Abstract
Background
Opioids are frequently prescribed for chronic low back pain (CLBP), but there is little prospective data on which patient subgroups may benefit. Psychiatric comorbidity, such as high levels of depression and anxiety symptoms (termed, comorbid negative affect [NA]) is a common presentation and may predict diminished opioid analgesia and/or increased opioid misuse.
Methods
We conducted a 6½-month prospective cohort study of oral opioid therapy, with an active drug/placebo run-in period, in 81 CLBP patients with low, moderate, and high levels of NA. Treatment included an opioid titration phase with a prescribing physician blinded to NA group assignment, and a 4-month continuation phase, during which subjects recorded daily pain levels using an electronic diary. The primary outcome was the percent improvement in average daily pain, summarized weekly.
Results
There was an overall 25% drop out rate. Despite the high NA group being prescribed a higher average daily dose of morphine equivalents, linear mixed model analysis revealed that the 24 study completers in each of the high and low NA groups had an average 21% vs. 39% improvement in pain, respectively (p<.01). The high NA group also had a significantly greater rate of opioid misuse (39% vs. 8%, p<.05), and significantly more and intense opioid side effects (p<.01).
Conclusions
These results indicate that the benefit and risk considerations in CLBP patients with high vs. low NA are distinctly different. Thus, negative affect is an important phenotypic variable to characterize at baseline, prior to deciding whether to prescribe opioids for CLBP.
Introduction
Despite the controversies regarding their effectiveness in chronic non-cancer pain,1 opioids are frequently prescribed for chronic low back pain (CLBP), a condition which affects 50 million adults in the United States.2 In a recent study of 1,860 patients with CLBP from clinics across the United States, 52% were prescribed opioids.3 While clinicians report significant inter-individual differences in opioid analgesia, there is a dearth of studies comparing whether certain patient subgroups are more or less likely to have significant pain improvement, side effects, and/or elevated rates of opioid misuse.4
While no such prospective studies of oral opioids have been conducted, using an intravenous opioid administration study design5 and in a post-hoc analysis of an oral opioid randomized controlled trial,6 we have shown that CLBP patients with high levels of comorbid negative affect (NA) have 40% diminished opioid analgesia compared with CLBP patients with low levels of NA. NA is a cluster of related, concurrent negative emotions and thoughts (such as high levels of depression, anxiety, and pain catastrophizing), which can occur in response to chronic pain.7 These psychological conditions, despite their distinct features, may be grouped, since depression, anxiety, and catastrophizing are highly co-morbid among patients with chronic pain, with correlations of .60 to .80, which points to an underlying construct of NA.8-13
High levels of NA are the most frequent presenting symptoms of a comorbid major depression or anxiety disorder, which afflict 30-50% of patients with CLBP receiving medical care.14,15 Given the phenomenology of depression-anxiety spectrum disorders, focusing on the sum total of NA symptoms as an indicator of significant psychopathology is endorsed by the National Institutes of Mental Health Research Domain Criteria (RDoC) initiative, which identifies ‘NA’ as one of the five primary neurobiological domains of mental illness.16,17 Our group and others also have shown in large cohorts with chronic musculoskeletal pain that high levels of depression and anxiety most frequently co-occur and is the most common clinical presentation of NA symptoms.11,13 High levels of NA afflict at least 40% of CLBP patients,14 and are associated with higher levels of pain, poor functioning, and worse treatment outcomes, such as with spine surgery, nerve blocks, physical therapy, or opioids.5,6,18-24
The prevalent subgroup of CLBP patients with high NA is prescribed opioids at an even greater rate, in part due to their treatment resistance.25-27 High NA in CLBP is also associated with greater opioid misuse,4 with rates ranging from 50-60%,28,29 and heightened craving for opioids;30,31 although, these studies are retrospective, cross-sectional, or from insurance claims databases. Consensus-based recommendations from the Food and Drug Administration define opioid misuse as, “the taking of medication with a therapeutic intent in a manner other than as prescribed,”32 which includes using opioids at higher doses and/or frequency than prescribed, obtaining opioids from multiple providers to improve pain, and/or concurrent use of illegal drugs. In our prior studies, pain intensity level was not a predictor of prescription opioid misuse.33,34 Rather, high NA was the most prevalent and powerful risk factor.28,35
Hence, it is important to understand prospectively and longitudinally whether high NA (an indicator of psychiatric comorbidity) is associated with treatment failure in CLBP patients initiating treatment with opioids. We hypothesized that in the treatment of CLBP, patients with high NA would have diminished opioid analgesia and greater rates of opioid misuse compared with CLBP patients with low NA.
Materials and Methods
Study design and population
This was a 6½-month prospective cohort study of oral opioid therapy, conducted from 2009-2012, with an active drug/placebo run-in period, in CLBP patients with low, moderate, and high levels of NA (ClinicalTrials.gov Identifier: NCT01502644). Inclusion criteria were: 1) Ages 21-75; 2) CLBP of at least six months duration with an average pain score of >3/10 (established with a one-week baseline pain observation period, using daily, electronic pain ratings); 3) LBP meeting Quebec Task Force Criteria for Grades I-III (low back pain [LBP] only to LBP with intermittent radicular pain [not constant or daily] and no neurological signs);36 4) No back surgery within the past year; 5) Having degenerative disc disease as a component of a pain syndrome of mixed etiology, confirmed by history, examination, and a previous lumbar MRI; 6) No opioid use or use of short-acting opioids only, and <90 mg/day in morphine equivalents. Those subjects taking opioids must also have agreed to a 2-week opioid washout period prior to beginning medication; 7) No pregnancy or intent to become pregnant during the study period; 8) No intent to begin new pain or psychiatric treatments during the study (such as pain medication, nerve blocks, physical therapy, or psychiatric medication), or increase any current medications; 9) No current, active substance use disorder and no history of an opioid substance use disorder (assessed with the Mini International Neuropsychiatric Interview);37 and 10) No active suicidality or psychosis (assessed by the Mini International Neuropsychiatric Interview as well).
Eligibility was determined by investigator ADW at the first visit through a review of a history and physical examination, and MRI findings confirming the presence of degenerative disc disease. Patients were included if this evaluation found that there was at least one degenerated, herniated, or torn lumbar disc with either a minimum Grade III disc degeneration,38 abnormal morphology,39 or a hyperintense zone.40 These inclusion criteria, used by the authors in previous studies,5,41,42 narrow the heterogeneity of CLBP phenotypes by including those with the commonly presenting mixed syndrome of low back pain with underlying degenerated discs, and possibly spinal stenosis or facet disease, and excluding those with pain due to purely nonspecific or myofascial causes.
Subject enrollment
Institutional Review Board approved procedures were used (Brigham and Women's Hospital, Boston, MA, USA). Volunteers were recruited from the Pain Medicine and Physiatry Spine clinics of Brigham and Women's Hospital. In addition, the hospital's research database of patients with CLBP ICD-9 diagnosis codes was queried to generate lists of potential subjects, and their corresponding treating providers. Letters were sent to those providers requesting permission to contact their specific patients. Upon provider approval, informational letters about the study were sent to these potential subjects. After telephone and medical record prescreening, subjects were enrolled at Visit 1 and signed written informed consent. The consent document included opioid therapy instructions regarding the appropriate use of prescription opioids.
Visit 1
The lead investigator (ADW) confirmed subject eligibility and subjects completed baseline self-report questionnaires, along with a history and physical exam. The primary predictor was the likely presence or absence of psychiatric comorbidity. This was determined by the levels of NA symptoms within the past week, which were assigned as low, moderate, or a high level to each subject by the combined depression and anxiety subscale scores of the Hospital Anxiety Depression Scale (HADS total score).43 We have used the HADS total score as an operational measure of NA and a grouping variable in previous CLBP treatment outcome studies.23 The HADS does not include somatic items which may be attributable to medical illness, and thus it is more appropriate for screening in chronic pain.43 High NA was defined as >8 on both the depression and anxiety subscales, Low NA was <6 on each subscale, and Moderate NA was all other scores in between these cutoffs.44 In CLBP specifically, scores meeting high NA criteria are highly correlated with a patient having a comorbid major depression or generalized anxiety disorder, and those meeting low NA criterion are highly unlikely to have psychiatric comorbidity.43,44 Recent reviews have concluded that the HADS functions best as a unidimensional measure of NA, while retaining excellent psychiatric case-finding ability.45,46
Other baseline measures included: the Brief Pain Inventory (BPI--for 24 hour pain and pain interference levels),47 the Oswestry Disability Index (for self-reported function),48 the Neuropathic Pain Questionnaire Short Form (for symptoms of burning, shooting, and sensitivity to touch),49 the Neuroticism Subscale of the NEO personality inventory,50 the Pain Catastrophizing Scale,51 and the Screener and Opioid Assessment for Patients with Pain, Revised (SOAPP-R, for estimating the risk of opioid misuse).33 These measures were chosen to conform to the IMMPACT Group (Initiative on Methods, Measurements, and Pain Assessment in Clinical Trials) recommendations for analgesic clinical studies.52
During Visit 1 a urine drug test (UDT) was collected to confirm that subjects were not taking opioids and not using illegal drugs. For those subjects prescribed opioids prior to enrollment, they underwent a 2-week weaning period and remained off of opioids for at least 7 days prior to beginning medication at Visit 2 (a period similar to other studies).53 Compliance with the wean was confirmed with a UDT at Visit 2.
Baseline pain observation period
In between Visits 1 and 2 subjects rated their average pain level (0-10) over the past 24 hours (Question 5 of the BPI) each day for seven days using an electronic diary, triggered by an alarm. These data were inspected and downloaded at Visit 2 and were averaged to determine the mean level of baseline pain for each subject. Subjects had to provide data for at least 4/7 days to continue in the study.
Visit 2
Subjects completed the HADS again to establish the stability of the self-reported NA symptoms, and to continue they could not have fallen out of the group (low, moderate, or high NA) to which they were assigned (no subjects changed group). UDT results were reviewed, and a psychiatrist administered a structured psychiatric interview to determine any Diagnostic and Statistical Manual-IV Axis 1 diagnosis, including a current substance use disorder.37
Active drug/placebo run-in
At Visit 2 subjects could choose to be prescribed morphine or oxycodone based on any prior experience with these drugs. In a double-blinded randomized order, subjects then received either placebo or morphine/oxycodone 1-2 tabs up to three times a day as needed for 1 week each (crossover), dispensed by the hospital research pharmacy (all pills looked identical). The hospital research pharmacy followed block randomization in groups of 8 and maintained the double blind until the conclusion of the study. The doses for morphine were immediate release morphine 15-30 mgs and for oxycodone 5-10 mgs three times a day as needed for pain. Subjects completed the electronic diary daily for the average pain rating as well as noting the number of medication doses they took each day. The run-in period allowed subjects to acclimate to opioid medication and to become familiar with the daily pain rating procedures. It also could clarify the role of placebo responses in predicting opioid analgesia. Subjects continued completing the electronic diaries daily in all remaining phases of the study, which were inspected at each study visit.
Opioid treatment periods
Visits 3-6, Titration of Opioids (weeks 1-3)
This open-label portion of the trial was conducted by a pain medicine physician-investigator (EM) blinded to group assignment who used a standardized titration schedule for morphine or oxycodone over a three week period. If a subject could not tolerate morphine or oxycodone during the run-in period (assessed at the weekly study visits), the blind was broken and they were allowed to switch to the other opioid for the rest of the study. The average pain intensity target was <4/10 and dosing was individually adjusted each week to maximize pain relief with acceptable side effects according to subject tolerability. At each weekly visit the subject completed the Brief Pain Inventory and a medication log (inspected by the study physician). In discussion with the subject, the dosing was adjusted using standardized parameters for long-acting and breakthrough medication for morphine or oxycodone. At the end of the titration period the maximum allowable daily dose in morphine equivalents was 30 mgs of short-acting plus 60 mgs of long-acting medication three times a day (270 mgs, no subjects reached the maximum possible dose).
Visits 7-10, Opioid continuation period (weeks 4-20)
Subjects remained at their individualized dose throughout this period, except that doses could be reduced (due to side effects, for example). They received monthly prescriptions for the short and long-acting opioids at monthly visits. They met with a study physician and completed questionnaires at each visit (including global impression of change ratings and an opioid side effects checklist used in our previous studies).54 In addition, at two and at four months into the continuation period opioid adherence measures were collected: the Current Opioid Misuse Measure (COMM—for self-reported misuse, score>13 highly predicts misuse using revised criteria),55 the Addiction Behaviors Checklist (for physician-rated misuse assessment, positive score>2),56 and a UDT. Subjects found misusing were continued in the study. We asked that subjects not begin any new pain or psychiatric treatments during the study and we tracked this issue at monthly visits.
Opioid tapering (visits 11-13, weeks 21-24)
Subjects returned for weekly visits during this period and the individualized opioid dose was lowered by approximately 25% each week. Subjects were off of opioids by the end of the study.
Statistical methods
Sample size calculations were based on a power of .80 and a 5% significance level using a two-tailed t test to test the hypothesis that opioids confer diminished analgesia in the high NA vs. in the low NA group. The primary outcome measure was the percent improvement in average daily pain during the opioid continuation period (measured with daily pain ratings on the electronic diary and calculated on a weekly basis), in comparison to the average baseline pain level. Based on our previous data from intravenous morphine administration in CLBP patients with low vs. high NA,5 20 subjects each were needed in the low and high groups to find a 33% difference in average percent improvement in pain between groups. The primary comparisons of interest were the outcomes differences between the low and high groups. Target study completer rates in these groups were 24 subjects/group so that we would be adequately powered using multiple comparison corrections to examine our main secondary outcome of interest, the rates of drug misuse between groups. Those in the moderate group continued in all phases of the study, since little is known about their opioid analgesic outcomes as well, and their data is presented for descriptive purposes only. Data from the moderate group was also included in multiple secondary “sensitivity analyses” to further interrogate our findings.
Statistical analyses were based on a “modified intent to treat principle,” such that subjects had to complete at least 50% of the opioid treatment period to have their data included for analysis. Otherwise, they were termed a “drop out” and these data were not included. Sources of missing data that were tracked include subject dropout, subjects missing study visits, or subjects not completing the electronic diaries at home. We also inspected the case report forms to understand if there was any pattern to the missing data. For the primary outcome measure we used linear mixed modeling (LMM) in SPSS version 22 (Chicago, IL, USA) to compare the low vs. high NA groups over time. LMM “borrows” information pertaining to the relationship between the weekly outcomes, such that subjects missing some weekly data (but not all) can still be used for analyses. Generally, in longitudinal trials this approach allows for inclusion in the analysis of the majority of subjects with any missing data.57 For the LMM model, Group, Group X week, average baseline pain, and opioid use at baseline (yes/no) were entered as fixed effects using an autoregressive covariance structure. Subject, intercept, and week were entered as random effects, using a compound symmetry covariance structure. This approach controls for possible differences in baseline pain level and opioid use between subjects. To further address the possible impacts of missing data (those who completed at least 50% but not 100% of the opioid treatment period) we imputed data using Last Observation Carried Forward (LOCF) and Baseline Observation Carried Forward (BOCF) methods as part of a secondary, sensitivity analysis. For LOCF we used the percent improvement in pain for the last week the subject was in the trial during the opioid continuation period, and the first week of this period for the BOCF value.
For the secondary outcome of rates of opioid misuse between groups, similar to our previous studies,58,59 we determined the Drug Misuse Index (DMI) at 2 and 4 months into the opioid continuation period. The DMI triangulates three domains (patient self-report [COMM], provider assessment of misuse [ABC], and UDT results) to determine adherence to opioids (adherence vs. misuse, a categorical outcome). A positive DMI is a positive finding of misuse on any of these 3 measures. Of note, positive misuse at two and four months was counted as one episode of misuse. Chi square was used to analyze the relationship between group and opioid misuse. Comparisons of demographics, pain history data, baseline questionnaires, and additional secondary outcome measures in relation to group were analyzed using Pearson correlations, Chi-square, and analysis of variance (ANOVA), depending on whether the variables were ordinal or numerical. Furthermore, sensitivity analyses using analysis of covariance (ANCOVA) were conducted to test if any baseline variables were significant univariate or covariate predictors when added to Group of total average percent improvement in pain during the opioid continuation period. We also analyzed HADS scores as a continuous predictor of pain treatment outcomes.
Results
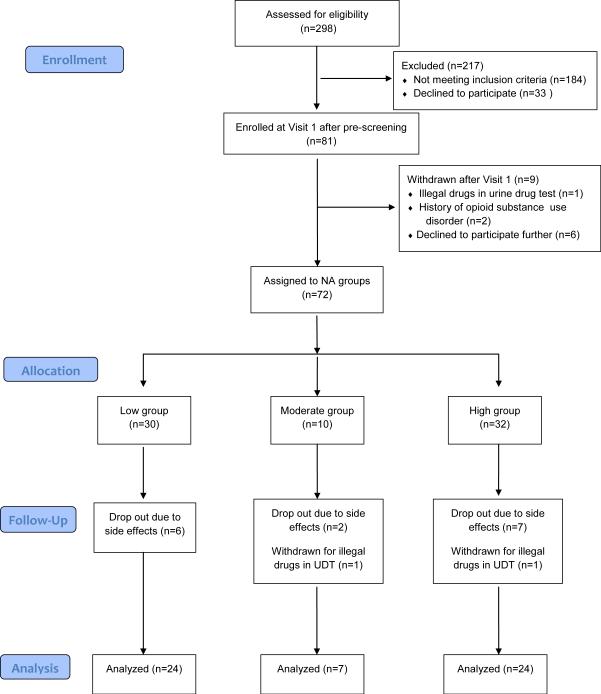
Figure 1 displays the study flow diagram. After assessing eligibility in 298 volunteers, 81 subjects enrolled in the study and 72 received medication during the run-in and/or opioid treatment phases. In these 72 subjects, there were 20%, 30%, and 25% rates of drop out/study withdrawal in the low, moderate, and high NA groups, respectively. The majority of these drop outs were due to side effects of opioid medication. Consequently, data analysis included 24 subjects each in the low and high groups and seven in the moderate group. There were no significant differences in average age, gender distribution, average baseline pain level and duration of pain between those who dropped out and those who completed the study. Amongst the 55 subjects with data suitable for analysis following a modified intent to treat principle: 10/55 finished at least half, but not the entire opioid continuation period, the distribution of these subjects was not skewed between NA groups (4 each in the low and high groups, and 2 in the moderate group), and no pattern to the missing data was found.
Figure 1.
Study Flow Diagram: This follows CONSORT (Consolidated Standards of Reporting Trials) guidelines for randomized and observational cohort studies. Note that 76% of subjects who began medication treatment completed the study. UDT=Urine Drug Test.
Table 1 displays the baseline characteristics of the three NA groups, with the moderate group being included for descriptive purposes only. The Low and High groups were similar on average age, gender and marital status distributions. The high NA group had a significantly lower percentage of those working. Average baseline pain, the duration of pain, and the percentages taking opioids or having intermittent radicular and other neuropathic pain complaints were similar between groups. Those on opioids prior to enrollment were taking an average of 28 mgs of morphine equivalents per day (range 5-80 mgs). The average baseline pain levels just prior to treatment and after any wean, were not significantly different between those taking/not taking opioids at baseline. While the low NA group tended to have intermittent radicular pain more frequently, the high NA group tended to have a more frequent composite presentation of neuropathic symptoms. The high NA group did report significantly greater pain interference, more disability, and were at a higher risk of opioid misuse. The high group also tended to have a more frequent past history of substance abuse. Every subject in the high group had a psychiatric diagnosis of some type of an affective disorder (Table 1, psychiatric diagnoses). The group of subjects with major depression is mainly composed of those with major depression with anxious features (90% of cases). The high NA group also had significantly greater pain catastrophizing and neuroticism scores, which were significantly correlated to total HADS scores (Pearson correlation coefficients .58 and .66, respectively, p's<.01).
Table 1.
Demographics and Baseline Pain
| History | Psychiatric | Group | Column1 | P value (Low vs. High) |
|---|---|---|---|---|
| Variable | Low NA (n=24) | Moderate NA (n=7) | High NA (n=24) | |
| Age (mean, years) | 55 | 54 | 49 | 0.08 |
| Gender (% female) | 67 | 43 | 58 | 0.38 |
| Work status (% working) | 42 | 29 | 12 | 0.03 |
| Marital status (% married) | 54 | 57 | 58 | 0.6 |
| Taking opioids at baseline (% yes) | 33 | 14 | 42 | 0.38 |
| Baseline pain (mean, 0-10) | 7.1 | 6.6 | 7.5 | 0.39 |
| Pain duration (mean, years) | 7.3 | 12.4 | 8 | 0.72 |
| Radicular pain (% yes)1 | 75 | 57 | 54 | 0.31 |
| Neuropathic pain (% yes)2 | 58 | 71 | 81 | 0.09 |
| Pain interference (mean, 0-10)3 | 5.6 | 6.6 | 7.5 | 0.002 |
| Function (% disability)4 | 35.6 | 40.1 | 50.3 | 0.007 |
| Opioid misuse risk score (mean, 0-96)5 | 10.7 | 14.7 | 24 | 0.0001 |
| Substance abuse history (% yes) | 12 | 14 | 21 | 0.35 |
| Co-morbid major depression (% yes)6 | 4 | 0 | 67 | 0.0001 |
| Generalized anxiety disorder (% yes) | 4 | 14 | 8 | 0.64 |
| Post-traumatic stress disorder (% yes) | 0 | 0 | 21 | 0.018 |
| Obsessive-compulsive disorder (% yes) | 0 | 0 | 8 | 0.61 |
| Panic disorder (% yes) | 0 | 0 | 8 | 0.61 |
| Bipolar disorder (% yes) | 0 | 0 | 8 | 0.61 |
| Dysthymia (% yes) | 0 | 0 | 46 | 0.001 |
| Adjustment disorder (% yes) | 0 | 29 | 12 | 0.2 |
| Depression symptoms (mean, 0-21)9 | 4 | 7.5 | 11.7 | 0.0001 |
| Anxiety symptoms (mean, 0-21)10 | 4.7 | 9.5 | 12.7 | 0.0001 |
| Pain catastrophizing (mean, 0-52)7 | 20.9 | 33 | 32.7 | 0.0001 |
| Neuroticism (mean, T score)8 | 42.4 | 44.9 | 60 | 0.0001 |
Intermittent only per history (not constant or daily)
Composite measure of burning, shooting, and sensitivity to touch symptoms, assessed with the Neuropathic Pain Questionnaire
Average of the pain interfence items on the Brief Pain Inventory
Oswestry Disability Index
Screener for Opioid Assessment in Patients with Pain, Revised (SOAPP-R). Score >17 predicts a high likihood of future opioid misuse
Results of the MINI (Mini-internationalneuropsychiatric-interview)
Pain Catastrophizing Scale
Neuroticism Subscale of the NEO Personality Inventory ‘T’ scores above 60= >85th percentile for neuroticism (NEO-FFI-Personality Inventory, Costa P, 1985)
Depression subscale of the HADS (Hospital Anxitey and Depression Scale)
Anxiety subscale of the HADS
For the run-in period, Table 2 displays the means of the percent improvement in pain per group for the active and placebo weeks. The high NA group had less analgesia with active drug or placebo than the low group, and there was a significant difference between groups for placebo analgesia (p=.025). During the run-in period, those in the low group used an average of 64 mgs/day of oral morphine equivalents, while the high group used 75 mgs/day (non-significant difference).
Table 2.
Secondary Outcomes
| Psychiatric Group | Column1 | ||
|---|---|---|---|
| Variable | Low NA (n=24) | Mod NA (n=7) | High NA (n=24) |
| Active drug run-in period analgesia (mean, % improvement in pain) | 18.3 (±29.2)6 | 26.4 (±39.9) | 6.8 (±14.2) |
| Placebo run-in period analgesia (mean, % improvement in pain) | 14.9 (±29.1) | 11.1 (±14.7) | −1.9 (±15.3) (p=.025)7 |
| Changes from beginning of study to end of continuation phase: | |||
| Maintenance opioid dose (mean, mgs daily morphine equivalents) | 75.6 (±44.6) | 72.2 (±52.9) | 94.7 (±47.8) |
| Negative affect (% change)1 | 27.5 (±45.6) | 4.2 (±24.6) | 31.1 (±27.1) |
| Pain interference (% change)2 | 39.5 (±42.7) | 30.4 (±25.7) | 20.2 (±27.6) (p=.0001) |
| Function (% change disability score)3 | 1 (±12.2) | 4.0 (±8.6) | 5.8 (±15.1) |
| Patient global impression of change (median distribution of 7 categories)4 | Much improved | Much improved | Minimally improved (p=.026) |
| Drug misuse index (% positive) | 8.3 | 14.3 | 39.1 (p=.013) |
| Opioid side effects (mean total, 0-18)5 | 4.1 (±3.8) | 4.7 (±.6) | 7.0 (±5.2) (p=.0001) |
| Opioid side effects (mean intensity, 0-10)5 | 1.8 (±1.6) | 2.3 (±2.0) | 3.1 (±2.0) (p=.0001) |
Measured with monthly administration of the Hospital Anxiety and Depression Scale (HADS)
Measured by averaging the pain interference items on the Brief Pain Inventory (BPI) collected monthly
Measured by averaging the Oswestry Disability Index Score (ODI) collected monthly
Measured by averaging the impression of change ratings collected monthly
Measured by the Opioid Side Effects Checklist collected monthly
± = 1 standard deviation
P values are comparisons of Low vs. High
During the opioid titration phase the high group was titrated to a higher average daily amount of morphine equivalents (94.7 vs. 75.6 mgs). For the continuation phase, Figure 2 displays the group differences in average percent improvement in pain by week and the estimated means for each group from the LMM analysis. The high group had significantly less average percentage improvement in pain (20.6% vs. 38.6%, p=.01, F=6.5, the 95% CI for the differences in mean analgesia between groups=3.7, 32.2). The moderate group had an average 30% improvement in pain during the continuation phase. Those with missing data in the low group had an average of 41.9% improvement in pain (n=4), while those in the high group with missing data had an average 14.9% improvement in pain (n=4).
Figure 2.
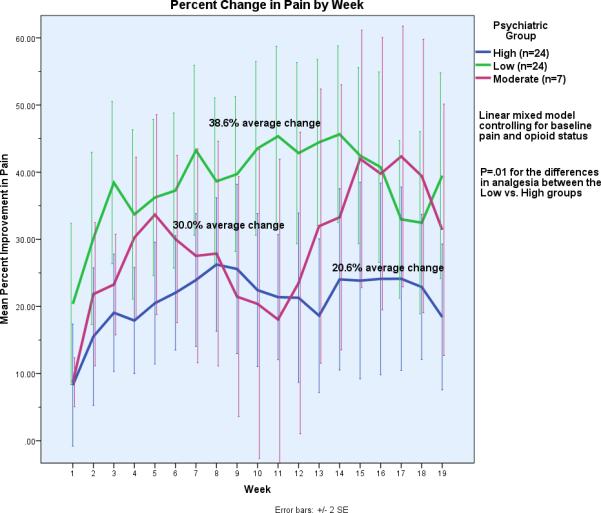
Percent Change in Pain by Week and by Group: These curves depict the average percent improvement in pain per group for each week of the trial. Weeks 1-3 are the titration weeks, and the end of week 3 through week 19 is the continuation (maintenance) period. Note that there are only seven subjects in Moderate group, and that the primary comparison is between the Low vs. High groups.
For the secondary outcomes (Table 2), the low NA group reported: 1) significantly greater change in pain interference (items 9A-G of the BPI, which includes general activity, walking, work, sleep, and social relations, p=.0001); 2) “Much improved” global impression of change vs. “minimally improved” in the high NA group, p=.026; 3) and lower total number of opioid side effects (p=.0001) as well as a lower intensity of side effects (p=.0001). Within the low and high groups, those on opioids at baseline did report a lower total number of side effects (p=.001). There were no differences in either group in self-reported changes in physical function. Both groups reported similar degrees of improvements in NA over the course of the study. The high NA group had a significantly greater rate of opioid misuse (39.1% vs. 8.3%, p=.013), and significantly more craving for opioids than the low NA group (item 11 of the SOAPP-R, p=.041).
Sensitivity Analyses
First, total scores on the HADS in all subjects (low, moderate, and high groups, n=55) were treated as a continuous predictor of average weekly percent improvement in pain during the opioid continuation period (outcome). Linear regression indicated that HADS score accounted for 15% of the variance in analgesic outcomes, such that a higher HADS score was associated with less improvement in pain with opioid treatment (R2=.15, stdBeta=−.38, p=.005). This result is consistent with the linear mixed modeling results in the low and high groups.
Next, Pearson Correlation Coefficients between analgesia for the active and placebo run-in periods vs. analgesia for the opioid continuation period (average percent improvement in pain) were .60 and .75, respectively (p's<.01). An analysis of partial correlation coefficients revealed that these correlations were not significantly different within psychiatric groups. In other words, regardless of NA group assignment, pain improvements during either the active or placebo run-in weeks were significant predictors of subsequent opioid analgesia. Furthermore, six subjects in the low NA group had >20% pain improvement during the placebo run-in week and a corresponding average 49.7% improvement in pain in the continuation period (vs. the 37.8% low group mean). This indicates that those six subjects with low NA who had heightened placebo analgesia were not the drivers of the robust analgesic responses for the group as a whole.
Using ANOVA, Table 3 displays the analysis of baseline pain history and demographic variables that were significant univariate predictors of analgesia during the continuation period (average percent improvement in pain), such as age, gender, working status, baseline pain, intermittent radicular pain, the composite neuropathic pain score, opioid misuse risk score (SOAPP scores), and the levels of pain catastrophizing or neuroticism. Baseline pain interference scores, SOAPP scores, and taking opioids prior to study entry were significant predictors (P's<.05). Additionally, opioid dose during the study and the specific psychiatric diagnosis were tested and neither were significant predictors of analgesia. Next, pain interference, SOAPP score, and opioid status at baseline were continued to the next stage of modeling and entered into separate ANCOVA models with group to test main and interaction effects which may predict analgesia during the continuation period. Pain interference and SOAPP score were not significant main or interaction effects.
Table 3.
Univariate Analysis of Baseline
| Predictors of Pain Improvement11 | Column1 | Column2 | Column3 |
|---|---|---|---|
| Variable (units) | R2 | F | Significance |
| Age (years) | 0.005 | 0.26 | 0.61 |
| Gender (female, male) | 0.001 | 0.06 | 0.82 |
| Work status (yes, no, retired) | 0.014 | 0.23 | 0.87 |
| Marital status (yes, no) | 0.001 | 0.07 | 0.8 |
| Taking opioids at baseline (yes, no) | 0.09 | 4.7 | 0.04* |
| Baseline pain (0-10) | 0 | 0.01 | 0.91 |
| Pain duration (years) | 0.01 | 0.57 | 0.46 |
| Radicular pain (yes, no)1 | 0.009 | 0.24 | 0.79 |
| Neuropathic pain (yes, no)2 | 0.04 | 0.94 | 0.4 |
| Pain interference (0-10)3 | 0.12 | 7.2 | .01* |
| Function (0-100%)4 | 0.003 | 0.16 | 0.67 |
| Opioid misuse risk score (0-96)5 | 0.17 | 9.9 | .003* |
| Substance abuse history (yes, no) | 0.015 | 0.78 | 0.38 |
| Pain catastrophizing (0-52)7 | 0.05 | 0.12 | 0.74 |
| Neuroticism (T score)11 | 0.02 | 1.2 | 0.28 |
Intermittent only per history (not constant or daily)
Composite measure of burning, shooting, and sensitivity to touch symptoms, assessed with the Neuropathic Pain Questionnaire
Average of the pain interfence items on the Brief Pain Inventory
Oswestry Disability Index
Screener for Opioid Assessment in Patients with Pain, Revised (SOAPP-R). Score >14 predicts a high likihood of future opioid misuse
6 Results of the MINI neuropsychiatric structured interview
Pain Catastrophizing Scale
Neuroticism Subscale of the NEO Personality Inventory ‘T’ scores above 60= >85th percentile for neuroticism
9 Depression subscale of the HADS
10 Anxiety subscale of the HADS
Average weekly percent improvement in pain during the opioid continuation period
Continued to ANCOVA stage of testing
However, opioid use at baseline was a significant confounder to analgesic responses in the Low NA group only. In the Low group the 33% of subjects on opioids at baseline had an average 22% improvement in pain during the opioid continuation period, while those not on opioids in this group had 48% improvement. In the High group those on opioids prior to study had an average 20% improvement in pain, while those not on opioids had 19% improvement. In an ANCOVA model with Group, Baseline Opioid Status, and Group X Opioids as predictors and average percent improvement in pain during the continuation period as the outcome, only Group (F= 5.35, p=.026) and the interaction term (Group X Opioids, F=5.34, p=.026) were significant (R2 for the model=.29). In addition, the adjusted means of percent improvement in pain in each group was lowered by~10% in the Low group, but not in the High group, compared to an ANOVA model with Group as the only predictor. These analyses indicate that opioid use at baseline affects analgesia responses in the Low group only, and that NA Group still remains a significant predictor of analgesia.
In terms of missing data in the 8 subjects who did not complete the entire opioid continuation period, imputation using LOCF into the LMM model resulted in the High group having 20.5% average percentage improvement in pain during the opioid continuation period vs. 39.8% in the Low group (p=.01, F=7.4, the 95% CI for the differences in mean analgesia between groups= 5.0, 33.6). For BOCF the High group had 20.1% vs. 40.1% average percentage improvement in pain in the Low group (p=.01, F=7.8, the 95% CI for the differences in mean analgesia between groups= 5.6, 34.3). Both imputation methods resulted in model estimates of analgesia between groups that were highly similar to the model without imputation.
Discussion
In this prospective cohort study of patients with CLBP, we found that psychiatric comorbidity (specifically, high NA) was a significant predictor of poor opioid treatment outcomes compared with CLBP patients with low NA, including almost 50% less improvement in pain, increased side effects, and 75% more opioid misuse. Subjects were carefully phenotyped to have the commonly presenting mixed CLBP syndrome of disc disease, facet, stenosis, and/or neuroforaminal components possibly contributing to their chronic pain. They were also phenotyped psychiatrically to fall into distinct groups of low, moderate, and high levels of NA (and no active substance use disorder), with corresponding higher rates of psychiatric comorbidity and higher levels of pain catastrophizing, and neuroticism in the high NA group. As a result, the low and high groups had a similar underlying pain condition and only differed significantly on their psychological characteristics.
Despite being prescribed a higher daily dose of opioid compared with the low NA group, patients in the high NA group experienced almost 50% less improvement in pain during the opioid continuation phase of the study (20.6% versus 38.6% improvement) and tended to have tolerance (diminishing analgesia over time). Interestingly, the high NA group reported a greater benefit to their mood (31% improvement in NA symptoms) than to their pain (19%). Although, the improvement in mood is far less than the 50% improvement in symptoms benchmark used to determine effective treatments for NA.60 Our findings do not suggest that opioids are a potential treatment for high NA or depression or anxiety disorders. While we have shown previously that patients with chronic pain prescribed opioids tend to self-medicate anxious and depressive feeling with opioids,30 we are unaware of any studies that have examined specifically whether CLBP patients with depression or anxiety disorders prescribed opioids obtain differential effects on their mood vs. pain.
Regarding other secondary outcomes, the high NA group rated opioids as less beneficial to them compared with the low group and reported less improvement in pain interference, with a corresponding greater incidence and intensity of opioid side effects. Furthermore, the high NA group had significantly more opioid misuse and reported more cravings for prescription opioids. We have shown that craving for prescription opioids is a risk factor predicting misuse and a potential psychological mechanism by which high NA may lead to prescription opioid misuse.31,59
Interrogation of our findings through sensitivity analyses revealed that they are robust. The active/placebo run-in period analysis addresses the issue that differences in placebo vs. active drug responses between groups might explain our findings. This unique crossover/cohort hybrid study provides a methodology for examining placebo responses in an otherwise uncontrolled study, with the understanding that a brief placebo-run in period is not equivalent to an efficacy study with a true “placebo arm.” In terms of other possible confounders, the ANCOVA analyses only uncovered prior opioid use as a significant covariate, which affected the Low group only. NA group remained a significant predictor of opioid analgesia. Perhaps the reason why the risk of opioid misuse at baseline (elevated SOAPP scores in the high NA group) was only a significant univariate predictor, was that there are several items regarding mood symptoms on the SOAPP. Thus, the SOAPP and HADS questionnaires do not form entirely separate constructs, and share a significant overlapping variance. Given the baseline differences in pain catastrophizing and neuroticism between groups, it was surprising that neither of these were significant univariate or multivariate predictors of average pain improvement during the continuation phase. However, our data and analysis indicate that if symptoms of depression and anxiety are controlled for, that neither pain catastrophizing or neuroticism are constructs predicting independently the responses to opioid therapy. These results, as well as numerous other published studies from our group,5,6,11,23,31 highlight the importance of NA being a strong, independent predictor of pain treatment responses. In addition, while catastrophizing and neuroticism are powerful predictors in patients with chronic pain of the levels of pain and function,19,61 fewer studies have prospectively examined these as predictors of outcomes for specific pain treatments. One of the relevant studies is by Smeets and colleagues who compared physical therapist-directed exercise or advice-based treatment for LBP, and they also found that pain catastrophizing levels did not predict clinical treatment responses.62
Our results indicate that the outcomes of opioid therapy in CLBP patients with a high or low degree of psychiatric comorbidity (as categorized by levels of NA) are distinctly different, with less benefit, more side effects, and greater risks in patients with high levels of NA. This prospective longitudinal study confirms findings from retrospective and cross-sectional studies by our group and others. Moreover, the five-month opioid treatment period in this study is substantially longer than the majority of oral opioid studies in CLBP, which typically average three months in length.63,64 This longer treatment period enabled a better study of longitudinal issues, such as the development of tolerance and opioid misuse, which cannot be well-studied in shorter trials. Our findings address the controversy over long-term opioid prescribing for chronic low back pain through showing prospectively that there may be subgroups who can potentially do well on this therapy long-term, and a distinct subgroup with psychiatric comorbidity who tends to do poorly.
Our data suggest that in deciding to prescribe opioids, it is prudent to assess for psychiatric comorbidity, and more specifically, high levels of NA. We have now demonstrated in three studies, each with a different study design and totaling over 250 subjects,5,6 that comorbid high NA predicts poor opioid treatment outcomes in patients with CLBP.
Rather than refusing to prescribe opioids for this subgroup, we suggest that comorbid high NA be identified and treated early in the course of CLBP, and preferably even before LBP becomes chronic. Large RCTs testing a variety of NA treatments, such as antidepressant medications,60 cognitive behavioral therapy,65 or fear of movement physical therapy66 have each shown efficacy for improving LBP, function, and mood. One implication of this work is that if high NA is identified and treated early in the course of LBP, one could prevent this vulnerable subgroup from being prescribed opioids if pain and function were improved through NA treatment. Furthermore, for those prescribed opioids, successful treatment of NA may improve opioid analgesia and reduce the chances of opioid misuse. These suppositions merit further testing in clinical studies.
There are a number of important limitations to consider in our study. First, while analgesia from opioids was lower in the high NA group, these participants may still have felt that the therapy was beneficial to them due to possible non-analgesic opioid effects and may have wished it to continue. Second, this study does not explore what effect the chronicity of high NA (from months to years in duration) has upon opioid analgesia. While our results are statistically significant, one could argue that the sample size is too small to be a definitive, confirmatory trial.
In summary, high NA predicts poor opioid treatment outcomes in CLBP. This finding provides an impetus for conducting further studies of patients with CLBP and high NA to improve opioid analgesia in this population and to reduce or prevent opioid misuse.
Acknowledgments
Funding source: This study was supported by the National Institute of Drug Abuse of the National Institutes of Health (K23 DA020682, Wasan, PI, Bethesda, MD, USA) and the Arthritis Foundation (Investigator Award; Wasan, PI, Atlanta, GA, USA).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Ballantyne J, Mao J. Opioid Therapy for Chronic Pain. NEJM. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 2.Fourney DR, Andersson G, Arnold PM, Dettori J, Cahana A, Fehlings MG, Norvell D, Samartzis D, Chapman JR. Chronic low back pain: A heterogeneous condition with challenges for an evidence-based approach. Spine (Phila Pa 1976) 2011;36:S1–9. doi: 10.1097/BRS.0b013e31822f0a0d. [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Stokes G, Lobosco S, Pike J, Sadosky AB, Ross E. Relationship between patient-reported chronic low back pain severity and medication resources. Clin Ther. 2011;33:1739–48. doi: 10.1016/j.clinthera.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Howe CQ, Sullivan MD. The missin 'P' in pain mangement: How the current opioid epidemic highlights the need for psychaitric services in chronic pain care. Gen Hosp Psychiatry. 2014;36:99–104. doi: 10.1016/j.genhosppsych.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Wasan AD, Davar G, Jamison RN. The Association between Negative Affect and Opioid Analgesia in Patients with Discogenic Low Back Pain. Pain. 2005;117:450–61. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Jamison RN, Edwards RR, Liu X, Ross EL, Michna E, Warnick M, Wasan AD. Relationship of Negative Affect and Outcome of an Opioid Therapy Trial Among Low Back Pain Patients. Pain Pract. 2013;3:173–81. doi: 10.1111/j.1533-2500.2012.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mounce C, Keogh E, Eccleston C. A Principal Components Analysis of Negative Affect-Related Constructs Relevant to Pain: Evidence for a Three Component Structure. J Pain. 2010;11:710–7. doi: 10.1016/j.jpain.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Turk DC. The Potential of Treatment Matching for Subgroups of Patients With Chronic Pain: Lumping Versus Splitting. Clin J Pain. 2005;21:44–55. doi: 10.1097/00002508-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Watson D, Clark AC. Affects separable and inseparable: On the hierarchical arrangements of the negative effects. J Pers Soc Psychol. 1992;62:489–505. [Google Scholar]
- 10.Fernandez E. Interactions between Pain and Affect, Anxiety, Depression, and Anger in Pain. Dallas, Advanced Psychological Resources. 2002:13–32. [Google Scholar]
- 11.Wasan AD, Anderson NK, Giddon DB. Differences in pain, psychological symptoms, and gender distribution among patients with left- vs right-sided chronic spinal pain. Pain Med. 2010;11:1373–80. doi: 10.1111/j.1526-4637.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen SA. Negative affect and sensitization to pain. Scand J Psychol. 2002;43:131–7. doi: 10.1111/1467-9450.00278. [DOI] [PubMed] [Google Scholar]
- 13.Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70:890–7. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dersh J, Gatchel RJ, Mayer DJ, Polatin PB, Temple OR. Prevalence of Psychiatric Disorders in Patients with Chronic Disabling Occupational Spinal DIsorders. Spine. 2006;31:1156–62. doi: 10.1097/01.brs.0000216441.83135.6f. [DOI] [PubMed] [Google Scholar]
- 15.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic Spinal Pain and Physical-Mental Comorbidity in the United States: Results from the National Comorbidity Survey Replication. Pain. 2005;113:331–39. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 17.Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119:631–9. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 18.Linton S. A Review of Psychological Risk Factors in Back and Neck Pain. Spine. 2000;25:1148–56. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- 19.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 2002;27:E109–20. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 20.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303:1295–302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- 21.Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: A systematic review and literature synthesis. Pain Med. 2009;10:639–53. doi: 10.1111/j.1526-4637.2009.00632.x. [DOI] [PubMed] [Google Scholar]
- 22.Daubs MD, Norvell DC, McGuire R, Molinari R, Hermsmeyer JT, Fourney DR, Wolinsky JP, Brodke D. Fusion versus nonoperative care for chronic low back pain: Do psychological factors affect outcomes? Spine (Phila Pa 1976) 2011;36:S96–109. doi: 10.1097/BRS.0b013e31822ef6b9. [DOI] [PubMed] [Google Scholar]
- 23.Wasan AD, Jamison RN, Pham L, Tipirneni N, Nedeljkovic SS, Katz JN. Psychopathology Predicts the Outcome of Medial Branch Blocks with Corticosteroid for Chronic Axial Low Back or Cervical Pain: A Prospective Cohort Study. BMC Musculoskelet Disord. 2009;10:1–9. doi: 10.1186/1471-2474-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karp JF, Yu L, Friedly J, Amtmann D, Pilkonis PA. Negative Affect and Sleep Disturbance May Be Associated With Response to Epidural Steroid Injections for Spine-Related Pain. Arch Phys Med Rehabil. 2014;95:309–15. doi: 10.1016/j.apmr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–93. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 26.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151:625–32. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31:564–70. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychological adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin J Pain. 2007;23:307–15. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- 29.Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10:304–11. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, Jamison RN. Craving of prescription opioids in patients with chronic pain: a longitudinal outcomes trial. J Pain. 2012;13:146–54. doi: 10.1016/j.jpain.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martel MO, Dolman AJ, Edwards RR, Jamison RN, Wasan AD. The Association Between Negative Affect and Prescription Opioid Misuse in Patients With Chronic Pain: The Mediating Role of Opioid Craving. J Pain. 2014;15:90–100. doi: 10.1016/j.jpain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SM, Dart RC, Katz NP, Paillare F, Adams EH, Comer SD, Degroot A, Edwards RR, Grant S, Haddox JD, Jaffe JH, Jones CM, Kleber HD, Kopecky EA, Markman JD, Montoya ID, O'Brien C, Roland CL, Savage SR, Stanton M, Strain EC, Vorsanger GJ, Wasan AD, Weiss RD, Turk DC, Dworkin RH. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:2287–96. doi: 10.1016/j.pain.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9:360–72. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–56. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martel MO, Wasan AD, Jamison RN, Edwards RR. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug Alcohol Depend. 2013;132:335–41. doi: 10.1016/j.drugalcdep.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werneke MW, Hart DL. Categorizing Patients With Occupational Low Back Pain by Use of the Quebec Task Force Classification System Versus Pain Pattern Classification Procedures: Discriminant and Predictive Validity. Phys. Ther. 2004;84:243–254. [PubMed] [Google Scholar]
- 37.Sheehan DV, Lecrubier Y, Sheehan KH. The Mini International Neuropsychiatric Interview (M.I.N.I.): the Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J. Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 38.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–78. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 39.Fardon D, Milette P. Nomenclature and Classification of Lumbar Disc Pathology: Recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and the American Society of Neuroradiology. Spine. 2001:E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 40.Aprill C, Bogduk N. High-Intensity Zone: A Diagnostic Sign of Painful Lumbar Disc on Magnetic Resonance Imaging. Brit J Radiol. 1992;65:361–69. doi: 10.1259/0007-1285-65-773-361. [DOI] [PubMed] [Google Scholar]
- 41.Wasan AD, Loggia ML, Chen LQ, Napadow V, Kong J, Gollub RL. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115:364–74. doi: 10.1097/ALN.0b013e318220e880. doi: 10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wasan AD, Kong J, Pham LD, Kaptchuk TJ, Edwards R, Gollub RL. The impact of placebo, psychopathology, and expectations on the response to acupuncture needling in patients with chronic low back pain. J Pain. 2010;11:555–63. doi: 10.1016/j.jpain.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. ACTA Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 44.Bjelland I, Dahl AA, Huag TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 45.Cosco TD, Doyle F, Ward M, McGee H. Latent structure of the Hospital Anxiety And Depression Scale: a 10-year systematic review. J Psychosom Res. 2012;72:180–4. doi: 10.1016/j.jpsychores.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Doyle F, Cosco T, Conroy R. Why the HADS is still important: Reply to Coyne & van Sonderen. J Psychosom Res. 2012;73:74. doi: 10.1016/j.jpsychores.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 48.Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–52. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 49.Backonja MM, Krause SJ. Neuropathic pain questionnaire--short form. Clin J Pain. 2003;19:315–16. doi: 10.1097/00002508-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Costa PT, McCrae RR. The NEO personality inventory manual. Orlando, Psychological Assessment Resources. 1985:1–131. [Google Scholar]
- 51.Sullivan MJ, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psych Assess. 1995;7:524–32. [Google Scholar]
- 52.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stuckl G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core Outcome Measures for Chronic Pain Clinical Trials: IMMPACT Recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Moulin DE, Iezzi A, Amireh R, Sharpe WKJ, Boyd D, Merskey H. Randomised Trial of Oral Morphine for Chronic Non-Cancer Pain. Lancet. 1996;347:143–47. doi: 10.1016/s0140-6736(96)90339-6. [DOI] [PubMed] [Google Scholar]
- 54.Jamison RN, Raymond SA, Slawsby EA, Nedeljkovic SS, Katz NP. Opioid Therapy for Chronic Noncancer Back Pain. Spine. 1998;23:2591–2600. doi: 10.1097/00007632-199812010-00014. [DOI] [PubMed] [Google Scholar]
- 55.Meltzer EC, Rybin D, Saitz R, Samet JH, Schwartz SL, Butler SF, Liebschutz JM. Identifying prescription opioid use disorder in primary care: Diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain. 2011;152:397–402. doi: 10.1016/j.pain.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu SM, Compton P, Bolus R, Schieffer B, Pham Q, Baria A, Van Vort W, Davis F, Shekelle P, Naliboff B. The Addiction Behaviors Checklist: Validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Sym Manage. 2006;32:342–52. doi: 10.1016/j.jpainsymman.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Mallinckrodt CH, Lane PW, Schnell D, Peng Y, Mancuso JP. Recommendations for the primary analysis of continuous endpoints in longitudinal clinical trials. Drug Information Journal. 2008;42:303–19. [Google Scholar]
- 58.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. Pain. 2010;150:390–400. doi: 10.1016/j.pain.2010.02.033. doi: 10.1016/j.pain.2010.02.033. Epub 2010 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wasan AD, Butler SF, Budman SH, Fernandez K, Wesiss R, Greenfield S, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin J Pain. 2009;25:193–98. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, Tu W. Optimized antidepressant therapy and pain self-managment in primary care patients with depression and musculoskeletal pain. JAMA. 2009;301:2099–2110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goubert L, Crombez G, Van Damme S. The Role of Neuroticism, Pain Catastrophizing, and Pain-Related Fear in Vigilance to Pain: a Structural Equations Approach. Pain. 2004;107:234–41. doi: 10.1016/j.pain.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Smeets RJ, Maher CG, Nicholas MK, Refshauge KM, Herbert RD. Do psychological characteristics predict response to exercise and advice for subacute low back pain? Arthritis Rheum. 2009;61:1202–9. doi: 10.1002/art.24731. [DOI] [PubMed] [Google Scholar]
- 63.Rauck RL, Nalamachu S, Wild JE, Walker GS, Robinson CY, Davis CS, Farr SJ. Single-Entity Hydrocodone Extended-Release Capsules in Opioid-Tolerant Subjects with Moderate-to-Severe Chronic Low Back Pain: A Randomized Double-Blind, Placebo-Controlled Study. Pain Med. 2014;15:975–85. doi: 10.1111/pme.12377. [DOI] [PubMed] [Google Scholar]
- 64.Hale M, Khan A, Kutch M, Li S. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain. Curr Med Res Opin. 2010;26:1505–18. doi: 10.1185/03007995.2010.484723. [DOI] [PubMed] [Google Scholar]
- 65.Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2009:CD007407. doi: 10.1002/14651858.CD007407.pub2. [DOI] [PubMed] [Google Scholar]
- 66.George SZ, Zeppieri G, Jr., Cere AL, Cere MR, Borut MS, Hodges MJ, Reed DM, Valencia C, Robinson ME. A randomized trial of behavioral physical therapy interventions for acute and sub-acute low back pain ( NCT00373867). Pain. 2008;140:145–57. doi: 10.1016/j.pain.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]