Abstract
It is estimated that 20000 to 30000 new patients are diagnosed with osteonecrosis annually accounting for approximately 10% of the 250000 total hip arthroplasties done annually in the United States. The lack of level 1 evidence in the literature makes it difficult to identify optimal treatment protocols to manage patients with pre-collapse avascular necrosis of the femoral head, and early intervention prior to collapse is critical to successful outcomes in joint preserving procedures. There have been a variety of traumatic and atraumatic factors that have been identified as risk factors for osteonecrosis, but the etiology and pathogenesis still remains unclear. Current osteonecrosis diagnosis is dependent upon plain anteroposterior and frog-leg lateral radiographs of the hip, followed by magnetic resonance imaging (MRI). Generally, the first radiographic changes seen by radiograph will be cystic and sclerotic changes in the femoral head. Although the diagnosis may be made by radiograph, plain radiographs are generally insufficient for early diagnosis, therefore MRI is considered the most accurate benchmark. Treatment options include pharmacologic agents such as bisphosphonates and statins, biophysical treatments, as well as joint-preserving and joint-replacing surgeries. the surgical treatment of osteonecrosis of the femoral head can be divided into two major branches: femoral head sparing procedures (FHSP) and femoral head replacement procedures (FHRP). In general, FHSP are indicated at pre-collapse stages with minimal symptoms whereas FHRP are preferred at post-collapse symptomatic stages. It is difficult to know whether any treatment modality changes the natural history of core decompression since the true natural history of core decompression has not been delineated.
Keywords: Osteonecrosis, Femoral head, Conservative treatment, Core decompression, Stem cells, Total hip arthroplasty
Core tip: This paper walks the reader through the most current evidence regarding the etiology, pathogenesis, treatment options and prognosis of patients presenting with osteonecrosis of the femoral head. We emphasize early diagnosis with magnetic resonance imaging, review surgical and non surgical treatment modalities and provide a personalized management algorithm according to the different stages of the disease.
INTRODUCTION
Osteonecrosis (ON) of the femoral head (ONFH) is the final common pathway of a series of derangements that result in a decrease in blood flow to the femoral head (FH) leading to cellular death, fracture, and collapse of the articular surface[1,2]. It typically affects relatively young, active people between 20 and 40 years and regularly follows an unrelenting course resulting in substantial loss of function. It is estimated that 20000 to 30000 new patients are diagnosed with ON annually accounting for approximately 10% of the 250000 total hip arthroplasties (THA) done annually in the United States[3]. Spontaneous regression of avascular necrosis is rare, with the vast majority of untreated patients progressing to THA and a collapse rate of 67% in asymptomatic patients and 85% of symptomatic hips[4]. Although many authors have suggested treatment based on patient age, symptoms, stage, and/or medical status, the orthopedic community has not yet adopted a uniform treatment algorithm[5-11]. The lack of level 1 evidence in the literature makes it difficult to identify optimal treatment protocols to manage patients with pre-collapse AVN of the FH, and early intervention prior to collapse is critical to successful outcomes in joint preserving procedures.
ETIOLOGY AND PATHOGENESIS
There have been a variety of traumatic and atraumatic factors that have been identified as risk factors for ON, but the etiology and pathogenesis still remains unclear. The estimated frequency of the most frequent risk factors for ONFH in the United States is: alcohol (20%-40%), corticosteroid therapy (35%-40%), and idiopathic (20%-40%)[12].
Most studies have attributed the disease process to the combined effects of genetic predisposition, metabolic factors, and local factors affecting blood supply such as vascular damage, increased intraosseous pressure, and mechanical stress[3,13,14]. This results in bone ischemia and infarction leading to bone death. The precipitating mechanism which leads to this pathway is variable though (Figure 1). Ischemia can result from external or internal vascular insult typically caused by direct trauma, vascular occlusion, direct cellular toxicity, or altered mesenchymal stem cell differentiation[15].
Figure 1.
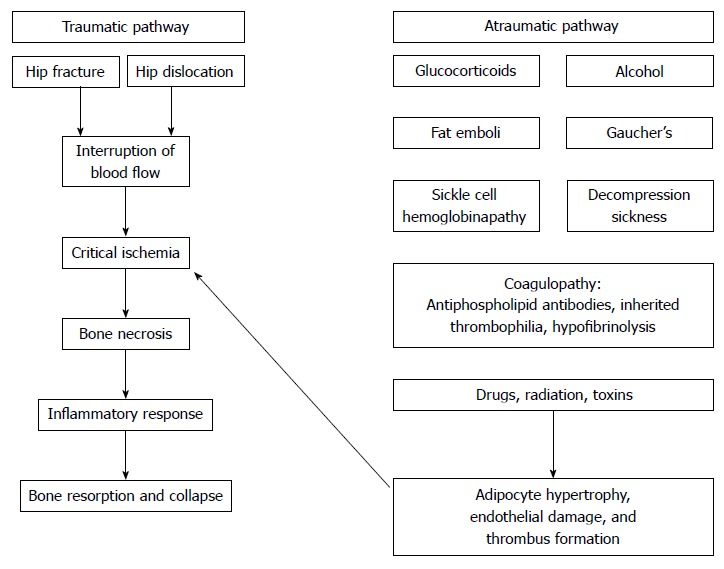
Mechanisms of osteonecrosis.
Several mechanisms leading to vascular occlusion have been proposed as possible underlying causes of necrosis. High doses of glucocorticoids prevalent in systemic diseases such as systemic lupus erythematosus as well as excessive alcohol intake have been associated with alterations in circulating lipids with resultant microemboli in the arteries supplying the bone[16]. In addition increased risk of fat emboli has also been attributed to the increase in bone marrow fat cell size which blocks venous flow. Therefore, fat emboli, adipocyte hypertrophy, and venous stasis have all been implicated as etiologic factors in this disease process. Vascular occlusion can also result from disease processes that increase intravascular coagulation and thrombus formation. Antiphospholipid antibodies, inherited thrombophilia, and hypofibrinolysis have all been associated with altered mechanisms in both the coagulation and fibrinolytic pathways. Occlusion can also occur as a result of red blood cell sickling and bone marrow hyperplasia as seen in sickle cell hemoglobinopathies or may be due to an accumulation of cerebroside-filled cells within the bone marrow as seen in Gaucher’s disease[17]. Decompression sickness associated with increased pressure can lead to nitrogen bubble formation that can also cause arteriolar occlusion and necrosis. This has also been shown to result in elevated plasma levels of plasminogen activator inhibitors leading to increased coagulation[18]. Trauma due to fracture or dislocation can lead to damage to the extraosseous blood supply. This is especially specific to fractures in the subcapital region of the femoral neck. Trauma at this location interrupts the anastomosis between the lateral epiphyseal vessels, which are branches from the medial femoral circumflex artery supplying, and the artery of the ligamentum teres leading to compromised blood flow to the FH. Lastly, direct cellular insult may result from irradiation, chemotherapy, or oxidative stress and may lead to a reduction in osteogenic differentiation and physiologic diversion of mesenchymal stem cells toward the adipocytic lineage[15].
DIAGNOSIS AND ASSESSMENT
Early diagnosis is crucial for optimal treatment of ON, as treatment success is related to the stage at which the care is initiated[13]. Current diagnostic modalities available include radiography, scintigraphy, functional evaluation of bone, magnetic resonance imaging (MRI), computer-assisted tomography, and histological studies.
Clinical presentation of ON typically is asymptomatic in early stages, although patients may develop groin pain that can radiate to the knee or ipsilateral buttock. On physical examination, patients usually present with a limited range of motion at the hip and complain of pain particularly with forced internal rotation. A detailed history can identify any associated risk factors (Table 1)[13]. ON must be suspected with presentation of pain in the hips, negative plain radiographs, and any of these risk factors, since plain radiographs may present as normal in the early stages of necrosis. Patients who have had a history of necrosis must be watched for bilateral ON, as bilaterality has been reported in up to 70%[19].
Table 1.
Risk factors for osteonecrosis of the femoral head
| Direct | Indirect |
| Femoral head/neck fracture | Chronic corticosteroid use |
| Hip dislocation | Excessive alcohol consumption |
| Slipped capital femora epiphysis | Coagulation disorders |
| Radiation | Hemoglobinopathies |
| Sickle cell disease | Dysbaric phenomena |
| Caisson disease | Autoinmune diseases |
| Myeloproliferative disorders | Smoking |
| Hyperlipidemia |
The two most common classifications used in the diagnosis of ON include the Ficat and Arlet and the Steinberg University of Pennsylvania systems (Tables 2 and 3)[20]. Ficat classification consists of four stages, based on standard radiographs. Stage I indicates normal imaging. Stage II indicates normal FH contour, but with evidence of bone-remodeling, such as cystic or osteosclerotic regions. Stage III indicates evidence of subchondral collapse, or flattening of the FH. Stage IV indicates a narrowing of the joint space with secondary degenerative changes in the acetabulum, such as cysts, osteophytes, and cartilage destruction. Hungerford[13] described the stage 0, silent hip (preclinical and preradiologic), in which AVN can be suggested if it has been already diagnosed in the contralateral femoral head. In this case bone marrow pressure and histology studies would be abnormal. Although the Ficat classification system has been well established, it is dependent on radiographic imaging and does not allow for quantitation of lesion size, making it impossible to measure disease progression[21]. Steinberg expands the Ficat system into six stages and includes quantification of involvement of the FH within each stage. They defined mild (less than 15% radiographic involvement of the head’s articular surface), moderate (15%-30% involvement of the head’s articular surface), and severe (greater than 30% involvement of the head’s articular surface) stages. In addition, the Association Research Circulation Osseous (ARCO) suggested a new classification system based on the combination of radiographic, MRI, bone scan and histologic findings. However, apparently these two classifications systems, Ficat and ARCO are still not enough reliable to assess the status of ONFH alone[22].
Table 2.
Ficat and arlet classification system
| Stage | Features |
| 0 | Normal radiographs (silent hip) |
| I | Slight abnormality as patchy/opaque areas, minor osteopenia |
| II | Sclerotic or cystic lesions |
| IIa: No crescent sign | |
| IIb: Crescent sign without flattening of the femoral head | |
| III | Flattening of the femoral head or femoral head collapse |
| IV | Femoral head collapse and osteoarthritis of the hip (joint space narrowing, osteophytes and acetabular changes) |
Table 3.
Steinberg staging system
| Stage | Features |
| 0 | Normal radiograph, bone scan and magnetic resonance imaging |
| I | Normal radiograph, abnormal bone scan and or magnetic resonance imaging |
| IA Mild (involves < 15% of femoral head) | |
| IB Moderate (involves 15% to 30% of femoral head) | |
| IC Severe (involves > 30% of femoral head) | |
| II | Cystic and sclerotic changes in the femoral head |
| IIA Mild (involves < 15% of femoral head) | |
| IIB Moderate (involves 15% to 30% of femoral head) | |
| IIC Severe (involves > 30% of femoral head) | |
| III | Subchondral collapse (crescent sign) without flattening of the femoral head |
| IIIA Mild (involves < 15% of femoral head) | |
| IIIB Moderate (involves 15% to 30% of femoral head) | |
| IIIC Severe (involves > 30% of femoral head) | |
| IV | Flattening of the femoral head/femoral head collapse |
| IVA Mild (involves < 15% of femoral head) | |
| IVB Moderate (involves 15% to 30% of femoral head) | |
| IVC Severe (involves > 30% of femoral head) | |
| V | Joint space narrowing and/or acetabular changes |
| VA Mild | |
| VB Moderate | |
| VC Severe | |
| VI | Advance degenerative joint disease |
Several studies have shown that the size of the necrotic segment in the FH is a fundamental parameter to determine the prognosis and treatment of this condition. Different methods are currently used to measure the size of the lesion. These include, the tradicional angular measurements methods described by Kerboul and Koo and Kim and the quantitative volumetric measurement performed by quantitative digital analysis[23,24]. A recent study[25] comparing the efficacy of these systems showed more accurate and reliable measurements using the volumetric measurement method[25]. However, simpler measurement systems, though less accurate, are more commonly utilized since volumetric measurements are technically too demanding for general use. In spite of that, the size of the necrotic region must be determined as part of a comprehensive evaluation of this condition.
Current ON diagnosis is dependent upon plain AP and frog-leg lateral radiographs of the hip, followed by MRI. The AP radiographs will usually demonstrate the primary area of involvement once changes can be viewed. Generally, the first radiographic changes seen by radiograph will be cystic and sclerotic changes in the FH. Subtle osteosclerotic or cystic changes in the subchondral regions may be missed because the anterior and posterior acetabular margins overlap the superior FH, therefore lateral frog-leg radiographs of the FH are necessary. Early delamination of the cartilage from the underlying bone will most likely be demonstrated by the crescent sign (Figures 2 and 3)[15]. Flattening of the FH can also be viewed by radiograph, but may only be visible in one view[15].
Figure 2.
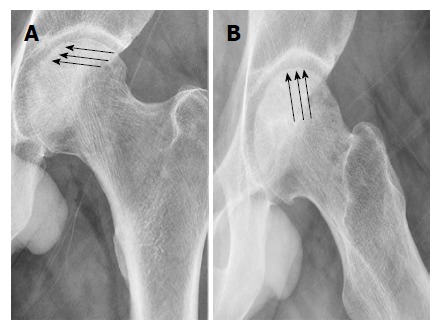
Left hip anteriorposterior and cross leg lateral X-rays showing (arrows) the crescent sing.
Figure 3.
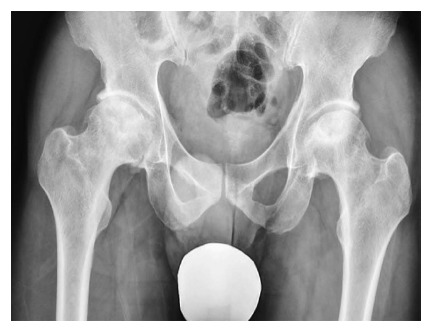
Bilateral osteonecrosis of the femoral head with flattening of the surface and early sings of osteoarthritis.
Although the diagnosis may be made by radiograph, plain radiographs are generally insufficient for early diagnosis; therefore MRI is considered the most accurate benchmark[5]. A single-density line on T1-weighted images and a high signal intensity line on T2-weighted images represent the early necrotic-viable bone interface and the hypervascular granulation tissue characterizing ON (Figure 4)[13]. However, recently subchondral insufficiency fractures of the FH have been proposed as a new concept regarding FH collapse with a reported incidence of 5%-10% of patients who underwent a hip replacement with a diagnosis of ONFH[26,27]. These entities must be differentiated since AVN represents an irreversible condition, which might lead to permanent joint failure and SIF may either completely resolve or progress toward epiphyseal collapse[28-30]. The characteristic finding of SIF on MRI is a low intensity band on T1 in association with bone marrow edema, however, this finding has also been described in ONFH. A recent study[30] demonstrated that the shape of the low intensity band (on T1-weighted MRI) is helpful for differentiation between the two diagnoses. The low intensity band seen in SIF is generally irregular, serpiginous, discontinuous, and convex to the articular surface, while the band in ONFH tends to be smooth, concave and well circumscribed. However, the shape of the low intensity band is not always diagnostic and further imaging may be required (MRI with gadolinium). Ultimately, both clinical and MRI characteristics need to be evaluated for the critical differentiation of both conditions (Table 4).
Figure 4.
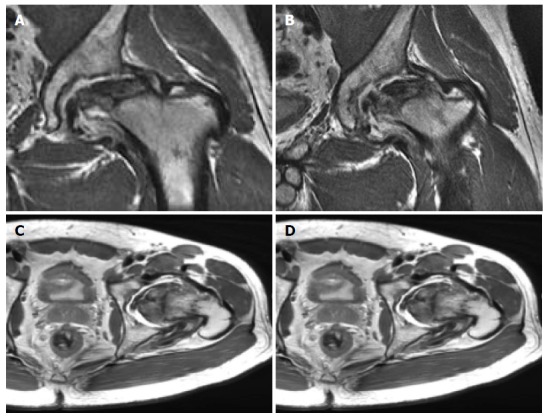
Magnetic resonance imaging of the left hip showing extensive avascular necrosis of the femoral head with collapse and a large area of devitalized bone demonstrating fibrocystic change. There is associated severe arthrosis of the left hip joint with a moderate effusion, synovitis and debris and a marked bone marrow edema pattern on both sides of the joint.
Table 4.
Clinical and imaging differences between osteonecrosis femoral head and subchondral insufficiency fracture
| SIF | ONFH | |
| Age/sex | Elderly/female | 30 s to 40 s |
| Etiology | Osteoporosis/obese | Steroid/alcohol |
| Bilateral | Rare | 50%-70% |
| Shape of the band | Iregular, disconnected | Smooth |
| High signal of the proximal | Yes | No |
| Segment on gadolimium MRI |
From Yamamoto T. In: Yamamoto T. Subchondral Insufficiency Fractures of the Femoral Head. Clinics in Orthropedic Surgery 2012: 4: 3. SIF: Subchondral insufficiency fracture; ONFH: Osteonecrosis femoral head; MRI: Magnetic resonance imaging.
Other functional tools for evaluating ON include measuring bone-marrow pressure, venography, and core biopsy. While these tests are specific and sensitive, they are invasive and only used when MRI, and radiograph reveal negative findings in a patient where ON is highly likely. Although CT scans can aid in distinguishing between late stages of ON before collapse of the FH, this modality is rarely used due to its high doses of radiation. Characteristic features that define the diagnosis of ON include: collapse of the FH, anterolateral sequestrum, or the crescent sign, or when a double-line sign is demonstrated through MRI on T2-weighted images, or there is a positive histologic finding upon bone biopsy.
Non-surgical management
The aim of treatment of AVN of the hip is to prevent collapse of the FH and may vary depending on the underlying etiology and stage of progression. Treatment options include pharmacologic agents, biophysical treatments, as well as joint-preserving and joint-replacing surgeries. Medical management of AVN has been increasingly used in early stages in attempt to delay the progression of the disease.
Pharmacological management of AVN includes lipid lowering agents, anticoagulants, vasoactive substances, and bisphosphonates. Increases in both the number and size of circulating fat cells have been associated with the development of ON of the hip, therefore lipid lowering agents, such as statins, which reduce the rate of adipogenesis, are beneficial. Statins have been shown to provide protective effects for patients receiving steroids. It is still unclear whether statins have the ability to reverse steroid-induced ON once it has already occurred[31,32]. Anticoagulants such as enoxaparin act through the inhibition of platelets aggregation thereby increasing blood flow to ischemic areas of the bone. These agents are primarily beneficial in patients with underlying coagulopathy disorders, such as thrombophilia or hypofrinolysis[9,33]. Prostacyclin is a vasoactive agent that improves blood flow through its vasodilator effects in the terminal vessels. Although prostacyclin has shown significant improvement in both clinical and radiologic outcomes in early stages of AVN, long term benefits have yet to be established[34].
Bisphosphonates significantly reduce the incidence of collapse of the FH in osteonecrotic hips by reducing osteoclast activity. Alendronate has been shown to prevent early collapse of the FH in Steinberg stages II and III non-traumatic ON at 24-28 mo follow up and has been reported to diminish the amount of pain at one year follow up when it is compared with placebo treatment[35,36]. Alendronate has been used as an adjunctive therapy with surgical procedures and has been found to reduce pain and the risk of collapse in early stages of ONFH[37]. Evidence for prevention of THR and reduction of AVN progression still remains controversial[38].
Biophysical treatments include extracorporeal shockwave therapy (ESWT), pulse electromagnetic therapy, and hyperbaric oxygen (HBO) therapy. ESWT has been shown to restore tissue oxygenation, reduce edema, and induce angiogenesis and may offer an alternative to the invasive modalities for FH necrosis in the earlier stages[39,40]. ESWT has also been associated with improvement in both pain and function, and has been found to result in a reduction of lesion size and bone marrow edema at 1-year follow up. Long term (8-9 years) improvement in pain and Harris Hip scores has also been demonstrated in the ESWT group treatment when compared with the core decompression group treatment[41]. Although not as commonly used, pulse electromagnetic therapy is believed to function by stimulating osteogenesis and angiogenesis however its role as early stage ON treatment has not yet been established[42]. HBO increases extracellular oxygen concentration and reduces cellular ischemia and edema by inducing vasoconstriction[43]. Studies have reported radiographic improvement in Steinberg stage I-AVN, as well as pain and ROM improvement in Ficat stage-II ON[39,44].
Conservative treatment of AVN may be effective in the earlier stages of the disease. Although medical management may improve pain and functional outcomes, randomized clinical trials are necessary with long term follow up to determine effectiveness of therapy.
Surgical treatment
Currently there is no consensus regarding the treatment of the different stages of ONFH in the adult population[7,10,45,46]. A recent survey of 753 members of the American Association of Hip and Knee Surgeons reported that total hip replacement was the most common intervention for treatment of post-collapse stages of ONFH, whereas core decompression was the most common procedure for symptomatic pre-collapse stages of ONFH. Other less frequently performed treatments include conservative management, vascularized and non-vascularized bone grafts, hemi-arthroplasty, osteotomy, and arthrodesis[47]. ONFH tends to affect younger patients, therefore a variety of joint preserving surgical procedures have been developed to delay the progression of the disease and afford pain relief[5,21,48,49].
The surgical treatment of ONFH can be divided into two major branches: FH sparing procedures (FHSP) and FH replacement procedures (FHRP). In general, FHSP are indicated at pre-collapse stages with minimal symptoms whereas FHRP are preferred at post-collapse symptomatic stages.
FHSP: FHSP aim to preserve the FH and include core decompression (CD), CD combined with different grafting procedures and/or biologic agents and rotational osteotomies. Since all these procedures cannot restore the sphericity of the FH their role in the management of post-collapse stages is very limited[50,51].
CD: CD of the FH is the most common procedure currently performed to treat early stages of ONFH with the goal of decompressing the FH pressure in order to restore normal vascular flow and ultimately relieve pain[5,52,53]. The technique of CD has varied in terms of surgical approaches, number of drillings, and trephine diameter. Small diameter drilling has been proposed as an alternative because it has the advantage of reaching the anterior portion of the FH (most frequently involved region in ONFH) (Figure 5). In addition, small diameter drilling has been associated with minimal morbidity, less risk of weakening the FH and the articular cartilage, and less risk of stress risers that ultimately can lead to a subtrochanteric fracture[54]. Although CD has been shown to delay the progression of ON, its role in complete reconstruction of the necrotic area has not yet been established[55].
Figure 5.
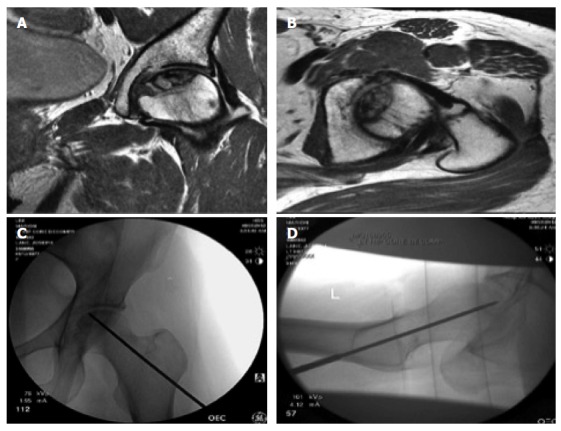
Core decompression of the left femoral head. Preoperative magnetic resonance imaging, above (coronal and axial views) and fluoroscopic imaging during the procedure below.
Bone grafting procedures: Non-vascularized bone grafts from different sources (allograft, autograft or artificial) have been used to fill the necrotic area in the FH. The grafting can be performed through the core decompression tract, which is the most common technique, but also through a window in the FH or in the femoral neck[56]. This latter technique, also referred to as the trapdoor procedure, requires a surgical dislocation of the hip in order to graft the defect through a cartilage window in the FH.
Vascularized bone grafting combines the benefit of core decompression along with an osteoinductive and osteoconductive graft in the devitalized FH. This procedure was popularized in the 1970’s coincidently with the emergence of microsurgical techniques[51]. The variability among the surgical techniques to perform this procedure has however confounded the uniformity of the published data.
The free vascularized fibular grafting (FVFG) has been shown to support the subchondral architecture as well as restore local circulation to the necrotic FH in treatment of ONFH. A study on 470 patients with a mean follow-up of 5.0 years showed an average Harris hip score improvement from 65.0 to 86.9, no radiographic changes in 57.3% of patients, improvement in 33.7% of patients, and necrosis progression in 9.0% of patients respectively. These results show that the modified technique of the use of FVFG for treatment of ONFH yields similar postoperative results in comparison to the traditional method. Although vascularized fibular grafting has shown promising results, especially in young patients with ONFH, the extensive surgical time, donor-site morbidity, prolonged rehabilitation, and an increased risk of a proximal femoral fracture has limited its use in practice[57-59].
Tantalum implants: Porous tantalum implants in combination with core decompression offers the advantage of providing structural support without the risk of autograft harvest or the infectious complications of bone allograft[60-62]. Veillete et al[62] reported an overall survival rate of 91.8% at twenty-four months, and 68.1% at forty-eight months after evaluating fifty-four patients with ONFH treated with core decompression and the insertion of a porous tantalum rod. Although these results appear promising, there are concerns about the origination of metal debris in the joint if a THR becomes necessary as well as a more complicated surgical technique. In addition, previous histologic studies demonstrated little bone ingrowth and insufficient mechanical support of the subchondral bone at the time of conversion from a tantalum rod to THR[63]. Long-term follow up is necessary in order to assess the functional and clinical outcomes of this technique.
Biological agents: There is considerable enthusiasm in the development of biological therapies that can enhance core decompression with osteogenic (mesenchymal stem cells) and/or osteoinductive agents (bone morphogenic protein) that have the potential to produce better results for larger lesions.
It has been hypothesized that there is an insufficient supply of progenitor cells in patients with AVN, which are required to enhance remodeling in areas of ON[64]. For this reason, newer treatment modalities have been developed to introduce stem cells to the areas of necrosis in order to prevent fracture and collapse of the FH. Since 2002, when Hernigou et al[65] first described a technique for injecting mesenchymal stem cells into an area of necrosis, four studies have prospectively evaluated the use of stem cells and core decompression. These studies presented consistent findings showing that patients treated with core decompression and stem cells achieved a significantly higher Harris Hip Score at final follow up. Gangji et al[66] reported in 2004 the results of a controlled, double blind study comparing core decompression with and without bone marrow aspirate. After 24 mo follow up the survival analysis revealed a significant difference in the time to collapse between both groups and a decreased of 35% of the necrotic lesion in the bone marrow graft group.
The instillation of stem cells into the osteonecrotic region of the FH can be performed through various methods. These include the direct instillation through the core tract, a selective femoral arterial perfusion, or the catheterization of the medial, lateral, or obturator artery. The direct instillation through the core tract is the most commonly performed procedure, however the catheterization of these vessels makes it difficult thereby requiring higher technical skills. However, it is important to maintain the final concentrate of cells when doing a direct instillation in order to effectively regenerate the osteonecrotic region (optimum effective dosage minimum necessary concentration 5 × 107 and CD 34 + 5 × 107 cells)[45,67]. This is another factor that has been shown to influence healing of necrotic areas in the FH[64,65]. Additionally, the relationship between the injected volume and the lesion volume needs to be studied. Although these previous studies confirm that bone marrow aspirate concentrate has the potential to induce bone repair in ONFH, the data is preliminary and many questions still need to be addressed[45,64-67].
Osteotomies: Two general types of osteotomies, angular intertrochanteric and rotational transtrochanteric, can be performed to remove the segment of necrotic bone away from the weight-bearing region in the hip[68-72].
The transtrochanteric rotational osteotomy (TRO) for treatment of ONFH was introduced by Sugioka[71] in 1972. The aim of this procedure is to rotate the necrotic region of the FH out of the weight bearing area of the acetabulum. Sugiota[71] reported promising clinical results with a success rate of 78% after 3-16 years. However, their results with this technically demanding procedure have not been reproduced[68-70]. Rotational osteotomies can provide a painless, mobile, and stable hip if there is an unloading of the necrotic area of the FH when it is rotated from the acetabular major bearing surface and if the depth of the necrosis is not bigger than one third of the head diameter[71]. Hisatome et al[72] reviewed 25 hips in 21 patients six years after Sugioka’s transtrochanteric anterior rotational osteotomy for ONFH. They concluded that although the collapse of a new weight-bearing region can be prevented, the progressive collapse of the transposed necrotic area induces anterior joint instability and subsequent arthritic changes.
Despite the promising results, patients who ultimately require conversion to THA after a proximal femoral osteotomy have a 17% intraoperative complication rate and an 82% survival rate of the implant after 10 years. Osteotomies are a reasonable option when they are performed by experienced surgeons in patients younger than 45 years with a Kerboull angle below 200° and no longer taking steroids.
FHRP
Although FHSP may provide good clinical results in patients with small pre-collapse lesions, these interventions are less predictable in patients with larger lesions or in FH collapse. These patients are therefore better candidates for FHRP.
Hemi-resurfacing arthroplasty and hemipolar/bipolar hip replacement: Hemi-resurfacing arthroplasty is a significant treatment option when the joint surface is still preserved and the articular cartilage is minimally damaged. Possible indications include a Ficat III, early stage Ficat IV, or early failure of a free vascularized fibula graft. With good patient selection and surgical technique this procedure can restore patient function although pain relief may not be as predictable as after THR[73]. Hemi-resurfacing arthroplasty causes little distortion of the anatomy, preserves bone, and produces minimal particle debris. Accurate evaluation of the acetabular articular cartilage and its longevity with this component poses a difficult challenge.
Hemi-arthroplasty replacements are an alternative treatment strategy as they preserve the acetabular bone stock. The major concerns with this procedure are the incidence of protrusion and polyethylene wear that can lead to particle-induced osteolysis and femoral stem loosening[74,75]. Nevertheless, either hemi-resurfacing arthroplasty or proximal femoral osteotomies are preferred to hemi-arthroplasty.
THA: Arthroplasty is typically reserved for patients with late-stage ONFH, as well as older patients and those with more advanced arthritis (Figures 6 and 7)[47]. Arthroplasty is the only treatment that has been proven to reduce pain and restore mobility. In the United States, it is estimated that approximately 10% of all THRs are done in symptomatic hip ON[6,49].
Figure 6.
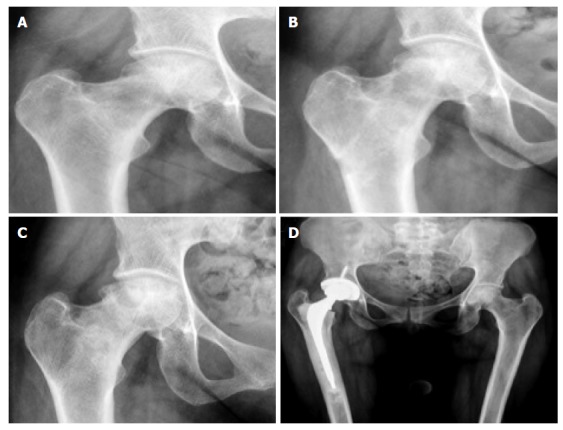
Right femoral head osteonecrosis. Flattening of femoral head progression in 24 mo ending up in a right total hip replacement.
Figure 7.
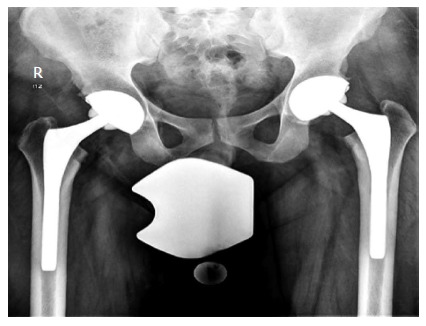
Bilateral total hip replacement in a patient with bilateral hip osteonecrosis of the femoral head.
There have been several studies which have shown poor results of THR for ONFH with failure rates between 37% and 53%, but more recent long term follow up studies have reported improved results compared with earlier reports[76-78]. The advances in the past two decades with the advent of surface bearings with low wear rates present promising results when used in patients with an advance stage of necrosis at mid-term follow up[79-81].
Kim et al[82] recently reported a 98% stem survivorship and an 85% cementless cup survivorship at 17.3 years of mean follow up. The most common reason for revision was due to cup wear or loosening. Although longer-term follow up studies are needed, promising stem and cup survivorship seems to be feasible.
Overall patients with ONFH present similar failure rates after THA than the general population. However a few ON risk factors, as renal failure and/or transplant and sickle cell disease, have been associated with worse outcomes[81]. Fortunately these risk factors are present in a small population of patients with ONFH and even in this high-risk group population the outcomes of THR have improved over time[83-85]. Many studies have also shown that the outcomes of primary THR are not affected by previous hip joint preserving procedures[86-91]. However, THAs performed after rotational or angular osteotomies have shown higher complication rates when compared to those who did not have a previous osteotomy because of the disturbed anatomy of the proximal femur after the TRO[86,92-94].
CONCLUSION
Clinical and MRI characteristics need to be evaluated for the critical diagnosis of ONFH (Table 4). The progression of ONFH has not been well established, therefore it is difficult to evaluate whether a specific treatment modality changes the natural course of the disease. Medical management and surgical intervention has demonstrated to provide symptomatic relief, and early intervention prior to collapse has been shown to be critical to successful outcomes in joint preserving procedures. Future research should be directed at delineating whether one treatment strategy can delay the progression of ONFH of the hip thereby preventing collapse and the need for THA. A proposed algorithm for the diagnosis and management of ONFH is given in Figure 8.
Figure 8.
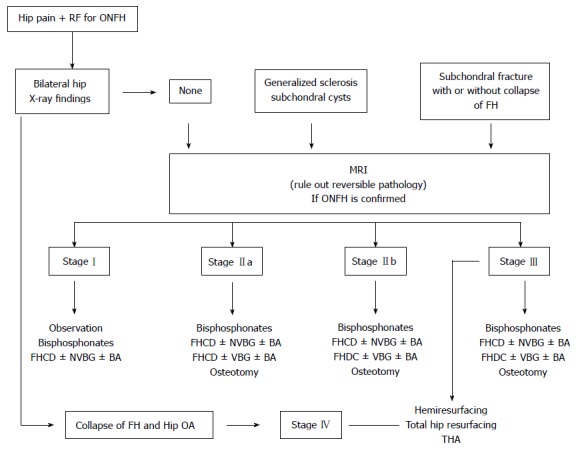
Algorithm for the management and treatment of patients with osteonecrosis of the femoral head. RF: Risk factors; ONFH: Osteonecrosis of the femoral head; FH: Femoral head; MRI: Magnetic resonance imaging; FHCD: Femoral head core decompression; NVBG: Non vascularized bone graft; BA: Biologic agents; VBG: Vascularized bone graft; OA: Osteoarthritis; THA: Total hip arthroplasty.
Footnotes
Conflict-of-interest statement: The authors of this manuscript report no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 9, 2015
First decision: June 3, 2015
Article in press: July 23, 2015
P- Reviewer: Cheung WH, Guo ZK, La Montagna G S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Herndon JH, Aufranc OE. Avascular necrosis of the femoral head in the adult. A review of its incidence in a variety of conditions. Clin Orthop Relat Res. 1972;86:43–62. doi: 10.1097/00003086-197207000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Mwale F, Wang H, Johnson AJ, Mont MA, Antoniou J. Abnormal vascular endothelial growth factor expression in mesenchymal stem cells from both osteonecrotic and osteoarthritic hips. Bull NYU Hosp Jt Dis. 2011;69 Suppl 1:S56–S61. [PubMed] [Google Scholar]
- 3.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 4.Musso ES, Mitchell SN, Schink-Ascani M, Bassett CA. Results of conservative management of osteonecrosis of the femoral head. A retrospective review. Clin Orthop Relat Res. 1986;(207):209–215. [PubMed] [Google Scholar]
- 5.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, Urbaniak JR. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–355. [PubMed] [Google Scholar]
- 6.Mont MA, Jones LC, Sotereanos DG, Amstutz HC, Hungerford DS. Understanding and treating osteonecrosis of the femoral head. Instr Course Lect. 2000;49:169–185. [PubMed] [Google Scholar]
- 7.Cheng EY, Thongtrangan I, Laorr A, Saleh KJ. Spontaneous resolution of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004;86-A:2594–2599. doi: 10.2106/00004623-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hungerford DS, Jones LC. Asymptomatic osteonecrosis: should it be treated? Clin Orthop Relat Res. 2004;(429):124–130. [PubMed] [Google Scholar]
- 9.Glueck CJ, Freiberg RA, Sieve L, Wang P. Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res. 2005;(435):164–170. doi: 10.1097/01.blo.0000157539.67567.03. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;(386):71–78. doi: 10.1097/00003086-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Jergesen HE, Khan AS. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J Bone Joint Surg Am. 1997;79:359–363. doi: 10.2106/00004623-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Bradway JK, Morrey BF. The natural history of the silent hip in bilateral atraumatic osteonecrosis. J Arthroplasty. 1993;8:383–387. doi: 10.1016/s0883-5403(06)80036-7. [DOI] [PubMed] [Google Scholar]
- 13.Hungerford DS. Bone marrow pressure, venography and core decompression in ischemic necrosis of the femoral head. In: Riley LH, editor. The Hip: Proceedings of the Seventh Open Scientific Meeting of The Hip Society. St Louis, MO: CV Mosby;; 1979. pp. 218–237. [Google Scholar]
- 14.Chang CC, Greenspan A, Gershwin ME. Osteonecrosis: current perspectives on pathogenesis and treatment. Semin Arthritis Rheum. 1993;23:47–69. doi: 10.1016/s0049-0172(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 15.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22:455–464. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 16.Jones JP. Fat embolism and osteonecrosis. Orthop Clin North Am. 1985;16:595–633. [PubMed] [Google Scholar]
- 17.Goldblatt J, Sacks S, Beighton P. The orthopedic aspects of Gaucher disease. Clin Orthop Relat Res. 1978;(137):208–214. [PubMed] [Google Scholar]
- 18.Miyanishi K, Kamo Y, Ihara H, Naka T, Hirakawa M, Sugioka Y. Risk factors for dysbaric osteonecrosis. Rheumatology (Oxford) 2006;45:855–858. doi: 10.1093/rheumatology/kel013. [DOI] [PubMed] [Google Scholar]
- 19.Boettcher WG, Bonfiglio M, Hamilton HH, Sheets RF, Smith K. Non-traumatic necrosis of the femoral head. I. Relation of altered hemostasis to etiology. J Bone Joint Surg Am. 1970;52:312–321. [PubMed] [Google Scholar]
- 20.Jawad MU, Haleem AA, Scully SP. In brief: Ficat classification: avascular necrosis of the femoral head. Clin Orthop Relat Res. 2012;470:2636–2639. doi: 10.1007/s11999-012-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, Steinberg ME. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt-Sody M, Kirchhoff C, Mayer W, Goebel M, Jansson V. Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications. Int Orthop. 2008;32:283–287. doi: 10.1007/s00264-007-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerboul M, Thomine J, Postel M, Merle d’Aubigné R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291–296. [PubMed] [Google Scholar]
- 24.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br. 1995;77:875–880. [PubMed] [Google Scholar]
- 25.Steinberg DR, Steinberg ME, Garino JP, Dalinka M, Udupa JK. Determining lesion size in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:27–34. doi: 10.2106/JBJS.F.00896. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Iwamoto Y, Schneider R, Bullough PG. Histopathological prevalence of subchondral insufficiency fracture of the femoral head. Ann Rheum Dis. 2008;67:150–153. doi: 10.1136/ard.2006.066878. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Bullough PG. Subchondral insufficiency fracture of the femoral head: a differential diagnosis in acute onset of coxarthrosis in the elderly. Arthritis Rheum. 1999;42:2719–2723. doi: 10.1002/1529-0131(199912)42:12<2719::AID-ANR31>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 29.Vande Berg BC, Malghem JJ, Lecouvet FE, Jamart J, Maldague BE. Idiopathic bone marrow edema lesions of the femoral head: predictive value of MR imaging findings. Radiology. 1999;212:527–535. doi: 10.1148/radiology.212.2.r99au03527. [DOI] [PubMed] [Google Scholar]
- 30.Ikemura S, Yamamoto T, Motomura G, Nakashima Y, Mawatari T, Iwamoto Y. MRI evaluation of collapsed femoral heads in patients 60 years old or older: Differentiation of subchondral insufficiency fracture from osteonecrosis of the femoral head. AJR Am J Roentgenol. 2010;195:W63–W68. doi: 10.2214/AJR.09.3271. [DOI] [PubMed] [Google Scholar]
- 31.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;(386):173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;(370):295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AJ, Mont MA, Tsao AK, Jones LC. Treatment of femoral head osteonecrosis in the United States: 16-year analysis of the Nationwide Inpatient Sample. Clin Orthop Relat Res. 2014;472:617–623. doi: 10.1007/s11999-013-3220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jäger M, Tillmann FP, Thornhill TS, Mahmoudi M, Blondin D, Hetzel GR, Zilkens C, Krauspe R. Rationale for prostaglandin I2 in bone marrow oedema--from theory to application. Arthritis Res Ther. 2008;10:R120. doi: 10.1186/ar2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 36.Nishii T, Sugano N, Miki H, Hashimoto J, Yoshikawa H. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin Orthop Relat Res. 2006;443:273–279. doi: 10.1097/01.blo.0000194078.32776.31. [DOI] [PubMed] [Google Scholar]
- 37.Kang P, Pei F, Shen B, Zhou Z, Yang J. Are the results of multiple drilling and alendronate for osteonecrosis of the femoral head better than those of multiple drilling? A pilot study. Joint Bone Spine. 2012;79:67–72. doi: 10.1016/j.jbspin.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Chen CH, Chang JK, Lai KA, Hou SM, Chang CH, Wang GJ. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:1572–1578. doi: 10.1002/art.33498. [DOI] [PubMed] [Google Scholar]
- 39.Reis ND, Schwartz O, Militianu D, Ramon Y, Levin D, Norman D, Melamed Y, Shupak A, Goldsher D, Zinman C. Hyperbaric oxygen therapy as a treatment for stage-I avascular necrosis of the femoral head. J Bone Joint Surg Br. 2003;85:371–375. doi: 10.1302/0301-620x.85b3.13237. [DOI] [PubMed] [Google Scholar]
- 40.Heller KD, Niethard FU. [Using extracorporeal shockwave therapy in orthopedics--a meta-analysis] Z Orthop Ihre Grenzgeb. 1998;136:390–401. doi: 10.1055/s-2008-1053674. [DOI] [PubMed] [Google Scholar]
- 41.Alves EM, Angrisani AT, Santiago MB. The use of extracorporeal shock waves in the treatment of osteonecrosis of the femoral head: a systematic review. Clin Rheumatol. 2009;28:1247–1251. doi: 10.1007/s10067-009-1231-y. [DOI] [PubMed] [Google Scholar]
- 42.Massari L, Fini M, Cadossi R, Setti S, Traina GC. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:56–60. doi: 10.2106/JBJS.F.00536. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee S, Issa K, Pivec R, Kapadia BH, Khanuja HS, Mont MA. Osteonecrosis of the hip: treatment options and outcomes. Orthop Clin North Am. 2013;44:463–476. doi: 10.1016/j.ocl.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Camporesi EM, Vezzani G, Bosco G, Mangar D, Bernasek TL. Hyperbaric oxygen therapy in femoral head necrosis. J Arthroplasty. 2010;25:118–123. doi: 10.1016/j.arth.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Hernigou P, Manicom O, Poignard A. Core decompression with marrow stem cells. Oper Tech Orthop. 2004;14:68. [Google Scholar]
- 46.Castro FP, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop (Belle Mead NJ) 2000;29:187–194. [PubMed] [Google Scholar]
- 47.McGrory BJ, York SC, Iorio R, Macaulay W, Pelker RR, Parsley BS, Teeny SM. Current practices of AAHKS members in the treatment of adult osteonecrosis of the femoral head. J Bone Joint Surg Am. 2007;89:1194–1204. doi: 10.2106/JBJS.F.00302. [DOI] [PubMed] [Google Scholar]
- 48.Aldridge JM, Urbaniak JR. Avascular necrosis of the femoral head: etiology, pathophysiology, classification, and current treatment guidelines. Am J Orthop (Belle Mead NJ) 2004;33:327–332. [PubMed] [Google Scholar]
- 49.Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250–261. doi: 10.5435/00124635-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am. 1995;77:674–680. doi: 10.2106/00004623-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long-term follow-up study of one hundred and three hips. J Bone Joint Surg Am. 1995;77:681–694. doi: 10.2106/00004623-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Ficat PAJ. Functional Investigation of Bone Under Normal Conditions. In: Ficat P, Arlet J, Hungerford DS, editors. Ischemia and Necroses of Bone. Baltimore: Williams and Wilkins; 1961. pp. 29–52. [Google Scholar]
- 53.Koo KH, Kim R, Ko GH, Song HR, Jeong ST, Cho SH. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br. 1995;77:870–874. [PubMed] [Google Scholar]
- 54.Al Omran A. Multiple drilling compared with standard core decompression for avascular necrosis of the femoral head in sickle cell disease patients. Arch Orthop Trauma Surg. 2013;133:609–613. doi: 10.1007/s00402-013-1714-9. [DOI] [PubMed] [Google Scholar]
- 55.Soohoo NF, Vyas S, Manunga J, Sharifi H, Kominski G, Lieberman JR. Cost-effectiveness analysis of core decompression. J Arthroplasty. 2006;21:670–681. doi: 10.1016/j.arth.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Seyler TM, Marker DR, Ulrich SD, Fatscher T, Mont MA. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res. 2008;466:1125–1132. doi: 10.1007/s11999-008-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vail TP, Urbaniak JR. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996;78:204–211. doi: 10.2106/00004623-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Tang CL, Mahoney JL, McKee MD, Richards RR, Waddell JP, Louie B. Donor site morbidity following vascularized fibular grafting. Microsurgery. 1998;18:383–386. doi: 10.1002/(sici)1098-2752(1998)18:6<383::aid-micr8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 59.Aluisio FV, Urbaniak JR. Proximal femur fractures after free vascularized fibular grafting to the hip. Clin Orthop Relat Res. 1998;(356):192–201. doi: 10.1097/00003086-199811000-00026. [DOI] [PubMed] [Google Scholar]
- 60.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22:26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, Poggie RA. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87 Suppl 2:22–27. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 62.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:48–55. doi: 10.2106/JBJS.F.00538. [DOI] [PubMed] [Google Scholar]
- 63.Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008;90:1282–1289. doi: 10.2106/JBJS.F.00847. [DOI] [PubMed] [Google Scholar]
- 64.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43:40–45. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;(405):14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87:896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 68.Dean MT, Cabanela ME. Transtrochanteric anterior rotational osteotomy for avascular necrosis of the femoral head. Long-term results. J Bone Joint Surg Br. 1993;75:597–601. doi: 10.1302/0301-620X.75B4.8331115. [DOI] [PubMed] [Google Scholar]
- 69.Langlais F, Fourastier J. Rotation osteotomies for osteonecrosis of the femoral head. Clin Orthop Relat Res. 1997;(343):110–123. [PubMed] [Google Scholar]
- 70.Tooke SM, Amstutz HC, Hedley AK. Results of transtrochanteric rotational osteotomy for femoral head osteonecrosis. Clin Orthop Relat Res. 1987;(224):150–157. [PubMed] [Google Scholar]
- 71.Sugioka Y. Transtrochanteric anterior rotational osteotomy of the femoral head in the treatment of osteonecrosis affecting the hip: a new osteotomy operation. Clin Orthop Relat Res. 1978;(130):191–201. [PubMed] [Google Scholar]
- 72.Hisatome T, Yasunaga Y, Takahashi K, Ochi M. Progressive collapse of transposed necrotic area after transtrochanteric rotational osteotomy for osteonecrosis of the femoral head induces osteoarthritic change. Mid-term results of transtrochanteric rotational osteotomy for osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2004;124:77–81. doi: 10.1007/s00402-003-0610-0. [DOI] [PubMed] [Google Scholar]
- 73.Mont MA, Rajadhyaksha AD, Hungerford DS. Outcomes of limited femoral resurfacing arthroplasty compared with total hip arthroplasty for osteonecrosis of the femoral head. J Arthroplasty. 2001;16:134–139. doi: 10.1054/arth.2001.28722. [DOI] [PubMed] [Google Scholar]
- 74.Kim KJ, Rubash HE. Large amounts of polyethylene debris in the interface tissue surrounding bipolar endoprostheses. Comparison to total hip prostheses. J Arthroplasty. 1997;12:32–39. doi: 10.1016/s0883-5403(97)90044-9. [DOI] [PubMed] [Google Scholar]
- 75.Cabanela ME. Femoral endoprostheses and total hip replacement for avascular necrosis. Semin Arhtroplasty. 1998;9:253–260. [Google Scholar]
- 76.Cornell CN, Salvati EA, Pellicci PM. Long-term follow-up of total hip replacement in patients with osteonecrosis. Orthop Clin North Am. 1985;16:757–769. [PubMed] [Google Scholar]
- 77.Stauffer RN. Ten-year follow-up study of total hip replacement. J Bone Joint Surg Am. 1982;64:983–990. [PubMed] [Google Scholar]
- 78.Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426–1434. [PubMed] [Google Scholar]
- 79.Issa K, Naziri Q, Maheshwari AV, Rasquinha VJ, Delanois RE, Mont MA. Excellent results and minimal complications of total hip arthroplasty in sickle cell hemoglobinopathy at mid-term follow-up using cementless prosthetic components. J Arthroplasty. 2013;28:1693–1698. doi: 10.1016/j.arth.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 80.Wang TI, Hung SH, Su YP, Feng CQ, Chiu FY, Liu CL. Noncemented total hip arthroplasty for osteonecrosis of the femoral head in elderly patients. Orthopedics. 2013;36:e271–e275. doi: 10.3928/01477447-20130222-13. [DOI] [PubMed] [Google Scholar]
- 81.Johannson HR, Zywiel MG, Marker DR, Jones LC, McGrath MS, Mont MA. Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: a systematic literature review. Int Orthop. 2011;35:465–473. doi: 10.1007/s00264-010-0979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim YH, Kim JS, Park JW, Joo JH. Contemporary total hip arthroplasty with and without cement in patients with osteonecrosis of the femoral head: a concise follow-up, at an average of seventeen years, of a previous report. J Bone Joint Surg Am. 2011;93:1806–1810. doi: 10.2106/JBJS.J.01312. [DOI] [PubMed] [Google Scholar]
- 83.Ilyas I, Moreau P. Simultaneous bilateral total hip arthroplasty in sickle cell disease. J Arthroplasty. 2002;17:441–445. doi: 10.1054/arth.2002.31084. [DOI] [PubMed] [Google Scholar]
- 84.Marulanda GA, Minniti CP, Ulrich SD, Seyler TM, Mont MA. Perioperative management for orthopaedic patients with sickle cell anaemia. J Orthop Surg (Hong Kong) 2009;17:346–350. doi: 10.1177/230949900901700321. [DOI] [PubMed] [Google Scholar]
- 85.Chang JS, Han DJ, Park SK, Sung JH, Ha YC. Cementless total hip arthroplasty in patients with osteonecrosis after kidney transplantation. J Arthroplasty. 2013;28:824–827. doi: 10.1016/j.arth.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 86.Kawasaki M, Hasegawa Y, Sakano S, Masui T, Ishiguro N. Total hip arthroplasty after failed transtrochanteric rotational osteotomy for avascular necrosis of the femoral head. J Arthroplasty. 2005;20:574–579. doi: 10.1016/j.arth.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 87.McGrath MS, Marker DR, Seyler TM, Ulrich SD, Mont MA. Surface replacement is comparable to primary total hip arthroplasty. Clin Orthop Relat Res. 2009;467:94–100. doi: 10.1007/s11999-008-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilbert RE, Cheung G, Carrothers AD, Meyer C, Richardson JB. Functional results of isolated femoral revision of hip resurfacing arthroplasty. J Bone Joint Surg Am. 2010;92:1600–1604. doi: 10.2106/JBJS.I.00698. [DOI] [PubMed] [Google Scholar]
- 89.Ball ST, Le Duff MJ, Amstutz HC. Early results of conversion of a failed femoral component in hip resurfacing arthroplasty. J Bone Joint Surg Am. 2007;89:735–741. doi: 10.2106/JBJS.F.00708. [DOI] [PubMed] [Google Scholar]
- 90.Beaulé PE, Schmalzried TP, Campbell P, Dorey F, Amstutz HC. Duration of symptoms and outcome of hemiresurfacing for hip osteonecrosis. Clin Orthop Relat Res. 2001;(385):104–117. doi: 10.1097/00003086-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 91.Cuckler JM, Moore KD, Estrada L. Outcome of hemiresurfacing in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2004;(429):146–150. doi: 10.1097/01.blo.0000150121.88033.50. [DOI] [PubMed] [Google Scholar]
- 92.Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79:247–253. doi: 10.1302/0301-620x.79b2.6982. [DOI] [PubMed] [Google Scholar]
- 93.Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76:252–257. [PubMed] [Google Scholar]
- 94.Shinar AA, Harris WH. Cemented total hip arthroplasty following previous femoral osteotomy: an average 16-year follow-up study. J Arthroplasty. 1998;13:243–253. doi: 10.1016/s0883-5403(98)90168-1. [DOI] [PubMed] [Google Scholar]


