Abstract
STUDY QUESTION
What are the pain characteristics among women, with no prior endometriosis diagnosis, undergoing laparoscopy or laparotomy regardless of clinical indication?
SUMMARY ANSWER
Women with surgically visualized endometriosis reported the highest chronic/cyclic pain and significantly greater dyspareunia, dysmenorrhea, and dyschezia compared with women with other gynecologic pathology (including uterine fibroids, pelvic adhesions, benign ovarian cysts, neoplasms and congenital Müllerian anomalies) or a normal pelvis.
WHAT IS KNOWN ALREADY
Prior research has shown that various treatments for pain associated with endometriosis can be effective, making identification of specific pain characteristics in relation to endometriosis necessary for informing disease diagnosis and management.
STUDY DESIGN, SIZE, DURATION
The study population for these analyses includes the ENDO Study (2007–2009) operative cohort: 473 women, ages 18–44 years, who underwent a diagnostic and/or therapeutic laparoscopy or laparotomy at one of 14 surgical centers located in Salt Lake City, UT or San Francisco, CA. Women with a history of surgically confirmed endometriosis were excluded.
PARTICIPANTS/MATERIALS, SETTING AND METHODS
Endometriosis was defined as surgically visualized disease; staging was based on revised American Society for Reproductive Medicine (rASRM) criteria. All women completed a computer-assisted personal interview at baseline specifying 17 types of pain (rating severity via 11-point visual analog scale) and identifying any of 35 perineal and 60 full-body front and 60 full-body back sites for which they experienced pain in the last 6 months.
MAIN RESULTS AND THE ROLE OF CHANCE
There was a high prevalence (≥30%) of chronic and cyclic pelvic pain reported by the entire study cohort regardless of post-operative diagnosis. However, women with a post-operative endometriosis diagnosis, compared with women diagnosed with other gynecologic disorders or a normal pelvis, reported more cyclic pelvic pain (49.5% versus 31.0% and 33.1%, P < 0.001). Additionally, women with endometriosis compared with women with a normal pelvis experienced more chronic pain (44.2 versus 30.2%, P = 0.04). Deep pain with intercourse, cramping with periods, and pain with bowel elimination were much more likely reported in women with versus without endometriosis (all P < 0.002). A higher percentage of women diagnosed with endometriosis compared with women with a normal pelvis reported vaginal (22.6 versus 10.3%, P < 0.01), right labial (18.4 versus 8.1%, P < 0.05) and left labial pain (15.3 versus 3.7%, P < 0.01) along with pain in the right/left hypogastric and umbilical abdominopelvic regions (P < 0.05 for all). Among women with endometriosis, no clear and consistent patterns emerged regarding pain characteristics and endometriosis staging or anatomic location.
LIMITATIONS, REASONS FOR CAUTION
Interpretation of our findings requires caution given that we were limited in our assessment of pain characteristics by endometriosis staging and anatomic location due to the majority of women having minimal (stage I) disease (56%) and lesions in peritoneum-only location (51%). Significance tests for pain topology related to gynecologic pathology were not corrected for multiple comparisons.
WIDER IMPLICATIONS OF THE FINDINGS
Results of our research suggest that while women with endometriosis appear to have higher pelvic pain, particularly dyspareunia, dysmenorrhea, dyschezia and pain in the vaginal and abdominopelvic area than women with other gynecologic disorders or a normal pelvis, pelvic pain is commonly reported among women undergoing laparoscopy, even among women with no identified gynecologic pathology. Future research should explore causes of pelvic pain among women who seek out gynecologic care but with no apparent gynecologic pathology. Given our and other's research showing little correlation between pelvic pain and rASRM staging among women with endometriosis, further development and use of a classification system that can better predict outcomes for endometriosis patients with pelvic pain for both surgical and nonsurgical treatment is needed.
STUDY FUNDING/COMPETING INTERESTS
Supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts NO1-DK-6-3428, NO1-DK-6-3427, and 10001406-02). The authors have no potential competing interests.
Keywords: endometriosis, epidemiology, laparoscopy, dysmenorrhea, dyspareunia
Introduction
A variety of pain symptoms are associated with endometriosis, including dysmenorrhea, dyspareunia, dysuria, dyschezia and chronic pelvic pain (Fauconnier and Chapron, 2005; Ballard et al., 2008). However, a clear characterization of pain typology and topology in populations with endometriosis, other gynecologic pathology, or a normal pelvis is lacking. Understanding the precise nature of the relationship between pain and endometriosis is important for the clinical management of affected women, given the body of evidence indicating that medical and surgical management for pain associated with endometriosis has been shown to be effective (American Society for Reproductive Medicine, 2014). Evaluating the relationship between pain and endometriosis, however, is challenging given that pain is difficult to measure and the mechanism by which endometriosis causes pain is not well understood (American Society for Reproductive Medicine, 2014). While previous studies have provided important data on the incidence of pelvic pain and endometriosis (Cox et al., 2007; Kang et al., 2007; Ballard et al., 2010; Hsu et al., 2011; Renner et al., 2012), little research has been done to assess both the typology and topology of pelvic pain, and pain beyond the pelvis, and endometriosis diagnosis and severity using operative findings and a standardized classification system (American Society for Reproductive Medicine, 1997).
Recent studies on clinical populations have reported posterior cul-de-sac and uterosacral lesions to be positively associated with dyspareunia, ovarian and peritoneal lesions with dysmenorrhea, bladder and peritoneal lesions with dysuria, and deep vaginal lesions with dyschezia in some but not all studies (Fauconnier and Chapron, 2005; Hsu et al., 2011; Khan et al., 2013). Additionally, depth of lesions has been linked with chronic, but not cyclic pelvic pain (Anaf et al., 2000; Koninckx et al., 2012), and stage of disease has been linked with severe dysmenorrhea in some (Buttram, 1979; Fedele et al., 1992; Vercellini et al., 1996; Muzii et al., 1997) but not all studies (Fedele et al., 1990; Marana et al., 1991; Porpora et al., 1999; Gruppo Italiano per lo Studio dell'Endometriosi, 2001, Chapron et al., 2003; Hsu et al., 2011). These characterizations underscore the need for further investigations on not only pain type but precise pain location with respect to endometriosis diagnosis, severity, and anatomical location (Renner et al., 2012), particularly among a sample of women undergoing surgical evaluations for various indications and with no prior endometriosis diagnosis. Given that the pathogenesis of endometriosis-associated pain is thought to involve diverse inflammatory and neuropathic mechanisms (Stratton and Berkley, 2011), identification of pain location with respect to anatomic sites of endometriosis is key to clarifying any precise relationships.
The objectives of this study, therefore, were to describe pain type, severity and location among (i) women undergoing a diagnostic laparoscopy or laparotomy irrespective of clinical indication; and (ii) women with an incident endometriosis diagnosis by endometriosis stage, anatomic location and depth.
Materials and Methods
Study populations
Data for this study was obtained from the Endometriosis, Natural History, Diagnosis, and Outcomes (ENDO) Study (Buck Louis et al., 2011). For this secondary analysis, we restricted the study population to the operative cohort. Briefly, the operative cohort comprised 473 women, ages 18–44 years, who underwent laparoscopy or laparotomy irrespective of clinical indication at one of 14 surgical centers located in Salt Lake City, UT, or San Francisco, CA, between 2007 and 2009. Surgical indications for laparoscopy/laparotomy included pelvic pain (44%), pelvic mass (16%), irregular menses (13%), fibroids (10%), tubal ligation (10%) and infertility (7%). Women with a history of surgically confirmed endometriosis (prevalent disease) or who could not communicate in English or Spanish were excluded. Other exclusion criteria included currently pregnant or breastfeeding ≥6 months, injectable hormones within the past 2 years, and a cancer diagnosis other than non-melanoma skin cancer.
Data collection
Prior to surgery, all women completed a computer-assisted personal interview to capture medical, gynecologic and reproductive history along with an intensive pain assessment.
Pain assessment
Women were asked whether they experienced pain lasting >6 months that was either cyclic (i.e. painful menstrual cramps not relieved by over-the-counter medications) or chronic (i.e. pain located in or near the bladder or vaginal canal not associated with menses) and for those answering yes, duration of pain (≥6 months–1 year, >1 year–2 years, or >2 years). Additionally, women were queried about 17 different sources or timing of pain (e.g. pain just before period, deep pain with intercourse, pain with urination, etc.) that they had experienced in the last 6 months (no minimum duration required) and to rate the severity of each using an 11-point visual analog scale (VAS), with 0 denoting no pain to 10 denoting the most severe pain imaginable using a standardized questionnaire (International Pelvic Pain Society, 2008). Information regarding pain medication use, for any indication on at least a monthly basis during the past 12 months, was also asked with women specifying over-the-counter, and/or narcotic prescription, and/or non-narcotic prescription, or no pain medication use. To specifically identify pain location, we utilized a computerized perineal/upper thigh, front body, and back body anatomical map that was programmed to allow women to point and click on any of 35 perineal/upper thigh sites and 60 front and back body sites for which they experience pain (International Pelvic Pain Society, 2008). Women only indicated areas where they experienced regular pain, if any. We utilized these data to generate color-coded figures reflecting the distribution of pain by anatomical site.
Endometriosis and other gynecologic pathology assessment
Surgeons completed a standardized operative report immediately after surgery to capture gynecologic and pelvic pathology. Endometriosis was diagnosed using the clinical gold standard of surgically visualized disease (American Society for Reproductive Medicine, 1997; Kennedy et al., 2005) while its severity was based upon the revised American Society for Reproductive Medicine's (rASRM) staging criteria (American Society for Reproductive Medicine, 1997). The rASRM staging system is based solely on surgical visualized disease volume and location and does not incorporate symptomatology. Stage of disease (I–IV: minimal, mild, moderate, severe) was automatically calculated via the rASRM weighted point score. Among the 473 study participants, 190 (40.2%) had a primary post-operative diagnosis of endometriosis, 147 (31.1%) had other gynecologic pathology (uterine fibroids [n = 58], pelvic adhesions [n = 30], benign ovarian cysts [n = 46], neoplasm [n = 3], and congenital Müllerian anomalies [n = 10]) and 136 (28.8%) had a normal pelvis.
Statistical analysis
In keeping with the study's aim to characterize pain type, severity, and location in relation to gynecologic condition, we utilized descriptive statistical techniques. Demographic, reproductive history, and pain characteristics (chronic and/or cyclic pain [yes/no]; pain type/severity via 17-item VAS questionnaire [any/none and mean ± SD on original scale of 0 to 10]; and pain medication intake [yes/no]) were compared between post-operative diagnosis (endometriosis, other gynecologic disorder, normal pelvis); and for women with endometriosis, stage (I, minimal; II, mild; III, moderate, IV, severe), location (peritoneum-only, ovary-only, cul de sac-only, or some such combination for a total of 7 categories), and location-specific lesion depth (deep or superficial for ovary and peritoneum; full or partial for cul de sac) using analysis of variance or nonparametric Wilcoxon–Mann–Whitney tests for continuous variables and chi-square or Fisher's exact tests for categorical variables. Values sharing a common superscript are significantly different at P < 0.05 via multiple comparison tests—Tukey procedure for analysis of variance and frequencies (Elliott and Reisch, 2006; Zar, 2009) and Dwass, Steel, Critchlow-Fligner procedure based on pairwise two-sample rankings for Wilcoxon comparisons. Given that pain characteristics in relation to endometriosis may be unique among women a history of subfertility, we compared pain characteristics by endometriosis diagnosis restricted to women reporting subfertility (i.e. women reporting having tried to get pregnant for 6 months or more, regardless of whether they ever achieved pregnancy).
We plotted the distribution of pain by location and endometriosis diagnosis and incorporated these data into color-coded pain location figures that reflect pain frequency for each of the 35 perineal and 60 front/back body associated anatomical locations by post-operative diagnosis, endometriosis stage, and anatomic location. For the color-coded figures, pain frequency was categorized as 0.0 to ≤4.9%, 5.0–9.9%, 10.0 to 14.9%, 15.0 to 19.9%, and ≥20.0%. These categories were selected a priori based on range of pain reporting and evenly distributed groups (vigintiles), with the exception of high pain reporting for front body and cul de sac in which groups were distributed by deciles >20% up to the maximal frequency. We ran chi-square or Fisher's exact tests to determine overall significant differences in reported pain location between groups. For any statistically significant comparison (i.e. P < 0.05), the Tukey procedure for multiple comparisons (Elliott and Reisch, 2006; Zar, 2009) was used to test significant pairwise differences (at both P < 0.05 and P < 0.01) in reported pain location between groups. All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Pain typology and endometriosis
Study participants were predominately white, non-Hispanic (74.8%), married (94.7%), college educated (80.2%), and with a mean age of 33.0 ± 7.0 year and body mass index of 28.0 ± 8.0. Irrespective of clinical indication for surgery or post-operative diagnosis, only 3% of the total study population (n = 473) reported no pain to any of the 17 types of pain, while 63.4% reported 6 or more types of pain (Supplementary Table SI). Women with endometriosis were more likely to be nulligravida/nulliparous compared with women with a normal pelvis and be of higher income and have a lower BMI compared with women with a normal pelvis or other gynecologic condition (Table I).
Table I.
Demographic characteristics of the study sample by endometriosis status.a
| Characteristic | Endometriosis (n = 190) |
Other gynecologic condition (n = 147) | Normal pelvis (n = 136) |
|---|---|---|---|
| Age (years; mean ± SD) | 32.0 ± 6.8 | 33.5 ± 7.2 | 33.8 ± 7.0 |
| Race/ethnicity | |||
| Hispanic | 24 (12.6) | 24 (16.3) | 15 (11.0) |
| Non-Hispanic white | 142 (74.7) | 102 (69.4) | 110 (80.9) |
| Non-Hispanic black | 1 (0.6) | 6 (4.1) | 1 (0.7) |
| Asian/Islander/Native | 13 (6.8) | 11 (7.5) | 4 (2.9) |
| Other/multiracial | 10 (5.3) | 4 (2.7) | 6 (4.4) |
| Married/living as married | 180 (95.2) | 138 (95.2) | 126 (93.3) |
| Education | |||
| ≤High school | 29 (15.3) | 25 (17.2) | 39 (28.7) |
| >College | 160 (84.7) | 120 (82.8) | 97 (71.3) |
| Household income | |||
| Within 180% of poverty or below | 29 (15.5) | 39 (27.1) | 39 (28.9) |
| Above poverty | 158 (84.5) | 105 (72.9) | 96 (71.1) |
| Nulligravida | 81 (42.6) | 54 (37.2) | 20 (14.7) |
| Nulliparous | 102 (54.0) | 76 (51.7) | 25 (18.4) |
| Ever use of Hormonal Medicationb | 176 (92.6) | 125 (85.0) | 126 (92.7) |
| BMI (mean ± SD) | 26.2 ± 7.0 | 29.2 ± 8.0 | 29.2 ± 8.8 |
Data are n (%) unless stated otherwise.
aIncludes 473 women in the ENDO operative cohort (excludes 22 women whose surgeries were canceled). Missing 4 observations for marital status, 7 for household income, 2 for gravidity, 1 for parity, 1 for chronic pain, 2 for cyclic pain, and 56 for pain medications.
bIncludes birth control pills, patch, shots, implants, vaginal rings, GnRH inhibitors and clomiphene.
With regards to pain characteristics, women with endometriosis were more likely to report experiencing cyclic pelvic pain (P < 0.001) compared with women with other gynecologic conditions or a normal pelvis; and chronic pelvic pain (P = 0.04) compared with women with a normal pelvis. Among women with chronic and/or cyclic pain, the duration of having had chronic or cyclic pain did not differ between groups (Table II). Dyspareunia (vaginal pain with intercourse, deep pain with intercourse, and burning vaginal pain after intercourse), dysmenorrhea (pain just before menstrual period, level of cramps with period, pain after period is over), dysuria (pain with urination), dyschezia (pain with bowel elimination), and ovulatory related pain (pain at ovulation [mid-cycle]) were all associated with endometriosis diagnosis, but abdominal pain or pain in other areas were not (Table II). There were no significant differences by disease stage (Table III). Women with endometriosis were more likely to use over-the-counter pain medications on a monthly basis compared with women with a normal pelvis or other gynecologic condition.
Table II.
Pain characteristics of the study sample by post-operative diagnosis.a
| Characteristic | Endometriosis (n = 190) |
Other gynecologic condition (n = 147) |
Normal pelvis (n = 136) |
P |
||||
|---|---|---|---|---|---|---|---|---|
| Chronic/cyclic pain (n [%]) | ||||||||
| Chronic pelvic pain | 84 (44.2)* | 57 (39.0) | 41 (30.2)* | 0.04 | ||||
| ≥6 months–1 year | 26 (31.3) | 17 (30.9) | 11 (26.8) | 0.77 | ||||
| >1 year–2 years | 18 (21.7) | 12 (21.8) | 6 (14.6) | |||||
| >2 years | 39 (47.0) | 26 (47.3) | 24 (58.5) | |||||
| Cyclic pelvic pain | 94 (49.5)*,† | 45 (31.0)* | 45 (33.1)† | <0.001 | ||||
| ≥6 months–1 year | 22 (23.4) | 8 (18.2) | 12 (26.7) | 0.58 | ||||
| >1 year–2 years | 20 (21.3) | 9 (20.5) | 5 (11.1) | |||||
| >2 years | 52 (55.3) | 27 (61.4) | 28 (62.2) | |||||
| Pain type and severityb (n [%]; mean ± SD) | P (n [%]) | P (mean ± SD) | ||||||
| Dyspareunia | ||||||||
| Vaginal pain with intercourse | 104 (54.7)*,† | 2.6 ± 3.0*,† | 61 (41.5)* | 1.8 ± 2.6* | 44 (32.4)† | 1.6 ± 2.8† | <0.001 | <0.001 |
| Deep pain with intercourse | 101 (53.2)*,† | 2.9 ± 3.3*,† | 56 (38.1)* | 2.1 ± 3.1* | 42 (30.9)† | 1.8 ± 3.0† | <0.001 | <0.001 |
| Burning vaginal pain after intercourse | 63 (33.2) | 1.3 ± 2.3 | 33 (22.5) | 0.9 ± 2.0 | 30 (22.1) | 0.9 ± 2.2 | 0.03 | 0.04 |
| Pelvic pain lasting hours or days after intercourse | 61 (32.1) | 1.5 ± 2.7 | 35 (23.8) | 1.2 ± 2.4 | 29 (21.3) | 1.2 ± 2.6 | 0.06 | 0.12 |
| Constant burning vaginal pain (regardless of intercourse) | 26 (13.7) | 0.5 ± 1.6 | 12 (8.2) | 0.3 ± 1.2 | 12 (8.8) | 0.4 ± 1.5 | 0.19 | 0.21 |
| Dysmenorrhea | ||||||||
| Pain just before period | 143 (75.3)* | 4.2 ± 3.2* | 91 (61.9)* | 3.0 ± 3.2* | 90 (66.2) | 3.4 ± 3.1 | 0.03 | 0.004 |
| Level of cramps with period | 173 (91.1)*,† | 6.5 ± 3.2*,† | 125 (85.0)* | 5.2 ± 3.4* | 108 (79.4)† | 5.3 ± 3.6† | 0.01 | <0.001 |
| Pain after period is over | 73 (38.4)* | 1.9 ± 2.9* | 39 (26.5)* | 1.2 ± 2.4* | 52 (38.2) | 1.5 ± 2.3 | 0.04 | 0.04 |
| Endometriosis (n = 190) | Other gynecologic condition (n = 147) | Normal pelvis (n = 136) |
P |
|||||
| P (n [%]) | P (mean ± SD) | |||||||
| Ovulatory related pain | ||||||||
| Pain at ovulation (mid-cycle) | 128 (67.4)*,† | 3.2 ± 3.0*,† | 72 (49.0)* | 2.4 ± 3.1* | 71 (52.2)† | 2.4 ± 2.9† | 0.001 | 0.01 |
| Dysuria | ||||||||
| Pain with urination | 43 (22.6)* | 1.0 ± 2.2* | 28 (19.1) | 0.7 ± 1.7 | 15 (11.0)* | 0.4 ± 1.4* | 0.03 | 0.02 |
| Dyschezia | ||||||||
| Pain with bowel elimination | 84 (44.2)* | 2.1 ± 3* | 48 (32.7) | 1.5 ± 2.7 | 35 (25.7)* | 1.2 ± 2.5* | 0.002 | 0.002 |
| Other pain | ||||||||
| Pain in groin when lifting | 50 (26.3) | 1.2 ± 2.3 | 40 (27.2) | 1.3 ± 2.5 | 27 (19.9) | 0.8 ± 2.1 | 0.29 | 0.24 |
| Pain when bladder is full | 101 (53.2) | 2.2 ± 2.8 | 75 (51) | 2.1 ± 2.9 | 58 (42.7) | 1.8 ± 2.6 | 0.16 | 0.26 |
| Abdominal pain | 97 (51.1) | 2.7 ± 3.3 | 73 (49.7) | 2.8 ± 3.5 | 60 (44.1) | 2.4 ± 3.2 | 0.45 | 0.52 |
| Low back pain | 137 (72.1) | 3.8 ± 3.3 | 100 (68) | 3.7 ± 3.4 | 92 (67.7) | 3.7 ± 3.3 | 0.62 | 0.92 |
| Muscle/joint pain | 97 (53.3) | 2.7 ± 3.1 | 64 (46) | 1.8 ± 2.5 | 61 (47.7) | 2.2 ± 3 | 0.39 | 0.05 |
| Migraine headache | 98 (53.6) | 3.4 ± 3.8 | 62 (44.6) | 2.9 ± 3.7 | 63 (49.2) | 3.5 ± 4.0 | 0.28 | 0.33 |
| Pain medicationsc (n [%]) | ||||||||
| Over-the-counter pain medications | 156 (82.1)*,† | 100 (68.0)* | 93 (68.4)† | 0.003 | ||||
| Narcotic prescription medications | 53 (30.1) | 33 (26) | 38 (33.3) | 0.46 | ||||
| Non-narcotic prescription medications | 31 (17.6) | 15 (11.8)* | 30 (26.3)* | 0.01 | ||||
| None | 8 (4.6) | 10 (7.9) | 6 (5.3) | 0.45 | ||||
aIncludes 473 women in the ENDO operative cohort (excludes 22 women whose surgeries were canceled). Missing 1 observation for chronic pain, 2 for cyclic pain, 0 for pain type, and 56 for pain medications. Statistical significance for differences was assessed via Wilcoxon–Mann–Whitney test for continuous variables and chi-square or Fisher's exact tests for categorical variables.
b11-point visual analog scale with 0 being no pain and 10 being the most severe pain imaginable, women reported any pain in the last 6 months (no minimum duration required). Mean ± SD for pain on original VAS scale (0 to 10).
cPain medications taken on at least a monthly basis for any indication in the past 12 months.
*,† Values that are significantly different P < 0.05 via multiple comparison tests (Tukey procedure for frequencies and Dwass, Steel, Critchlow-Fligner procedure [based on pairwise two-sample rankings] for means).
Table III.
Pain characteristics by endometriosis stage.a
| Characteristic | Stage I (n = 107) |
Stage II (n = 27) |
Stage III (n = 23) |
Stage IV (n = 28) |
P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chronic/cyclic pain (n [%]) | ||||||||||
| Chronic pelvic pain | 45 (42.1) | 14 (51.9) | 12 (52.2) | 11 (39.3) | 0.64 | |||||
| Cyclic pelvic pain | 53 (49.5) | 11 (40.7) | 12 (52.2) | 15 (53.6) | 0.78 | |||||
| Pain type and severityb (n [%]; mean ± SD) | P (n [%]) | P (mean ± SD) | ||||||||
| Dyspareunia | ||||||||||
| Vaginal pain with intercourse | 63 (58.9) | 2.7 ± 3.1 | 15 (55.6) | 3.1 ± 3.5 | 9 (39.1) | 1.5 ± 2.4 | 14 (50.0) | 2.4 ± 2.9 | 0.35 | 0.31 |
| Deep pain with intercourse | 60 (56.1) | 3.1 ± 3.4 | 16 (59.3) | 3.1 ± 3.4 | 7 (30.4) | 1.8 ± 3.0 | 15 (53.6) | 2.9 ± 3.4 | 0.14 | 0.27 |
| Burning vaginal pain after intercourse | 36 (33.6) | 1.4 ± 2.6 | 11 (40.7) | 1.6 ± 2.2 | 5 (21.7) | 0.6 ± 1.3 | 10 (35.7) | 1.1 ± 2.0 | 0.55 | 0.43 |
| Pelvic pain lasting hours or days after intercourse | 39 (36.5) | 1.6 ± 2.7 | 9 (33.3) | 2.3 ± 3.6 | 4 (17.4) | 0.6 ± 1.6 | 6 (21.4) | 0.9 ± 2.1 | 0.19 | 0.16 |
| Constant burning vaginal pain (regardless of intercourse) | 13 (12.2) | 0.4 ± 1.5 | 6 (22.2) | 0.9 ± 2.1 | 0 (0) | 0 ± 0 | 6 (21.4) | 0.9 ± 1.9 | 0.07 | 0.07 |
| Dysmenorrhea | ||||||||||
| Pain just before menstrual period | 77 (72) | 4.0 ± 3.3 | 24 (88.9) | 5.0 ± 3.1 | 17 (73.9) | 4.0 ± 2.8 | 22 (78.6) | 4.1 ± 3.2 | 0.32 | 0.47 |
| Level of cramps with period | 95 (88.8) | 6.3 ± 3.3 | 24 (88.9) | 7.1 ± 3.4 | 23 (100) | 6.9 ± 2.7 | 26 (92.9) | 6.4 ± 3.0 | 0.37 | 0.50 |
| Pain after period is over | 38 (35.5) | 1.6 ± 2.7 | 12 (44.4) | 2.2 ± 3.2 | 8 (34.8) | 1.8 ± 3.2 | 11 (39.3) | 2.4 ± 3.5 | 0.84 | 0.74 |
| Ovulatory related pain | ||||||||||
| Pain at ovulation (mid-cycle) | 70 (65.4) | 3.2 ± 3.1 | 21 (77.8) | 3.1 ± 2.6 | 17 (73.9) | 3.7 ± 3.1 | 17 (60.7) | 2.5 ± 2.9 | 0.47 | 0.46 |
| Dysuria | ||||||||||
| Pain with urination | 25 (23.4) | 1.0 ± 2.3 | 8 (29.6) | 1.1 ± 1.9 | 4 (17.4) | 0.7 ± 1.8 | 4 (14.3) | 0.8 ± 2.3 | 0.52 | 0.59 |
| Stage I (n = 107) | Stage II (n = 27) | Stage III (n = 23) | Stage IV (n = 28) |
P |
||||||
| P (n [%]) | P (mean ± SD) | |||||||||
| Dyschezia | ||||||||||
| Pain with bowel elimination | 42 (39.3) | 2.0 ± 3.0 | 13 (48.2) | 2.1 ± 3.1 | 12 (52.2) | 2.1 ± 2.4 | 13 (46.4) | 2.6 ± 3.2 | 0.61 | 0.80 |
| Other pain | ||||||||||
| Pain in groin when lifting | 28 (26.2) | 1.3 ± 2.4 | 9 (33.3) | 1.3 ± 2 | 4 (17.4) | 0.8 ± 1.8 | 6 (21.4) | 0.9 ± 2.2 | 0.58 | 0.61 |
| Pain when bladder is full | 60 (56.1) | 2.3 ± 2.8 | 11 (40.7) | 2.3 ± 3.3 | 12 (52.2) | 2.1 ± 2.7 | 14 (50) | 1.9 ± 2.6 | 0.55 | 0.84 |
| Abdominal pain | 52 (48.6) | 2.7 ± 3.4 | 14 (51.9) | 2.6 ± 3.1 | 13 (56.5) | 3.0 ± 3.3 | 14 (50) | 2.3 ± 3.2 | 0.92 | 0.92 |
| Low back pain | 84 (78.5) | 4.2 ± 3.2 | 18 (66.7) | 3.0 ± 3.1 | 14 (60.9) | 2.9 ± 3.2 | 18 (64.3) | 3.5 ± 3.6 | 0.18 | 0.12 |
| Muscle/joint pain | 55 (55.0) | 2.8 ± 3.2 | 14 (51.9) | 2.4 ± 2.9 | 9 (39.1) | 2.6 ± 3.5 | 15 (55.6) | 2.4 ± 2.8 | 0.57 | 0.89 |
| Migraine headache | 59 (57.8) | 3.8 ± 3.9 | 14 (51.9) | 3.0 ± 3.6 | 10 (43.5) | 3.1 ± 4.1 | 12 (44.4) | 2.4 ± 3.0 | 0.46 | 0.31 |
| Pain medicationsc (n [%]) | ||||||||||
| Over-the-counter pain medications | 81 (83.5) | 23 (88.5) | 23 (100) | 24 (96.0) | 0.08 | |||||
| Narcotic prescription medications | 30 (30.9) | 8 (30.8) | 9 (39.1) | 4 (16.0) | 0.35 | |||||
| Non-narcotic prescription medications | 18 (18.6) | 5 (19.2) | 3 (13.0) | 4 (16.0) | 0.92 | |||||
| None | 8 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0.09 | |||||
aIncludes 185 women in the ENDO operative cohort with endometriosis diagnosis and automatically calculated rASRM weighted point score for staging (all 190 women had staging based on operating surgeons' empiric assessment). Missing 0 observations for chronic pain, 0 for cyclic pain, 0 for pain type and 14 for pain medications. Statistical significance for differences was assessed via Wilcoxon–Mann–Whitney test for continuous variables and chi-square or Fisher's exact tests for categorical variables. Values sharing a common superscript are significantly different P < 0.05 via multiple comparison tests (Tukey procedure for frequencies and Dwass, Steel, Critchlow-Fligner procedure [based on pairwise two-sample rankings] for means).
b11-point visual analog scale with 0 being no pain and 10 being the most severe pain imaginable, women reported any pain in the last 6 months (no minimum duration required). Mean ± SD for pain on original VAS scale (0 to 10).
cPain medications taken on at least a monthly basis for any indication in the past 12 months.
In regards to differences in pain typology by lesion location and location-specific lesion depth among women diagnosed with endometriosis, no clear patterns emerged with the vast majority of comparisons resulting in non-significance (Supplementary Tables SII–SVI). The only strong signals detected were superficial versus deep ovarian lesions linked with chronic pain (65.8 versus 40.5%, respectively P = 0.03), and dyspareunia (notably, lasting pelvic pain after intercourse, 47.4 versus 16.2%, respectively P = 0.001) (Supplementary Table SIV); and deep versus superficial peritoneal lesions linked with dyschezia or pain with bowel elimination (60.8 versus 34.6%, P = 0.003) (Supplementary Table SV).
The relationship between pain characteristics and endometriosis among subfertile women suggested that women with subfertility who were diagnosed with endometriosis were more likely to experience cyclic (but not chronic) pelvic pain including increased severity of pain just before and with menstrual period and ovulatory related pain compared with subfertile women who were not diagnosed with endometriosis.
Pain location and endometriosis
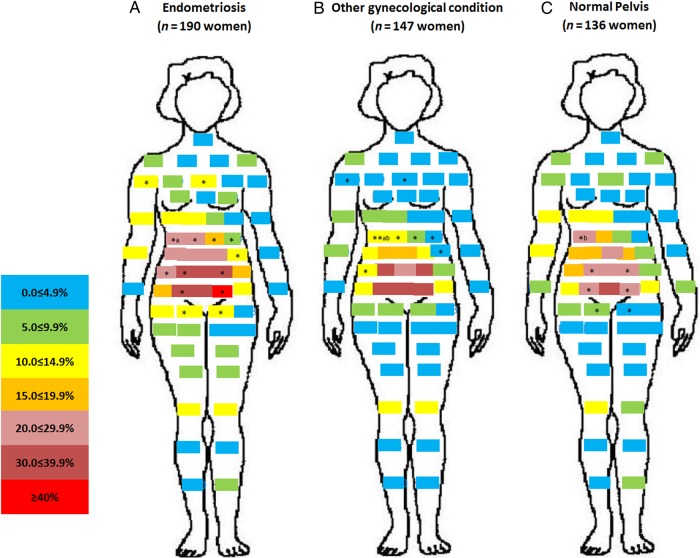
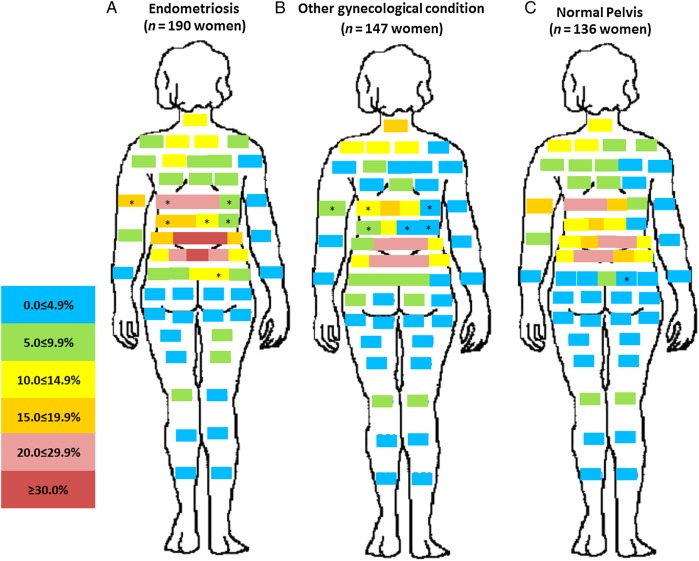
A unique pattern emerged for the distribution of pain by post-operative diagnosis. Relatively minimal pain was reported for women with other gynecologic pathology or a normal pelvis when compared with women with endometriosis (Fig. 1). Of note is the high percentage of women with endometriosis reporting vaginal pain (22.6%, P < 0.01) or right- (18.4%, P < 0.05) and left-sided (15.3%, P < 0.01) labial pain near the vagina, compared with women diagnosed with a normal pelvis. Front and back pain maps showed that while there was no difference in pain in the upper torso, legs, or arms between women with versus without endometriosis, women with endometriosis in comparison to women with a normal pelvis reported significantly more pain in the hypogastric/umbilical abdominopelvic region (Figs 2 and 3).
Figure 1.
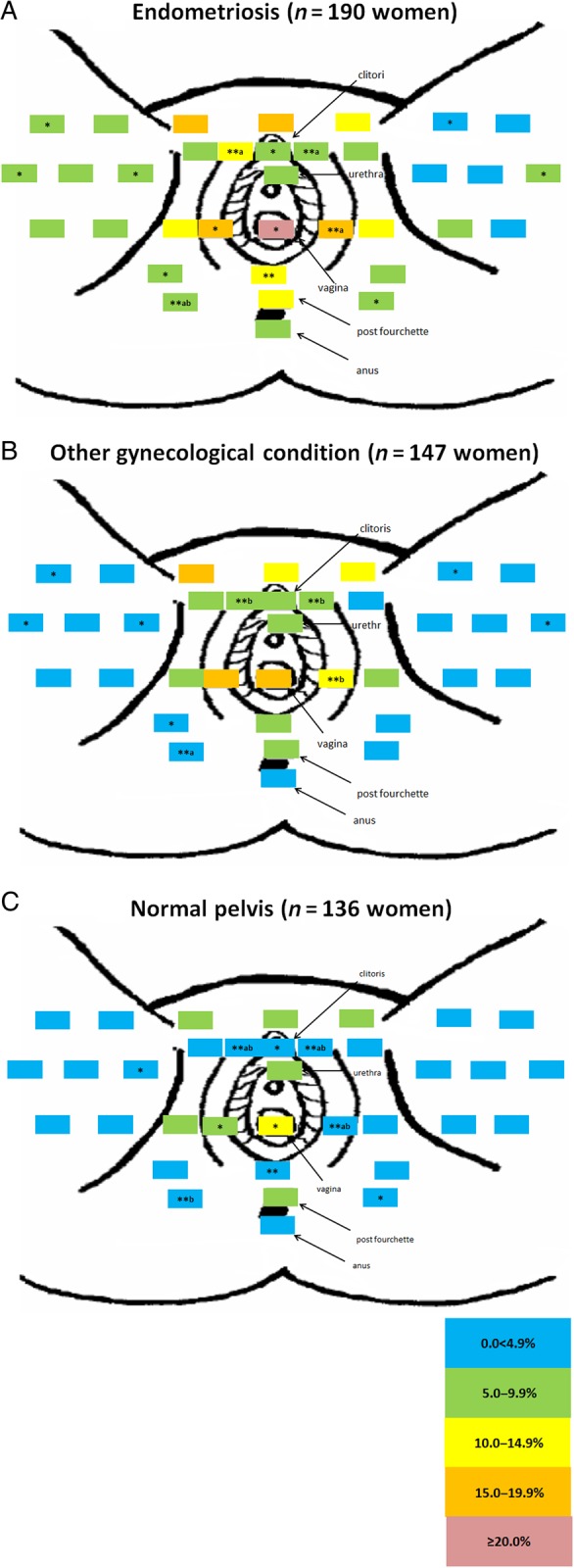
Percent distribution of anatomical site-specific pain in the perineal area by post-operative diagnosis. (A = endometriosis, B = other gynecologic pathology and C = normal pelvis). Significant differences (*P < 0.05 and **P < 0.01) between pair-wise pain report frequencies were conducted using the Tukey procedure for multiple comparisons (Elliott and Reisch, 2006; Zar, 2009). Women only indicated areas where they experienced regular pain, if any. To delineate significant differences between more than two groups (endometriosis, other gynecologic pathology and normal pelvis), values sharing a common superscript (a or b) are significantly different at *P < 0.05 and **P < 0.01. Study sample for these analyses includes all women undergoing a diagnostic and/or therapeutic laparoscopy or laparotomy regardless of clinical indication who participated in the ENDO Study (n = 473). There were no missing data in regards to pain location by post-operative diagnosis. Primary post-operative diagnosis among women with gynecologic pathology included uterine fibroids (n = 58), pelvic adhesions (n = 30), benign ovarian cysts (n = 46), neoplasm (n = 3) and congenital Müllerian anomalies (n = 10). Primary reason for surgery among women with a post-operative diagnosis of a normal pelvis included tubal ligation (n = 36), pelvic pain (n = 42), pelvic mass (n = 9), infertility (n = 16), and menstrual irregularities (n = 32), missing (n = 1).
Figure 2.
Percent distribution of anatomical site-specific pain in the front of the body by post-operative diagnosis. (A = endometriosis, B = other gynecologic pathology and C = normal pelvis). For other information see Fig. 1.
Figure 3.
Percent distribution of anatomical site-specific pain in the rear of the body by post-operative diagnosis. (A= endometriosis, B= other gynecologic pathology and C= normal pelvis). For other information see Fig. 1.
When looking at the distribution of pain by disease stage, we observed no significant differences in reporting of perineal or front/back body pain by minimal (58%), mild (15%), moderate (12%), or severe (15%) stages with the notable exception of increased anal pain with increased staging (4.7% for minimal, 7.4% for mild, 17.4% for moderate, and 17.9% for severe, P = 0.04) (data not shown).
While we were limited in power for our comparisons made between pain location and anatomic lesion location due to the fact that women could have lesions in more than one location, pain maps failed to signify any clear differences between perineal, front, or back body pain and anatomic lesion location (data not shown). Stratifying women by location-specific lesion depth, we found no notable differences in pain location by ovarian lesion depth (Supplementary Figures S1A and B, S4a and b), but did find that deep versus superficial peritoneum lesions were associated with significantly higher peri-anal pain and abdominopelvic pain (Supplementary Figures S2A and B, S5a and b), while women with complete versus partial cul de sac obliteration reported significantly more pain involving the entire central perineum (Supplementary Figure S3A and B), and abdominopelvic area, albeit not significantly (Supplementary Figure 6a and b).
Discussion
Our unique study comprising women with no previous diagnosis of endometriosis undergoing gynecologic surgery for any indication provides the first empirical evidence known to us reflecting a higher prevalence of chronic and cyclic pelvic pain, notably dyspareunia, dysmenorrhea, and dyschezia and in the peri-vaginal and abdominopelvic regions, in women with endometriosis compared to either women with other gynecologic pathology or a post-operative diagnosis of a normal pelvis. Our detailed pain assessment tools build on emerging research in this area (Hsu et al., 2011; Renner et al., 2012) and may benefit future researchers and/or clinicians interested in better understanding how dimensions of pain may contribute to the diagnostic picture and/or symptom management of endometriosis and other gynecologic disorders. Our inability to detect a clear, strong, and consistent pattern between pain characteristics and endometriosis staging or lesion location is consistent with past research using a similar pain assessment tool (Hsu et al., 2011) and should be corroborated by future research among larger samples of women with more diversity in staging and lesion location.
The associations we found between endometriosis and dysmenorrhea and dyspareunia are supported by past studies. Multiple studies report an increased risk for endometriosis in women with reported dysmenorrhea (Cramer et al., 1986; Vercellini et al., 1996; Fauconnier and Chapron, 2005; Dai et al., 2012), and increased dyspareunia among women with endometriosis (Mahmood et al., 1991; Fedele et al., 1992; Al-Badawi et al., 1999; Ferrero et al., 2005; Ballard et al., 2008). It is hypothesized that cyclic recurrent micro-bleeding within endometriotic lesions with consequent inflammation may be the cause of severe dysmenorrhea among women with endometriosis (Fauconnier and Chapron, 2005). Mechanistic explanations for how endometriosis may cause dyspareunia include tension on the infiltrated uterosacral ligament during intercourse (Fauconnier et al., 2002), as evidenced by the shortened distance between nerve fibers and ectopic endometrial growths in women with dyspareunia compared with those without (Tulandi et al., 2001). Our finding that women with subfertility who were diagnosed with endometriosis were more likely to experience dysmenorrhea compared with subfertile women who were not diagnosed with endometriosis should be further explored among women who report failure to conceive after 12 months or more of unprotected intercourse.
Studying the relationship between endometriosis and other types of pain including dysuria, dyschezia, and abdominal pain is challenging, since women with endometriosis-related pain may have pain generated by comorbid pain conditions such as the painful bladder syndrome, migraine, and irritable bowel syndrome (Stratton and Berkley, 2011; Tirlapur et al., 2013). While pathophysiologic relationships have been found between rectovaginal, urinary tract, and intestinal endometriosis and dyschezia, dysuria, and abdominal pain, respectively (Gabriel et al., 2011; Kruse et al., 2012), associations of these pain types with endometriosis restricted to the pelvic area are, as yet, not extensively characterized (Ballard et al., 2010). Our finding of higher dyschezia among women with endometriosis compared with unaffected women warrants further research to help inform pathophysiology.
We found no association between pain type and stage of disease, which is not inconsistent with previous studies (Gruppo Italiano per lo Studio dell'Endometriosi, 2001; Vercellini et al., 2007). Specifically, in a sample of 469 women with surgically visualized endometriosis who reported pain lasting ≥6 months, disease stage was not associated with presence or severity of dysmenorrhea, non-menstrual pain, and dyspareunia (Gruppo Italiano per lo Studio dell’ Endometriosi, 2001). In another study involving 1054 women with surgically visualized endometriosis, a marginal association was found between disease severity and dysmenorrhea (odds ratio (OR): 1.33, 95% CI: 1.04–1.71) and non-menstrual pain (OR: 1.02, 95% CI: 1.00–1.03) but not deep dyspareunia (Vercellini et al., 2007). Lack of a consistent or strong association between pain and disease stage may be due to the hypothesis that pain intensity is most likely determined by the interaction between endometriotic lesions and sensory afferent nerve fibers rather than simply type and extent of implants alone (Vercellini et al., 2007). Additionally, classification schemes for staging endometriosis, including the rASRM that was used in our study, have previously been reported to not be predictive of fertility (Guzick et al., 1997) and it may be that this empirically designed staging system is also poorly correlated with pain symptomatology. This poor correlation is supported by previous research. While the rASRM system upstages based on increasing size of ovarian endometrioma, previous research has shown an inverse relationship between endometrioma diameter and dysmenorrhea and non-menstrual pelvic pain (Vercellini et al., 2007). Alternative staging systems validated with outcomes should be explored (Adamson and Pasta, 2010).
While we did find evidence for peri-vaginal and hypogastric/umbilical abdominopelvic pain for women with versus without endometriosis, we found no clear pattern between pain typology or topology and anatomic lesion site among women with a post-operative endometriosis diagnosis, a finding consistent with previous research. Two recent studies have used preoperative pain mapping to pinpoint pain location as it relates to visualized and/or histologically confirmed endometriosis (Hsu et al., 2011; Renner et al., 2012), one of which examined pelvic pain location as it related to endometriosis diagnosis (Renner et al., 2012) and the other to endometriosis location. In Renner et al.'s study among 159 women reporting chronic pelvic pain, 117 women with and 42 women without surgically visualized endometriosis, pelvic pain among women with versus without endometriosis was most frequently reported in the vesicouterine pouch (near the anterior vaginal fornix) (Renner et al., 2012). In Hsu et al.'s study among 96 women with histologically confirmed endometriosis, no associations were found between lesion location and dyspareunia or dyschezia or pain location within five pelvic region locations (Hsu et al., 2011). Comparison between these two studies and ours is difficult given that we used the rASRM classification system to assess endometriosis location while the others created study-specific pictograms to identify endometriosis location (Hsu et al., 2011; Renner et al., 2012) and only one used self versus physician-reported pain documentation (Hsu et al., 2011). Despite study differences, similar results were found with respect to increased peri-vaginal pain among women diagnosed with versus without surgically visualized endometriosis (Renner et al., 2012) and no apparent relationship among women with endometriosis between pain typology or topology and lesion location (Hsu et al., 2011). This null relationship between pain location and endometriosis location was also found in another study looking at three pain areas (left-sided, right-sided and low back) and site of endometriosis among 113 women with visualized disease (Ballard et al., 2010), but conversely found a significant correlation with disease depth (mirroring our results for peritoneal lesion depth).
Our finding of increased vaginal or vulvar pain in women with endometriosis corroborates the work of previous authors and may be biologically plausible. Despite the fact that the vulva is innervated by the pudendal nerves, there appears to be some hypersensitivity of the perineum in women with endometriosis. Both peptidergic and sympathetic nerve fibers that innervate blood vessels near endometriotic lesions can sprout new axons that can become sensitized. Though input from peripheral afferent fibers at the level of the dorsal root are concentrated in the segment associated with its corresponding body part, branches of nerve fibers can extend to other segments (intersegmental nerve connections) (Stratton and Berkley, 2011). This may explain why pain can exist in places other than the pelvis in women with endometriosis. Given that up to 18% of American women experience pain symptoms consistent with a vulvodynia diagnosis at some point in their lifetime (Nguyen et al., 2013) but that vulvodynia by definition is vulvar discomfort in the absence of gross anatomic or neurologic findings (Haefner et al., 2005), further research should try to delineate vulvar pain associated with endometriosis compared with vulvar pain attributed to other causes (Vincent, 2011).
Our study had several strengths including gold-standard assessment of endometriosis (American Society for Reproductive Medicine, 1997) among a relatively large sample of women and the assessment of pelvic pain via multiple standardized instruments (International Pelvic Pain Society, 2008), including pain mapping using women's recording of pain on pictograms versus physician recording as derived from clinical examination. Interpretation of our findings, however, requires caution given that this is an exploratory analysis involving multiple comparisons. Additionally, given lag time between onset of symptoms and diagnosis of endometriosis, women may have been taking pain medications to relieve endometriosis-related pain; however, women would not have been prescribed pain medications due to endometriosis diagnosis. While it is possible that pain medication use may have attenuated scores in pain assessment, formal assessment of pain was done in a standardized fashion to improve overall assessment. Finally, we were unable to assess pelvic pain in relation to deep infiltrating endometriosis or endometriosis outside of the pelvic cavity; though this has been examined by others in detail (Faucconnier et al., 2002; Dai et al., 2012). In regards to the generalizability of our findings, we believe that our very limited exclusion criteria (Buck Louis et al., 2010) and broad sampling frame drawing women from fourteen surgical centers in two states, would be representative of other surgical patients undergoing a diagnostic and/or therapeutic laparoscopy or laparotomy. Indeed, based on one review article, 40% of gynecologic laparoscopies are due to chronic pelvic pain, a finding very close to what we observed in our study (Howard, 1993) but an often-cited statistic that was in need of revisiting with a more contemporary clinical population.
In summary, our findings suggest that while women with endometriosis report more site-specific pain particularly involving the vaginal and abdominopelvic area than women with other or no gynecologic pathology, chronic and/or cyclic pelvic pain is high among all women presenting for diagnostic or therapeutic laparoscopy, even those with an apparent normal pelvis. Further research should be done to determine causes of pelvic pain outside of gynecologic pathology. Concordant with previous research, we found little association between pain typology or topology and rASRM disease stage or location, suggesting the need for further development of classification systems that can better predict outcomes for endometriosis patients with pelvic pain for both surgical and nonsurgical treatment (Adamson, 2011). Given that endometriosis is a chronic disease requiring lifelong management (American Society for Reproductive Medicine, 2014), future adequately powered research in this emerging area linking precise pain location with endometriosis anatomic location should be conducted in hopes that surgical and medical treatments for pain associated with endometriosis may become more effective.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
K.C.S., Z.C., L.S. and G.M.B.L. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. C.M.P. and G.M.B.L. supervised the study. Z.C., C.M.P., J.B.S. and G.M.B.L. were involved in the study concept and design. Z.C., C.M.P. and G.M.B.L. were responsible for acquisition of data. K.C.S., S.L.M., Z.C., L.S. and G.M.B.L. did the analysis and interpretation of data. K.C.S., S.L.M., Z.C., C.M.P, H.T.S., E.B.J., A.O.H., J.B.S., L.S. and G.M.B.L. drafted the manuscript and critically revised the manuscript for important intellectual content.
Funding
This work was funded by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (contract numbers N01-DK-6-3428, N01-DK-6-3427, and 10001406-02). Ethicon Endo-Surgery kindly donated the Harmonic Ace 36P shears and scalpel blades for use in the study through a signed materials transfer agreement with the University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Conflict of interest
None declared.
Supplementary Material
References
- Adamson GD. Endometriosis classification: an update. Curr Opin Obstet Gynecol 2011;23:213–220. [DOI] [PubMed] [Google Scholar]
- Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril 2010;94:1609–1615. [DOI] [PubMed] [Google Scholar]
- Al-Badawi IA, Fluker MR, Bebbington MW. Diagnostic laparoscopy in infertile women with normal hysterosalpingograms. J Reprod Med 1999;44:953–957. [PubMed] [Google Scholar]
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine (rASRM) classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril 2014;101:927–935. [DOI] [PubMed] [Google Scholar]
- Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, Peny MO, Noel JC. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod 2000;15:1744–1750. [DOI] [PubMed] [Google Scholar]
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382–1391. [DOI] [PubMed] [Google Scholar]
- Ballard K, Lane H, Hudelist G, Banerjee S, Wright J. Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain. Fertil Steril 2010;94:20–27. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 2011;96:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttram VC., Jr Conservative surgery for endometriosis in the infertile female: a study of 206 patients with implications for both medical and surgical therapy. Fertil Steril 1979;31:117–123. [DOI] [PubMed] [Google Scholar]
- Chapron C, Fauconnier A, Dubuisson JB, Barakat H, Vieira M, Breart G. Deep infiltrating endometriosis: relation between severity of dysmenorrhoea and extent of disease. Hum Reprod 2003;18:760–766. [DOI] [PubMed] [Google Scholar]
- Cox L, Ayers S, Nala K, Penny J. Chronic pelvic pain and quality of life after laparoscopy. Eur J Obstet Gynecol Reprod Biol 2007;132:214–219. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986;255:1904–1908. [PubMed] [Google Scholar]
- Dai Y, Leng JH, Lang JH, Li XY, Zhang JJ. Anatomical distribution of pelvic deep infiltrating endometriosis and its relationship with pain symptoms. Chin Med J (Engl) 2012;125:209–213. [PubMed] [Google Scholar]
- Elliott A, Reisch J. Implementing a multiple comparison test for proportions in a 2xc crosstabulation in SAS. Proceedings of the 31st Annual SAS Users Group International Conference, San Francisco, California Cary, NC: SAS Institute Inc 2006 26–29 March 2006 Paper 204–31. Available from: http://www2.sas.com/proceedings/sugi31/204-31.pdf. [Google Scholar]
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update 2005;11:595–606. [DOI] [PubMed] [Google Scholar]
- Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Bréart G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril 2002;78:719–726. [DOI] [PubMed] [Google Scholar]
- Fedele L, Parazzini F, Bianchi S, Arcaini L, Candiani GB. Stage and localization of pelvic endometriosis and pain. Fertil Steril 1990;53:155–158. [DOI] [PubMed] [Google Scholar]
- Fedele L, Bianchi S, Bocciolone L, Di Nola G, Parazzini F. Pain symptoms associated with endometriosis. Obstet Gynecol 1992;79:767–769. [PubMed] [Google Scholar]
- Ferrero S, Esposito F, Abbamonte LH, Anserini P, Remorgida V, Ragni N. Quality of sex life in women with endometriosis and deep dyspareunia. Fertil Steril 2005;83:573–579. [DOI] [PubMed] [Google Scholar]
- Gabriel B, Nassif J, Trompoukis P, Barata S, Wattiez A. Prevalence and management of urinary tract endometriosis: a clinical case series. Urology 2011;78:1269–1274. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppo Italiano per lo Studio dell'Endometriosi. Relationship between stage, site and morphological characteristics of pelvic endometriosis and pain. Hum Reprod 2001;16:2668–2671. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Silliman NP, Adamson GD, Buttram VC Jr, Canis M, Malinak LR, Schenken RS. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine's revised classification of endometriosis. Fertil Steril 1997;67:822–829. [DOI] [PubMed] [Google Scholar]
- Haefner HK, Collins ME, Davis GD, Edwards L, Foster DC, Hartmann ED, Kaufman RH, Lynch PJ, Margesson LJ, Moyal-Barracco M et al. The vulvodynia guideline. J Low Genit Tract Dis 2005;9:40–51. [DOI] [PubMed] [Google Scholar]
- Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv 1993;48:357–387. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol 2011;118:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Pelvic Pain Society. History and Physical Form. http://www.pelvicpain.org/Professional/Documents-and-Forms (Accessed 22 April 2015).
- Kang SB, Chung HH, Lee HP, Lee JY, Chang YS. Impact of diagnostic laparoscopy on the management of chronic pelvic pain. Surg Endosc 2007;21:916–919. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E and Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod 2005;20:2698–2704. [DOI] [PubMed] [Google Scholar]
- Khan KN, Kitajima M, Fujishita A, Hiraki K, Matsumoto A, Nakashima M, Masuzaki H. Pelvic pain in women with ovarian endometrioma is mostly associated with coexisting peritoneal lesions. Hum Reprod 2013;28:109–118. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril 2012;98:564–571. [DOI] [PubMed] [Google Scholar]
- Kruse C, Seyer-Hansen M, Forman A. Diagnosis and treatment of rectovaginal endometriosis: an overview. Acta Obstet Gynecol Scand 2012;91:648–657. [DOI] [PubMed] [Google Scholar]
- Mahmood TA, Templeton AA, Thomson L, Fraser C. Menstrual symptoms in women with pelvic endometriosis. Br J Obstet Gynaecol 1991;98:558–563. [DOI] [PubMed] [Google Scholar]
- Marana R, Muzii L, Caruana P, Dell'Acqua S, Mancuso S. Evaluation of the correlation between endometriosis extent, age of the patients and associated symptomatology. Acta Eur Fertil 1991;22:209–212. [PubMed] [Google Scholar]
- Muzii L, Marana R, Pedulla S, Catalano GF, Mancuso S. Correlation between endometriosis-associated dysmenorrhea and the presence of typical or atypical lesions. Fertil Steril 1997;68:19–22. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Veasley C, Smolenski D. Latent class analysis of comorbidity patterns among women with generalized and localized vulvodynia: preliminary findings. J Pain Res 2013;18:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain . J Am Assoc Gynecol Laparosc 1999;6:429–434. [DOI] [PubMed] [Google Scholar]
- Renner SP, Boosz AS, Burghaus S, Maihöfner C, Beckmann MW, Fasching PA, Jud SM. Visual pain mapping in endometriosis. Arch Gynecol Obstet 2012;286:687–693. [DOI] [PubMed] [Google Scholar]
- Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update 2011;17:327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirlapur SA, Kuhrt K, Chaliha C, Ball E, Meads C, Khan KS. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg 2013;11:233–237. [DOI] [PubMed] [Google Scholar]
- Tulandi T, Felemban A, Chen MF. Nerve fibers and histopathology of endometriosis-harboring peritoneum. J Am Assoc Gynecol Laparosc 2001;8:95–98. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril 1996;65:299–293. [PubMed] [Google Scholar]
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 2007;22:266–271. [DOI] [PubMed] [Google Scholar]
- Vincent K. Pelvic pain in women: clinical and scientific aspects. Curr Opin Support Palliat Care 2011;5:143–149. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis, 5th edn Upper Saddle River, NJ: Pearson Prentice-Hall, 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.