Abstract
The follicular variant of papillary thyroid carcinoma usually presents as an encapsulated tumor and less commonly as a partially/non-encapsulated infiltrative neoplasm. The encapsulated form rarely metastasizes to lymph node, whereas infiltrative tumor often harbors nodal metastases. The molecular profile of the follicular variant was shown to be close to the follicular adenoma/carcinoma group of tumors with a high RAS and very low BRAF mutation rates. A comprehensive survey of oncogenic mutations in the follicular variant of papillary thyroid carcinoma according to its encapsulated and infiltrative forms has not been performed. Paraffin tissue from 28 patients with encapsulated and 19 with infiltrative follicular variant were subjected to mass spectrometry genotyping encompassing the most significant oncogenes in thyroid carcinomas: 111 mutations in RET, BRAF, NRAS, HRAS, KRAS, PIK3CA, AKT1 and other related genes. There was no difference in age, gender, tumor size and angioinvasion between encapsulated or infiltrative tumors. Infiltrative carcinomas had a much higher frequency of extrathyroid extension, positive margins and nodal metastases than encapsulated tumors (P<0.05). The BRAF 1799T>A mutation was found in 5 of 19 (26%) of the infiltrative tumor and in none of the encapsulated carcinomas (P=0.007). In contrast, RAS mutations were observed in 10 of 28 (36%) of the encapsulated group (5 NRAS_Q61R, 3 HRAS_Q61, 1 HRAS_G13C and 1 KRAS_Q61R) and in only 2 of 19 (10%) of infiltrative tumors (P=0.09). One encapsulated carcinoma showed a PAX8/PPARγ rearrangement, whereas two infiltrative tumors harbored RET/PTC fusions. Encapsulated follicular variant of papillary thyroid carcinomas have a molecular profile very close to follicular adenomas/carcinomas (high rate of RAS and absence of BRAF mutations). Infiltrative follicular variant has an opposite molecular profile closer to classical papillary thyroid carcinoma than to follicular adenoma/carcinoma (BRAF>RAS mutations). The molecular profile of encapsulated and infiltrative follicular variant parallels their biological behavior (ie, metastatic nodal and invasive patterns).
Keywords: BRAF, follicular variant, genotyping, molecular, papillary thyroid carcinoma, RAS
The papillary thyroid carcinoma family of tumor is defined histologically by the presence of clear, irregular, overlapping nuclei with grooves and pseudoinclusions. In addition to the aforementioned nuclear features, classical (‘garden variety’) papillary thyroid carcinoma is composed of a variable mixture of papillae and follicles. In contrast, the follicular variant of papillary thyroid carcinoma is almost exclusively arranged in follicles lined by cells with characteristic papillary carcinoma nuclei.1 The follicular variant of papillary carcinoma presents usually as an encapsulated tumor and less commonly as a partially/non-encapsulated infiltrative neoplasm.2 Encapsulated follicular variant of papillary carcinoma is a frequent diagnosis in current surgical pathology practice, being the most common variant of papillary carcinoma. The diagnosis of encapsulated follicular variant is difficult at the cytopathological, frozen section and histological level. This is reflected in considerable interobserver variability.3,4 Recent studies have shown that the encapsulated form rarely metastasize to lymph node (5% of cases), whereas infiltrative follicular variant often harbors lymph node (LN) metastases (65% of patients).2 The encapsulated follicular variant is therefore closer in its invasive and nodal metastatic pattern to the follicular adenoma/carcinoma group of tumors, whereas infiltrative follicular variant behaves similar to classical papillary thyroid carcinoma.2
In addition, the molecular profile of the follicular variant was shown to be close to the follicular adenoma/carcinoma group of tumors with a high prevalence of RAS and a very low BRAF mutation rate.5 However, a comprehensive survey of oncogenic mutations in the follicular variant of papillary thyroid carcinoma according to its encapsulated and non-encapsulated forms has not been performed. In conjunction with available histological and follow-up data, we show that the delineation of the molecular profile of the follicular variant of papillary thyroid carcinoma according to its subtypes helps better classify these tumors into clinically relevant entities.
Materials and methods
Patient Population and Inclusion Criteria
The institutional database was searched for all cases with a diagnosis of thyroid carcinomas treated at Memorial Sloan-Kettering Cancer Center between January 1980 and December 2002. Additional cases were supplied from the personal file of one of us (RAG). The slides from the cases included in the study were examined by two head and neck pathologists with special interest in thyroid neoplasia (RAG and MR). The pathologists were blinded to the clinical outcome of all patients studied. The follicular variant of papillary thyroid carcinomas were defined as follicular lesions almost entirely composed of follicles (up to 99% of the neoplasm) lined by cells having the characteristic nuclear features of papillary carcinoma (ie, clear, irregular and overlapping nuclei with grooves and pseudoinclusions). These nuclear changes were either diffuse or multifocal (encompassing at least 60% of the tumor even when multifocal). The nuclear changes were well developed (ie, at least enlarged, irregular hypochromatic nuclei with overlapping and occasional grooves). All follicular variant of papillary thyroid carcinomas (encapsulated and infiltrative) had ≤1% papillary formations. Follicular variant of papillary thyroid carcinomas were designated as encapsulated if totally surrounded by a fibrous capsule and as infiltrative if partially or totally unencapsulated with invasive tongues of tumor-infiltrating nonneoplastic thyroid parenchyma (Figures 1 and 2). Only the follicular variant of papillary thyroid carcinomas of ≥1 cm in greatest size were analyzed. Tumors with high mitotic activity (≥5 mitosis/10 high power fields, × 400) and/or tumor necrosis were excluded as well as those containing ≥30% tall cells. The latter cell type was defined as having a height at least twice its width with an oncocytic cytoplasm. The study was approved by the institutional review board of the Memorial Sloan-Kettering Cancer Center.
Figure 1.
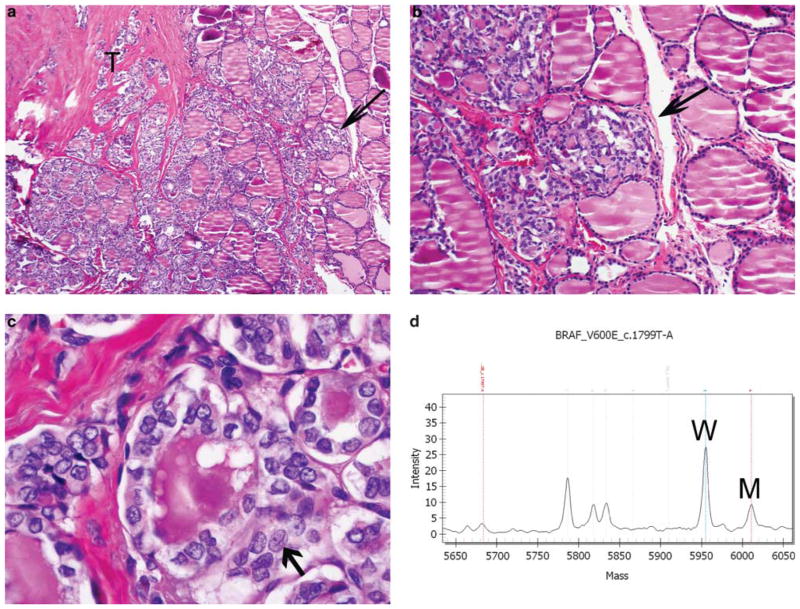
Infiltrative follicular variant of papillary thyroid carcinoma. (a) Low-power view showing tumor (T) growing in a follicular pattern with marked fibrosis and an infiltrating advancing front (arrow). (b) Medium power showing tongues of tumor-infiltrating nonneoplastic thyroid without interposition of a capsule (arrow). (c) High-power view of the nuclear features of papillary thyroid carcinoma: hypochromatic, irregular and overlapping nuclei with grooves (the latter indicated by an arrow). (d) Mass spectrometry traces for BRAF mutation from the tumor. Note the mutant BRAF_T1799A (indicated by M) and wild-type (W) peaks.
Figure 2.
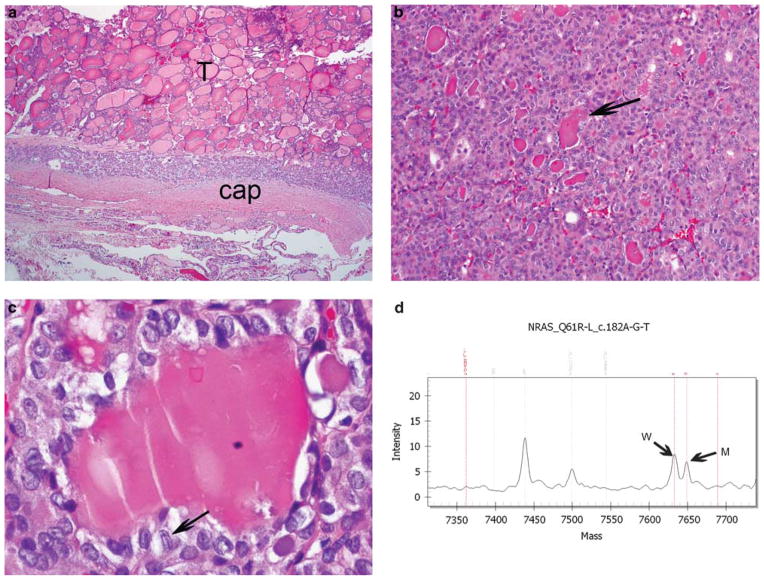
Encapsulated follicular variant of papillary thyroid carcinoma. (a) Low-power view showing tumor (T) surrounded by its capsule (cap) with no invasion. (b) Medium-power view revealing follicular growth with densely eosinophilic colloid characteristic of the follicular variant of papillary thyroid carcinoma. (c) High-power view of the nuclear features of papillary thyroid carcinoma: hypochromatic, irregular and overlapping nuclei with grooves (the latter indicated by an arrow). (d) Mass spectrometry traces for NRAS codon 61 mutation from the tumor. Note the mutant NRAS 182G (indicated by M) and wild-type (W) peaks.
Histopathological Analysis
The largest dimension of the tumor was recorded as dictated during gross examination. The mitotic rate of the tumor was determined by counting 10 contiguous high power fields (×400) using an Olympus microscope (U-DO model BX-40; Olympus America, Melville, NY, USA). Using that microscope type, these 10 high power fields corresponded to 2.4 mm2. Mitotic counts were performed in a focused manner, examining areas that appeared to show greater proliferative activity. Tumor necrosis was defined by a ‘comedo-like’ appearance composed of degenerating cytoplasm and punctuate, karyorrhectic nuclear debris. The presence of fibroblastic stromal reaction, hemorrhage or an identifiable needle track in the necrotic area was attributable to reaction induced by previous fine needle aspiration, and was therefore not regarded as spontaneous tumor necrosis. When present, the numbers of foci of capsular and vascular invasion were noted. Only complete penetration of the capsule by tumor was regarded as capsular invasion, as described by Lang et al.6 The presence of vascular invasion was noted only when such foci were present within or beyond the capsule in accordance with criteria outlined by the Armed Forces Institute of Pathology (AFIP) fascicle.1 Only when the invasive focus protruded into the lumen of the vessel in a polypoid manner covered by endothelial cells, or when it was attached to the vessel wall or associated with thrombus formation, was it considered true vascular invasion. Areas of vascular invasion that were closely adjacent to one another were counted as separate foci. The foci of capsular and vascular invasion were subdivided into two categories: focal (<4 invasive foci) and extensive (≥4 foci).
Clinical Parameters
The patient’s electronic medical records were reviewed for the age at diagnosis, type of surgery and adjuvant treatment, including radioactive iodine therapy. The current disease status was based on a combination of physical examination, biochemical serum markers (thyroid stimulating hormone and thyroglobulin), radioactive iodine dosimetry, cross-sectional imaging and/or positron emission tomography (PET) scanning. The date of initial surgery and last date of follow-up were recorded. The status at last follow-up was recorded as follows: no evidence of disease; alive with disease; dead of other causes; and dead of disease.
Genotyping by Mass Spectrometry
Four sections of 10 μm from each formalin-fixed paraffin-embedded tissue block were subjected to DNA extraction using the PUREGene Genomic DNA purification kit (Gentra, Minneapolis, MN, USA). Mutation detection was performed as described previously.7 We used mass spectrometry Sequenom-based genotyping assay (Sequenom Mass array; Sequenom, San Diego CA, USA), which is especially suited for high-throughput genotyping, to interrogate 111 known mutations in 16 different genes: BRAF, RET, NRAS, HRAS, KRAS, PIK3CA, MAP2K1, AKT1, MET, IKBKB, PIK3R5, PRKCZ, RHEB, RPS6KA3, RPS6KB1 and FRAP1. Of note, BRAFK600E was also included in the detection platform. As the mass spectrometry genotyping assays for codons 12 and 13 of HRAS were not informative, we designed primers for this region and sequenced all the tumors that were wild type for BRAF, RAS mutations or for RET/PTC rearrangements.7
Screening for RET/PTC and PAX8/PPARγ Rearrangements
We used tumor cDNA as template for quantitative polymerase chain reaction (PCR) to analyze for unbalanced expression of exons 10 and 11 relative to 12 and 13 of RET, which flank the rearrangement site in intron 11. Samples with 12–13 >10–11 expression were screened for specific RET recombination events using primers bracketing the fusion point of RET/PTC1, RET/PTC2 and RET/PTC3, respectively, as previously described.7,8 We screened for the PAX8/PPARγ fusion by reverse transcriptase-PCR, using primers for the different possible transcripts of PAX8/PPARγ as previously described.9 PCR products were resolved by electrophoresis in a 2% agarose gel and selected cases were sequenced. RET and PAX8/PPARγ rearrangements were analyzed for tumors that were wild type for RAS and BRAF.
Statistical Analysis
Two-tailed Fisher’s exact test was used to assess the relation between categorical variables. The P-value of <0.05 was considered as significant.
Results
Clinical and Histopathological Features
A total of 47 cases were included in the study (19 infiltrative and 28 encapsulated follicular variant). All captured cases of infiltrative follicular variant with adequate tissue were analyzed, whereas the encapsulated tumors were randomly selected. This randomization was performed in view of the large number of encapsulated follicular variant of papillary thyroid carcinomas in the database.
Table 1 lists the clinical and pathological features according to the invasive growth patterns of the follicular variant of papillary thyroid carcinoma (encapsulated vs infiltrative). There was no significant difference in age, gender, tumor size, mitotic rate or vascular invasion between patients with encapsulated tumors and those with infiltrative neoplasms. In contrast, patients with encapsulated carcinomas had a significantly lower rate of extrathyroid extension (4 vs 53%, respectively, P=0.0001) and positive margins (4 vs 26%, respectively, P=0.03). The presence of any invasion (capsular or vascular) was observed in 6 of 29 (21%) encapsulated follicular variant (2 tumors showed both capsular and vascular invasion, 2 tumors showed capsular invasion only and 2 tumors showed vascular invasion alone). Of 29 encapsulated carcinomas, 23 (79%) were totally noninvasive. The lymph node metastatic rate was significantly higher in patients who had infiltrative tumors (10 of 19 patients; 53%) compared with patients who had encapsulated neoplasms (2 of 28 patients; 7%; P=0.001). All 23 patients who had noninvasive, encapsulated follicular variant of papillary thyroid carcinoma lacked evidence of lymph node metastases, except one case with a cervical node micrometastasis. This micrometastatic deposit was controlateral to the noninvasive encapsulated papillary thyroid carcinoma, follicular variant, and ipsilateral to two papillary microcarcinomas that showed a histology similar to the micrometastatic focus. In all, 23 patients had lymph node tissue available for microscopic examination. In these 23 patients, the metastatic lymph node rate was significantly higher for patients who had infiltrative tumors (10 of 13 patients; 77%) than for patients who had encapsulated carcinomas (2 of 10 patients; 20%; P=0.01).
Table 1.
Clinical and pathological characteristics according to the histological subvariants of the follicular variant of papillary thyroid carcinoma (encapsulated and nonencapsulated)
| No. of patients (%)
|
|||
|---|---|---|---|
| Characteristic | Encapsulated papillary thyroid carcinoma, follicular variant (n=28 patients) | Infiltrative papillary thyroid carcinoma, follicular variant (n=19 patients) | P-valuea |
| Age, years | |||
| Median | 47 | 50 | 0.7 |
| ≤45 | 13 (46) | 10 (53) | |
| 445 | 15 (54) | 9 (47) | |
| Gender | 1 | ||
| Female | 17 (61) | 11 (58) | |
| Male | 11 (39) | 8 (42) | |
| Tumor size (cm) | |||
| Median | 2.9 | 2 | 0.12 |
| ≤4 | 21 (75) | 18 (95) | |
| 44 | 7 (25) | 1 (5) | |
| Vascular invasion | 1 | ||
| Absent | 24 (86) | 16 (84) | |
| Present | 4 (14) | 3 (16) | |
| Capsular invasion | NA | ||
| Absent | 24 (86) | NA | |
| Present | 4 (14) | ||
| Extrathyroid extension | 0.0001 | ||
| Absent | 27 (96) | 9 (47) | |
| Present | 1 (4) | 10 (53) | |
| Margins | 0.03 | ||
| Absent | 27 (96) | 14 (74) | |
| Present | 1 (4) | 5 (26) | |
| Lymph node metastases | 0.001 | ||
| Absent | 26 (93) | 9 (47) | |
| Present | 2 (7) | 10 (53) | |
| Distant metastases | |||
| Absent | 27 (96) | 16 (84) | 0.28 |
| Present | 1 (4) | 3 (16) | |
| Thyroid surgery | 1 | ||
| Lobectomy | 10 (36) | 6 (32) | |
| Total thyroidectomy | 18 (64) | 13 (68) | |
| Radioactive iodine therapyb | 0.54 | ||
| No | 13 (50) | 7 (39) | |
| Yes | 13 (50) | 11 (61) | |
NA, not applicable.
Fisher’s exact test, two-tailed values.
Radioactive iodine status was available in 26 encapsulated tumors and 18 infiltrative carcinomas.
Of the 28 patients with encapsulated follicular variant, 10 patients underwent a lobectomy, and total thyroidectomy was performed in the remaining 18 patients. Of the 19 patients with infiltrative tumors, 6 underwent lobectomy and 13 underwent total thyroidectomy. Two patients with encapsulated follicular variant of papillary thyroid carcinoma underwent a formal neck dissection (1 selective and the other a central compartment dissection). Formal neck dissection was also performed in 9 infiltrative follicular variant of papillary thyroid carcinomas subdivided as follows: modified neck dissection (n=4), extended neck dissection (n=4) and selective neck dissection (n=1). Radioactive iodine therapy information was available in 26 encapsulated tumors. Of these 26, radioactive iodine was administered in 13 (50%). Of the 18 patients with infiltrative carcinomas and known radioactive iodine status, 11 (61%) received radioactive iodine therapy.
Of the 23 encapsulated follicular variant of papillary thyroid carcinomas with adequate follow-up (median: 5.4 years, up to 12 years), only one patient developed recurrence. This 66-year-old male who presented with bone metastasis had an encapsulated tumor with extensive capsular and vascular invasion. He developed recurrences and died of disease approximately 3 years after initial diagnosis. None of the 19 encapsulated noninvasive follicular variant of papillary thyroid carcinomas with adequate follow-up (median: 5.4 years, up to 12 years) recurred. Of the 19 infiltrative carcinomas, 3 had distant metastases. All were present at presentation (one patient with bone and two with lung metastasis). Of the 16 infiltrative tumors with adequate follow-up (10 years, up to 19 years), one patient was alive with disease and the remainder free of disease. This patient with persistent disease had lung metastases at presentation (Figure 3). The other case with lung metastasis at presentation was lost for follow-up, whereas the patient with bone metastasis at presentation had his single clavicular metastasis resected and was free of disease 7 years after his surgery.
Figure 3.
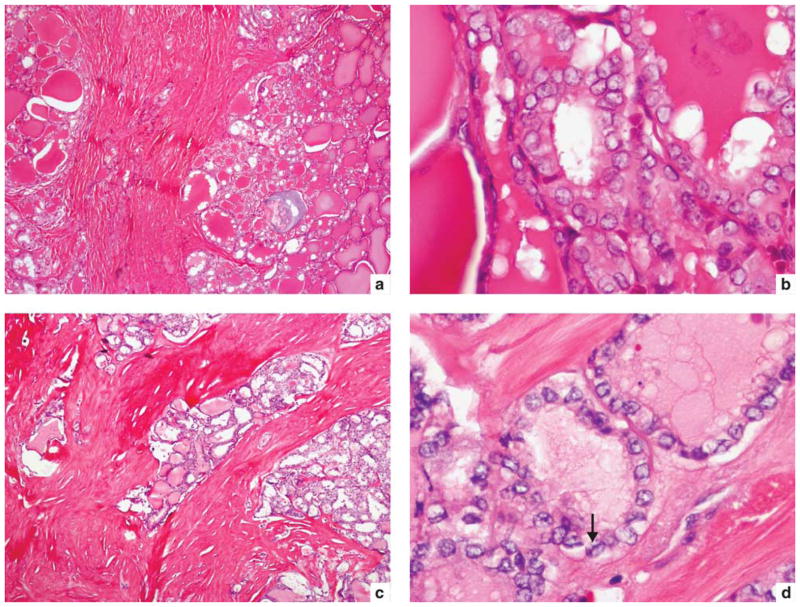
Infiltrative follicular variant of papillary thyroid carcinoma metastatic to lung at presentation. (a, b) A 35-year-old male with BRAF_T1799A mutation. Medium power of primary tumor (a) shows tumor growing in a follicular pattern with marked fibrosis. High power (b) reveals clear, irregular and overlapping nuclei. (c, d) A 53-year-old male with no mutations detected. Medium power of primary tumor (c) shows tongues of infiltrating tumor with marked fibrosis. High power of follicle lined by clear, irregular and overlapping nuclei with grooves (arrow).
Genotyping Analysis
Overall, genotyping abnormalities were found in 11 (39%) of 28 encapsulated follicular variant of papillary thyroid carcinomas and in 9 (47%) of 19 infiltrative tumors. Table 2 lists the genotyping data according to the histological subtypes of the tumor. There was a statistically higher BRAF 1799T>A positivity rate in infiltrative carcinomas (26%) than in encapsulated tumors (0%) (P=0.007; Figure 1). In contrast, there was a trend toward higher RAS positivity rate in encapsulated follicular variant of papillary thyroid carcinomas (36%) when compared with infiltrative tumors (10%; P=0.09). Within encapsulated follicular variant of papillary thyroid carcinomas, the most common RAS mutations were NRAS 182A>G (Q61R) (n=5) followed by HRAS 182A>G (Q61R) (n=2), HRAS 181C>A (Q61K) (n=1), HRAS 37G>T (G13C) (n=1) and KRAS 37G>T (G13C) (n=1; Figure 2). Infiltrative follicular variant of papillary thyroid carcinoma showed only NRAS 182A>G mutations (n=2). In tumors that were wild type for RAS and BRAF, one encapsulated follicular variant of papillary thyroid carcinoma contained a PAX8/PPARγ rearrangement (Figure 4), whereas two infiltrative tumors harbored a RET/PTC recombination. One of the infiltrative carcinoma harbored a RET/PTC3 fusion, whereas the other showed a RET/PTC recombination event not detected by the specific reverse transcriptase-PCR reactions for RET/PTC 1, 2 and 3. Within encapsulated follicular variant of papillary thyroid carcinomas, there was no correlation between the type of mutation and age, gender and vascular invasion. There was however a trend toward higher tumor size in RAS-mutated neoplasms (median: 4.25 cm for RAS-mutated tumors vs 2.25 cm in non-RAS-mutated tumors, P=0.06). Within infiltrative follicular variant of papillary thyroid carcinomas, there was no correlation between the type of mutation and age, gender, size, extrathyroid extension and nodal metastases. Within the whole patient population, RAS-mutated tumors had a significantly higher tumor size (median: 3.5 cm) than non-RAS-mutated papillary thyroid carcinomas, follicular variant (median: 2 cm, P=0.018). In addition, within the same group, there was a trend toward higher extrathyroid extension rate in BRAF-mutated papillary thyroid carcinomas, follicular variant (3/5, 60%) than in non-BRAF mutated tumors (8/42, 19%; P=0.075). Within encapsulated follicular variant of papillary thyroid carcinomas, there was no correlation between genotype and outcome. The encapsulated follicular variant of papillary thyroid carcinoma with distant metastasis had a NRAS 182A>G mutation. There was also no correlation between genotype and outcome in infiltrative follicular variant of papillary thyroid carcinomas. Of the two patients with infiltrative follicular variant of papillary thyroid carcinoma and lung metastasis at presentation, one had a BRAF 1799T>A mutation, whereas the other case did not show any genetic aberration. The infiltrative case with bone metastasis at presentation showed an NRAS 182A>G mutation.
Table 2.
Molecular genotype according to the histological subvariants of follicular variant of papillary thyroid carcinoma (encapsulated and nonencapsulated)
| No. of patients (%)
|
|||
|---|---|---|---|
| Genotype | Encapsulated papillary thyroid carcinoma, follicular variant (n=28 patients) | Infiltrative papillary thyroid carcinoma, follicular variant (n=19 patients) | P-valuea |
| BRAF mutation | 0/28 | 5/19 (26) | 0.007 |
| RAS mutation | 10/28 (36) | 2/19 (10) | 0.09 |
| PAX8/PPARγ rearrangement | 1/28 (3.5) | 0/19 | 1 |
| RET/PTC recombination | 0/28 | 2/19 (10) | 0.15 |
| Total number of genotypic aberrations | 11/28 (39) | 9/19 (47) | 0.76 |
Fisher’s exact test, two-tailed values.
Figure 4.
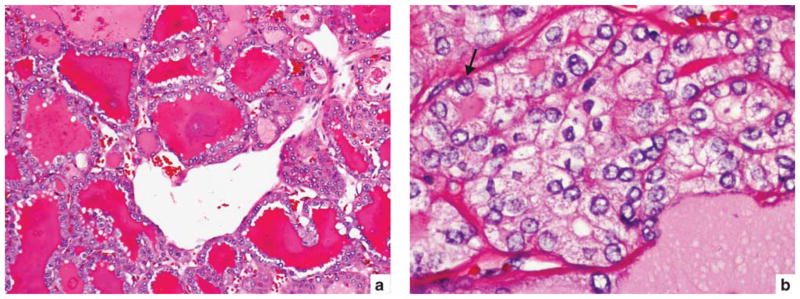
A 36-year-old male with encapsulated follicular variant of papillary thyroid carcinoma harboring a PAX8/PPARγ rearrangement. (a) Medium power showing follicles with densely eosinophilic colloid characteristic of the follicular variant of papillary thyroid carcinoma. (b) High power showing clear, irregular and overlapping nuclei with grooves (arrow).
Discussion
On the basis of the above data, encapsulated follicular variant of papillary thyroid carcinomas have a genotypic profile very close to the one observed in follicular adenoma/carcinoma. Indeed, RAS mutations were found in 36% of encapsulated follicular variant of papillary thyroid carcinomas. This rate is very similar to the overall RAS mutation frequency range observed in non-oncocytic follicular adenomas (24–53%) and carcinomas (18–52%).10–15 Furthermore, as described for follicular adenoma and carcinomas, NRAS codon 61 mutations were the most common RAS abnormality observed in encapsulated follicular variant of papillary thyroid carcinomas.10 In contrast to RAS mutations, BRAF 1799T>A mutations and RET/PTC fusions were not found in encapsulated follicular variant of papillary thyroid carcinomas. This is again consistent with a follicular adenoma/carcinoma molecular profile, as these alterations are characteristically absent in follicular adenomas and carcinomas. 16 Only one encapsulated follicular variant harbored a PAX8/PPARγ rearrangement in our series. This is closer to the findings of Zhu et al,17 who did not detect PAX8/PPARγ alteration in follicular variant of papillary thyroid carcinomas, but at odds with Castro et al,16 who detected this rearrangement in 37.5% of follicular variant of papillary thyroid carcinomas. These discrepant results could be due to differences in genotyping technologies or to different definitions and thresholds for the histological diagnosis of follicular variant of papillary thyroid carcinomas. It is interesting to note that the sole tumor with PAX8/PPARγ rearrangement in our study showed angioinvasion. Nikiforova et al10 have shown that this fusion gene correlates with the presence of angioinvasion in follicular carcinomas and Castro et al16 showed the same relationship in the follicular variant of papillary thyroid carcinomas.
Although encapsulated follicular variant had a molecular profile very similar to the one observed in follicular adenoma/carcinoma, most of the genetic alterations found in infiltrative follicular variant of papillary thyroid carcinomas are in between follicular adenomas/carcinomas and classical papillary thyroid carcinomas, although closer to the latter. Indeed, BRAF mutations were present in 26% of these tumors. This is definitely much higher than the 0% rate observed in follicular adenomas/carcinomas but lower than classical papillary carcinomas (57%).5 The RAS mutation frequency in infiltrative follicular variant of papillary thyroid carcinomas (10%) was closer to classical papillary carcinomas (0%) than to follicular carcinomas (average 40%).18 The absence of PAX8/PPARγ rearrangement in infiltrative follicular variant of papillary thyroid carcinoma is much more consistent with a classical papillary thyroid carcinoma genotype rather than a follicular adenoma/carcinoma molecular profile. Indeed, that alteration is virtually absent in papillary carcinomas (0–1%)10,19–21 but present in a significant proportion of follicular adenomas/carcinomas.10 RET/PTC fusions were detected in 10% of infiltrative follicular variant of papillary thyroid carcinomas. This rate is in between the 0% RET/PTC frequency observed in follicular adenoma/carcinomas and the 26% RET/PTC fusion rate reported in classical papillary thyroid carcinomas.5
As shown in our previous study on the subject, patients who had infiltrative follicular variant of papillary thyroid carcinoma in this study had a significantly greater frequency (P<0.05) of extrathyroid extension, and positive margins than patients who had encapsulated follicular variant of papillary thyroid carcinoma. This superior potential of infiltrative follicular variant of papillary thyroid carcinoma in invading the extrathyroid stroma was reflected by its higher rate of regional lymph node metastases. Indeed, patients with infiltrative follicular variant of papillary thyroid carcinoma had a metastatic lymph node rate of 53% compared with 7% for patients with encapsulated follicular variant of papillary thyroid carcinoma (P=0.001). This strong and significant correlation between lymph node metastases and infiltrative follicular variant of papillary thyroid carcinoma was maintained when only specimens (23 tumors) that contained lymph node tissue were analyzed. This difference in lymph node disease could not be explained by differences in tumor size or age at presentation, because the latter two variables were similar in both the encapsulated and infiltrative group. In this study, the metastatic lymph node pattern of encapsulated follicular variant of papillary thyroid carcinomas (7%) was much closer to that reported in follicular carcinomas (on the order of 5–10%), whereas infiltrative follicular variant of papillary thyroid carcinomas had a metastatic lymph node pattern within the range reported for classic papillary carcinomas (on the order of 45–65%).17,22
With regard to prognosis, patients who had invasive tumors, whether encapsulated or not, had a rare but real potential for adverse outcome. One patient who had an encapsulated follicular variant of papillary thyroid carcinoma with extensive capsular and vascular invasion harbored distant metastasis at presentation, developed recurrences and died of disease approximately 3 years after initial diagnosis. Interestingly, this patient did not develop any regional nodal disease, consistent with the behavior of follicular carcinoma rather than papillary carcinoma.23 In contrast, none of the 19 patients with adequate follow-up who had noninvasive, encapsulated follicular variant of papillary thyroid carcinoma developed recurrences, or died of disease. These data are in congruence with our previous reports stating the indolent nature of encapsulated non-invasive follicular variant of papillary thyroid carcinoma.2,24 Indeed, encapsulated follicular variant of papillary thyroid carcinoma appears to behave similar to follicular adenoma/carcinoma, its metastatic potential governed by the presence or absence of capsular or vascular invasion. In addition to the correlation between the overall molecular genotype of follicular variant of papillary thyroid carcinoma subtype and their respective behavior, we found a relationship between some individual mutations and clinicopathological features. In accordance with previous articles on follicular variant of papillary thyroid carcinomas, the presence of RAS significantly correlated with large tumor size within our whole patient population. (P<0.05).16 In addition, within the same group, there was a trend toward higher extrathyroid extension rate in BRAF-mutated follicular variant of papillary thyroid carcinomas (60%) than in non-BRAF-mutated tumors (19%; P=0.075). The association between BRAF and higher extrathyroid extension rate in papillary carcinoma has been shown by us and others.5,25
In conclusion, this study shows that encapsulated and infiltrative follicular variant of papillary thyroid carcinomas have different genotypic profiles. Encapsulated follicular variant of papillary thyroid carcinoma has a BRAF and RAS mutation pattern very similar to follicular adenoma/carcinoma, whereas infiltrative follicular variant of papillary thyroid carcinoma has a BRAF and RAS genotype in between follicular adenoma/carcinoma and classical papillary carcinoma, although closer to the latter (Figure 5). The molecular profile of each follicular variant of papillary thyroid carcinoma subtype mirrors their histopathological features and metastatic behavior. This strongly argues for follicular variant of papillary thyroid carcinoma being two diseases: (1) encapsulated follicular variant of papillary thyroid carcinoma with a genotypic, invasive and behavioral profile very close to follicular adenoma/carcinoma and (2) infiltrative follicular variant of papillary thyroid carcinoma with invasive and behavioral features very close to classical papillary carcinoma and a molecular profile in between follicular adenoma/carcinoma and classical papillary carcinoma, although closer to the latter.
Figure 5.
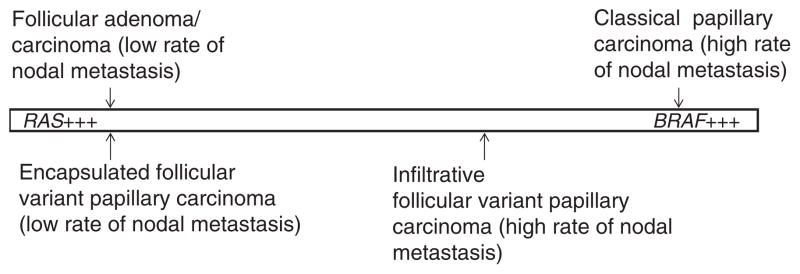
BRAF and RAS mutational patterns of follicular variant of papillary thyroid carcinoma according to its encapsulated and infiltrative subtypes. The mutational pattern of encapsulated follicular variant of papillary thyroid carcinoma is similar to follicular adenoma/carcinoma, whereas infiltrative follicular variant of papillary thyroid carcinoma has a BRAF and RAS genotype in between follicular adenoma/carcinoma and classical papillary carcinoma, although closer to the latter. The mutational patterns of encapsulated and infiltrative follicular variant of papillary thyroid carcinomas parallel their lymph node metastatic rate.
These findings may have important clinical implications. As encapsulated follicular variant of papillary thyroid carcinoma has a genotypic profile as well as invasive and behavioral features very similar to follicular adenoma/carcinoma, one may reconsider reclassifying it as an entity close to the follicular adenoma/carcinoma group. The same criteria that are used to decide whether follicular tumors are biologically benign or malignant (ie, capsular and vascular invasion) would be applied to the evaluation of encapsulated follicular variant of papillary thyroid carcinoma. In practical terms, a lack of capsular and vascular invasion should denote an extremely indolent clinical behavior in encapsulated follicular variant of papillary thyroid carcinoma. If this reclassification is achieved, it will have a major effect on the diagnosis and management of patients with follicular variant of papillary thyroid carcinoma. In noninvasive, encapsulated follicular variant of papillary thyroid carcinoma, pathologists will be spared the frustrating and very subjective exercise of deciding whether a tumor has the nuclear features of papillary carcinoma. More importantly, countless numbers of patients with noninvasive, encapsulated follicular variant of papillary thyroid carcinoma will be treated conservatively akin minimally invasive follicular carcinoma. These individuals will be spared unnecessary and aggressive surgical and radioactive iodine therapy with their attached morbidity and financial costs.
Footnotes
Part of this work was accepted as an abstract at the United States and Canadian Academy of Pathology Meeting in Washington, DC, March 2010.
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- 1.Rosai J, Carcangiu ML, Delellis RA. Tumors of the thyroid gland. In: Rosai J, Sobin LH, editors. Atlas of Tumor Pathology. Vol. 5. Armed Forces Institute of Pathology; New York: 1992. pp. 161–182. [Google Scholar]
- 2.Liu J, Singh B, Tallini G, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–1264. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336–1340. doi: 10.1097/01.pas.0000135519.34847.f6. [DOI] [PubMed] [Google Scholar]
- 4.Elsheikh TM, Asa SL, Chan JK, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008;130:736–744. doi: 10.1309/AJCPKP2QUVN4RCCP. [DOI] [PubMed] [Google Scholar]
- 5.Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 6.Lang W, Choritz H, Hundeshagen H. Risk factors in follicular thyroid carcinomas. A retrospective follow-up study covering a 14-year period with emphasis on morphological findings. Am J Surg Pathol. 1986;10:246–255. [PubMed] [Google Scholar]
- 7.Ricarte-Filho JCM, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imkamp F, von Wasielewski R, Musholt TJ, et al. Rearrangement analysis in archival thyroid tissues: punching microdissection and artificial RET/PTC 1-12 transcripts. J Surg Res. 2007;143:350–363. doi: 10.1016/j.jss.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforova MN, Biddinger PW, Caudill CM, et al. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPARgamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 11.Lemoine NR, Mayall ES, Wyllie FS, et al. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989;4:159–164. [PubMed] [Google Scholar]
- 12.Suarez HG, du Villard JA, Severino M, et al. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene. 1990;5:565–570. [PubMed] [Google Scholar]
- 13.Esapa CT, Johnson SJ, Kendall-Taylor P, et al. Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol (Oxf) 1999;50:529–535. doi: 10.1046/j.1365-2265.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- 14.Motoi N, Sakamoto A, Yamochi T, et al. Role of ras mutation in the progression of thyroid carcinoma of follicular epithelial origin. Pathol Res Pract. 2000;196:1–7. doi: 10.1016/S0344-0338(00)80015-1. [DOI] [PubMed] [Google Scholar]
- 15.Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990;4:1474–1479. doi: 10.1210/mend-4-10-1474. [DOI] [PubMed] [Google Scholar]
- 16.Castro P, Rebocho AP, Soares RJ, et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:213–220. doi: 10.1210/jc.2005-1336. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Z, Gandhi M, Nikiforova MN, et al. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(Suppl 2):S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikiforova MN, Biddinger PW, Caudill CM, et al. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Marques AR, Espadinha C, Catarino AL, et al. Expression of PAX8-PPARgamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2002;87:3947–3952. doi: 10.1210/jcem.87.8.8756. [DOI] [PubMed] [Google Scholar]
- 21.French CA, Alexander EK, Cibas ES, et al. Genetic and biologic subgroups of early stage follicular thyroid cancer. Am J Pathol. 2003;162:1053–1060. doi: 10.1016/S0002-9440(10)63902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passler C, Prager G, Scheuba C, et al. Follicular variant of papillary thyroid carcinoma: a long-term follow-up. Arch Surg. 2003;138:1362–1366. doi: 10.1001/archsurg.138.12.1362. [DOI] [PubMed] [Google Scholar]
- 23.Baloch ZW, LiVolsi VA. Encapsulated follicular variant of papillary thyroid carcinoma with bone metastases. Mod Pathol. 2000;13:861–865. doi: 10.1038/modpathol.3880153. [DOI] [PubMed] [Google Scholar]
- 24.Rivera M, Tuttle RM, Patel S, et al. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern) Thyroid. 2009;19:119–127. doi: 10.1089/thy.2008.0303. [DOI] [PubMed] [Google Scholar]
- 25.Rivera M, Ricarte-Filho J, Knauf J, et al. Molecular and morphologic characterization of thyroid carcinomas according to extra-thyroid extension status. Mod Pathol. 2010;23(Suppl 1S):132A. Abst 584. [Google Scholar]