Abstract
Medial tibial stress syndrome (MTSS) is a debilitating overuse injury of the tibia sustained by individuals who perform recurrent impact exercise such as athletes and military recruits. Characterised by diffuse tibial anteromedial or posteromedial surface subcutaneous periostitis, in most cases it is also an injury involving underlying cortical bone microtrauma, although it is not clear if the soft tissue or cortical bone reaction occurs first. Nuclear bone scans and magnetic resonance imaging (MRI) can both be used for the diagnosis of MTSS, but the patient’s history and clinical symptoms need to be considered in conjunction with the imaging findings for a correct interpretation of the results, as both imaging modalities have demonstrated positive findings in the absence of injury. However, MRI is rapidly becoming the preferred imaging modality for the diagnosis of bone stress injuries. It can also be used for the early diagnosis of MTSS, as the developing periosteal oedema can be identified. Retrospective studies have demonstrated that MTSS patients have lower bone mineral density (BMD) at the injury site than exercising controls, and preliminary data indicates the BMD is lower in MTSS subjects than tibial stress fracture (TSF) subjects. The values of a number of tibial geometric parameters such as cross-sectional area and section modulus are also lower in MTSS subjects than exercising controls, but not as low as the values in TSF subjects. Thus, the balance between BMD and cortical bone geometry may predict an individual's likelihood of developing MTSS. However, prospective longitudinal studies are needed to determine how these factors alter during the development of the injury and to find the detailed structural cause, which is still unknown. Finite element analysis has recently been used to examine the mechanisms involved in tibial stress injuries and offer a promising future tool to understand the mechanisms involved in MTSS. Contemporary accurate diagnosis of either MTSS or a TSF includes a thorough clinical examination to identify signs of bone stress injury and to exclude other pathologies. This should be followed by an MRI study of the whole tibia. The cause of the injury should be established and addressed in order to facilitate healing and prevent future re-occurrence.
Keywords: Medial tibial stress syndrome, Tibia, Injury, Shin splints, Fatigue injury, Strain gauge, Cortical bone geometry, Bone mineral density, Finite element model
Core tip: Medial tibial stress syndrome (MTSS) is an overuse injury characterised by diffuse tibial anteromedial or posteromedial surface subcutaneous periostitis, usually in conjunction with underlying cortical bone microtrauma. Nuclear bone scans or magnetic resonance imaging findings need to be considered in conjunction with clinical symptoms and patient history for an accurate diagnosis. Compared to exercising controls, MTSS patients have low bone mineral density and low values of a number of tibial cortical bone geometric parameters such a cross-sectional area. Recent research includes the development of computational models for studying tibial stress injuries. These models offer a tool to study the exact causes of MTSS, which are still unknown.
BACKGROUND
Medial tibial stress syndrome (MTSS) is a debilitating overuse injury of the tibia sustained by individuals who perform recurrent impact exercise such as athletes and military recruits. It is characterised by diffuse tibial anteromedial or posteromedial surface subcutaneous periostitis, most often on the medial border near the junction of the mid and distal thirds of the tibia[1]. Although the injury was identified in runners as early as 1913, when it was termed “spike soreness”, it was believed to be a type of tibial stress fracture (TSF) rather than a separate entity[2].
Devas[3] (1958) was one of the first physicians to study “shin soreness” in athletes, although like earlier researchers, he believed it to be a type of TSF. Using both clinical observations and plane radiographs, Devas described shin soreness as “a type of stress fracture involving a disruption of the periosteum over a varying distance”. He noted there was tibial tenderness, soft tissue “thickening” of the subcutaneous surface of the tibia and periosteal oedema, with radiological changes either late onset, or not visible at all. In 1966, after soliciting the views of a large number of physicians and other individuals involved in sports medicine, “shin splints” was defined by the American Medical Association as “pain and discomfort in leg from repetitive running on hard surface or forcible excessive use of foot flexors; diagnosis should be limited to musculotendinous inflammations, excluding fatigue fracture or ischemic disorder”[4]. The following year, Slocum[5] presented a detailed review of the injury, highlighting the fact that shin splints was a specific syndrome with its own clinical symptoms and aetiology.
In the late 1960s and during the 1970s, advancements in nuclear medicine techniques led to the development of Triple Phase Bone Scintigraphy (TPBS), or nuclear bone scans, as a diagnostic tool. The technique enables inflammation and increased bone metabolism to be visualised after injection of a radioisotope and could be used in conjunction with a clinical diagnosis for positive identification of MTSS, or shin splint syndrome as it was then still called. However, despite these advances, the term “shin splints” was still being used as a generic expression for general pain in the tibia and for various lower limb injuries such as compartment syndrome. For this reason, the term “MTSS” was coined in the early 1980s[6] and was subsequently adopted by nuclear medicine experts[7,8] as well as some researchers and clinicians.
In the 1980s, a number of nuclear medicine studies led to more specific diagnostic criteria for MTSS. This included identifying the appearance of MTSS on nuclear bone scans, which consisted of an elongated uptake of radionuclide, visually seen as a “double stripe” pattern, differing from the localised fusiform pattern characteristic of a TSF[7-10]. This was later followed by studies where tibial stress injuries were identified and classified using magnetic resonance imaging (MRI), which has the advantage of depicting periosteal and bone marrow oedema[11,12]. However, despite these studies and more recent research into the aetiology of the injury, MTSS, but more commonly the term “shin splints”, is sometimes still used as a generic expression for tibial pain; however, this is gradually changing as the mechanisms of the injury are further understood.
CORTICAL BONE FATIGUE IN MTSS
MTSS was initially believed to be an anteromedial and/or posteromedial subcutaneous soft tissue injury only with an associated periostitis; a reasonable assumption given that no fracture or microfractures could be visualised on plane radiographs or computed tomography (CT) images. This is unlike a TSF, where a small partial cortical bone fracture can sometimes be identified at the site of pain and oedema, occasionally on a radiograph but more readily on CT, depending on the views imaged. However, it is now known that MTSS involves cortical bone microfractures associated with the periostitis, if not in all cases, then certainly in the majority of cases.
Johnell et al[13] first demonstrated microtrauma was a cause of MTSS from bone biopsies obtained from chronic MTSS patients undergoing fasciotomy after failing to respond to conservative treatment, and bone biopsies from control subjects at autopsy or who were undergoing surgery for other injuries. They found MTSS patients had increased osteoblastic activity and vascular ingrowth along with the inflammatory changes to the soft tissue, while none of the non-injured controls demonstrated these changes. As the majority, but not all, MTSS patients had bone changes on biopsy (22 of 35 patients), the authors concluded MTSS was caused by microfractures in most, but not in all cases[13]. Although a limitation of this study was the bone biopsies were all extracted from the same region, the medial surface of the tibia, which may not have been the exact injury site in some patients so some of the bone changes may have been missed, it clearly demonstrated that microtrauma was a cause of MTSS.
Bone fatigue was examined in a number of studies published in the 1970s and 1980s; although this research was not for the specific purpose of understanding MTSS aetiology, it provided critical insights on how microcracks develop in cortical bone. Carter, Caler, Hayes and others performed a series of investigations on cortical bone samples which were tested under cyclic loading in order to understand the biological mechanisms of fatigue failure in cortical bone.
Using bovine femora cortical bone specimens under fully reversed loading (cyclic loading where the mean stress is zero), they found that tensile cyclic loads result in tensile stresses which cause failure at osteon cement lines, i.e., the osteons debond from the surrounding interstitial bone, whereas compressive cyclic loads cause oblique microcracks to develop along the planes of high shear (tangential) stress, which are oblique to the loading direction, and these microcracks are influenced to some extent by the vascular canals and lacunae[14,15]. Thus, cortical bone under cyclic loading fails in both tension and compression; however, the mode of failure differs in each case. It was also found that the tensile failure will occur first, before any compressive failure occurs[16], which differs from most engineering materials, where cyclic loading results only in tensile failure.
Cortical bone specimen tests also demonstrated load frequency had a strong influence on the number of cycles to failure: a higher frequency resulted in less damage, but did not affect the total time to failure[17]. Importantly, the number of cycles to failure in cortical bone was affected by the strain range (amplitude) but not by the mean strain or the maximum strain; bone specimens subjected to a smaller strain range had a longer fatigue life[15,17].
As summarised by Martin and Burr[18], microcracks in cortical bone under cyclic tensile loading initially develop and propagate through the thickness of the lamellae: in areas of cortical bone under tension, the primary crack develops transversely, and are accompanied by secondary cracks which develop longitudinally, i.e., in the direction of the lamellae, which helps dissipate energy and thus slow the advancement of the primary (transverse) crack. The secondary cracks create interlamellar tensile and shear stresses which separate the lamellae, later resulting in debonding of the osteons.
Forwood and Parker[19] observed some of these effects in their study using whole-bone specimens to examine cortical bone fatigue microdamage in rats. Tibiae harvested from 60 rats were loaded in torsion at a number of different loading cycles. The authors found that lower levels of cyclic loading caused cracks to develop parallel to and traversing the lamellae, whereas higher levels of cyclic loading resulted in cracks through the full thickness of the cortex, invading across and through the Haversian canals or osteons[19].
Li et al[20] conducted an in vivo experiment where 20 rabbits were induced to run and jump over a period of 60 d by subjecting them to an electrical impulse at various intervals. Using radiographic and histological analyses on this group and a control (non-exercising) group, the authors found osteoclastic reabsorption occurred before the presence of any cracks in the cortical bone. Furthermore, only some rabbits developed cracks in the bone after the period of exercise, suggesting that in the majority of cases, the rabbit tibiae rapidly adapted to changes in the applied stress. Unlike the studies on cortical bone specimens, these in vivo tests may account for adaptive remodelling in living cortical bone.
The above research on cortical bone cyclic testing, both in vitro and in vivo studies, provided invaluable data on the development of fatigue injury in cortical bone. Like TSFs, cortical bone microtrauma occurring in MTSS is likely the result of tensile failure causing osteon debonding at the cement lines as the tibial microstructure is unable to repair quickly enough through adaptive bone remodelling. However, unlike a TSF, this microdamage clearly does not extend beyond the microscopic lamellae structure, at least in many cases, so that crack development is arrested in MTSS before a macroscopic partial fracture transversing the osteons occurs.
THE CAUSES OF MTSS
There are different theories on the exact cause of MTSS, although none of these theories have yet been proven. A number of previous studies have involved linking a specific muscle or muscle groups to MTSS based on the anatomical location in relation to patient symptoms. However, there have been conflicting results from these studies, leading experts to have different opinions to the exact cause of the injury.
Holder and Michael[7] performed TPBS on five male and five female athletes with clinically diagnosed posteromedial tibial pain, where the location of the injury in the ten patients was a combination of the lower, middle and upper thirds of the tibia[7]. Based on a concurrent analysis by the authors where lower leg musculature on cadavers was examined and EMG studies performed, they concluded that the proximal tibia and fibula origins of the soleus was largely responsible for the injury due to the location of radionuclide uptake[21]. However, there was no data presented showing the results of individual patient nuclear bone scans and the exact location of symptoms in those patients; hence, it is difficult to understand how the authors came to this conclusion.
Beck and Osternig[22] dissected the legs of 50 cadavera and concluded that either the soleus or flexor digitorum longus (FDL) was responsible for MTSS based on muscle attachment sites, but the tibialis posterior was not. In their study, the soleus and FDL both had origins from the posteromedial border of the tibia, which is one of the injury sites of MTSS (48% ± 11% and 35% ± 7.9% of the tibial length from the medial malleolus respectively), whereas no fibres from the tibialis posterior did. Based on their work and results of previous studies, they concluded that the soleus was most likely responsible for MTSS, and the cause was a traction-induced longitudinal periostitis at the injury site.
In a later study, Saxena et al[23] also conducted a dissection analysis, finding the origin of the tibialis posterior includes a portion of the lower third of the tibia in all cadavera examined. They therefore concluded that the tibialis posterior may be the cause the type of MTSS which occurs in the lower third of the tibia, since this muscle correlates to the location of the symptoms. However, a significant limitation in their study was there were only ten cadavers in their sample. The findings in this study were contradictory to Beck and Osternig, who concluded that the tibialis posterior was probably not involved in MTSS, as few tibialis posterior muscle fibres in their fifty cadavera arose from the tibial posteromedial border.
Matin proposed that the disruption of Sharpey’s fibres, which extend from the soleus-muscle-tendon complex to the cortical bone, could result in increased remodelling in the bone, therefore producing a longitudinal elongated pattern of injury[8]. In this hypothesis, the periosteal irritation from the Sharpey’s fibres result in an osteoblastic response in the cortical bone[9].
The apparent contrary findings in some of these previous studies, where the injury has been attributed to different muscles or other tissues, may be because there are different types of MTSS, each with their own specific aetiology. One of the current authors (Oakes[24]) first proposed this in 1988, where, based on the bone fatigue studies which had been conducted at the time and his own extensive clinical observations, MTSS could be classified into two main categories, where the first type was associated with external cortical bone microfractures, and both types may also be seen together to form a third type of MTSS. This has been previously described by the authors[24,33], but is also outlined below:
Type I: Distal tibial tenderness which when overt, can result in subcutaneous periostitis or oedema on the anteromedial surface of the mid to distal third of the tibia (Figure 1) due to microtrauma caused by microcracks between the Haversian systems or osteons in the underlying superficial cortical bone. Oakes postulated this was caused by “tibial flexion from contraction of the two heads of the Gastrocnemius and the Soleus muscle causing tibial bending moments during the push-off phase of running”[33].
Figure 1.
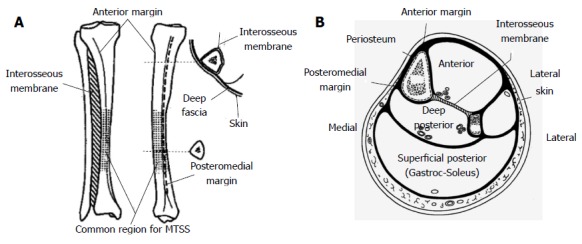
Anterior and medial views of the tibia with the main features shown, with the larger insert demonstrating the deep fascial attachments (A) and schematic section through the tibia illustrating the four compartments of the leg and their fascial coverings (B). The wide subcutaneous medial surface of the tibia can be seen. Images adapted from Oakes[24].
Type II: Posteromedial linear pain and tenderness, principally from the strong deep fascia of the posterior calf muscle compartment attaching to the linear posteromedial border of the tibia (Figure 1), but also due to the tibial origin of the FDL. Franklyn et al[33] proposed this was caused by “tension in the tibial attachment of the deep fascia in conjunction with the origins of the powerful action of the soleus and gastrocnemius muscles proximally”.
Type III: A combination of the two types observed in committed middle and long distance runners, or in young immature bone where growth is not complete and BMD is low.
Despite these different theories, clinical and research studies on the cause of MTSS, the fact that the detailed structural cause is still unknown highlights the need for prospective longitudinal investigations.
DOES PERIOSTITIS OR CORTICAL BONE MICROTRAUMA OCCUR FIRST IN MTSS?
It is apparent from the current evidence available that MTSS involves cortical bone microtrauma in the majority of cases. However, it is not clear if cortical bone microcracks cause tibial periostitis or if tibial periostitis results in cortical bone microcracks. In the first instance, it is theorised that underlying cortical bone microtrauma developing over a period of time eventually results in a periosteal soft tissue reaction in the region of the microcracks. In the second case, muscle fibre traction is postulated to cause periostitis which may or may not lead to cortical bone microcracks.
In dissection studies on the human tibia in situ, the soleus, FDL and tibialis posterior were all purported to be associated with MTSS. Although the authors of these studies did not specifically discuss the relationship between these muscles and cortical bone microtrauma, it is apparent the general consensus is that muscle fibre traction via Sharpey’s fibres results in tibial periostitis at the injury site, thus implying that either the periostitis occurs first, or there is a periosteal reaction in the absence of cortical bone microtrauma (since microtrauma was not discussed in these papers).
Matin[8] believed that the radionuclide deposition at the injury site of his patients was due to the periosteal response from the early developing bone abnormality and that Sharpey’s fibres were the cause. In other words, the early underlying cortical bone microtrauma initiates periostitis at the injury site through the Sharpey’s fibres; thus suggesting the bone response occurs first.
Based on their MRI study of 14 patients with 18 symptomatic legs, Fredericson et al[12] postulated that periosteal oedema occurs prior to the formation of cortical bone microcracks, as only periosteal oedema was detected in their patients with the mild injuries, or the MTSS, while those with more severe injuries had both periosteal oedema and either a partial fracture, or marrow oedema indicating bone microtrauma.
While the literature on cadaveric dissection supports muscle fibre traction as a potential cause of MTSS, there is also evidence for cortical bone microtrauma causing the injury, and in fact, it is known that cortical bone microtrauma occurs from impact exercise at the early stages of training. For example, Etherington et al[25] studied a cohort of 40 male military recruits over 10 wk of basic training, 26 of whom completed the training, and measured a number of parameters including the velocity of ultrasound in the heel. The authors found there was a mean decrease in the ultrasonic velocity from pre to post training in recruits who completed the training uninjured, signifying that either trabecular thinning due to bone remodelling or loss of trabeculae due to the development of microfractures. However, as the bone markers measured indicated there was an overall reduction in bone turnover, the decrease in ultrasonic velocity was likely due to microfractures rather than active bone remodelling. Thus, cortical bone microtrauma occurs prior to the development of any clinical injury, and could be a precursor to periostitis.
NUCLEAR MEDICINE AND MRI
Prior to the advent of nuclear medicine techniques, MTSS could only be diagnosed early by a clinical examination and a detailed patient history, as radiographs, if not occult, would not show any visible radiological signs of the injury for at least 3-4 wk. However, this changed in the 1980s, after TPBS had been developed, as a clinical examination could be supplemented by medical imaging to confirm the diagnosis and exclude other conditions with similar symptoms.
Nuclear medicine studies have shown that patients with MTSS have increased uptake of radionuclide in the cortical bone, showing a characteristic longitudinal “double stripe” pattern[10]. Accuracies of 75% or greater have been found for nuclear bone scans[10,26,27], although it has been criticised for resulting in false positives: it has been argued that increased radionuclide uptake is not specific to a particular pathology, but instead due to increased activity of the patient[27-29]. However, nuclear bone scanning indicates there is a bone osteoclastic/osteoblastic response and an uptake of radionuclide may be due to a number of reasons including an increased cortical bone vascularity associated with bone metastases and/or increased physical activity of the patient. Thus, while nuclear bone scanning is an important diagnostic tool, the results need to be considered in conjunction with the patient’s clinical symptoms for a correct interpretation of the findings.
MRI has more recently emerged as the preferred imaging modality for the diagnosis of both MTSS and TSFs. This was first reported by Fredericson et al[12], who found that MRI was more effective than other imaging modalities for the diagnosis, and also the early diagnosis, of tibial stress injuries. In a study involving 14 runners with 18 symptomatic legs (4 had bilateral symptoms) who sustained either a tibial stress reaction, MTSS or a TSF, the authors compared radiology, nuclear bone scans and MRI, concluding that MRI was anatomically specific and more sensitive in its correlation with the clinical symptoms and signs of bone stress injuries than TPBS.
The primary limitation of the study was the small number of patients analysed: out of 18 tibiae, two were found to have no pathology; thus there were a total of 16 painful tibiae. Also, although all tibial stress reactions were on the posteromedial border, the location along the tibia differed, comprising of patients with proximal, midshaft and distal leg pain. However, from this work, the authors also developed a four-level MRI classification system for tibial stress injuries, where Grades 1 and 2 were diffuse injuries (MTSS) while Grades 3 and 4 were localised injuries (TSFs). Pomeranz[11] (2001) later modified this classification system by separating Group 4 into two different types: Group 4a (partial cortical fracture) and Group 4b (complete cortical fracture). Table 1 demonstrates the modified grading system, which has been further adapted by Oakes.
Table 1.
Clinical features and magnetic resonance imaging findings in the four grades of tibial stress injury
| Grade | Clinical exam | MRI |
| 1 | Periosteal tenderness at the distal 1/3 to 1/2 of the anteromedial tibial surface. Requires firm palpation with thumb | Periosteal oedema: mild to moderate on T2-weighted images. Marrow normal on T1 and T2-weighted images |
| 2 | Tenderness as above | Periosteal oedema: moderate to severe on T2-weighted images Marrow oedema on STIR or T2-weighted images. T1 normal |
| Requires less firm palpation with thumb and may have linear tenderness along the posteromedial tibial border | ||
| 3 | Tenderness as above | Periosteal oedema: moderate to severe on T2-weighted images. Marrow oedema on T1 and STIR-T2-weighted images |
| Requires less firm palpation and may have linear tenderness as above | ||
| May have subcutaneous anteromedial tibial oedema | ||
| 4 | Tenderness as above | Periosteal oedema: moderate to severe on T2-weighted images. Marrow oedema on T1-STIR or T2-weighted images |
| Requires less firm palpation and may have linear tenderness as above | Fracture line clearly visible as low fuzzy incomplete (4a) or complete (4b) line | |
| A discrete region of maximal tenderness/thickening (early callus formation) over the fracture site will be palpable. Obvious tibial subcutaneous oedema is usually present | May see oedema in proximal tibial origins of Tibialis Posterior, FDL and Soleus |
In a later study by the Bergman et al[30] group it was found that MRI can demonstrate a positive stress reaction in individuals performing intense exercise; this is similar to nuclear bone scans where radionuclide uptake had previously been observed in individuals due to intense exercise. In 21 asymptomatic elite university runners, the authors found nine athletes had Grades 1-3 abnormalities on MRI, indicating a tibial stress reaction was present, yet on follow-up, none of these individuals developed a bone stress injury. This not only highlights the importance of assessing MRI (or nuclear bone scan) findings in conjunction with a detailed clinical examination and patient history, but demonstrates cortical bone microcracks can develop in response to intense impact training and do not always signify a current or subsequent bone stress injury with overt microcracks.
Figure 2 demonstrates T2-weighted images of a 17-year-old patient who sustained MTSS after playing hockey on a synthetic turf surface (Astro Turf®) for approximately 2 mo. Her treating sports physician (Oakes) recommended a series of MRI scans. The periosteal oedema can be visualised on the medial cortex.
Figure 2.
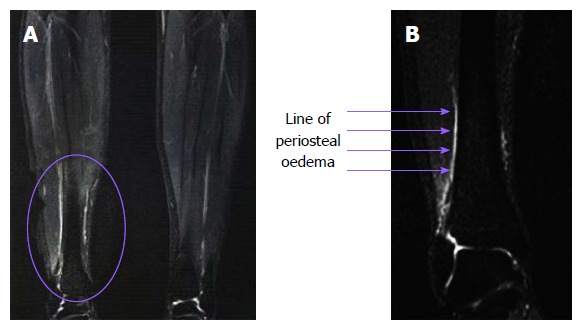
Coronal T2-weighted magnetic resonance imaging images of a 17-year-old female hockey player who was training on a concrete pitch covered with Astro Turf® for approximately 2 mo and was subsequently diagnosed with medial tibial stress syndrome. A white longitudinal line of periosteal oedema on the medial cortex can clearly be seen on the enlarged view (right), which was consistent with the region of pain and tenderness.
BMD AND CORTICAL BONE GEOMETRY CHANGES IN MTSS
Since the studies were published on cortical bone microtrauma and MTSS, there has been more recent research demonstrating MTSS patients have other changes to the cortical bone. This work has involved either BMD measurements or detailed tibial cortical bone geometry studies.
BMD
The initial research on MTSS and BMD was performed by Magnusson et al[31], who measured BMD in 18 male professional athletes who sustained chronic MTSS diagnosed both clinically and by nuclear bone scanning, 18 male age and sex matched professional control athletes (exercising 3-15 h/wk) who were not injured, and 16 age and sex matched male control subjects who were recreational athletes (0 to 5 h per week) using Duel Energy X-ray Absorptiometry (DEXA). The MTSS patients were diagnosed both clinically and by a nuclear bone scan, and all had medial diffuse pain at the junction of the middle and distal thirds of the tibia (it was not stated if all patients had posteromedial pain, although this was implied in their introductory discussion).
The authors demonstrated that athletes with chronic MTSS had a localised lower BMD at the injury site than both the athletic control and the control subjects, and the low BMD was bilateral, even when the injury was unilateral. Conversely, in the proximal and distal tibial regions, where the BMD was also measured, it was found that the MTSS subjects had higher BMD than the two groups of control subjects (Table 2); thus, leading the authors to conclude that MTSS is associated with low regional BMD. In a subsequent study, the authors found that after recovery from the injury, the BMD returns to normal[32]. This observation suggests that the low BMD is not inherent, or pre-existing, but develops in conjunction with the symptoms.
Table 2.
Bone mineral density in male medial tibial stress syndrome patients and an athletic control group[31]
| BMD (g/cm2) | MTSS | Athletic control | Significance |
| Proximal | 1.29 | 1.48 | < 0.01b |
| 33% level (injury site) | 1.43 | 1.85 | < 0.001b |
| Distal | 1.32 | 1.33 | > 0.05 |
Statistically significant. Although there are differences in the proximal tibia, the difference is much greater at the injury site. Select data presented for comparison with the two other BMD studies discussed. BMD: Bone mineral density; MTSS: Medial tibial stress syndrome.
The study by Magnusson et al[31,32] had significant limitations related to exercise exposure. First, there was considerable variation in the amount of exercise performed per week in the professional athlete control group (3-15 h/wk), while individuals in the recreational exercise control group performed some exercise (0-5 h/wk); hence, they were not a real sedentary control group. Second, the individuals who exercised performed a wide variety of activities including both impact (e.g., running) and non-impact activities (e.g., weightlifting and swimming), which may have affected the BMD results. Last, in both control groups there were individuals with both manual and non-manual occupations, further diversifying exercise exposure of individuals in the groups.
The current authors conducted a preliminary study where BMD was compared between female chronic MTSS and TSF patients[33]. BMD was measured in three locations in the tibia: proximally, distally and at the injury site (the junction of the mid and distal thirds of the tibia); these locations were similar to three of the five locations BMD was measured in the Magnusson study. Patients were diagnosed both clinically and by a nuclear bone scan. They had been performing impact exercise at least 3-4 times per week with a 2-year minimum training history (although the majority had a much longer training history) prior to the analysis. It was found that at all three sites, the BMD was lower in the MTSS patients than the TSF patients, although it was only statistically significant at the injury site (Table 3). The main limitation with our preliminary study was that the subject numbers were not large: there were only five TSF patients (10 tibiae) and ten MTSS patients (20 tibiae). Nevertheless, the patient numbers were sufficient to demonstrate statistical significance.
Table 3.
Bone mineral density in female tibial stress fracture and medial tibial stress syndrome patients[33]
| BMD (g/cm2) | MTSS (n = 20) | TSF (n = 10) | Significance |
| Proximal | 1.21 | 1.27 | 0.136 |
| 33% level (injury site) | 1.46 | 1.63 | 0.013a |
| Distal | 0.90 | 0.94 | 0.403 |
Statistically significant. BMD: Bone mineral density; MTSS: Medial tibial stress syndrome; TSF: Tibial stress fracture.
In another BMD study on MTSS patients, Ozgürbüz et al[34] found that the BMD did not differ between MTSS patients and aerobic controls in several different bones, including the tibia at three different sites. The study contained a total of 22 subjects, where 11 subjects were MTSS patients and 11 subjects were aerobic controls, and each group comprised of both males and females. MTSS was diagnosed clinically by two different physicians and the MTSS patients had a history of the injury from 3-10 wk.
The strength of this study was the control group, which contained subjects who were all performing impact exercise rather than a mix of subjects performing impact and non-impact exercise. However, there were some significant limitations: MTSS patients were only diagnosed clinically and there was no information provided on the assessment criteria used in the diagnosis. More importantly, the patients had only sustained MTSS for a period of 3-10 wk (5 wk on average); therefore, they were not chronic MTSS patients. Thus, it is unlikely that these patients would yet have experienced any changes to the cortical bone in such a short time period, which is the most likely explanation why the authors found that BMD did not differ between the MTSS subjects and the aerobic controls. Interestingly, the BMD values measured by Ozgürbüz were considerably lower than the values found in the other BMD studies, for example, at the injury site (a similar location in the tibia in all the BMD studies), the BMD values were Ozgürbüz 0.315 (MTSS) and 0.323 (aerobic control), Franklyn and Oakes 1.46 (MTSS), and Magnusson 1.43 (MTSS) and 1.85 (aerobic control). This clearly requires further examination.
Thus, it can be concluded that BMD is lower in chronic MTSS patients than in aerobic controls, but this is not the case for other regions of the tibia, while patients with acute MTSS do not appear to have low regional BMD. In addition, BMD is lower in patients with MTSS than TSF patients. It is probable that the low BMD in MTSS patients occurs in conjunction with the symptoms. A longitudinal study, where BMD is measured at periodic intervals in an exercising cohort, and where both male and female subjects are included but analysed as separate groups, is needed to confirm these findings.
Cortical bone geometry
In previous research, low values of various cortical bone geometric factors have been associated with TSFs[35-37], but there is only one previous study where detailed cortical bone geometry has been analysed in MTSS patients[38]. In this research, it was found that the MTSS subjects had lower values of some geometric parameters than aerobic control subjects, but not as low as TSF subjects, and these differences were not the same in males and females[38]. Significant parameters in males included cortical bone cross-sectional area, polar moment of area, second moments of area and section moduli, indicating that males with MTSS are less adapted to axial loads, torsion, maximum and minimum bending and pure bending. Females sustaining MTSS had smaller section moduli than aerobic controls, indicating less adaptation to pure bending, but other geometric parameters did not differ. Although MTSS patients had lower values of geometric bone parameters than aerobic controls, they were not as low as the values in the TSF groups, indicating that there may be some different mechanisms involved in each of these injuries.
Although this research was limited in that it was not a longitudinal study, the aerobic control group in the study had higher values of the significant cortical bone geometric parameters, suggesting these parameters increase in response to impact exercise and in fact, longitudinal studies in the literature on both humans and animals demonstrate that cortical bone geometric parameters increase in response to exercise[39,40]. Thus, it is probable that bone geometric factors also alter in conjunction with the development of the injury, although a longitudinal study using periodic CT or MRI scans is needed to confirm these findings. While CT has traditionally been the best imaging modality for the calculation of tibial geometric factors due to its superior depiction of cortical bone, new generation MRI scanners now show improved bone resolution (Figure 3); therefore, may be an alternative choice due to the lack of ionising radiation. However, validation studies comparing geometric parameter computations on the same individuals scanned using both CT and MRI would be initially needed to elucidate any significant differences between the two imaging modalities.
Figure 3.

Comparison of computed tomography with a new generation magnetic resonance imaging image. (A) typical CT image (B) enlarged CT showing the high resolution cortical bone depiction and (C) MRI image for comparison. CT: Computed tomography; MRI: Magnetic resonance imaging.
BMD and cortical bone geometry
In summary, previous studies on BMD and cortical bone geometric parameters demonstrate that patients with MTSS have lower BMD and lower values of various cortical bone geometric factors than aerobic control subjects. MTSS patients appear to also have lower BMD than TSF individuals, but higher values of cortical bone geometric factors. These findings suggest that both BMD and cortical bone geometry may both contribute to the likelihood of sustaining a TSF or MTSS, but the balance between the two factors may predict an individual’s likelihood of developing one of these specific injuries.
IS MTSS A PRECURSOR TO A TSF?
There are conflicting views as to whether MTSS is a precursor to a TSF and thus they are on a continuum of injury[12], or if they are two separate entities with common aetiology and risk factors, but differences in predisposition and development of the injury[8,41].
It can be argued that MTSS and TSFs are on a continuum as MTSS is most commonly found in the same location as TSFs, at the junction of the mid and distal thirds of the tibia, but this is not always the case as MTSS is also observed in other locations in the tibia[12,42], suggesting it is a separate injury. Clinical examination of patients with TSFs demonstrates that in addition to the small pronounced area of focal pain overlying the fracture location, there is often overt anteromedial subcutaneous pitting oedema on palpation along a region of the tibia, indicating that the diffuse region of microcracks may have progressed to a macrocrack at one location. However, not all cases of MTSS lead to a TSF; if they were one injury on a continuum, all MTSS patients would eventually sustain a TSF with continued exposure to the same impact forces, yet this does not occur.
Both MTSS and TSFs occur from microcracks developing in cortical bone as the anterior cortex of the tibia cycles from overt compression loading on heel-strike to tension loading at push-off, and both injuries involve an alteration in cortical bone geometry[38] and BMD[31-33]. However, cortical bone geometry and BMD also differs between TSF and MTSS patients[33,38], indicating there may be different specific biomechanism involved in each case.
While it is clear that MTSS and TSFs have commonality with regards to the development of microcracks in the cortical bone, changes in BMD and alteration to the cortical bone geometry, it is yet to be proven if they are one injury or two separate entities. Opinions in the literature differ but the issue is unlikely to be resolved until longitudinal studies are performed.
STRAIN GAUGE ANALYSES AND COMPUTER MODELLING
Earlier papers on MTSS predominately focused on defining the injury and describing the most appropriate techniques for diagnosis, with some authors hypothesising potential causes of the injury, while recent research has centred on reviews of the literature[1,43,44], risk factors[42,45-48], interventions[49,50] and treatment options[51,52]. However, studies investigating the aetiology of the injury are limited, and future research should focus on the exact mechanisms of MTSS, which may lead to the development of improved interventions. Some techniques which may be employed in future work are in vivo strain gauge experiments and finite element (FE) analysis.
Surgically-bonded strain gauges have been used in previous TSF research in order to examine the relationship between loading conditions and stress or strain in the bone in vivo[53-57]. While these studies have provided information on the stress or strain experienced by the tibia under different types of impact exercise, in all these studies, the subjects had no pathology, and the stress or strain experienced by the tibia is likely to differ between these non-injured subjects and individuals with MTSS or a TSF. Conducting this type of experimental work on injured subjects would provide invaluable data pertaining to the injured tibia; however, there are obviously ethical and other considerations in performing this type of analysis which may preclude this type of study from being conducted, especially on subjects who are injured.
An alternative technique for analysing stress or strain in bone is by the use of computational techniques such as the FE method. FE analysis has a number of advantages over strain gauges in that the entire stress or strain in the bone can be computed; therefore, regions of peak stress or strain can be easily identified. In addition, the loading conditions on the model can easily be altered so the direct relationship between applied load and stress or strain in the bone can be determined, and the model geometry can also be changed.
Several FE models have more recently been developed in order to better understand tibial stress injuries; however, these studies have focused on TSFs rather than MTSS. Sonoda et al[58] developed a subject-specific tibiofibula FE model based a on 20-year-old female, 165 cm in height and 52 kg in weight, applying loading conditions from the literature on the model. The subject had no pathology; however, they simulated small tibial fractures in the model to represent TSFs, finding that that the (von Mises) stresses in the anterior border, where the TSF was most severe, ranged from approximately 63 MPa to approximately 75 MPa. Edwards et al[59] developed a generic tibial FE model based on a publicly available dataset which they used to develop separate models for each of their 10 male subjects (approximately 24.9-year-old 1.7 m, 70.1 kg) by scaling the tibial length based on the subject's body weight and then using gait data from the subjects to determine the loads to apply to the models. The authors used a probabilistic model for TSFs to determine when failure would occur and found the peak (maximum principal) strain to be approximately 3670 (approximately 68 MPa) on the tibial anterior surface.
The stresses predicted in these FE models are considerably higher than those measured in the strain gauge studies, where values of stress on the anteromedial border ranged from approximately 14 MPa[53] to approximately 28 MPa[54] (by converting the measured strains into stress using a Young’s modulus of 18600 MPa), highlighting the fact that the tibial stresses will be higher in injured individuals at the injury site, and the need for more studies examining the stress and strain in the tibia of both TSF and MTSS patients.
More recently, the current authors developed an FE model based on a female athletic patient who sustained chronic MTSS with the input loads to the model derived from gait analysis data from the same patient[33]. The model was used to analyse the relationship between loads while running and stresses in the tibia. While the analysis is still being finalised, the results show the magnitude of stress in the tibia is higher in the MTSS patient than the tibial stresses in the subjects from the strain gauge studies; a similar finding to the FE models representing TSF patients (Figure 4). Additionally, the results indicate the magnitude and position of the high tensile stress region is predominately affected by the combination of the input loads, while the distribution of the high stresses (diffuse or localised) appear to be more influenced by the specific bone geometry of the subject. However, these preliminary findings require further analysis.
Figure 4.
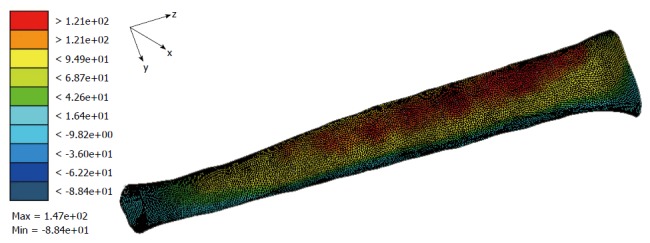
Preliminary finite element analysis by the current authors. Maximum values of principal stresses in the model were significantly higher than those measured by strain gauge analysis, but similar to some other FE models in the literature. FE: Finite element.
Previous strain gauge studies have provided invaluable data on the stress and strain state of the tibia under loading, but as these were all performed on uninjured subjects, the results are not necessarily transferable to individuals with bone stress injuries; indeed, the FE modelling which has been conducted to date indicates they are not. Performing strain gauge experiments on MTSS patients may provide a critical insight into the strain experienced by the tibia when injured; however, there are obviously ethical considerations in surgically bonding strain gauges to the bone of injured individuals. Hence, further computational modelling might provide the key to better understanding the stresses and strains in the tibia in injured individuals.
ADVICE FOR THE TREATING PHYSICIAN
In the last few decades, the diagnosis of MTSS has changed, predominately due to the advances in medical imaging technology. In the 1980s and 1990s, physicians were reliant on plain film radiology and nuclear bone scans to verify their clinical findings. Plain radiographs were often normal in the early stages of a suspected TSF (e.g., 3-4 wk post-symptoms or 4-6 wk post-injury), but a nuclear bone scan may be positive, demonstrating early uptake of radionuclide in the region of increased vascularity of the overt fracture not readily seen on plain radiographs, such as a fractured navicular in a running athlete or a fractured scaphoid in a gymnast. Nuclear bone scans were particularly useful to the clinician in that a positive scan with a localised radionuclide uptake (i.e., “hot spot”) was objective evidence of a fracture; however, the anatomical specificity was poor, especially with the small bones of the carpus. CT imaging could be used in conjunction with radiography and a nuclear bone scan for cases where a TSF was suspected, as small overt fractures could often be observed, such as small fracture in the navicular, other tarsal bones, the carpals and the sesamoids of the foot, and avascular necrosis of these bones could also be identified.
Plain radiology was not particularly useful for an early diagnosis of MTSS as the inflammatory reaction associated with the periostitis and cortical microfracture formation could not generally be observed for 4-6 wk post-injury, even though symptoms and signs are usually present at 3-4 wk post-injury.
The advent of MRI and developments in this imaging modality over the last 10-15 years has given the treating physician an alternative option involving no ionising radiation. MRI exams now demonstrate excellent anatomical resolution of both bone and soft tissue. Physicians could use it to follow patients at various points in time, and it was particularly useful for clinical trials, as the long-term response of bone and soft tissues to both normal and excess loading conditions could be determined.
For the practicing physician, the current contemporary diagnosis of both MTSS and a TSF involves a combination of both a clinical examination and medical imaging. The clinical exam should include an assessment of both legs (while the patient is standing) for alignment, length, any deformity and foot stance. Foot pronation, indicating weak invertors, may signify an alignment problem associated with a TSF or MTSS. While the patient is seated, the physician should palpate the tibia for tenderness, especially the anterior border and posteromedial longitudinal borders of the tibia where the deep fascia attaches, as well as the whole of the subcutaneous anteromedial surface. Particular note should be made of regions with more acute tenderness, especially the distal one-third of the tibia, and its distribution (local or diffuse). The leg should also be examined for any subcutaneous oedema, which indicates periostitis is present and probable associated microfractures. The three compartments of the leg (anterior, peroneal and posterior) should be palpated for tenderness, with “tightness” in the muscle compartment of the leg indicating the patient may have compartment syndrome. A weakness in one or more muscle compartments or in a myotome may indicate lumbar spinal nerve compression or other isolated motor nerve pathologies including rare entrapment syndromes.
A full strength/power assessment of all the muscles of the leg should be performed as well as a full vascular and neural exam. Range of motion in the ankle joint, especially ankle joint dorsiflexion or extension, should be checked to exclude a tight/short gastroc-soleus-tendon complex; if short, it would increase anteromedial tibial loading on running. Similarly, excess forefoot pronation may indicate tibialis anterior/posterior weakness and thus greater tibial torque on running.
Bone pain and tenderness, especially in a non-athletic patient, should be regarded with special care, as bone tumours or infection must be initially excluded. For these patients, plain radiographs of the whole tibia are mandatory.
Where other pathologies have been excluded and the patient has clinical indications of a tibial bone stress injury, an MRI exam should be performed of the whole tibia, where the findings and classification of the injury have presented earlier in this review.
Treatment of the patient with a confirmed MTSS (or a TSF) will vary according to the cause. While non or reduced weight bearing should be generally prescribed, issues such as leg alignment and forefoot pronation need to be addressed in order to facilitate healing and prevent future re-occurrence.
CONCLUSION
MTSS is an overuse fatigue injury involving tibial periostitis in conjunction with cortical bone oedema and microtrauma, although the cortical bone response may not occur in all individuals. The two main mechanisms of injury appear to be a traction-induced periostitis, where the cause is likely to be the soleus and/or the FDL, and microtrauma comprising of oedema and microcracks in the cortical bone which result in debonding of the osteons and subcutaneous periostitis on the surface of the tibia. While there are numerous studies in the literature on risk factors, interventions and treatment for MTSS in addition to a number of review papers, studies examining the aetiology are limited, therefore the exact causal mechanisms are still not understood.
It is apparent that prospective longitudinal studies are required where athletes or military recruits are monitored by CT or MRI and DEXA in order to quantify precise changes in cortical bone geometry and simultaneously monitor both BMD and cortical bone oedema during the development of MTSS. However, this type of research requires a large cohort where a definite minimum number of individuals will reliably sustain the injury, and consent to perform a large number of scans, some with ionising radiation. This may not occur in the near future as the current focus in many universities and research organisations is for shorter research studies which lead to the development of quick clinical outcomes. Surgically-bonded strain gauges on the tibia offer an alternative approach, although there are ethical considerations with conducting these types of experiments. FE analysis is another technique which should be explored for future studies, as it can be used to examine stresses in the whole tibia under different loading conditions.
Contemporary accurate diagnosis of either MTSS or a TSF includes a comprehensive clinical examination to identify signs of bone stress injury and to exclude other pathologies. This should be followed by an MRI study of the whole tibia. The possible cause of the injury should be established and addressed in order to facilitate healing and prevent future long-term re-occurrence.
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge Mr Jeff Copeland for compiling and formatting the references and photographing the MRI images.
Footnotes
Conflict-of-interest statement: Both authors, Dr. Melanie Franklyn and Associate Professor Barry Oakes, declare that there is no conflict of interest for this work. They have received no funds from any commercial party in relation to this work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 4, 2015
First decision: April 27, 2015
Article in press: July 27, 2015
P- Reviewer: Ohishi T, Zak L S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Moen MH, Tol JL, Weir A, Steunebrink M, De Winter TC. Medial tibial stress syndrome: a critical review. Sports Med. 2009;39:523–546. doi: 10.2165/00007256-200939070-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hutchins CP. Explanation of spike soreness in runners. Am Phys Ed Rev. 1913;18:31–35. [Google Scholar]
- 3.Devas MB. Stress fractures of the tibia in athletes or shin soreness. J Bone Joint Surg Br. 1958;40-B:227–239. doi: 10.1302/0301-620X.40B2.227. [DOI] [PubMed] [Google Scholar]
- 4.American Medical Association. Committee on the Medical Aspects of Sports. Subcommittee on Classification of Sports Injuries. Standard nomenclature of athletic injuries. Chicago: American Medical Association; 1966. p. 126. Available from: https://books.google.com.au/books/about/Standard_nomenclature_of_athletic_injuri.html?id=UPY7AAAAIAAJ&redir_esc=y. [Google Scholar]
- 5.Slocum DB. The shin splint syndrome. Medical aspects and differential diagnosis. Am J Surg. 1967;114:875–881. doi: 10.1016/0002-9610(67)90410-2. [DOI] [PubMed] [Google Scholar]
- 6.Mubarak SJ, Gould RN, Lee YF, Schmidt DA, Hargens AR. The medial tibial stress syndrome. A cause of shin splints. Am J Sports Med. 1982;10:201–205. doi: 10.1177/036354658201000402. [DOI] [PubMed] [Google Scholar]
- 7.Holder LE, Michael RH. The specific scintigraphic pattern of “shin splints in the lower leg”: concise communication. J Nucl Med. 1984;25:865–869. [PubMed] [Google Scholar]
- 8.Matin P. Basic principles of nuclear medicine techniques for detection and evaluation of trauma and sports medicine injuries. Semin Nucl Med. 1988;18:90–112. doi: 10.1016/s0001-2998(88)80003-5. [DOI] [PubMed] [Google Scholar]
- 9.Deutsch AL, Coel MN, Mink JH. Imaging of stress injuries to bone. Radiography, scintigraphy, and MR imaging. Clin Sports Med. 1997;16:275–290. doi: 10.1016/s0278-5919(05)70022-3. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman CM, Hemingway DL. Scintigraphy of shin splints. Clin Nucl Med. 1980;5:31. doi: 10.1097/00003072-198001000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Pomeranz SJ. Instructional lectures on MRI. Australian MRI workshop course lecture notes on assessing chronic bone injury. Melbourne, Australia, June 11-15. Available from: http://www.proscan.com/fw/main/Education-Foundation-1148.html.
- 12.Fredericson M, Bergman AG, Hoffman KL, Dillingham MS. Tibial stress reaction in runners. Correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med. 1995;23:472–481. doi: 10.1177/036354659502300418. [DOI] [PubMed] [Google Scholar]
- 13.Johnell O, Rausing A, Wendeberg B, Westlin N. Morphological bone changes in shin splints. Clin Orthop Relat Res. 1982;167:180–184. [PubMed] [Google Scholar]
- 14.Carter DR, Hayes WC. Compact bone fatigue damage: a microscopic examination. Clin Orthop Relat Res. 1977;127:265–274. [PubMed] [Google Scholar]
- 15.Carter DR, Caler WE, Spengler DM, Frankel VH. Fatigue behavior of adult cortical bone: the influence of mean strain and strain range. Acta Orthop Scand. 1981;52:481–490. doi: 10.3109/17453678108992136. [DOI] [PubMed] [Google Scholar]
- 16.Carter DR, Hayes WC. Compact bone fatigue damage--I. Residual strength and stiffness. J Biomech. 1977;10:325–337. doi: 10.1016/0021-9290(77)90005-7. [DOI] [PubMed] [Google Scholar]
- 17.Caler WE, Carter DR. Bone creep-fatigue damage accumulation. J Biomech. 1989;22:625–635. doi: 10.1016/0021-9290(89)90013-4. [DOI] [PubMed] [Google Scholar]
- 18.Martin RB, Burr DB. Structure, Function, and Adaption of Compact Bone. 1st ed. New York: Raven Press; 1989. pp. 189–192. [Google Scholar]
- 19.Forwood MR, Parker AW. Microdamage in response to repetitive torsional loading in the rat tibia. Calcif Tissue Int. 1989;45:47–53. doi: 10.1007/BF02556660. [DOI] [PubMed] [Google Scholar]
- 20.Li GP, Zhang SD, Chen G, Chen H, Wang AM. Radiographic and histologic analyses of stress fracture in rabbit tibias. Am J Sports Med. 1985;13:285–294. doi: 10.1177/036354658501300501. [DOI] [PubMed] [Google Scholar]
- 21.Michael RH, Holder LE. The soleus syndrome. A cause of medial tibial stress (shin splints) Am J Sports Med. 1985;13:87–94. doi: 10.1177/036354658501300202. [DOI] [PubMed] [Google Scholar]
- 22.Beck BR, Osternig LR. Medial tibial stress syndrome. The location of muscles in the leg in relation to symptoms. J Bone Joint Surg Am. 1994;76:1057–1061. doi: 10.2106/00004623-199407000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Saxena A, O’Brien T, Bunce D. Anatomic dissection of the tibialis posterior muscle and its correlation to medial tibial stress syndrome. J Foot Surg. 1990;29:105–108. [PubMed] [Google Scholar]
- 24.Oakes BW. Tibial pain or shin soreness (“shin splints”)-its cause, differential diagnosis and management. In: Draper J, editor. Second Report on the National Sports Research Program. Canberra, Australia: Australian Sports Commission;; 1986. pp. 47–51. [Google Scholar]
- 25.Etherington J, Keeling J, Bramley R, Swaminathan R, McCurdie I, Spector TD. The effects of 10 weeks military training on heel ultrasound and bone turnover. Calcif Tissue Int. 1999;64:389–393. doi: 10.1007/pl00005820. [DOI] [PubMed] [Google Scholar]
- 26.Gaeta M, Minutoli F, Scribano E, Ascenti G, Vinci S, Bruschetta D, Magaudda L, Blandino A. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235:553–561. doi: 10.1148/radiol.2352040406. [DOI] [PubMed] [Google Scholar]
- 27.Allen MJ. Shin pain. In: Hutson MA. Sports Injuries. Recognition and Management. 2nd ed. Oxford University Press; 1996. pp. 151–154. [Google Scholar]
- 28.Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65:1245–1251. [PubMed] [Google Scholar]
- 29.Wallensten R. Results of fasciotomy in patients with medial tibial syndrome or chronic anterior-compartment syndrome. J Bone Joint Surg Am. 1983;65:1252–1255. [PubMed] [Google Scholar]
- 30.Bergman AG, Fredericson M, Ho C, Matheson GO. Asymptomatic tibial stress reactions: MRI detection and clinical follow-up in distance runners. AJR Am J Roentgenol. 2004;183:635–638. doi: 10.2214/ajr.183.3.1830635. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson HI, Westlin NE, Nyqvist F, Gärdsell P, Seeman E, Karlsson MK. Abnormally decreased regional bone density in athletes with medial tibial stress syndrome. Am J Sports Med. 2001;29:712–715. doi: 10.1177/03635465010290060701. [DOI] [PubMed] [Google Scholar]
- 32.Magnusson HI, Ahlborg HG, Karlsson C, Nyquist F, Karlsson MK. Low regional tibial bone density in athletes with medial tibial stress syndrome normalizes after recovery from symptoms. Am J Sports Med. 2003;31:596–600. doi: 10.1177/03635465030310042001. [DOI] [PubMed] [Google Scholar]
- 33.Franklyn M, Oakes B. Tibial Stress Injuries: Aetiology, Classification, Biomechanics and the Failure of Bone. In: Zaslav KR, editor. An International Perspective on Topics in Sports Medicine and Sports Injury. Rijeka: InTech; 2012. pp. 509–534. [Google Scholar]
- 34.Ozgürbüz C, Yüksel O, Ergün M, Işlegen C, Taskiran E, Denerel N, Karamizrak O. Tibial bone density in athletes with medial tibial stress syndrome: a controlled study. J Sports Sci Med. 2011;10:743–747. [PMC free article] [PubMed] [Google Scholar]
- 35.Beck TJ, Ruff CB, Mourtada FA, Shaffer RA, Maxwell-Williams K, Kao GL, Sartoris DJ, Brodine S. Dual-energy X-ray absorptiometry derived structural geometry for stress fracture prediction in male U.S. Marine Corps recruits. J Bone Miner Res. 1996;11:645–653. doi: 10.1002/jbmr.5650110512. [DOI] [PubMed] [Google Scholar]
- 36.Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437–444. doi: 10.1016/s8756-3282(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 37.Popp KL, Hughes JM, Smock AJ, Novotny SA, Stovitz SD, Koehler SM, Petit MA. Bone geometry, strength, and muscle size in runners with a history of stress fracture. Med Sci Sports Exerc. 2009;41:2145–2150. doi: 10.1249/MSS.0b013e3181a9e772. [DOI] [PubMed] [Google Scholar]
- 38.Franklyn M, Oakes B, Field B, Wells P, Morgan D. Section modulus is the optimum geometric predictor for stress fractures and medial tibial stress syndrome in both male and female athletes. Am J Sports Med. 2008;36:1179–1189. doi: 10.1177/0363546508314408. [DOI] [PubMed] [Google Scholar]
- 39.Haapasalo H, Kontulainen S, Sievänen H, Kannus P, Järvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27:351–357. doi: 10.1016/s8756-3282(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 40.Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, Akeson WH. The effect of prolonged physical training on the properties of long bone: a study of Wolff’s Law. J Bone Joint Surg Am. 1981;63:780–787. [PubMed] [Google Scholar]
- 41.Aoki Y, Yasuda K, Tohyama H, Ito H, Minami A. Magnetic resonance imaging in stress fractures and shin splints. Clin Orthop Relat Res. 2004;421:260–267. doi: 10.1097/01.blo.0000126333.13806.87. [DOI] [PubMed] [Google Scholar]
- 42.Yates B, White S. The incidence and risk factors in the development of medial tibial stress syndrome among naval recruits. Am J Sports Med. 2004;32:772–780. doi: 10.1177/0095399703258776. [DOI] [PubMed] [Google Scholar]
- 43.Craig DI. Medial tibial stress syndrome: evidence-based prevention. J Athl Train. 2008;43:316–318. doi: 10.4085/1062-6050-43.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tweed JL, Avil SJ, Campbell JA, Barnes MR. Etiologic factors in the development of medial tibial stress syndrome: a review of the literature. J Am Podiatr Med Assoc. 2008;98:107–111. [PubMed] [Google Scholar]
- 45.Hubbard TJ, Carpenter EM, Cordova ML. Contributing factors to medial tibial stress syndrome: a prospective investigation. Med Sci Sports Exerc. 2009;41:490–496. doi: 10.1249/MSS.0b013e31818b98e6. [DOI] [PubMed] [Google Scholar]
- 46.Plisky MS, Rauh MJ, Heiderscheit B, Underwood FB, Tank RT. Medial tibial stress syndrome in high school cross-country runners: incidence and risk factors. J Orthop Sports Phys Ther. 2007;37:40–47. doi: 10.2519/jospt.2007.2343. [DOI] [PubMed] [Google Scholar]
- 47.Sharma J, Golby J, Greeves J, Spears IR. Biomechanical and lifestyle risk factors for medial tibia stress syndrome in army recruits: a prospective study. Gait Posture. 2011;33:361–365. doi: 10.1016/j.gaitpost.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Moen MH, Bongers T, Bakker EW, Zimmermann WO, Weir A, Tol JL, Backx FJ. Risk factors and prognostic indicators for medial tibial stress syndrome. Scand J Med Sci Sports. 2012;22:34–39. doi: 10.1111/j.1600-0838.2010.01144.x. [DOI] [PubMed] [Google Scholar]
- 49.Winters M, Eskes M, Weir A, Moen MH, Backx FJ, Bakker EW. Treatment of medial tibial stress syndrome: a systematic review. Sports Med. 2013;43:1315–1333. doi: 10.1007/s40279-013-0087-0. [DOI] [PubMed] [Google Scholar]
- 50.Loudon JK, Dolphino MR. Use of foot orthoses and calf stretching for individuals with medial tibial stress syndrome. Foot Ankle Spec. 2010;3:15–20. doi: 10.1177/1938640009355659. [DOI] [PubMed] [Google Scholar]
- 51.Moen MH, Rayer S, Schipper M, Schmikli S, Weir A, Tol JL, Backx FJ. Shockwave treatment for medial tibial stress syndrome in athletes; a prospective controlled study. Br J Sports Med. 2012;46:253–257. doi: 10.1136/bjsm.2010.081992. [DOI] [PubMed] [Google Scholar]
- 52.Galbraith RM, Lavallee ME. Medial tibial stress syndrome: conservative treatment options. Curr Rev Musculoskelet Med. 2009;2:127–133. doi: 10.1007/s12178-009-9055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanyon LE, Hampson WG, Goodship AE, Shah JS. Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand. 1975;46:256–268. doi: 10.3109/17453677508989216. [DOI] [PubMed] [Google Scholar]
- 54.Ekenman I, Halvorsen K, Westblad P, Fellander-Tsai L, Rolf C. Local bone deformation at two predominant sites for stress fractures of the tibia: an in vivo study. Foot Ankle Int. 1998;19:479–484. doi: 10.1177/107110079801900711. [DOI] [PubMed] [Google Scholar]
- 55.Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18:405–410. doi: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 56.Milgrom C, Finestone A, Levi Y, Simkin A, Ekenman I, Mendelson S, Millgram M, Nyska M, Benjuya N, Burr D. Do high impact exercises produce higher tibial strains than running? Br J Sports Med. 2000;34:195–199. doi: 10.1136/bjsm.34.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milgrom C, Finestone A, Simkin A, Ekenman I, Mendelson S, Millgram M, Nyska M, Larsson E, Burr D. In-vivo strain measurements to evaluate the strengthening potential of exercises on the tibial bone. J Bone Joint Surg Br. 2000;82:591–594. doi: 10.1302/0301-620x.82b4.9677. [DOI] [PubMed] [Google Scholar]
- 58.Sonoda N, Chosa E, Totoribe K, Tajima N. Biomechanical analysis for stress fractures of the anterior middle third of the tibia in athletes: nonlinear analysis using a three-dimensional finite element method. J Orthop Sci. 2003;8:505–513. doi: 10.1007/s00776-003-0671-5. [DOI] [PubMed] [Google Scholar]
- 59.Edwards WB, Taylor D, Rudolphi TJ, Gillette JC, Derrick TR. Effects of running speed on a probabilistic stress fracture model. Clin Biomech (Bristol, Avon) 2010;25:372–377. doi: 10.1016/j.clinbiomech.2010.01.001. [DOI] [PubMed] [Google Scholar]


