;23:1–18. doi: 10.1097/JSM.0b013e31827f5f93. [DOI] [PubMed] [Google Scholar]
- 62.Hartmann DA, Underly RG, Watson AN, Shih AY. A murine toolbox for imaging the neurovascular unit. Microcirculation. 2014 doi: 10.1111/micc.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helmy A, De Simoni MG, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Progress in neurobiology. 2011;95:352–372. doi: 10.1016/j.pneurobio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. Journal of neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- 66.Ho KM, Honeybul S, Yip CB, Silbert BI. Prognostic significance of blood-brain barrier disruption in patients with severe nonpenetrating traumatic brain injury requiring decompressive craniectomy. Journal of neurosurgery. 2014:1–6. doi: 10.3171/2014.6.JNS132838. [DOI] [PubMed] [Google Scholar]
- 67.Hochstadter E, Stewart TC, Alharfi IM, Ranger A, Fraser DD. Subarachnoid Hemorrhage Prevalence and Its Association with Short-Term Outcome in Pediatric Severe Traumatic Brain Injury. Neurocritical care. 2014 doi: 10.1007/s12028-014-9986-7. [DOI] [PubMed] [Google Scholar]
- 68.Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochemical and biophysical research communications. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 69.Huh JW, Franklin MA, Widing AG, Raghupathi R. Regionally distinct patterns of calpain activation and traumatic axonal injury following contusive brain injury in immature rats. Developmental neuroscience. 2006;28:466–476. doi: 10.1159/000094172. [DOI] [PubMed] [Google Scholar]
- 70.Huh JW, Widing AG, Raghupathi R. Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Experimental neurology. 2008;213:84–92. doi: 10.1016/j.expneurol.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inglese M, Bomsztyk E, Gonen O, Mannon LJ, Grossman RI, Rusinek H. Dilated perivascular spaces: hallmarks of mild traumatic brain injury. AJNR American journal of neuroradiology. 2005;26:719–724. [PMC free article] [PubMed] [Google Scholar]
- 72.Izzy S, Muehlschlegel S. Cerebral vasospasm after aneurysmal subarachnoid hemorrhage and traumatic brain injury. Current treatment options in neurology. 2014;16:278. doi: 10.1007/s11940-013-0278-x. [DOI] [PubMed] [Google Scholar]
- 73.Jalloh I, Carpenter KL, Grice P, Howe DJ, Mason A, Gallagher CN, Helmy A, Murphy MP, Menon DK, Carpenter TA, Pickard JD, Hutchinson PJ. Glycolysis and the pentose phosphate pathway after human traumatic brain injury: microdialysis studies using 1,2-C glucose. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014 doi: 10.1038/jcbfm.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji X, Tian Y, Xie K, Liu W, Qu Y, Fei Z. Protective effects of hydrogen-rich saline in a rat model of traumatic brain injury via reducing oxidative stress. The Journal of surgical research. 2012;178:e9–16. doi: 10.1016/j.jss.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 75.Jiang JY, Lyeth BG, Kapasi MZ, Jenkins LW, Povlishock JT. Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta neuropathologica. 1992;84:495–500. doi: 10.1007/BF00304468. [DOI] [PubMed] [Google Scholar]
- 76.Jilka SR, Scott G, Ham T, Pickering A, Bonnelle V, Braga RM, Leech R, Sharp DJ. Damage to the Salience Network and interactions with the Default Mode Network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Experimental neurology. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kabadi SV, Faden AI. Neuroprotective strategies for traumatic brain injury: improving clinical translation. International journal of molecular sciences. 2014;15:1216–1236. doi: 10.3390/ijms15011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. Journal of neurosurgery. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 80.Kay A, Teasdale G. Head injury in the United Kingdom. World journal of surgery. 2001;25:1210–1220. doi: 10.1007/s00268-001-0084-6. [DOI] [PubMed] [Google Scholar]
- 81.Kelman C, Reavey-Cantwell J. Endovascular management of cerebral vasospasm. Neurosurgical focus. 2014;37:1. doi: 10.3171/2014.V2.FOCUS14172. [DOI] [PubMed] [Google Scholar]
- 82.Kim H, Kim GD, Yoon BC, Kim K, Kim BJ, Choi Y, Czosnyka M, Oh BM, Kim DJ. Quantitative analysis of computed tomography images and early detection of cerebral edema for pediatric traumatic brain injury patients: retrospective study. BMC medicine. 2014;12:186. doi: 10.1186/s12916-014-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kochanek PM, Bramlett H, Dietrich WD, Dixon CE, Hayes RL, Povlishock J, Tortella FC, Wang KK. A novel multicenter preclinical drug screening and biomarker consortium for experimental traumatic brain injury: operation brain trauma therapy. The Journal of trauma. 2011;71:S15–24. doi: 10.1097/TA.0b013e31822117fe. [DOI] [PubMed] [Google Scholar]
- 84.Koponen S, Taiminen T, Portin R, Himanen L, Isoniemi H, Heinonen H, Hinkka S, Tenovuo O. Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. The American journal of psychiatry. 2002;159:1315–1321. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- 85.Korczyn AD. Why have we failed to cure Alzheimer’s disease? Journal of Alzheimer’s disease : JAD. 2012;29:275–282. doi: 10.3233/JAD-2011-110359. [DOI] [PubMed] [Google Scholar]
- 86.Kramer AH, Le Roux P. Red Blood Cell Transfusion and Transfusion Alternatives in Traumatic Brain Injury. Current treatment options in neurology. 2012 doi: 10.1007/s11940-012-0167-8. [DOI] [PubMed] [Google Scholar]
- 87.Kramer DR, Winer JL, Pease BA, Amar AP, Mack WJ. Cerebral vasospasm in traumatic brain injury. Neurology research international. 2013;2013:415813. doi: 10.1155/2013/415813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larner SF, Hayes RL, McKinsey DM, Pike BR, Wang KK. Increased expression and processing of caspase-12 after traumatic brain injury in rats. Journal of neurochemistry. 2004;88:78–90. doi: 10.1046/j.1471-4159.2003.02141.x. [DOI] [PubMed] [Google Scholar]
- 89.Larner SF, Hayes RL, Wang KK. Unfolded protein response after neurotrauma. Journal of neurotrauma. 2006;23:807–829. doi: 10.1089/neu.2006.23.807. [DOI] [PubMed] [Google Scholar]
- 90.Lazaridis C, Czosnyka M. Cerebral blood flow, brain tissue oxygen, and metabolic effects of decompressive craniectomy. Neurocritical care. 2012;16:478–484. doi: 10.1007/s12028-012-9685-1. [DOI] [PubMed] [Google Scholar]
- 91.Le Heron CJ, Wright SL, Melzer TR, Myall DJ, MacAskill MR, Livingston L, Keenan RJ, Watts R, Dalrymple-Alford JC, Anderson TJ. Comparing cerebral perfusion in Alzheimer’s disease and Parkinson’s disease dementia: an ASL-MRI study. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:964–970. doi: 10.1038/jcbfm.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee HC, Chuang HC, Cho DY, Cheng KF, Lin PH, Chen CC. Applying cerebral hypothermia and brain oxygen monitoring in treating severe traumatic brain injury. World neurosurgery. 2010;74:654–660. doi: 10.1016/j.wneu.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 93.Levy AS, Orlando A, Salottolo K, Mains CW, Bar-Or D. Outcomes of a Nontransfer Protocol for Mild Traumatic Brain Injury with Abnormal Head Computed Tomography in a Rural Hospital Setting. World neurosurgery. 2013 doi: 10.1016/j.wneu.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 94.Li S, Sun Y, Shan D, Feng B, Xing J, Duan Y, Dai J, Lei H, Zhou Y. Temporal profiles of axonal injury following impact acceleration traumatic brain injury in rats--a comparative study with diffusion tensor imaging and morphological analysis. International journal of legal medicine. 2013;127:159–167. doi: 10.1007/s00414-012-0712-8. [DOI] [PubMed] [Google Scholar]
- 95.Li S, Yang L, Selzer ME, Hu Y. Neuronal endoplasmic reticulum stress in axon injury and neurodegeneration. Annals of neurology. 2013;74:768–777. doi: 10.1002/ana.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liao GP, Olson SD, Kota DJ, Hetz RA, Smith P, Bedi S, Cox CS., Jr Far-red tracer analysis of traumatic cerebrovascular permeability. The Journal of surgical research. 2014;190:628–633. doi: 10.1016/j.jss.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ling G, Bandak F, Armonda R, Grant G, Ecklund J. Explosive blast neurotrauma. Journal of neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- 98.Liu HD, Li W, Chen ZR, Zhou ML, Zhuang Z, Zhang DD, Zhu L, Hang CH. Increased expression of ferritin in cerebral cortex after human traumatic brain injury. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;34:1173–1180. doi: 10.1007/s10072-012-1214-7. [DOI] [PubMed] [Google Scholar]
- 99.Liu S, Yin F, Zhang J, Qian Y. The role of calpains in traumatic brain injury. Brain injury : [BI] 2014;28:133–137. doi: 10.3109/02699052.2013.860479. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Liu Z, Li X, Luo B, Xiong J, Gan W, Jiang M, Zhang Z, Schluesener HJ, Zhang Z. Accumulation of connective tissue growth factor+ cells during the early phase of rat traumatic brain injury. Diagnostic pathology. 2014;9:141. doi: 10.1186/1746-1596-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: recreating a battlefield injury in the laboratory. Journal of neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- 102.Maas AI. Neuroprotective agents in traumatic brain injury. Expert opinion on investigational drugs. 2001;10:753–767. doi: 10.1517/13543784.10.4.753. [DOI] [PubMed] [Google Scholar]
- 103.Magnuson J, Leonessa F, Ling GS. Neuropathology of explosive blast traumatic brain injury. Current neurology and neuroscience reports. 2012;12:570–579. doi: 10.1007/s11910-012-0303-6. [DOI] [PubMed] [Google Scholar]
- 104.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 105.Mangat HS. Severe traumatic brain injury. Continuum. 2012;18:532–546. doi: 10.1212/01.CON.0000415426.76524.e1. [DOI] [PubMed] [Google Scholar]
- 106.Marchesi VT. Alzheimer’s disease and CADASIL are heritable, adult-onset dementias that both involve damaged small blood vessels. Cellular and molecular life sciences : CMLS. 2014;71:949–955. doi: 10.1007/s00018-013-1542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marmarou CR, Liang X, Abidi NH, Parveen S, Taya K, Henderson SC, Young HF, Filippidis AS, Baumgarten CM. Selective vasopressin-1a receptor antagonist prevents brain edema, reduces astrocytic cell swelling and GFAP, V1aR and AQP4 expression after focal traumatic brain injury. Brain research. 2014 doi: 10.1016/j.brainres.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mata-Mbemba D, Mugikura S, Nakagawa A, Murata T, Ishii K, Li L, Takase K, Kushimoto S, Takahashi S. Early CT findings to predict early death in patients with traumatic brain injury: Marshall and Rotterdam CT scoring systems compared in the major academic tertiary care hospital in northeastern Japan. Academic radiology. 2014;21:605–611. doi: 10.1016/j.acra.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 109.Mata-Mbemba D, Mugikura S, Nakagawa A, Murata T, Kato Y, Tatewaki Y, Li L, Takase K, Ishii K, Kushimoto S, Tominaga T, Takahashi S. Intraventricular Hemorrhage on Initial Computed Tomography as Marker of Diffuse Axonal Injury after Traumatic Brain Injury. Journal of neurotrauma. 2014 doi: 10.1089/neu.2014.3453. [DOI] [PubMed] [Google Scholar]
- 110.McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta neuropathologica. 2014;127:29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain : a journal of neurology. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. Journal of neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- 113.Miyauchi T, Wei EP, Povlishock JT. Evidence for the therapeutic efficacy of either mild hypothermia or oxygen radical scavengers after repetitive mild traumatic brain injury. Journal of neurotrauma. 2014;31:773–781. doi: 10.1089/neu.2013.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyauchi T, Wei EP, Povlishock JT. Therapeutic targeting of the axonal and microvascular change associated with repetitive mild traumatic brain injury. Journal of neurotrauma. 2013;30:1664–1671. doi: 10.1089/neu.2013.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molino Y, Jabes F, Lacassagne E, Gaudin N, Khrestchatisky M. Setting-up an in vitro model of rat blood-brain barrier (BBB): a focus on BBB impermeability and receptor-mediated transport. Journal of visualized experiments : JoVE. 2014:e51278. doi: 10.3791/51278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, Mallucci GR. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Science translational medicine. 2013;5:206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 117.Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, Halliday M, Morgan J, Dinsdale D, Ortori CA, Barrett DA, Tsaytler P, Bertolotti A, Willis AE, Bushell M, Mallucci GR. Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration. Nature. 2012;485:507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 119.Munoz P, Humeres A, Elgueta C, Kirkwood A, Hidalgo C, Nunez MT. Iron mediates N-methyl-D-aspartate receptor-dependent stimulation of calcium-induced pathways and hippocampal synaptic plasticity. The Journal of biological chemistry. 2011;286:13382–13392. doi: 10.1074/jbc.M110.213785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Munoz P, Zavala G, Castillo K, Aguirre P, Hidalgo C, Nunez MT. Effect of iron on the activation of the MAPK/ERK pathway in PC12 neuroblastoma cells. Biological research. 2006;39:189–190. doi: 10.4067/s0716-97602006000100021. [DOI] [PubMed] [Google Scholar]
- 121.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. The Journal of cell biology. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World journal of cardiology. 2014;6:638–652. doi: 10.4330/wjc.v6.i7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Newcomb JK, Pike BR, Zhao X, Banik NL, Hayes RL. Altered calpastatin protein levels following traumatic brain injury in rat. Journal of neurotrauma. 1999;16:1–11. doi: 10.1089/neu.1999.16.1. [DOI] [PubMed] [Google Scholar]
- 124.Nirula R, Millar D, Greene T, McFadden M, Shah L, Scalea TM, Stein DM, Magnotti LJ, Jurkovich GJ, Vercruysse G, Demetriades D, Scherer LA, Peitzman A, Sperry J, Beauchamp K, Bell S, Feiz-Erfan I, O’Neill P, Coimbra R. Decompressive craniectomy or medical management for refractory intracranial hypertension: an AAST-MIT propensity score analysis. The journal of trauma and acute care surgery. 2014;76:944–952. doi: 10.1097/TA.0000000000000194. discussion 952–945. [DOI] [PubMed] [Google Scholar]
- 125.Nisenbaum EJ, Novikov DS, Lui YW. The presence and role of iron in mild traumatic brain injury: an imaging perspective. Journal of neurotrauma. 2014;31:301–307. doi: 10.1089/neu.2013.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Fitzsimmons R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183. doi: 10.1227/NEU.0b013e318212bc7b. discussion 183. [DOI] [PubMed] [Google Scholar]
- 127.Omalu B, Hammers JL, Bailes J, Hamilton RL, Kamboh MI, Webster G, Fitzsimmons RP. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurgical focus. 2011;31:E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 128.Padayachy LC, Rohlwink U, Zwane E, Fieggen G, Peter JC, Figaji AA. The frequency of cerebral ischemia/hypoxia in pediatric severe traumatic brain injury. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2012;28:1911–1918. doi: 10.1007/s00381-012-1837-2. [DOI] [PubMed] [Google Scholar]
- 129.Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. Journal of neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 130.Pandya JD, Pauly JR, Sullivan PG. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Experimental neurology. 2009;218:381–389. doi: 10.1016/j.expneurol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 131.Paradis A, Zhang L. Role of endothelin in uteroplacental circulation and fetal vascular function. Current vascular pharmacology. 2013;11:594–605. doi: 10.2174/1570161111311050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park E, Eisen R, Kinio A, Baker AJ. Electrophysiological white matter dysfunction and association with neurobehavioral deficits following low-level primary blast trauma. Neurobiology of disease. 2013;52:150–159. doi: 10.1016/j.nbd.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 133.Pechmann A, Anastasopoulos C, Korinthenberg R, van Velthoven-Wurster V, Kirschner J. Decompressive Craniectomy after Severe Traumatic Brain Injury in Children: Complications and Outcome. Neuropediatrics. 2014 doi: 10.1055/s-0034-1393707. [DOI] [PubMed] [Google Scholar]
- 134.Peek-Asa C, McArthur D, Hovda D, Kraus J. Early predictors of mortality in penetrating compared with closed brain injury. Brain injury : [BI] 2001;15:801–810. doi: 10.1080/02699050010025768. [DOI] [PubMed] [Google Scholar]
- 135.Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. The Journal of clinical psychiatry. 2013;74:180–188. doi: 10.4088/JCP.12011co1c. quiz 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, Huang JH. The Spectrum of Neuro-behavioral Sequelae Following Repetitive Mild Traumatic Brain Injury: A Novel Mouse Model of Chronic Traumatic Encephalopathy (CTE) Journal of neurotrauma. 2014 doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pop V, Badaut J. A neurovascular perspective for long-term changes after brain trauma. Translational stroke research. 2011;2:533–545. doi: 10.1007/s12975-011-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free radical research. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- 139.Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Molecular and cellular biology. 2008;28:5106–5119. doi: 10.1128/MCB.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Raghupathi R, Margulies SS. Traumatic axonal injury after closed head injury in the neonatal pig. Journal of neurotrauma. 2002;19:843–853. doi: 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- 141.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Annals of neurology. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 142.Razumovsky A, Tigno T, Hochheimer SM, Stephens FL, Bell R, Vo AH, Severson MA, Marshall SA, Oppenheimer SM, Ecker R, Armonda RA. Cerebral hemodynamic changes after wartime traumatic brain injury. Acta neurochirurgica Supplement. 2013;115:87–90. doi: 10.1007/978-3-7091-1192-5_19. [DOI] [PubMed] [Google Scholar]
- 143.Rigg JL, Mooney SR. Concussions and the military: issues specific to service members. PM & R : the journal of injury, function, and rehabilitation. 2011;3:S380–386. doi: 10.1016/j.pmrj.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 144.Ringger NC, Tolentino PJ, McKinsey DM, Pike BR, Wang KK, Hayes RL. Effects of injury severity on regional and temporal mRNA expression levels of calpains and caspases after traumatic brain injury in rats. Journal of neurotrauma. 2004;21:829–841. doi: 10.1089/0897715041526177. [DOI] [PubMed] [Google Scholar]
- 145.Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, McIntosh TK. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. The American journal of pathology. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- 146.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature reviews Molecular cell biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 147.Rosenbaum BP, Kelly ML, Kshettry VR, Weil RJ. Neurologic disorders, in-hospital deaths, and years of potential life lost in the USA, 1988–2011. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2014 doi: 10.1016/j.jocn.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 148.Rostami E, Engquist H, Enblad P. Imaging of cerebral blood flow in patients with severe traumatic brain injury in the neurointensive care. Frontiers in neurology. 2014;5:114. doi: 10.3389/fneur.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rubino S, Zaman RA, Sturge CR, Fried JG, Desai A, Simmons NE, Lollis SS. Outpatient follow-up of nonoperative cerebral contusion and traumatic subarachnoid hemorrhage: does repeat head CT alter clinical decision-making? Journal of neurosurgery. 2014:1–6. doi: 10.3171/2014.6.JNS132204. [DOI] [PubMed] [Google Scholar]
- 151.Rubovitch V, Shachar A, Werner H, Pick CG. Does IGF-1 administration after a mild traumatic brain injury in mice activate the adaptive arm of ER stress? Neurochemistry international. 2011;58:443–446. doi: 10.1016/j.neuint.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 152.Ruhe A, Fejer R, Gansslen A, Klein W. Assessing postural stability in the concussed athlete: what to do, what to expect, and when. Sports health. 2014;6:427–433. doi: 10.1177/1941738114541238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Russell KL, Berman NE, Gregg PR, Levant B. Fish oil improves motor function, limits blood-brain barrier disruption, and reduces Mmp9 gene expression in a rat model of juvenile traumatic brain injury. Prostaglandins, leukotrienes, and essential fatty acids. 2014;90:5–11. doi: 10.1016/j.plefa.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saito T, Shibasaki K, Kurachi M, Puentes S, Mikuni M, Ishizaki Y. Cerebral capillary endothelial cells are covered by the VEGF-expressing foot processes of astrocytes. Neuroscience letters. 2011;497:116–121. doi: 10.1016/j.neulet.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 155.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. Journal of neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sanelli PC, Pandya A, Segal AZ, Gupta A, Hurtado-Rua S, Ivanidze J, Kesavabhotla K, Mir D, Mushlin AI, Hunink MG. Cost-Effectiveness of CT Angiography and Perfusion Imaging for Delayed Cerebral Ischemia and Vasospasm in Aneurysmal Subarachnoid Hemorrhage. AJNR American journal of neuroradiology. 2014 doi: 10.3174/ajnr.A3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochimica et biophysica acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schaible EV, Windschugl J, Bobkiewicz W, Kaburov Y, Dangel L, Kramer T, Huang C, Sebastiani A, Luh C, Werner C, Engelhard K, Thal SC, Schafer MK. 2-Methoxyestradiol confers neuroprotection and inhibits a maladaptive HIF-1alpha response after traumatic brain injury in mice. Journal of neurochemistry. 2014;129:940–954. doi: 10.1111/jnc.12708. [DOI] [PubMed] [Google Scholar]
- 160.Scheper W, Nijholt DA, Hoozemans JJ. The unfolded protein response and proteostasis in Alzheimer disease: preferential activation of autophagy by endoplasmic reticulum stress. Autophagy. 2011;7:910–911. doi: 10.4161/auto.7.8.15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schiera G, Bono E, Raffa MP, Gallo A, Pitarresi GL, Di Liegro I, Savettieri G. Synergistic effects of neurons and astrocytes on the differentiation of brain capillary endothelial cells in culture. Journal of cellular and molecular medicine. 2003;7:165–170. doi: 10.1111/j.1582-4934.2003.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shahlaie K, Keachie K, Hutchins IM, Rudisill N, Madden LK, Smith KA, Ko KA, Latchaw RE, Muizelaar JP. Risk factors for posttraumatic vasospasm. Journal of neurosurgery. 2011;115:602–611. doi: 10.3171/2011.5.JNS101667. [DOI] [PubMed] [Google Scholar]
- 163.Shin SS, Pathak S, Presson N, Bird W, Wagener L, Schneider W, Okonkwo DO, Fernandez-Miranda JC. Detection of white matter injury in concussion using high-definition fiber tractography. Progress in neurological surgery. 2014;28:86–93. doi: 10.1159/000358767. [DOI] [PubMed] [Google Scholar]
- 164.Shohami E, Biegon A. Novel Approach to the Role of NMDA Receptors in Traumatic Brain Injury. CNS & neurological disorders drug targets. 2014;13:567–573. doi: 10.2174/18715273113126660196. [DOI] [PubMed] [Google Scholar]
- 165.Small GW, Kepe V, Siddarth P, Ercoli LM, Merrill DA, Donoghue N, Bookheimer SY, Martinez J, Omalu B, Bailes J, Barrio JR. PET scanning of brain tau in retired national football league players: preliminary findings. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:138–144. doi: 10.1016/j.jagp.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 166.Smith DH, Chen XH, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. Journal of neurosurgery. 2003;98:1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- 167.Smith DW, Bailes JE, Fisher JA, Robles J, Turner RC, Mills JD. Internal jugular vein compression mitigates traumatic axonal injury in a rat model by reducing the intracranial slosh effect. Neurosurgery. 2012;70:740–746. doi: 10.1227/NEU.0b013e318235b991. [DOI] [PubMed] [Google Scholar]
- 168.Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. Journal of neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. The Journal of trauma. 2002;53:219–223. doi: 10.1097/00005373-200208000-00004. discussion 223–214. [DOI] [PubMed] [Google Scholar]
- 170.Sutton RL, Lescaudron L, Stein DG. Unilateral cortical contusion injury in the rat: vascular disruption and temporal development of cortical necrosis. Journal of neurotrauma. 1993;10:135–149. doi: 10.1089/neu.1993.10.135. [DOI] [PubMed] [Google Scholar]
- 171.Szmydynger-Chodobska J, Chung I, Kozniewska E, Tran B, Harrington FJ, Duncan JA, Chodobski A. Increased expression of vasopressin v1a receptors after traumatic brain injury. Journal of neurotrauma. 2004;21:1090–1102. doi: 10.1089/0897715041651033. [DOI] [PubMed] [Google Scholar]
- 172.Szmydynger-Chodobska J, Fox LM, Lynch KM, Zink BJ, Chodobski A. Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. Journal of neurotrauma. 2010;27:1449–1461. doi: 10.1089/neu.2010.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tavazzi B, Signoretti S, Lazzarino G, Amorini AM, Delfini R, Cimatti M, Marmarou A, Vagnozzi R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery. 2005;56:582–589. doi: 10.1227/01.neu.0000156715.04900.e6. discussion 582–589. [DOI] [PubMed] [Google Scholar]
- 174.Truettner JS, Hu B, Alonso OF, Bramlett HM, Kokame K, Dietrich WD. Subcellular stress response after traumatic brain injury. Journal of neurotrauma. 2007;24:599–612. doi: 10.1089/neu.2006.0186. [DOI] [PubMed] [Google Scholar]
- 175.Turner RC, Dodson SC, Rosen CL, Huber JD. The science of cerebral ischemia and the quest for neuroprotection: navigating past failure to future success. Journal of neurosurgery. 2013;118:1072–1085. doi: 10.3171/2012.11.JNS12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Turner RC, Naser ZJ, Bailes JE, Smith DW, Fisher JA, Rosen CL. Effect of slosh mitigation on histologic markers of traumatic brain injury: laboratory investigation. Journal of neurosurgery. 2012;117:1110–1118. doi: 10.3171/2012.8.JNS12358. [DOI] [PubMed] [Google Scholar]
- 177.Ueda Y, Walker SA, Povlishock JT. Perivascular nerve damage in the cerebral circulation following traumatic brain injury. Acta neuropathologica. 2006;112:85–94. doi: 10.1007/s00401-005-0029-5. [DOI] [PubMed] [Google Scholar]
- 178.van der Eerden AW, Khalilzadeh O, Perlbarg V, Dinkel J, Sanchez P, Vos PE, Luyt CE, Stevens RD, Menjot de Champfleur N, Delmaire C, Tollard E, Gupta R, Dormont D, Laureys S, Benali H, Vanhaudenhuyse A, Galanaud D, Puybasset L, Consortium N. White matter changes in comatose survivors of anoxic ischemic encephalopathy and traumatic brain injury: comparative diffusion-tensor imaging study. Radiology. 2014;270:506–516. doi: 10.1148/radiol.13122720. [DOI] [PubMed] [Google Scholar]
- 179.Wang Y, Arun P, Wei Y, Oguntayo S, Gharavi R, Valiyaveettil M, Nambiar MP, Long JB. Repeated blast exposures cause brain DNA fragmentation in mice. Journal of neurotrauma. 2014;31:498–504. doi: 10.1089/neu.2013.3074. [DOI] [PubMed] [Google Scholar]
- 180.Wilberger JE, Jr, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. Journal of neurosurgery. 1991;74:212–218. doi: 10.3171/jns.1991.74.2.0212. [DOI] [PubMed] [Google Scholar]
- 181.Wilson CD, Shankar JJ. Diagnosing Vasospasm After Subarachnoid Hemorrhage: CTA and CTP. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2014;41:314–319. doi: 10.1017/s031716710001725x. [DOI] [PubMed] [Google Scholar]
- 182.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nature reviews Neuroscience. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu FF, Sun S, Ho AS, Lee D, Kiang KM, Zhang XQ, Wang AM, Wu EX, Lui WM, Liu BY, Leung GK. Effects of progesterone vs. dexamethasone on brain oedema and inflammatory responses following experimental brain resection. Brain injury : [BI] 2014:1–8. doi: 10.3109/02699052.2014.943289. [DOI] [PubMed] [Google Scholar]
- 184.Xu ZS, Yao A, Chu SS, Paun MK, McClintic AM, Murphy SP, Mourad PD. Detection of mild traumatic brain injury in rodent models using shear wave elastography: preliminary studies. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2014;33:1763–1771. doi: 10.7863/ultra.33.10.1763. [DOI] [PubMed] [Google Scholar]
- 185.Yeh DD, Schecter WP. Primary blast injuries--an updated concise review. World journal of surgery. 2012;36:966–972. doi: 10.1007/s00268-012-1500-9. [DOI] [PubMed] [Google Scholar]
- 186.Yeoh S, Bell ED, Monson KL. Distribution of blood-brain barrier disruption in primary blast injury. Annals of biomedical engineering. 2013;41:2206–2214. doi: 10.1007/s10439-013-0805-7. [DOI] [PubMed] [Google Scholar]
- 187.Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochemistry international. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 188.Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PloS one. 2012;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang YP, Cai J, Shields LB, Liu N, Xu XM, Shields CB. Traumatic brain injury using mouse models. Translational stroke research. 2014;5:454–471. doi: 10.1007/s12975-014-0327-0. [DOI] [PubMed] [Google Scholar]
- 190.Zhong N, Ramaswamy G, Weisgraber KH. Apolipoprotein E4 domain interaction induces endoplasmic reticulum stress and impairs astrocyte function. The Journal of biological chemistry. 2009;284:27273–27280. doi: 10.1074/jbc.M109.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cross-References & Further Reading
- Kozlowski Dorothy A, Leigh Leasure J, Schallert Timothy. The Control of Movement Following Traumatic Brain Injury. Compr Physiol. 2013;3:121–139. doi: 10.1002/cphy.c110005. [DOI] [PubMed] [Google Scholar]
- Pober Jordan S. Physiology and Pathobiology of Microvascular Endothelium. Compr Physiol 2011, Supplement 9: Handbook of Physiology, The Cardiovascular System, Microcirculation. :37–55. doi: 10.1002/cphy.cp020402. First published in print 2008. [DOI] [Google Scholar]
- Lang Florian, Hoffmann Else K. Role of Ion Transport in Control of Apoptotic Cell Death. Compr Physiol. 2012;2:2037–2061. doi: 10.1002/cphy.c110046. [DOI] [PubMed] [Google Scholar]
- Günzel Dorothee, Fromm Michael. Claudins and Other Tight Junction Proteins. Compr Physiol. 2012;2:1819–1852. doi: 10.1002/cphy.c110045. [DOI] [PubMed] [Google Scholar]
- Stamenović Dimitrije, Wang Ning. Stress Transmission within the Cell. Compr Physiol. 2011;1:499–524. doi: 10.1002/cphy.c100019. [DOI] [PMC free article] [PubMed] [Google Scholar]