Summary
Encapsulated thyroid tumors of follicular cell origin with high-grade features (EFHG) are unusual neoplasms. In current classification schemes, they are called atypical adenomas or follicular, papillary, or poorly differentiated carcinoma. When noninvasive, EFHG create a major therapeutic/diagnostic dilemma stemming from their rarity, low-stage, high-grade appearance, and lack of long-term follow-up studies. All cases of EFHG were defined as encapsulated tumors of follicular cell origin with at least 5 mitoses per 10 high-power fields and/or tumor necrosis. Available tissues were subjected to a thyroid carcinoma platform for mass spectrometry high-throughput genotyping, which consisted of 111 known mutations in 16 different genes: BRAF, RET, NRAS, HRAS, KRAS, PIK3CA, AKT1, and other related genes. Twenty-five cases met the selection criteria. Tumor necrosis was present in 56.0% (n = 14). Extensive vascular invasion was identified in 24.0% (n = 6). Eight (32%) of 25 tumors were noninvasive. Twenty-two patients (88%) were free of disease (median follow up: 8.5 years). All 8 noninvasive tumor did not recur despite focal/extensive tumor necrosis in 3 cases and a median follow-up of 11.9 years. EFHG with no vascular invasion did not recur. In patients without distant metastases at presentation (n = 24), 33% (2/6) of patients with extensive angioinvasion relapsed, whereas none of 18 with absent/focal vascular invasion recurred (P = .054). Mutations were found in 10 (45%) of 22 cases tested: 8 had NRAS codon 61, 1 KRAS codon 61, and 1 had coexistent BRAF V600E and AKT1. There was a higher frequency of RAS (9/22, 41%) than BRAF mutations (1/22, 4.5%) (P = .009). Noninvasive EFHG have an indolent behavior even in the presence of extensive tumor necrosis. EFHG with absent vascular invasion have an excellent prognosis despite the frequent occurrence of tumor necrosis. NRAS mutations are the most frequent oncogenic event in EFHG.
Keywords: Thyroid, Encapsulated, Follicular, Mitosis, Necrosis, Molecular, Clinicopathologic
1. Introduction
Encapsulated thyroid tumors of follicular cell origin are generally thought to behave in an indolent fashion. The classification of these tumors is based on the recognition of a combination of cytologic and invasive features. The latter include invasion of the tumor capsule and angioinvasion. These tumors are designated as the follicular variant of papillary carcinoma when they contain nuclear features of classical papillary thyroid carcinoma (ie, grooves, pseudoinclusions and clearing). Encapsulated follicular variant of papillary carcinoma without significant vascular and capsular invasion have been shown to have an indolent course with infrequent recurrences and metastases [1]. When the nuclear features of papillary thyroid carcinoma (PTC) are absent, the presence of capsular or vascular invasion categorize the tumor as follicular carcinoma. Follicular carcinomas showing minimal capsular invasion recur or metastasize infrequently [2]. When the nuclear features of PTC are absent in a noninvasive tumor, the diagnosis of follicular adenoma is rendered.
On occasion, increased mitotic activity and/or tumor necrosis has been noted in encapsulated thyroid tumors. In noninvasive tumors without nuclear features of PTC, necrosis, and high mitotic rate can be a source of difficulty in making a benign diagnosis. Indeed, these high-grade features seem at odds with what otherwise could be regarded as an adenoma. In the first half of the 20th century, the term atypical adenoma was proposed to define noninvasive, nonpapillary encapsulated follicular-derived neoplasms with unusual disturbing histologic features. These “atypical adenomas” were defined on the basis of worrisome architecture (eg, solid hypercellular growth) or a combination of hypercellularity and increased mitotic activity [3,4]. No recurrences were reported after long-term follow-up (up to 20 years) in “atypical adenomas” defined based on architecture [3]. Unfortunately, the latter studies included tumors with very low mitotic rate or in which the number of mitoses was not mentioned [3,4]. Hence, the outcome of encapsulated non-invasive follicular-derived tumors with high-grade features (ie, high mitotic rate and/or tumor necrosis) has not been reported. This is important because several studies support the notion that the presence of tumor necrosis and increased mitoses are adverse prognostic indicators in thyroid malignancies [5–8]. In fact, patients with invasive thyroid carcinomas harboring increased mitoses and/or necrosis have a greater chance of becoming refractory to radioactive iodine and to die of disease [9]. The presence of tumor necrosis and/or increased mitotic activity in a non-invasive tumor can create major anxiety on the part of the patient and physicians (pathologist and clinician) involved in his/her care. This uncertainty in regard to outcome obviously creates a major therapeutic dilemma.
To address these issues, 25 cases of encapsulated follicular-derived lesions with increased mitoses and/or necrosis were studied extensively at the histological and molecular level. In addition to the noninvasive cases, we have included similar high-grade but invasive encapsulated tumors in order to assess the value of invasive features while stratifying for mitosis and necrosis. The purpose of this study is not to create another entity or a new terminology but, rather, to help pathologists and clinicians understand the behavior and treat these rare tumors.
2. Material and methods
2.1. Patient population and inclusion criteria
The institutional database was searched for all cases with a diagnosis of thyroid carcinomas treated at Memorial Sloan-Kettering Cancer Center between January 1980 and December 2000. Additional cases were supplied from the personal file of one of us (R.A.G). The slides from the cases included in the study were examined by 2 head and neck pathologists with special interest in thyroid neoplasia (R.A.G. and M.R.). The pathologists were blinded to the clinical outcome of all patients studied. This study was restricted to cases containing tumors which were found to be entirely encapsulated, of thyroid follicular cell origin showing at least 5 mitoses per 10 high-power fields and/or spontaneous tumor necrosis. Tumors with any papillary growth or extrathyroidal extension were excluded from the study. Inadequately sampled tumor (<1 tumor section per centimeter) were also excluded. The study was approved by the institutional review board of Memorial Sloan-Kettering Cancer Center.
2.2. Histopathologic analysis
The largest dimension of the tumor was recorded as dictated during gross examination. The mitotic rate of the tumor was determined by counting 10 contiguous high-power fields (400×) using an Olympus microscope (U-DO model BX-40; Olympus America Inc., Melville, NY). Using that microscope type, these 10 high-power fields corresponds to 2.4 mm2. Mitotic counts were performed in a focused fashion, examining areas that appeared to show greater proliferative activity. Spontaneous tumor necrosis was classified as absent, focal (≤5% of the tumor area), or extensive (>5% of the tumor area). Tumor necrosis was defined by a “comedo-like” appearance composed of degenerating cytoplasm and punctuate, karyorectic nuclear debris. The presence of fibroblastic stromal reaction, hemorrhage or an identifiable needle track in the necrotic area was attributable to reaction induced by prior fine needle aspiration and was therefore not regarded as spontaneous tumor necrosis. When present, the numbers of foci of capsular and vascular invasion were noted. Only complete penetration of the capsule by tumor was regarded as capsular invasion as described by Lang et al [10]. The presence of vascular invasion was noted only when such foci were present within or beyond the capsule in accordance with criteria outlined by the Armed Forces Institute of Pathology fascicle [11]. Only when the invasive focus protruded into the lumen of the vessel in a polypoid fashion covered by endothelial cells or when attached to the vessel wall or associated with thrombus formation was it accepted as true vascular invasion. Areas of vascular invasion that were closely adjacent to one another were counted as separate foci. The foci of capsular and vascular invasion were subdivided into 2 categories: focal (<4 invasive foci) and extensive (≥4 foci). Growth patterns were divided into follicular and solid/trabecular/insular (STI) and quantified. The cytoplasm of the tumor cells were subdivided into oncocytic and nononcocytic. The nuclei were described as those of papillary carcinoma if nuclear grooves, pseudoinclusions or optical clearing were identified in the majority of cells. If the nuclei had a convoluted appearance with partial clearing while lacking grooves and pseudoinclusions as described in the Turin proposal then they were reported as convoluted [9]. The nuclei were called oncocytic if they were vesicular and contained prominent central nucleoli. Finally, those tumor cells containing normochromatic nuclei with even granular chromatin were designated as containing follicular nuclei as seen in typical follicular adenomas.
2.3. Clinical parameters
The patient's electronic medical records were reviewed for the age at diagnosis, type of surgery, and adjuvant treatment including radioactive iodine (RAI) therapy. The current disease status was based on a combination of physical examination, biochemical serum markers (thyroid stimulating hormone and thyroglobulin), RAI dosimetry, cross-sectional imaging and/or positron emission tomography scanning. The date of initial surgery and last date of follow-up were recorded. The status at last follow-up was recorded as follows: no evidence of disease, alive with disease, dead of other causes, and dead of disease.
2.4. DNA extraction and genotyping of thyroid cancer genetic alterations by mass spectrometry
Four sections of 10 µm from each formalin-fixed, paraffin-embedded tissue block were subjected to DNA extraction using the PUREGene Genomic DNA purification kit (Gentra, Inc, Minneapolis, MN). Mutation detection was performed as described previously [12]. We used mass spectrometry Sequenom–based genotyping assay (Sequenom Mass array, Sequenom, San Diego CA), which is especially suited for high throughput genotyping, to interrogate 111 known mutations in 16 different genes: BRAF, RET, NRAS, HRAS, KRAS, PIK3CA, MAP2K1, AKT1, MET, IKBKB, PIK3R5, PRKCZ, RHEB, RPS6KA3, RPS6KB1, and FRAP1. As the mass spectrometry genotyping assays for codons 12 and 13 of HRAS were not informative, we designed primers for this region and sequenced all the tumors that were wild type for BRAF and RAS mutations [12].
2.5. Screening for RET/PTC and PAX8/PPAR rearrangements
We used tumor cDNA as template for quantitative polymerase chain reaction (PCR) to analyze for unbalanced expression of exons 10 to 11 relative to 12 to 13 of RET, which flank the rearrangement site in the intron 11. Samples with 12–13 > 10–11 expression were screened for specific RET recombination events using primers bracketing the fusion point of RET/PTC1, RET/PTC2, and RET/PTC3 as previously described [12,13]. We screened for the PAX8-PPARγ fusion by reverse transcriptase–PCR, using primers for the different possible transcripts of PAX8/PPARγ as previously described [14]. PCR products were resolved by electrophoresis in a 2% agarose gel and selected cases were sequenced. RET and PAX8-PPARγ rearrangements were analyzed for tumors that were wild type for RAS.
2.6. Statistical analysis
Two-tailed Fisher exact test was used to assess the relation between categorical variables. P < .05 was considered significant.
3. Results
3.1. Clinical and histopathologic features
Slides from 980 patients were obtained for review, of which 19 satisfied the selection criteria. Six additional cases were supplied from the personal files of R.A.G., for a total of 25 cases. A minimum of one section per centimeter of tumor was examined in each patient with an average of 12 tumor sections examined per case. Seventeen women and 8 men were present in the cohort with a median age of 49 years (range, 16–84 years). The mean and median tumor size for all cases was 3.6 and 3.5 cm, respectively (range, 2.0–7.0). The median tumor size for noninvasive and invasive tumors was 3.6 cm (mean, 4.3 cm) and 3.2 cm (mean, 3.4 cm), respectively. Six tumors (n = 6) exceeded 4.0 cm.
A follicular pattern of growth was seen in 18 (72.0%) of 25 cases, with all of them demonstrating microfollicular growth. An STI growth pattern was seen in 21 (84%) of 25 tumors, with a predominance of this growth pattern seen in 9 (36%) of 25 of the cases. Nononcocytic and oncocytic cytoplasm was seen in n = 23 and n = 2 cases, respectively. A predominance of nuclei with the features of papillary carcinoma was identified in 4 (16.0%) of 25 cases. A predominance of convoluted nuclei was present in 7 (28%) of the cases, whereas 2 (8%) had oncocytic (ie, vesicular round nuclei with prominent central nucleolus) nuclei. The remaining cases (n = 12, 48.0%) had a predominance of follicular nuclei similar to those seen in typical follicular adenomas. Seventeen cases fulfilled the cytologic, architectural and proliferative features required for the diagnosis of poorly differentiated thyroid carcinoma (PDTC) according to the Turin proposal. Of those, 13 were invasive and thus fulfill all the criteria required for the diagnosis of PDTC according to the Turin proposal [8]. Based on current classification schemes, the remaining 12 cases would have been categorized as follicular adenoma (n = 5) albeit with very atypical features, follicular variant of papillary thyroid carcinoma (n = 6) and follicular carcinoma (n = 1).
Mitoses numbering at least 5 per 10 high-power fields were seen in 64.0% of cases (n = 16). Necrosis was present in 56.0% (n = 14) and extensive necrosis was identified in 32.0% (n = 8) of the cases examined. Eight (32%) of 25 tumors were completely noninvasive. Three (37.5%) of the 8 noninvasive tumors showed necrosis, including 1 case showing extensive necrosis (Fig. 1). Extensive vascular invasion (≥4 foci) was identified in 24.0% (n = 6) of the cases studied and focal angioinvasion (<4 foci) in 32% (n = 8). Extensive capsular invasion was seen in 4% of tumors (n = 1) and focal capsular invasion in 44% (n = 11). The surgical margins were negative in all cases with assessable margins (n = 24).
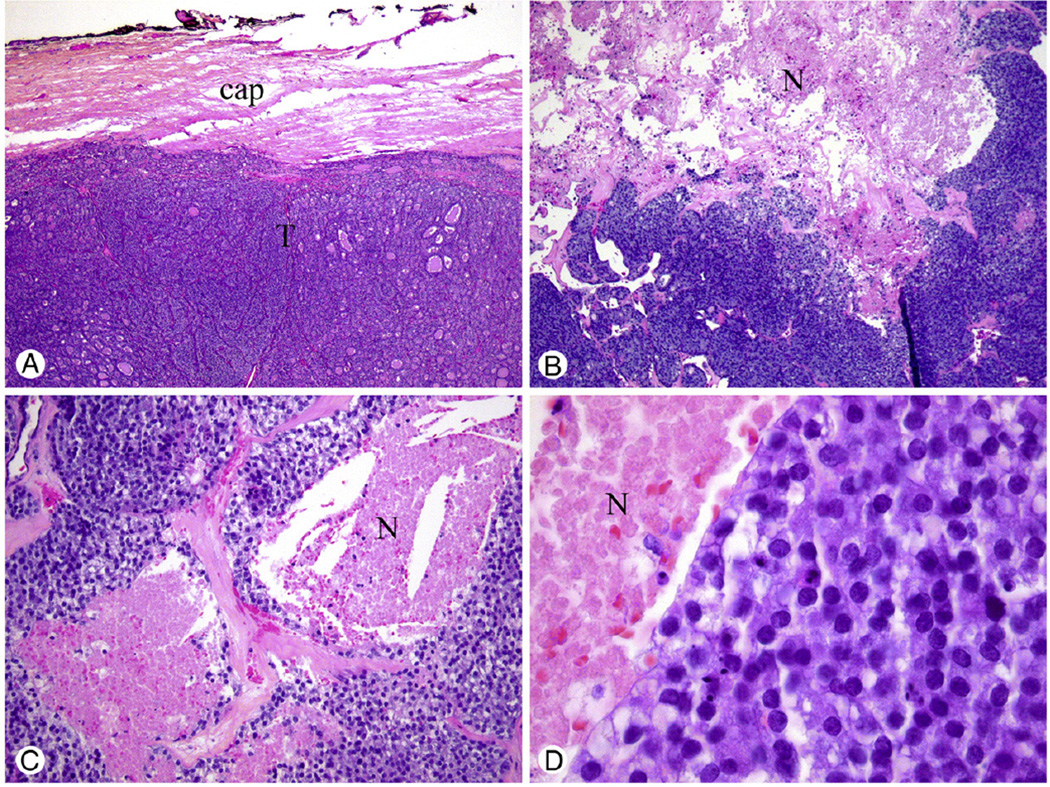
Fig. 1.
Encapsulated noninvasive tumor of follicular cell origin with extensive necrosis treated by lobectomy alone without radioactive iodine therapy. The patient did not recur after a follow-up of 13 years. A, Low power of hypercellular tumor (T) with its capsule (cap) free of invasion. B, Medium power showing extensive necrosis (N) surrounded by a tumor with a solid/nesting growth pattern. C, Tumor necrosis (N) was defined by a “comedo-like” appearance composed of degenerating cytoplasm and punctuate, karyorrhectic nuclear debris. D, High-power view showing round normochromatic tumor nuclei (resembling follicular adenoma/carcinoma nuclei) adjacent to necrosis (N).
Eighty-eight percent (n = 22) of the patients were free of disease with a median and mean time of follow up of 8.5 years and 8.9 years respectively. None of the 8 noninvasive tumors recurred over a median follow-up of 11.9 years. Neoplasms without vascular invasion did not recur or metastasize. One patient with persistent metastatic thyroid carcinoma died of acute myelogenous leukemia after 6.5 years (Table 1, patient 15). This patient presented with metastatic disease to the pelvis and right proximal femur after which she underwent total thyroidectomy showing a 2.0-cm encapsulated tumor with focal necrosis and focal capsular and vascular invasion. One patient died of unrelated causes and was free of recurrent or metastatic carcinoma of thyroid origin (patient 19). Two patients were alive with disease at last follow-up (4.9 and 13.4 years, respectively), with one patient confirmed to have distant metastases to the lungs (patient #23), and the other had recurrent neck disease without distant metastases (patient #25). Both patients (#23 and #25) had extensive vascular invasion in their primaries. In patients without distant metastases at presentation (n = 24), 2 (33%) of 6 individuals with extensive angioinvasion relapsed, while none of the 18 patients with focal or absent vascular invasion recurred (P = .054). In the same subgroup, all 7 tumors with focal vascular invasion did not recur despite the presence of tumor necrosis in 5 (71%) of 7 cases and a median follow up of 6.91 years. None of the patients developed metastases to regional lymph nodes during follow-up.
Table 1.
Clinicopathologic and molecular features of 25 cases of encapsulated follicular derived tumor with high grade features
| # | Age | Sex | Tumor size (cm) |
1° Growth pattern (%) a |
2° Growth pattern (%) a |
Cytoplasm type b |
Nuclei (F/P/C/H) c |
# of Mitoses |
Necrosis (N/F/E) d |
CI | VI | Surgery | RAI therapy |
Status↑ | F/U | Mutation status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | M | 7.0 | STI(100) | NA | NON-O | F | 6 | N | 0 | 0 | Lobectomy | none | NED | 14.3 | B-RAF V600E AKT1 E17K |
| 2 | 49 | F | 2.2 | F(60) | STI(40) | NON-O | F | 5 | E | 0 | 0 | Lobectomy | none | NED | 13.3 | NRAS Q61 R |
| 3 | 43 | M | 3.5 | F(70) | STI(30) | NON-O | F | 4 | F | 0 | 0 | TT | none | NED | 14.4 | Wild type |
| 4 | 32 | M | 5.5 | F(95) | STI(5) | NON-O | F | 7 | N | 0 | 0 | TT | none | NED | 13.8 | Wild type |
| 5 | 32 | F | 6.5 | F(100) | NA | NON-O | P | 6 | N | 0 | 0 | Lobectomy | none | NED | 8.5 | NRAS Q61R |
| 6 | 41 | F | 2.6 | F(100) | NA | NON-O | F | 7 | N | 0 | 0 | TT | none | NED | 1.3 | Wild type |
| 7 | 50 | F | 3.7 | F(95) | STI(5) | NON-O | P | 8 | N | 0 | 0 | TT | yes | NED | 10.5 | PAX8/PPARγ |
| 8 | 76 | F | 3.5 | STI(60) | F(40) | NON-O | C | 4 | F | 0 | 0 | TT | yes | NED | 4.8 | Wild type |
| 9 | 84 | F | 4.5 | STI(100) | NA | NON-O | C | 5 | N | 1 | 0 | TT | none | NED | 2.0 | NRAS Q61 R |
| 10 | 50 | M | 2.3 | STI(100) | NA | O | O | 7 | N | 1 | 0 | Lobectomy | none | NED | 10.3 | NRAS Q61R |
| 11 | 36 | F | 5.6 | F(60) | STI(40) | O | O | 7 | N | 1 | 0 | Lobectomy | none | NED | 5.4 | Wild type |
| 12 | 70 | F | 3.8 | STI(100) | NA | NON-O | C | 9 | E | 0 | 1 | TT | yes | NED | 4.7 | KRASQ61R |
| 13 | 16 | M | 4.0 | F(100) | NA | NON-O | C | 4 | F | 0 | 1 | Lobectomy | none | NED | 12.8 | Wild type |
| 14 | 58 | M | 2.1 | STI(100) | NA | NON-O | F | 3 | E | 1 | 1 | Lobectomy | none | NED | 3.2 | Wild type |
| 15 | 57 | M | 2.0 | F(90) | STI(10) | NON-O | F | 3 | E | 2 | 1 | TT | yes | DWD | 6.5 | ND |
| 16 | 52 | F | 2.3 | F(50) | STI(50) | NON-O | F | 1 | F | 1 | 2 | TT | yes | NED | 6.9 | NRAS Q61R |
| 17 | 28 | F | 3.5 | F(60) | STI(40) | NON-O | P | 5 | N | 3 | 2 | Lobectomy | none | NED | 13.2 | Wild type |
| 18 | 36 | M | 3.5 | F(95) | STI(5) | NON-O | F | 8 | N | 1 | 3 | Lobectomy | none | NED | 3.2 | Wild type |
| 19 | 49 | F | 3.8 | F(60) | STI(40) | NON-O | C | 5 | E | 1 | 3 | TT | none | DOC | 11.2 | ND |
| 20 | 26 | F | 2.6 | F(90) | STI(10) | NON-O | F | 6 | F | 2 | 5 | Lobectomy | none | NED | 8.5 | PAX8/PPARγ |
| 21 | 35 | F | 2.9 | STI(100) | NA | NON-O | F | 2 | E | 3 | 5 | Lobectomy | none | NED | 23.4 | NRAS Q61K |
| 22 | 29 | F | 3.2 | F(60) | STI(40) | NON-O | C | 4 | E | 1 | 6 | Incomplete lobectomy | none | NED | 15.3 | ND |
| 23 | 72 | F | 7.0 | STI(60) | F(40) | NON-O | F | 6 | F | 2 | 10 | TT | yes | AWD | 4.9 | NRAS Q61R |
| 24 | 33 | F | 2.1 | F(100) | NA | NON-O | P | 8 | N | 5 | 11 | TT | yes | NED | 6.3 | Wild type |
| 25 | 69 | F | 3.2 | STI(100) | NA | NON-O | C | 4 | E | 1 | 25 | TT | yes | AWD | 13.4 | NRAS Q61R |
Abbreviations: CI, number of foci of capsular invasion; VI, number of foci of vascular invasion; TT, Total thyroidectomy; ↑, Status at last follow up; NED, no evidence of disease; DOD, died of disease; AWD, alive with disease; DOC, died of other causes; FU, follow-up (years); ND, not done.
Patient 15 was the only individual who had distant metastases at presentation.
STI, solid/trabecular/insular growth pattern; F, follicular growth pattern; NA, not applicable.
Non-O, nononcocytic; O, oncocytic.
F, follicular nuclei; P, classical papillary carcinoma nuclei; C, convoluted nuclei; O, oncocytic nuclei (ie, vesicular nucleus with prominent central nucleolus).
N, none; F, focal; E, extensive.
Lobectomy was performed in 12 (48%) of 25 cases, while a total thyroidectomy in 13 (52%) of 25 patients. Eight of the 25 (32%) patients received RAI, including all patients with recurrent/metastatic disease. In patients with noninvasive tumors, 5 (62.5%) of 8 were subjected to total thyroidectomy and 2 of these (25%) received RAI therapy. For data summary, see Table 1.
3.2. Genetics of EFHG tumors
Tissue blocks were available from 22 of the 25 primary tumors included in the study. All tissue blocks yielded informative results. Table 1 shows the genotyping results of the patients. Overall, mutations were found in 10 (45%) of 22 cases. RAS mutations were found in 9 (41%) of 22 cases, and all but one of them (8/9) were in NRAS (Fig. 2). RAS mutations (41% of cases) were more frequent than BRAF mutations (1 [4.5%] of 22 patients) (P = .009). In addition, 1 tumor had a mutation encoding the AKT1E17 K substitution, which was associated with the BRAFV600E aberration. This case had a majority of follicular type nuclei with the minority nuclei having PTC nuclear features in the form of irregular hypochromatic nuclei with occasional grooves. Interestingly, the tissue section that was genotyped was particularly rich in PTC nuclei. Older patients (>45 years) had a significantly higher mutation rate (8 [67%] of 12) than younger individuals (2 [20%] of 10) (P = .042). Two tumors were found to have a PAX8-PPARγ rearrangements (nos. 7 and 20). Both tumors had a predominant follicular growth with follicular type nuclei seen in one of the patients along with vascular invasion, whereas the other patient had papillary type nuclei and no invasion. No RET rearrangement was found. There was no correlation between mutations frequency or type of mutations and sex, tumor size, predominant growth pattern, mitotic rate, necrosis, nuclear and cytoplasmic phenotypes, extent of capsular and vascular invasion, and outcome.
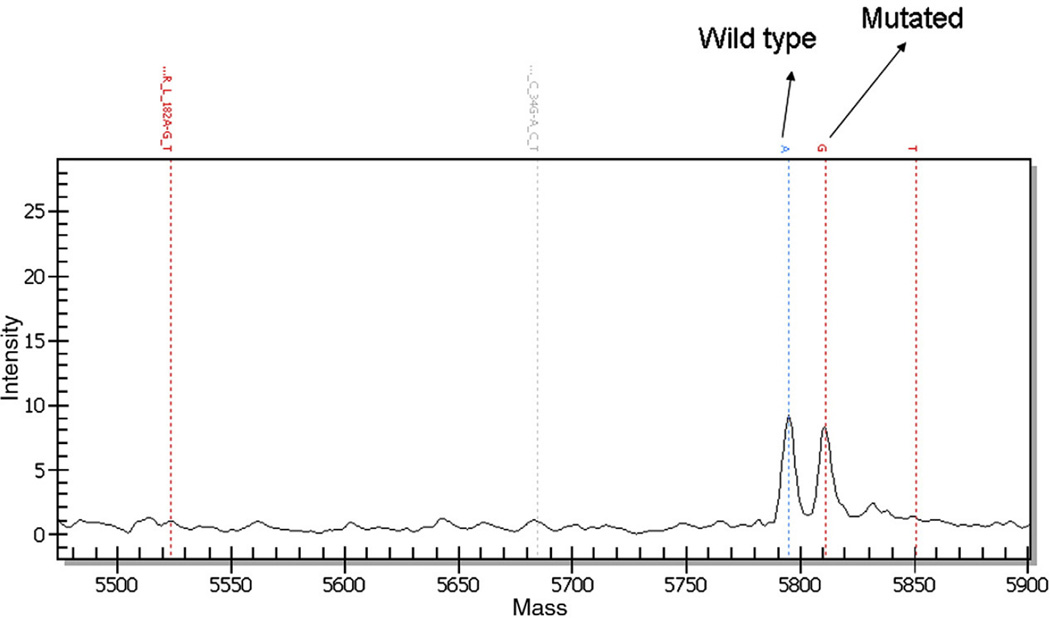
Fig. 2.
Mass spectrometry traces for NRAS codon 61 mutation from tumor in Fig. 1. Note the mutant NRAS 182G peak.
4. Discussion
Noninvasive EFHG are quite rare. Indeed, in this series derived from a major cancer center, these tumors constituted <1% of thyroid carcinomas. It is not possible to compare the incidence and demographics of these neoplasms to previous studies [3,4] on “atypical adenomas” since our definition for worrisome histology differ significantly. Indeed, our definition is based on mitosis and tumor necrosis rather than architectural grading. All noninvasive EFHG tumors showed no evidence of disease after a median follow up of 11.9 years. This excellent outcome occurred despite an average size of 4.3 cm, a median age of 48 years, and the presence of tumor necrosis in 3 (37.5%) of 8 noninvasive tumors. It is also noteworthy that one encapsulated non-invasive tumor treated by lobectomy alone without RAI therapy did not recur despite the presence of extensive tumor necrosis after a follow-up of 13 years. However, we recommend that a thorough examination of such tumors be performed before an indolent outcome is suggested. Our noninvasive EFHG had an average of 13 tumor sections per primary neoplasm to avoid inadequate sampling, which may cause the pathologist to miss foci of invasion. This was demonstrated in a study by Lang et al [4] in which additional submitted sections revealed foci of invasion in 3 tumors from a group of 33 tumors previously diagnosed as atypical adenomas.
Complete encapsulation and extent of angioinvasion appears to be a key factor in determining the biology of thyroid tumors. Surgically removing these tumors at a time when they are still encapsulated and noninvasive may interrupt the biology of a tumor that might otherwise evolve into an aggressive infiltrative carcinoma. Indeed, encapsulation is a significant predictor of better overall survival even in poorly differentiated thyroid carcinomas defined on the basis of high mitotic activity and/or tumor necrosis (P = .001) [6]. In the present series, as one might expect, tumors with no vascular invasion did not recur or metastasize. Conversely, two cases showing extensive angioinvasion are alive with disease, one of them with metastatic lung disease. Extensive vascular invasion has been shown to increase the risk of relapse in other encapsulated tumors of the thyroid. For instance, in the study of encapsulated Hurthle cell neoplasms by Ghossein et al [15], 47% of cases exhibiting ≥4 foci of vascular invasion metastasized. The importance of extent of vascular invasion in encapsulated thyroid tumors has also been observed by Collini et al [16]. In their article, they describe 18 encapsulated non-Hurthle follicular carcinomas in which 2 of 3 patients with extensive capsular angioinvasion (>4 foci) relapsed [16]. None of their tumors with focal angioinvasion recurred despite a median follow-up of 11 years [16]. It is interesting to note that one of our patients presented with metastatic thyroid carcinoma to the bone from a 2.0-cm tumor showing only one focus of vascular and 2 foci of capsular invasion. It has been a personal observation of one of the authors (R.A.G.) that minimally invasive carcinomas that metastasize tend to do so in almost all instances at presentation. In addition, an unusual histologic finding of extensive intratumoral fibrosis has been seen in these minimally invasive tumors with distant metastases. Despite the small number of patients in this study, our data is in congruence with the one of Collini et al. and others [10,15,16]. Indeed, if we exclude our case with distant metastases at presentation, 2 (33%) of our 6 patients with extensive angioinvasion relapsed while none of the 18 patients with focal or absent vascular invasion recurred (P = .054). In this subgroup of patients without distant disease at presentation, tumors with focal angioinvasion did not relapse despite the presence of necrosis in 5 (71%) of 7 cases and a median follow-up of 6.9 years.
It is unclear whether the tumors reported in this study should be regarded as low-stage, poorly differentiated thyroid carcinomas. Genotyping of the genes encoding effectors that signal along two key pathways involved in thyroid cancer pathogenesis (ie, RET-RAS-BRAF-MAPK and PIK3CA/AKT) primarily revealed the presence of RAS mutations in EFHG tumors. The mutational rate in this study was significantly higher in patients older than 45 (8 [67%] of 12) than in younger individuals (2 [20%] of 10) (P = .042). This is congruent with previous articles that report a higher frequency of BRAF as well as RAS mutations in older age [17,18]. In accordance with previous studies on adenomas and differentiated thyroid carcinoma, we had very few (1/22) mutations in the PIK3CA/AKT pathway [19]. Two of the patients in the series herein displayed PAX8-PPARγ rearrangement. Both patients had a predominantly follicular growth pattern. Follicular type nuclei were seen in one of the patients along with vascular invasion, whereas the other patient had papillary type nuclei and no invasion. The former would be classified by many authors as a follicular carcinoma and the latter as a papillary carcinoma follicular variant. The presence of PAX8-PPARγ in these 2 cases is not a surprise since this rearrangement has been previously described in both follicular carcinoma and papillary carcinoma follicular variant [20]. Because RET rearrangements are rare in tumors with a follicular growth pattern, the lack of RET rearrangement in our series is expected in view of the fact that many of these tumors would have been classified as “atypical follicular adenoma,” papillary thyroid carcinoma follicular variant, or follicular carcinoma. [21]. More interestingly, there was a significantly higher rate of RAS mutation (9 [41%] of 22) than BRAF mutations (1 [4.5%] of 22) (P = .009). This is congruent with our findings of a much higher prevalence of RAS than BRAF mutations in poorly differentiated thyroid carcinomas (encapsulated and non-encapsulated) using the same genotyping platform [12]. Interestingly, in that previous study, we showed that poorly differentiated thyroid carcinomas with RAS-mutations had a better outcome than those with BRAF [12]. The paucity of BRAF mutation in EFHG tumors could also be related to the lack of classical papillary carcinoma phenotype in the tumors we analyzed. All but one of the RAS mutations present in this study were located at codon 61 of NRAS. Vasko et al [22] found NRAS codon 61 mutations in atypical adenomas and minimally invasive (encapsulated) follicular carcinomas at a similar frequency (23.3 % and 22.2%, respectively) but none in typical adenomas. Their frequency is approximately similar to the NRAS mutation rate in our non-invasive tumors (25%), but lower than the NRAS aberrations that we found in invasive encapsulated tumors (46%). The similarity in mutation rate in noninvasive tumors is remarkable in view of the fact that different morphological criteria were used to define these lesions. In contrast to our study, Vasko et al used cytologic atypia (mainly nuclear features suggestive of papillary carcinoma) rather than proliferative features to define their atypical non-invasive tumors [22]. The role of NRAS in the progression of thyroid carcinomas is further supported by the work of Vitagliano et al [23]. These authors showed that poorly differentiated thyroid carcinomas develop in 25% of transgenic mice harboring the NRASGln61Lys mutation [23]. Furthermore, Garcia-Rostan et al [24] found a significant increase in NRAS mutations along the spectrum of thyroid carcinoma progression from well differentiated to poorly differentiated and anaplastic carcinoma. This group defined poorly differentiated carcinoma on the basis of growth pattern as well as mitosis and necrosis. Their morphological criteria for poorly differentiated carcinoma are therefore very close to ours. Finally, Tzen et al [25] found P53 mutations in the pleomorphic cells of 2 atypical follicular adenomas. However, their cases did not have significant mitotic activity and tumor necrosis. Based on the above data, one could speculate that noninvasive follicular derived tumors with worrisome histology (whether cytologic atypia or mitosis/necrosis) are preinvasive precursors of thyroid carcinomas or low stage poorly differentiated carcinomas. Unfortunately, there is little information on the prevalence of N-ras mutations in “atypical” adenomas since the different morphologic subtypes of follicular adenomas have been very rarely analyzed separately at the molecular level. Clearly, more studies are needed to clarify the true nature of noninvasive EFHG.
Whatever the position of these tumors in the classification scheme of thyroid carcinomas, the data in this article suggest that noninvasive tumors with high-grade features (mitosis/necrosis) are indolent. From a practical point of view, we believe that encapsulated noninvasive follicular-derived tumors with necrosis should still be labeled as thyroid carcinomas (with a mention that they harbor tumor necrosis) despite their probable good prognosis. This is due to the extreme rarity of this lesion (only 3 cases in these series) and the fact that tumor necrosis is a very powerful indicator of poor behavior in thyroid carcinomas in general [9]. A note should therefore be added to the pathology report stipulating the probable indolent behavior with the caveat that experience is limited. This can allow the clinician to proceed with appropriate initial therapy and staging. In the case of a tumor with increased mitotic activity but without necrosis, a thorough search is indicated for capsular or vascular invasion. If the tumor is noninvasive after extensive sampling, we advocate labeling it as a follicular adenoma [26]. This is because mitotic activity is not as strong predictor of outcome as tumor necrosis [9].
In summary, we present 25 cases with long-term follow-up of encapsulated follicular derived lesions with increased mitoses and/or necrosis showing varying degrees of capsular and/or vascular invasion. Tumors with extensive angioinvasion are at increased risk of recurrence/metastasis. In contrast, an indolent outcome is expected in the adequately sampled noninvasive tumors despite the presence of necrosis. Excellent behavior is also seen in invasive tumor without vascular invasion. The above data strongly suggest that the marked adverse effect that tumor necrosis and high mitotic rate have on survival in invasive thyroid carcinomas [5–9] is inexistent in encapsulated noninvasive tumors. As stated earlier, surgical removal of these noninvasive tumors with necrosis and high mitotic activity may have interrupted their biology and prevented their progression into high-grade invasive carcinomas. Although this genotypic analysis in conjunction with some of the published molecular data suggest that noninvasive encapsulated tumors with disturbing histology (whether cytologic atypia or mitosis/necrosis) are undergoing malignant transformation, there are very few articles on the subject to confirm this impression. Additional molecular studies are needed to define the position of these tumors in the classification of thyroid neoplasms of follicular cell origin.
Footnotes
This work was presented in part at the annual meeting of the United States and Canadian Academy of Pathology in March 2009.
References
- 1.Liu J, Singh B, Tallini G, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–1264. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 2.Van Heerden JA, Hay I, Goellner J, et al. Follicular thyroid carcinoma with capsular invasion alone: a nonthreatening malignancy. Surgery. 1992;112:1130–1136. [PubMed] [Google Scholar]
- 3.Hazard JB, Kenyon R. Atypical adenoma of the thyroid. AMA Arch Pathol. 1954;58:554–563. [PubMed] [Google Scholar]
- 4.Lang W, Georggi A, Stauch G, Kienzle E. The differentiation of atypical adenomas and encapsulated follicular carcinomas in the thyroid gland. Virchows Arch A Path Anat Histol. 1980;385:125–141. doi: 10.1007/BF00427399. [DOI] [PubMed] [Google Scholar]
- 5.Akslen LA, Livolsi VA. Prognostic significance of histologic grading compared with subclassification of papillary thyroid carcinoma. Cancer. 2000;88:1902–1908. [PubMed] [Google Scholar]
- 6.Hiltzik D, Carlson DL, Tuttle MR, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis. Cancer. 2006;106:1286–1295. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 7.Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: The Turin proposal for the use of uniform diagnostic criteria and an algorothmic diagnostic approach. Am J Surg Pathol. 2007;31:1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 8.Volante M, Landolfi S, Chiusa L, et al. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid Patterns. A clinicopathologic study of 183 patients. Cancer. 2004;100:950–957. doi: 10.1002/cncr.20087. [DOI] [PubMed] [Google Scholar]
- 9.Rivera M, Ghossein RA, Schoder H, Gonez D, Larson SM, Tuttle MR. Histopathologic characterization of radioactive iodine refractory FDG-PET positive thyroid carcinoma. Cancer. 2008;113:48–56. doi: 10.1002/cncr.23515. [DOI] [PubMed] [Google Scholar]
- 10.Lang W, Choritz H, Hundeshagen H. Risk factors in follicular thyroid carcinomas. A retrospective follow-up study covering a 14-year period with emphasis on morphological findings. Am J Surg Pathol. 1986;10:246–255. [PubMed] [Google Scholar]
- 11.Rosai J, Carcangiu ML, Delellis RA. Tumors of the thyroid gland. In: Rosai J, Sobin LH, editors. Atlas of tumor pathology. Vol. 5. New York: Armed Forces Institute of Pathology; 1992. pp. 161–182. [Google Scholar]
- 12.Ricarte-Filho JCM, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imkamp F, von Wasielewski R, Musholt TJ, Musholt PB. Rearrangement analysis in archival thyroid tissues: punching microdissection and artificial RET/PTC 1–12 transcripts. J Surg Res. 2007;143:350–363. doi: 10.1016/j.jss.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Ghossein R, Hiltzik D, Carlson DL, et al. Prognostic factors of recurrence in encapsulated hurthle cell carcinoma of the thyroid gland: a clinicopathologic study of 50 cases. Cancer. 2006;106:1669–1676. doi: 10.1002/cncr.21825. [DOI] [PubMed] [Google Scholar]
- 16.Collini P, Sampierto G, Pilotti S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non-hurthle cell follicular carcinoma of the thyroid gland: a clinicopathologic study of 18 consecutive cases from a single institution with a 11-year median follow-up. Histopathology. 2004;44:35–39. doi: 10.1111/j.1365-2559.2004.01729.x. [DOI] [PubMed] [Google Scholar]
- 17.Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 19.Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 20.Castro P, Rebocho AP, Soares RJ, et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:213–220. doi: 10.1210/jc.2005-1336. [DOI] [PubMed] [Google Scholar]
- 21.DeLellis RA. Pathology and genetics of thyroid carcinoma. J Surg Oncol. 2006;94:662–669. doi: 10.1002/jso.20700. [DOI] [PubMed] [Google Scholar]
- 22.Vasko V, Ferrand M, Di Cristofaro J, Carayon P, Henry JF, de Micco C. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab. 2003;88:2745–2752. doi: 10.1210/jc.2002-021186. [DOI] [PubMed] [Google Scholar]
- 23.Vitagliano D, Portella G, Troncone G, et al. Thyroid targeting of the N-RAS (Gln61Lys) oncogene in transgenic mice results in follicular tumors that progress to poorly differentiated carcinomas. Oncogene. 2006;25:5467–5474. doi: 10.1038/sj.onc.1209527. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rostan G, Zhao H, Camp RL, et al. RAS mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21:3226–3235. doi: 10.1200/JCO.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 25.Tzen CY, Huang YW, Fu YS. Is atypical follicular adenoma of the thyroid a preinvasive malignancy? Hum Pathol. 2003;34:666–669. doi: 10.1016/s0046-8177(03)00241-7. [DOI] [PubMed] [Google Scholar]
- 26.LiVolsi VA, Baloch ZW. Follicular neoplasms of the thyroid: view, biases, and experiences. Adv Anat Pathol. 2004;11:279–287. doi: 10.1097/01.pap.0000138143.34505.02. [DOI] [PubMed] [Google Scholar]