Abstract
Sodium bicarbonate cotransporters (NBCs) are involved in the pH regulation of salivary glands. However, the roles and regulatory mechanisms among different NBC isotypes have not been rigorously evaluated. We investigated the roles of two different types of NBCs, electroneutral (NBCn1) and electrogenic NBC (NBCe1), with respect to pH regulation and regulatory mechanisms using human submandibular glands (hSMGs) and HSG cells. Intracellular pH (pHi) was measured and the pHi recovery rate from cell acidification induced by an NH4Cl pulse was recorded. Subcellular localization and protein phosphorylation were determined using immunohistochemistry and co-immunoprecipitation techniques. We determined that NBCn1 is expressed on the basolateral side of acinar cells and the apical side of duct cells, while NBCe1 is exclusively expressed on the apical membrane of duct cells. The pHi recovery rate in hSMG acinar cells, which only express NBCn1, was not affected by pre-incubation with 5 μM PP2, an Src tyrosine kinase inhibitor. However, in HSG cells, which express both NBCe1 and NBCn1, the pHi recovery rate was inhibited by PP2. The apparent difference in regulatory mechanisms for NBCn1 and NBCe1 was evaluated by artificial overexpression of NBCn1 or NBCe1 in HSG cells, which revealed that the pHi recovery rate was only inhibited by PP2 in cells overexpressing NBCe1. Furthermore, only NBCe1 was significantly phosphorylated and translocated by NH4Cl, which was inhibited by PP2. Our results suggest that both NBCn1 and NBCe1 play a role in pHi regulation in hSMG acinar cells, and also that Src kinase does not regulate the activity of NBCn1.
Introduction
The ability to maintain intracellular pH (pHi) homeostasis is critical, and dysregulated pHi is connected with several diseases [1]. Furthermore, pHi can influence various metabolic reactions and vascular functions [2, 3]. There are two major types of proteins that regulate pHi, namely, Na+-H+ exchangers (NHEs) and Na+-HCO3 - cotransporters (NBCs). Moreover, bicarbonate ion (HCO3 -), which functions as a buffer that provides optimal pH, is one of the crucial ions in epithelial cells and fluctuations in the concentration of HCO3 - in final fluids is associated with several epithelial diseases [4]. HCO3 - ions in saliva are also protective against enamel erosion under low pH conditions [5]. Thus, bicarbonate transporter is an important protein in the epithelia, especially salivary glands.
NBCs are classified into either electrogenic (NBCe1) or electroneutral (NBCn1) types according to their net transport activity [6]. Human submandibular glands (hSMGs) express two NBC variants, namely NBCe1-B and NBCn1, which were originally cloned as pancreatic and Cl--independent electroneutral NBCs, respectively [7–9]. NBCe1s are further divided into NBCe1-A, -B and -C. NBCe1-A and NBCe1-B are identical except for their N-terminal domains, which are 41 and 85 amino acids in length, respectively [10]. NBCe1-C is the longest of the three NBCe1 variants, and is identical to NBCe1-B except for a unique 61 C-terminal amino acid sequence, which replaces the 46 amino acids of the C-terminus of NBCe1-B [11]. Despite significant research, the role of NBCs in pH regulation as well as the regulatory mechanisms and subcellular localizations of the different NBCs isotypes in hSMG remain elusive.
Protein phosphorylation, a common mechanism of protein regulation, is mediated via the addition of phosphate groups onto serine, threonine, or tyrosine residues. In addition to NBCs, CFTR [12] and neuronal channels such as potassium channels [13] and NMDA receptors [14] are controlled by Src family tyrosine kinases (SFK). In renal epithelial cells, non-receptor tyrosine kinase proline-rich tyrosine kinase 2 (Pyk2) increases the activity of NBCe1 by autophosphorylation and interactions with Pyk2-Src family kinases [15]. However, the role and regulatory mechanisms of the specific isotypes of NBCe1 and NBCn1 in hSMG remain poorly understood. Interestingly, we found that the Src kinase inhibitor PP2 alters pHi regulation in an HSG cell line originating from hSMG duct cells.
In the present study, we studied the expression of NBCn1 and NBCe1-B in hSMG and HSG cells. We also examined whether NBCn1 in hSMG cells plays a role in pHi regulation and investigated its regulatory mechanism via tyrosine phosphorylation compared with NBCe1-B.
Materials and Methods
Source of Human Submandibular glands (hSMGs)
Human submandibular glands (hSMGs) were obtained from patients who underwent resection of their submandibular gland as part of their treatment for oral tumors. The patient group included both males and females ranging from 37 to 82 years of age. Tissues were kept in cold physiological saline while transporting the dissected gland from the hospital to the laboratory for analysis. Some of the tissues were fixed with 4% paraformaldehyde for immunohistochemistry studies while the remainder were prepared for physiological experiments. All patients gave written informed consent for participation in this study. The collection and use of human tissue was performed according to ethical guidelines and the study protocol was approved by the Institutional Review Board of Seoul National University Dental Hospital (CRI11023G).
hSMG acinar cell preparation
hSMG acinar cells were prepared as described previously [16]. Briefly, after trimming fat and connective tissues, tissues were minced with scissors in an ice-cold Ca2+-free incubation solution containing 130 mM NaCl, 4.5 mM KCl, 1 mM NaH2PO4·2H2O, 1 mM MgCl2, 10 mM D-glucose, and 10 mM HEPES at pH7.4. The minced tissue was then incubated for 60 min at 37°C in a Ca2+-free incubation solution containing 2 mg/mL trypsin inhibitor (Sigma, St. Louis, MO, USA), 0.04 mg/mL collagenase P (Worthington, Lakewood, UK), and 1% BSA. During the incubation period, the tissue was mechanically dissociated by repeated pipetting with different sizes of 1 mL pipet tips at 20 min intervals. The isolated acinar cells and cell clusters were then filtered through a 200 μm nylon mesh to remove large debris and harvested by centrifugation.
Cell culture
HSG cells, which were isolated from human submandibular gland intercalated duct cells [17], were cultured in Dulbecco’s modified Eagle’s medium (Welgene, Republic of Korea) supplemented with 10% fetal bovine serum (Welgene) and 1% penicillin/streptomycin (Gibco, Carlsbad, CA, USA) at 37°C in a 5% CO2 atmosphere.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from hSMG acinar cells obtained from the hSMG acinar cell preparation, whole hSMG, and HSG cells using Trizol (Invitrogen, Carlsbad, CA, USA). Reverse transcription reactions were performed using 1 μg total RNA to generate cDNA (Invitrogen). PCR was performed with 1 μL of cDNA and specific primers (Table 1). The cycling parameters were as follows: 32 cycles of denaturation at 95°C for 30s, annealing for 30s, and extension at 72°C for 30s, followed by a final extension at 72°C for 10 min. Products from RT-PCR reactions were sequenced to confirm their identity.
Table 1. List of DNA primers sequences designed for RT-PCR.
| Target gene | Forward primers | Reverse primers | Length | GenBank no. |
|---|---|---|---|---|
| NBCe1-A | ACCTTGGGGAGAGAGGAAGA | TCCTTCCACTCCATCTCCTG | 214 bp | NM_003759.3 |
| NBCe1-B/C | TGGAGGATGAAGCTGTCCTG | TGCAGCAGGAGAGATGAGAG | 266 bp | NM_001134742.1 / NM_001098484.2 |
| NBCe1-A/B/C | AGCATGACCTCAGCTTCCTG | CAGCATGATGTGTGGCGTTC | 253 bp | NM_003759.3 / NM_001098484.2 |
| NBCe1-A/B/C | AGCATGACCTCAGCTTCCTG | CAGCATGATGTGTGGCGTTC | 156 bp | NM_001134742.1 |
| NBCn1 | CCCAGTCTGCTCCTGGAAAC | ACCCTGTAAGGAGGACAGCA | 234 bp | NM_003615.4 |
| AQP5 | TCCATTGGCCTGTCTGTCAC | CACTCAGGCTCAGGGAGTTG | 211 bp | NM_001651.3 |
| GAPDH | CATCACTGCCACCCAGAAGA | GTCAAAGGTGGAGGAGTGGG | 349 bp | NM_001289745.1 |
pHi measurements
Intracellular pH (pHi) measurements were performed as described previously [18]. HSG cells were loaded with 2 μM BCECF-AM (Molecular Probes, Eugene, OR, USA) for 30 min at 37°C in DMEM medium (10% FBS and 1% penicillin/streptomycin) and washed twice with PBS. Isolated hSMG acinar cells were incubated with 2 μM BCECF-AM for 30 min at room temperature in normal HEPES solution with 0.2% BSA and washed twice with normal HEPES. The standard HCO3 --buffered solution consisted of 10 mM D-glucose, 10 mM HEPES, 115 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 25 mM NaHCO3, and equilibrated with 95% O2 + 5% CO2 gas. For intracellular acidification, cells were perfused with 20 mM NH4Cl in a Na+-free bath solution. In Na+-free HCO3 --buffered solutions, NaCl, and/or NaHCO3 were replaced with equimolar concentrations of N-methyl- D-glucamine Cl and choline-HCO3 -, respectively. All solutions were adjusted to pH 7.4 at 37°C. pHi recordings were obtained using a MetaFlour imaging system and BCECF was excited at 440 and 490 nm with an emission wavelength of 530 nm. The resulting excitation/emission ratios were converted into pH values using a nigericin-based calibration technique. S1 Fig shows calibration curves. Specifically, cells were perfused with calibration solutions containing 10 mM NaCl, 130 mM KCl, 0.8 mM MgCl2, 20 mM HEPES, and 0.005 mM nigericin corresponding to pH 6.2, 6.6, 7.0, 7.4, and 7.8.
Immunofluorescence
hSMG tissues were cut into small pieces, fixed with 4% paraformaldehyde for at least 48 hours at 4°C, and embedded in paraffin. After microdissecting the block into 10 μm thick sections, the tissues were deparaffinized with Histo-Clear II and rehydrated with a graded series of ethanol (100%, 90%, 80%, and 70%). Sections were incubated in pepsin antigen retrieval solution for 15 minutes at 37°C and permeabilized in PBS containing 0.1% Triton X-100. After permeabilization, the tissues were covered with a blocking solution consisting of PBS with 20% normal donkey serum for 1 h at room temperature. Next, the cells were stained with either rabbit anti-NBCe1 (ab78326, Abcam) or rabbit anti-NBCn1 (ab82335, Abcam) followed by Alexa Flour 468 donkey anti-rabbit IgG (Invitrogen) at a dilution of 1:400 to detect NBCe1 and NBCn1, respectively. Finally, tissues were mounted with Vectashield mounting medium (Vector Laboratories) and visualized using a laser scanning confocal microscope (LSM 700, Carl Zeiss).
HSG cells were grown on cover-glass bottom dishes, fixed in ice-cold methanol for 15 minutes at -20°C, and then washed in ice-cold PBS three times for 5 minutes. The samples were then incubated in PBS containing 1% Triton X-100 for 10 minutes at room temperature, followed by washing in PBS three times and re-incubating in blocking solution consisting of PBS with 10% normal donkey serum for 1 hour at room temperature. The primary and secondary antibodies as well as methods for detection and visualization were the same as those described for hSMG tissues.
Plasmid Construction, Transfection, and Co-immunoprecipitation
NBCe1-B was cloned in pFlag-CMV-2 vector for expression in HSG cells. The NBCn1 construct in pcDNA3.1 was a gift from Dr. Jeong Hee Hong and Dr. Shmuel Muallem. pFlag-CMV-2-NBCe1-B, pcDNA3.1-NBCn1, and empty vectors were transfected into HSG cells using Lipofectamine 2000 (Invitrogen). Co-immunoprecipitation experiments were performed as described previously [18].
Reagents
5-(N-ethyl-N-isopropyl) amiloride (EIPA) and 4,4-diisothiocyanostilbene-2,2-disulfonic acid (DIDS) were obtained from Sigma-Aldrich.
Statistical analysis
Data are presented as the mean ± SEM and n is the number of experimental repeats with different hSMG sample preparations and HSG cultures. Differences between means were evaluated by ANOVA followed by post-hoc test for determining statistical analysis where appropriate. Statistical significance of experiments with multiple comparisons was assessed by analysis of variance; p < 0.05 was considered significant. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Results
Expression of NBCe1 and NBCn1 in human submandibular glands (hSMGs) and an HSG cell line
We used RT-PCR to investigate the expression of various isotypes of sodium bicarbonate cotransporters (NBCs) in human submandibular glands (hSMGs) and HSG cells originating from hSMG ducts. Three types of primers were designed for NBCe1, one to detect NBCe1-A only at 214 bp, another to detect both NBCe1-B and -C at 266 bp, and the other to detect each of the NBCe1 isoforms (NBCe1-A/B/C, Table 1) at 253 bp and 156 bp corresponding to NBCe1-A/B and NBCe1-C, respectively. The NBCe1 mRNA transcripts, except NBCe1-A, were expressed in whole hSMGs and HSG cell lines, but appeared as a faint band in hSMG acini (Fig 1A). With respect to the NBCe1-A/B/C primer, only the 253 bp product was detected, indicating that only NBCe1-B, but not NBCe1-C, was expressed at the mRNA level in hSMG duct cells and HSG cells. NBCe1-B/C mRNA transcripts were also detected, indicating that NBCe1-B is expressed at the mRNA level in both hSMG ducts and HSG cells. These findings were consistent with the results of our previous study [18]. On the other hand, NBCn1 mRNA transcripts were detected in hSMG acinar cells, whole hSMGs, and the HSG cell line. In these assays, AQP5 was used as an acinar cell marker [19].
Fig 1. NBCe1 and NBCn1 are expressed in human submandibular gland (hSMG) and HSG cells.
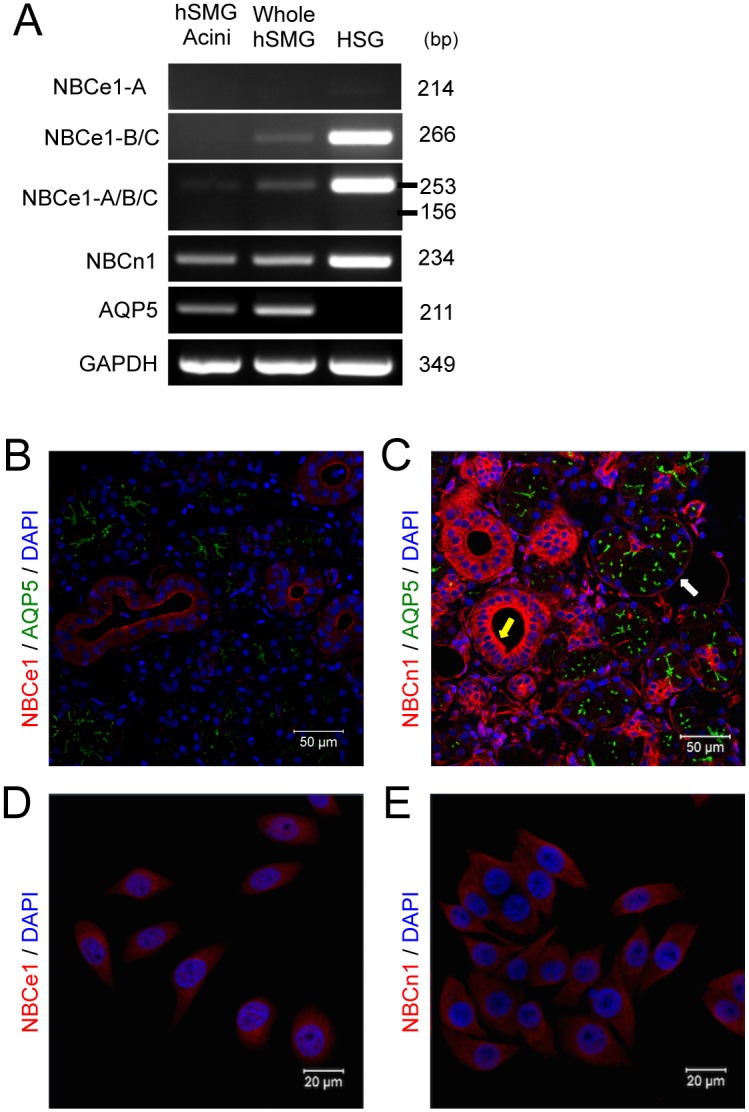
(A) NBCe1 and NBCn1 mRNA transcripts in hSMG and HSG cells. Aquaporin 5 (AQP5) was used as a marker for acinar cells. (B and C) hSMG tissue sections were stained with NBCe1, NBCn1, and AQP5 antibodies. (Bar = 50 μm). AQP5 was used as a marker for acinar cells. White and yellow arrows indicate acinar cells and duct cells, respectively. NBCe1-B is expressed in human submandibular gland (hSMG) duct cells, whereas NBCn1 is expressed in acinar (white arrow) and duct cells (yellow arrow). (D and E) HSG cells were stained with antibodies for NBCe1 and NBCn1. (Bar = 20 μm).
To confirm the protein expression and localization of NBCe1-B and NBCn1 in hSMGs and HSG cells, immunostaining was performed with NBCe1 and NBCn1 antibodies. NBCe1 was strongly expressed on the apical side of all hSMG duct cells (Fig 1B), whereas NBCn1 was expressed on the basal side of acinar cells (white arrows, Fig 1C) and on the lateral and possibly basal membrane of duct cells (yellow arrow, Fig 1C). NBCe1 and NBCn1 were diffusely located in both the cytosol and membrane in HSG cells (Fig 1D and 1E). Taken together, these data demonstrated that NBCe1-B is expressed in hSMG ducts and HSG cells, whereas NBCn1 is expressed in the acinar and duct cells of hSMGs and HSG cells.
Intracellular pH (pHi) regulation and the effect of Src tyrosine kinase in hSMG acini and HSG cells
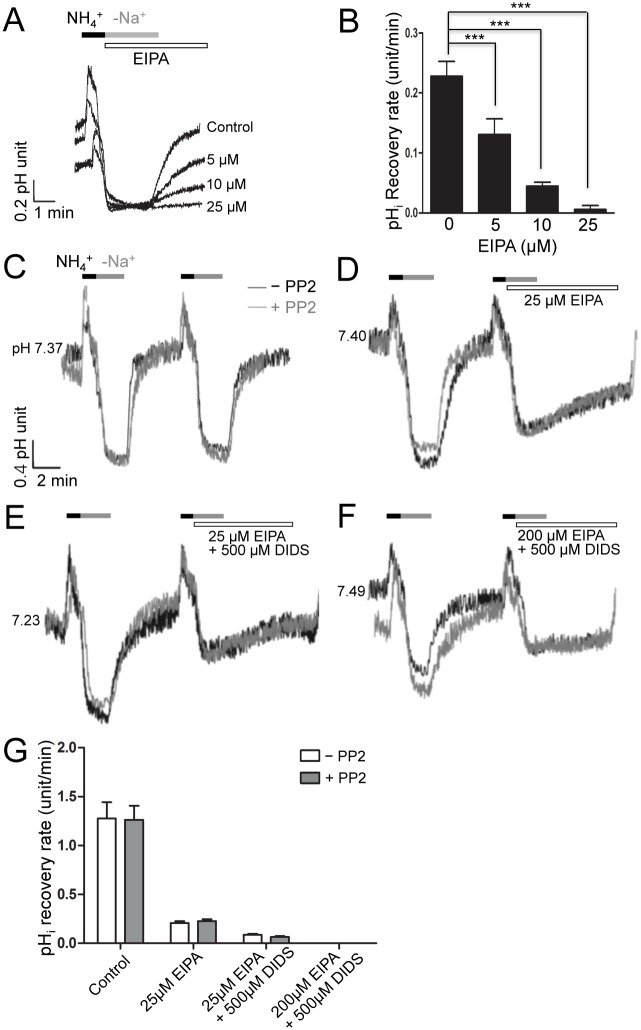
To evaluate the activity of NBCs on pHi recovery, Na+-H+ exchanger (NHE) activity should be blocked, as NHEs also regulate intracellular pH. We first examined NHE activity on Na+-dependent pHi recovery from cell acidification induced by an NH4 + pulse in hSMG acinar cells in a HEPES buffered solution (Fig 2A). The pHi recovery rate mediated by NHE was inhibited by 5-(N-ethyl-N-isopropyl) amiloride (EIPA) in a concentration dependent manner. In addition, the pHi recovery rate of 0.228 ± 0.010 pH units/min (n = 6) in resting states was completely inhibited by 25 μM EIPA, a specific NHE inhibitor (n = 6, Fig 2B).
Fig 2. Src tyrosine kinase does not affect pHi recovery of hSMG acinar cells.
(A and B) The intracellular pH recovery patterns of hSMG acinar cells in the absence or presence of several concentrations of EIPA in HEPES-buffered solution (HBS) were measured and the pHi recovery rates were summarized. (C-F) The pHi recovery patterns of hSMG acinar cells following an NH4 +-pulse (blank bar) were recorded in a bicarbonate-buffered bath solution (BBS). The cells were pretreated for 20 min with 5 μM PP2, a Src tyrosine kinase inhibitor (grey trace) or incubated in normal BBS (black trace). The effects of treatment with EIPA and DIDS are shown using horizontal bars. (G) Summary of pHi recovery rates. The data are presented as the mean ± S.E.
We measured pHi in an HCO3 --buffered solution (BBS) to investigate NBCs activity. The pHi of the unstimulated cell was 7.36 ± 0.05 (n = 16) in BBS. When the cell was exposed to NH4Cl, the pHi was increased to 9.16 ± 0.13 (n = 16), and then the cell was acidified to pH 6.31 ± 0.04 (n = 16). As shown in Fig 2C, pre-incubation of cells for 20 min with 5 μM PP2, a Src tyrosine kinase inhibitor, had little effect on pHi recovery in hSMG acinar cells in BBS. Further, the pHi recovery rate (grey lines, 1.263 ± 0.142 pH units/min, n = 12) was not significantly different from that of control cells (black lines, 1.277 ± 0.166 pH units/min, n = 12). Continued recovery of pHi was observed in the presence of 25 μM EIPA (0.207 ± 0.018 pH units/min, n = 9, Fig 2D), indicating that remnant pHi recovery was mediated by NBCn1. In addition, the pHi recovery rate of PP2 pretreated hSMG acinar cells (grey lines, 0.228 ± 0.018 pH units/min, n = 8) was not significantly different from control cells. The pHi recovery mediated by NBCn1 was further inhibited by 25 μM EIPA and 4,4-diisothiocyanostilbene-2,2-disulfonic acid (DIDS) (0.087 ± 0.011 pH units/min, n = 8, Fig 2E), and was completely blocked in the presence of high concentrations of EIPA and DIDS (n = 8, Fig 2F). Together, these data suggested that NBCn1 regulates intracellular pH in hSMG acinar cells, but is not affected by Src tyrosine kinase. Fig 2G summarizes the results of these experiments.
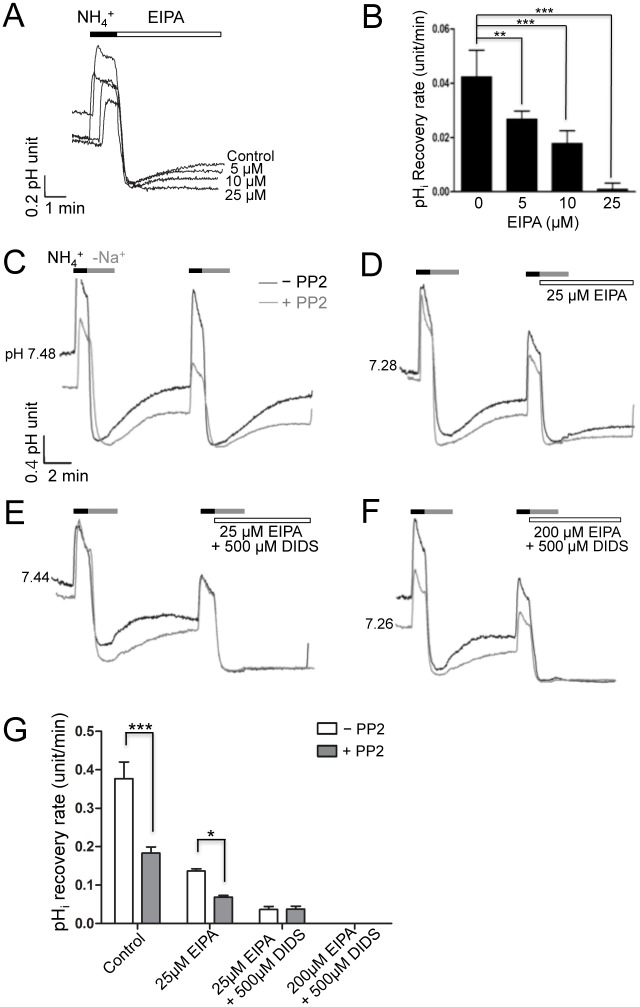
In addition to hSMG acinar cells, we also studied the activities of NBCs in an HSG cell line. Specifically, we first confirmed the effective concentration of EIPA needed to block NHE activity in HSG cells by measuring its effect on the rate of pHi recovery in a HEPES buffered solution (Fig 3A). We found that the pHi recovery rate of 0.042 ± 0.004 pH units/min (n = 5) was completely inhibited by 25 μM EIPA (n = 6, Fig 3B).
Fig 3. pHi recovery of HSG cells is inhibited by PP2.
(A and B) The pHi of HSG cells in HBS was obtained in the absence or presence of 5, 10, or 25 μM EIPA and the results were summarized (C-F) pHi measurements were performed using HSG cells in BBS, and the effects of pre-treatment with 5 μM PP2 for 20 min were evaluated (grey trace). (G) Summary of pHi recovery rates in HSG cells. The data are presented as the mean ± S.E. (error bars) (*, P < 0.05; ***, P < 0.001)
We next measured pHi in BBS to examine the effect of PP2 on NBCe1-B and NBCn1 activities in HSG cells using the same technique as for hSMG acinar cells. The pHi recovery rate in HSG cells (0.377 ± 0.043 pH units/min, n = 9, Fig 3C, black line) was less than that of hSMG acinar cells (Fig 2C), and was decreased by ~50% upon pre-incubation with 5 μM PP2 (0.183 ± 0.016 pH units/min, n = 9, Fig 3C, grey line). When the cells were exposed to 25 μM EIPA to inhibit the NHE activity, the pHi recovery rate of PP2 pretreated HSG cells (0.069 ± 0.004 pH units/min, n = 10, Fig 3D, grey line) was significantly different from control treated cells (0.136 ± 0.006 pH units/min, n = 9, Fig 3D, black line). In addition, the pHi recovery by NBCe1-B and NBCn1 was inhibited by 25 μM EIPA supplemented with DIDS (0.037 ± 0.008 pH units/min, n = 7, Fig 3E) and was completely blocked by a combination of DIDS and 200 μM EIPA (n = 5, Fig 3F). The pHi recovery rates of HSG cells are summarized in Fig 3G.
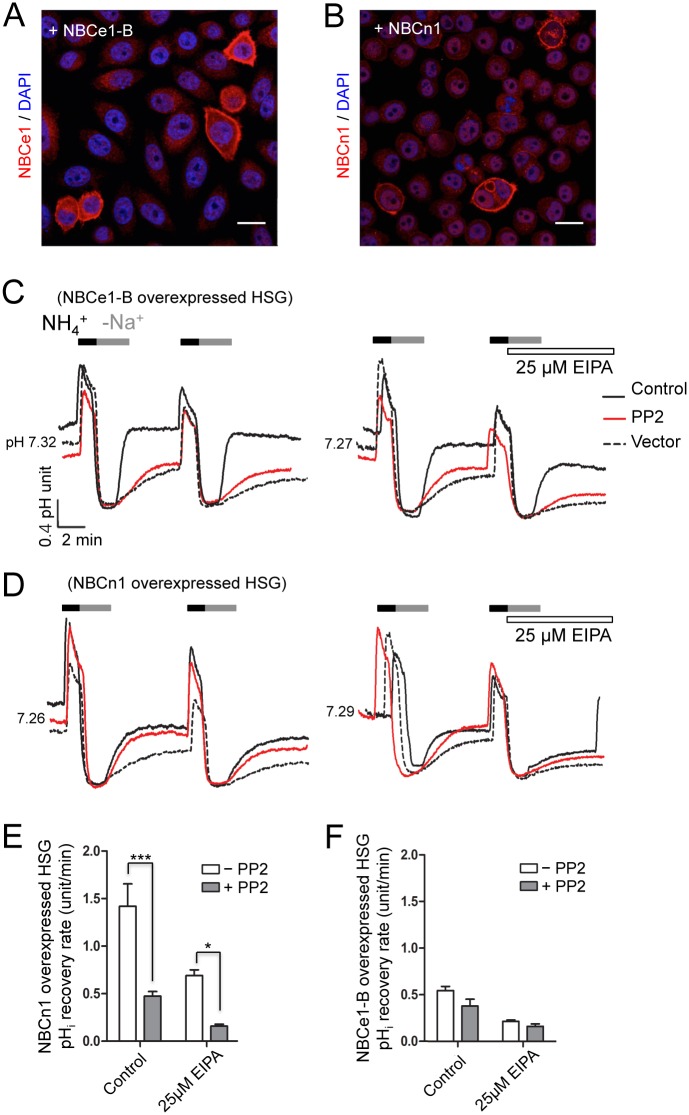
Overexpression of NBCe1-B and NBCn1 in HSG cells
To establish definitively whether Src kinase modulate the activity of NBCe1-B or NBCn1, HSG cells overexpressing either NBCe1-B or NBCn1 were generated and the subsequent effect on intracellular pH was measured. Overexpression of NBCe1-B and NBCn1 was confirmed via immunocytochemistry (Fig 4A and 4B). Overexpression of NBCe1-B increased the pHi recovery rate (1.419 ± 0.235 pH units/min, n = 12, Fig 4C, black trace), but this was suppressed by PP2 (0.474 ± 0.048 pH units/min, n = 12, red trace). Moreover, addition of 25 μM EIPA to NBCe1-B overexpressing cells shifted the pHi recovery rate to 0.690 ± 0.060 pH units/min (n = 11), which was decreased to 0.159 ± 0.021 pH units/min (n = 11) upon pre-treatment with PP2, suggesting that NBCe1-B is affected by PP2.
Fig 4. Transfected NBCe1-B is affected by PP2.
(A and B) Flag-NBCe1-B and NBCn1 were transfected into HSG cells and overexpression was confirmed by immunofluorescence assay. (C and D) pHi recovery rates were recorded in HSG cells overexpressing NBCe1-B or NBCn1. Horizontal bars indicate all applications. (E and F) Graphical summary of pHi recovery rates. The data are presented as the mean ± S.E. (error bars) (*, P < 0.05; ***, P < 0.001).
Overexpression of NBCn1 in HSG cells also increased the pHi recovery rate (0.493 ± 0.067 pH units/min, n = 10, Fig 4D, black trace) compared with control cells (0.377 ± 0.043 pH units/min, dotted trace), indicating that NBCn1 regulates pHi similar to NBCe1-B. On the other hand, the pHi recovery rate induced by NBCn1 overexpression was not significantly decreased by PP2 (0.381 ± 0.073 pH units/min, n = 8, red trace). Likewise, upon incubation with 25 μM EIPA, a pHi recovery rate of 0.218 ± 0.012 pH units/min (n = 3) was noted, which decreased to 0.162 ± 0.027 pH units/min (n = 4) in the presence of PP2; however, this difference was not significant, suggesting that NBCn1 is not regulated by Src kinase. The pHi recovery rates of HSG cells overexpressing NBCe1-B and NBCn1 are summarized in Fig 4E and 4F. The above results were consistent with the shown in Figs 2 and 3, indicating that Src kinase regulates the activity of NBCe1-B only, and not that of NBCn1.
NBCe1-B tyrosine residue phosphorylation and NBCe1-B translocation by Src kinase
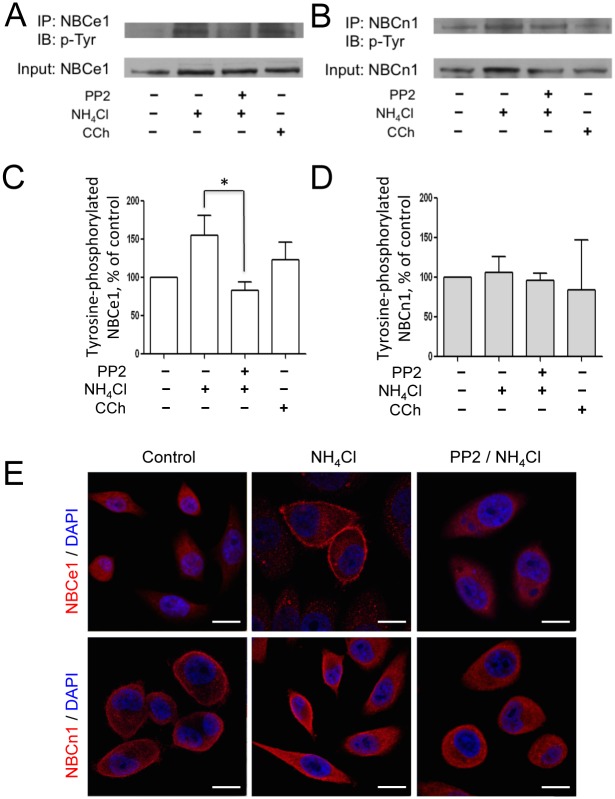
We next hypothesized that Src tyrosine kinase may affect NBC activity by phosphorylating tyrosine residues on NBCe1-B or NBCn1. To investigate this possibility, we performed co-immunoprecipitation assays using HSG cells. The degree of NBCe1-B tyrosine phosphorylation was significantly increased by NH4Cl, but was suppressed by PP2 (n = 4, Fig 5A and 5C). Contrary to NBCe1-B, tyrosine on NBCn1 was not affected either by NH4Cl or PP2, indicating that Src tyrosine kinase has no effect on NBCn1 phosphorylation (n = 4, Fig 5B and 5D). Carbachol was used as a positive control to stimulate NBCs.
Fig 5. PP2 inhibits tyrosine phosphorylation and translocation of NBCe1-B, but NBCn1, in HSG cells.
(A and B) Cell lysates were subjected to immunoprecipitation with NBCe1 and NBCn1 antibodies and evaluated by Western blotting with a phosphotyrosine antibody. The cells were pre-incubated in 20 mM of NH4Cl for 2 mins, in 5 μM of PP2 for 20 mins, and in 50 μM of CCh for 5 mins. The input control comprised 5% of the lysates. (C and D) Phosphorylated NBCe1 and NBCn1 were quantified based on protein band intensities. The data are shown as the mean ± S.E. (error bars) (n = 4; *, P < 0.05). (E) Locations of NBCe1 and NBCn1 in response of ammonium pulse in the presence or absence of PP2 were confirmed using immunocytochemistry. (Bar = 20 μm).
Immunocytochemistry analysis also showed that the NBCe1-B, which was located at the plasma membrane by NH4Cl, remained in the cytosol when Src tyrosine kinase was inhibited by PP2 pre-treatment (Fig 5E). On the other hand, NBCn1 was constitutively expressed on the plasma membrane side. Consistent with our result thus far, these findings suggested that Src tyrosine kinase influences only the activity of NBCe1-B, and not that of NBCn1.
Discussion
In the present study, we identified the expression and cellular localization of specific isoforms of NBCe1 and NBCn1 in human submandibular glands (hSMG) by RT-PCR and immunohistochemistry. Intracellular pH measurement and co-immunoprecipitation studies confirmed the activities of NBCs, especially NBCe1-B and NBCn1 in hSMG acinar and HSG cells. In addition, our data demonstrated that only NBCe1-B activity is regulated by Src tyrosine kinase.
We first asked which NBC isoforms are expressed in hSMGs. NBC expression was previously demonstrated in an HSG cell line originating from hSMG ducts. We confirmed that all of the NBC isoforms in hSMG ducts were expressed in an identical pattern in HSG cells. Specifically, RT-PCR and immunofluorescence studies were used to determine the isoforms of NBCe1 or NBCn1 expressed in hSMGs and HSG cells and their cellular localization. Our immunohistochemical studies demonstrated that NBCn1 was expressed at the basolateral membrane of acinar cells in hSMGs (Fig 1). Consistent with this data, NBCn1 is also expressed on the basolateral side of the ParC5 rat parotid acinar cell line [20, 21], suggesting that NBCn1 may be involved in HCO3 - influx and pHi regulation in acinar cells as well as in other secretory epithelial cells [22–24], including the murine duodenum [25]. In our experiments, NBCn1 appeared to function as a major pHi regulator in hSMG acinar cells. Specifically, decreased pHi in hSMG acinar cells evoked by an ammonium pulse recovered rapidly to prestimulus levels, which was inhibited by high concentrations of EIPA, an inhibitor of NBCn1 [24, 26] (Fig 2). On the other hand, NBCn1, referred to as NBC3 (GeneBank accession no. 047033) in Ref. 28, was expressed on the luminal side of hSMG duct cells, which was consistent with previous work on human salivary glands [27, 28]. Indeed, apical NBCn1 likely functions as an intracellular pH regulator and HCO3 - salvage mechanism to maintain acidic saliva in human salivary glands in a resting state [26, 29].
In rat parotid glands, NBCe1-B is expressed at the basolateral membrane of acinar cells [30], and is also located at the basolateral side of human parotid acinar cells as an acid extruder for intracellular pH regulation and HCO3 - ion absorption from the basolateral side [31]. However, unlike cells of the parotid gland, NBCe1-B is expressed on the apical membrane of hSMG duct cells as shown in Fig 1B, suggesting that NBCe1-B may also function as an HCO3 - salvage mechanism in resting state cells, similar to NBCn1.
Resting saliva, in which submandibular and sublingual glands play a dominant role, contain low HCO3 - concentrations [32, 33]. NBCe1 was expressed on the apical membrane of hSMG ducts (Fig 1B), and appeared to play a salvage role by absorbing HCO3 -. On the other hand, stimulated saliva in which the parotid glands play a dominant role contains high concentrations of HCO3 -. Indeed, in the human parotid glands, NBCe1 is also expressed at the basolateral membrane in acinar cells [31], which may enable parotid glands to accumulate more HCO3 -.
Although the specific mechanisms of NBCs activities were not fully identified in this study, several studies have investigated the signaling molecules that regulate the activities of NBCs such as Ca2+ [34], cAMP [35], PKA [36], and PKC [37]. In addition, a few other studies have looked into whether CO2-induced renal NBC activity is modulated by Src kinase [38, 39]. The activity of Src kinase is increased by CO2, low intracellular pH, and metabolic acidosis [40, 41]; however, there is currently no evidence regarding which specific NBC isoform is regulated by Src kinase and whether Src kinase directly phosphorylates tyrosine residues on NBCs or if it is a part of an upstream signaling cascade. Thus, in this study, we examined whether PP2, a Src kinase inhibitor, can inhibit pHi recovery mediated by two different types of NBCs. Our data demonstrated that the activity of NBCe1-B is regulated by Src kinase in hSMG acinar and HSG cells, while that of NBCn1 is not. pHi recovery was not affected by PP2 in hSMG acinar cells in which only NBCn1 was expressed (Fig 2C). On the other hand, when HSG cells containing both NBCe1-B and NBCn1 were exposed to PP2, the pHi recovery rate was significantly decreased (Fig 3C), indicating that NBCe1-B might be modulated by Src kinase. In the presence of 25 μM EIPA, the pHi recovery rate was affected by PP2 pre-treatment when both NBCe1 and NBCn1 were functionally expressed (Fig 3D). As shown in Fig 2, expression of NBCe1-B differs between hSMG acinar cells and HSG cells, in that only hSMG acinar cells express NBCn1, which is not affected by PP2. Indeed, because the HSG cell line only expresses NBCe1-B, we were able to confirm that only NBCe1-B is regulated by Src tyrosine kinase, while NBCn1 is not. The data in Fig 3C and 3D demonstrate a significant regulation of EIPA-sensitive pHi recovery, which is regulated by NHE in HSG cells, suggesting this process is influenced by Src tyrosine kinase. The result was further confirmed by the overexpression of either NBCn1 or NBCe1-B in HSG cells. Specifically, the pHi recovery rate in cells overexpressing NBCn1 was not changed by PP2 (Fig 4D), while overexpression of NBCe1-B led to a decreased pHi recovery rate following pre-treatment with PP2 (Fig 4C). Taken together, these data indicate that only NBCe1-B is modulated by Src tyrosine kinase (Fig 4C).
In the present study, we noted that phosphorylation of NBCe1 tyrosine residues by an NH4Cl pulse was suppressed by PP2 using co-immunoprecipitation experiments (Fig 5A), and that only phosphorylated NBCe1 was able to translocate from the cytoplasm to the plasma membrane (Fig 5E). However, NH4Cl did not affect the phosphorylation status of NBCn1 tyrosine residues, nor was the phospho-tyrosine of NBCn1 dephosphorylated by PP2 (Fig 5B). Carbachol (CCh) was used as a positive control in the experiments shown in Fig 5, since CCh stimulates NBCs via ERKs and a PKC-dependent pathway, which is independent of Src [42]. A schematic model summarizing the regulatory mechanisms of NBCe1-B and NBCn1 is presented in Fig 6.
Fig 6. Schematic model of NBCe1-B and NBCn1 regulation by Src kinase.
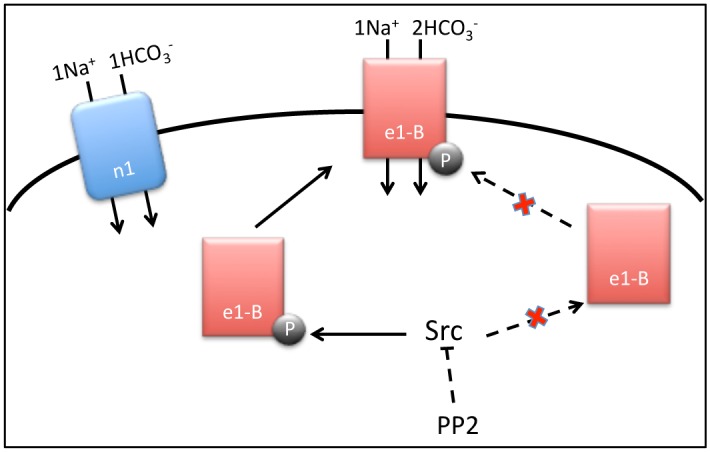
NBCe1-B and NBCn1 mediate intracellular pH in an HSG cell line and human submandibular glands, especially on the apical side of duct cells. NBCe1-B is phosphorylated by Src kinase and translocates to the plasma membrane, whereas the NBCn1 is not regulated by Src kinase. The effect of Src kinase is inhibited by PP2. Arrows indicate activation and bars indicate inhibition.
It was investigated the relationship between NBC and Src kinase from several previous studies in renal cells [38, 39]. In these studies, the NBC was identified as NBCe1-A, which is known to be expressed renal cells. Moreover, these studies only showed differences in activities of NBC by Src kinase. In the present study, we not only characterized the effects of Src kinase on NBC activity, but also the phosphorylation status of tyrosine residues on activated NBCs due to Src kinase activity. In addition, we were concerned as to why NBCn1 did not associate with Src kinase, and also which molecule is responsible for NBCn1 activation. We hypothesized that another kinase may be responsible for NBCn1 activation, since it has an abundance of putative serine phosphorylation sites compared with tyrosine phosphorylation sites (analyzed by NetPhos 2.0, http://www.cbs.dtu.dk). Moreover, differences in membrane trafficking regulation of electrogenic type and electroneutral type NBC have been observed [21].
In conclusion, we confirmed that electrogenic NBCe1-B and electroneutral NBCn1 are expressed in hSMGs. We found that NBCe1 is localized to the apical membrane of duct cells while NBCn1 is expressed on the basolateral side of acinar cells and the apical side of duct cells. We also demonstrated that NBCe1-B is modulated and phosphorylated by Src kinase, whereas NBCn1 is not regulated by Src kinase. Taken together, our results suggest that Na+-HCO3 - cotransporters, especially NBCe1-B and NBCn1, play an important role in hSMG pHi regulation and are regulated by different mechanisms. Future work will focus on the molecules that regulate NBCn1 activity and their respective mechanisms of action.
Supporting Information
(A) Fluorescence ratio (490/440 nm) changes during exposure to nigericin-containing solutions at pH 6.2, 6.6, 7.0, 7.4, and 7.8. (B) Dependence of fluorescence ratio on intracellular pH (n = 16).
(TIF)
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea, through the Oromaxillofacial Dysfunction Research Center for the Elderly (No. 2014050477) at Seoul National University in Korea.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a grant from the National Research Foundation of Korea, through the Oromaxillofacial Dysfunction Research Center for the Elderly (No. 2014050477) at Seoul National University in Korea (EN YS JB SC MK NK SH KP).
References
- 1. Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF. Regulation and roles of bicarbonate transporters in cancer. Frontiers in physiology. 2014;5:130 10.3389/fphys.2014.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nature reviews Molecular cell biology. 2010;11(1):50–61. 10.1038/nrm2820 . [DOI] [PubMed] [Google Scholar]
- 3. Schulz E, Munzel T. Intracellular pH: a fundamental modulator of vascular function. Circulation. 2011;124(17):1806–7. 10.1161/CIRCULATIONAHA.111.061226 . [DOI] [PubMed] [Google Scholar]
- 4. Kurtz I. NBCe1 as a model carrier for understanding the structure-function properties of Na(+)-coupled SLC4 transporters in health and disease. Pflugers Archiv: European journal of physiology. 2014;466(8):1501–16. 10.1007/s00424-014-1448-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catalan MA, Scott-Anne K, Klein MI, Koo H, Bowen WH, Melvin JE. Elevated incidence of dental caries in a mouse model of cystic fibrosis. PloS one. 2011;6(1):e16549 10.1371/journal.pone.0016549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. The Journal of experimental biology. 2009;212(Pt 11):1697–706. 10.1242/jeb.028563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annual review of physiology. 2005;67:445–69. 10.1146/annurev.physiol.67.041703.084745 . [DOI] [PubMed] [Google Scholar]
- 8. Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiological reviews. 2012;92(1):39–74. 10.1152/physrev.00011.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiological reviews. 2013;93(2):803–959. 10.1152/physrev.00023.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, et al. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. The Journal of biological chemistry. 1998;273(28):17689–95. . [DOI] [PubMed] [Google Scholar]
- 11. Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na(+)-HCO(-)(3) cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. American journal of physiology Cell physiology. 2000;278(6):C1200–11. . [DOI] [PubMed] [Google Scholar]
- 12. Billet A, Luo Y, Balghi H, Hanrahan JW. Role of tyrosine phosphorylation in the muscarinic activation of the cystic fibrosis transmembrane conductance regulator (CFTR). The Journal of biological chemistry. 2013;288(30):21815–23. 10.1074/jbc.M113.479360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bae SH, Kim DH, Shin SK, Choi JS, Park KS. Src regulates membrane trafficking of the Kv3.1b channel. FEBS letters. 2014;588(1):86–91. 10.1016/j.febslet.2013.11.010 . [DOI] [PubMed] [Google Scholar]
- 14. Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nature reviews Neuroscience. 2004;5(4):317–28. 10.1038/nrn1368 . [DOI] [PubMed] [Google Scholar]
- 15. Espiritu DJ, Bernardo AA, Robey RB, Arruda JA. A central role for Pyk2-Src interaction in coupling diverse stimuli to increased epithelial NBC activity. American journal of physiology Renal physiology. 2002;283(4):F663–70. 10.1152/ajprenal.00338.2001 . [DOI] [PubMed] [Google Scholar]
- 16. Jin M, Hwang SM, Davies AJ, Shin Y, Bae JS, Lee JH, et al. Autoantibodies in primary Sjogren's syndrome patients induce internalization of muscarinic type 3 receptors. Biochimica et biophysica acta. 2012;1822(2):161–7. 10.1016/j.bbadis.2011.11.012 . [DOI] [PubMed] [Google Scholar]
- 17. Shirasuna K, Sato M, Miyazaki T. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer. 1981;48(3):745–52. Epub 1981/08/01. . [DOI] [PubMed] [Google Scholar]
- 18. Bae JS, Koo NY, Namkoong E, Davies AJ, Choi SK, Shin Y, et al. Chaperone stress 70 protein (STCH) binds and regulates two acid/base transporters NBCe1-B and NHE1. The Journal of biological chemistry. 2013;288(9):6295–305. 10.1074/jbc.M112.392001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gresz V, Kwon TH, Hurley PT, Varga G, Zelles T, Nielsen S, et al. Identification and localization of aquaporin water channels in human salivary glands. American journal of physiology Gastrointestinal and liver physiology. 2001;281(1):G247–54. . [DOI] [PubMed] [Google Scholar]
- 20. Perry C, Baker OJ, Reyland ME, Grichtchenko II. PKC{alpha}{beta}{gamma}- and PKC{delta}-dependent endocytosis of NBCe1-A and NBCe1-B in salivary parotid acinar cells. American journal of physiology Cell physiology. 2009;297(6):C1409–23. 10.1152/ajpcell.00028.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perry C, Quissell DO, Reyland ME, Grichtchenko II. Electrogenic NBCe1 (SLC4A4), but not electroneutral NBCn1 (SLC4A7), cotransporter undergoes cholinergic-stimulated endocytosis in salivary ParC5 cells. American journal of physiology Cell physiology. 2008;295(5):C1385–98. 10.1152/ajpcell.00153.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. American journal of physiology Renal physiology. 2003;285(1):F152–65. 10.1152/ajprenal.00307.2002 . [DOI] [PubMed] [Google Scholar]
- 23. Praetorius J, Kim YH, Bouzinova EV, Frische S, Rojek A, Aalkjaer C, et al. NBCn1 is a basolateral Na+-HCO3- cotransporter in rat kidney inner medullary collecting ducts. American journal of physiology Renal physiology. 2004;286(5):F903–12. 10.1152/ajprenal.00437.2002 . [DOI] [PubMed] [Google Scholar]
- 24. Pushkin A, Yip KP, Clark I, Abuladze N, Kwon TH, Tsuruoka S, et al. NBC3 expression in rabbit collecting duct: colocalization with vacuolar H+-ATPase. The American journal of physiology. 1999;277(6 Pt 2):F974–81. . [DOI] [PubMed] [Google Scholar]
- 25. Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, Singh AK, et al. The electroneutral Na(+):HCO(3)(-) cotransporter NBCn1 is a major pHi regulator in murine duodenum. The Journal of physiology. 2012;590(Pt 14):3317–33. 10.1113/jphysiol.2011.226506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo X, Choi JY, Ko SB, Pushkin A, Kurtz I, Ahn W, et al. HCO3- salvage mechanisms in the submandibular gland acinar and duct cells. The Journal of biological chemistry. 2001;276(13):9808–16. 10.1074/jbc.M008548200 . [DOI] [PubMed] [Google Scholar]
- 27. Roussa E. H+ and HCO3- transporters in human salivary ducts. An immunohistochemical study. The Histochemical journal. 2001;33(6):337–44. . [DOI] [PubMed] [Google Scholar]
- 28. Gresz V, Kwon TH, Vorum H, Zelles T, Kurtz I, Steward MC, et al. Immunolocalization of electroneutral Na(+)-HCO cotransporters in human and rat salivary glands. American journal of physiology Gastrointestinal and liver physiology. 2002;283(2):G473–80. 10.1152/ajpgi.00421.2001 . [DOI] [PubMed] [Google Scholar]
- 29. Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, et al. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3- salvage transporter human Na+-HCO3- cotransport isoform 3. The Journal of biological chemistry. 2002;277(52):50503–9. 10.1074/jbc.M201862200 . [DOI] [PubMed] [Google Scholar]
- 30. Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thevenod F. Immunolocalization of anion exchanger AE2 and Na(+)-HCO(-)(3) cotransporter in rat parotid and submandibular glands. The American journal of physiology. 1999;277(6 Pt 1):G1288–96. . [DOI] [PubMed] [Google Scholar]
- 31. Park K, Hurley PT, Roussa E, Cooper GJ, Smith CP, Thevenod F, et al. Expression of a sodium bicarbonate cotransporter in human parotid salivary glands. Archives of oral biology. 2002;47(1):1–9. . [DOI] [PubMed] [Google Scholar]
- 32. Roussa E. Channels and transporters in salivary glands. Cell and tissue research. 2011;343(2):263–87. 10.1007/s00441-010-1089-y . [DOI] [PubMed] [Google Scholar]
- 33. Dawes C, Dong C. The flow rate and electrolyte composition of whole saliva elicited by the use of sucrose-containing and sugar-free chewing-gums. Archives of oral biology. 1995;40(8):699–705. Epub 1995/08/01. . [DOI] [PubMed] [Google Scholar]
- 34. Muller-Berger S, Ducoudret O, Diakov A, Fromter E. The renal Na-HCO3-cotransporter expressed in Xenopus laevis oocytes: change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflugers Archiv: European journal of physiology. 2001;442(5):718–28. . [DOI] [PubMed] [Google Scholar]
- 35. Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, et al. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3- cotransporter subtype pNBC1. American journal of physiology Gastrointestinal and liver physiology. 2003;284(1):G37–45. 10.1152/ajpgi.00209.2002 . [DOI] [PubMed] [Google Scholar]
- 36. Gross E, Fedotoff O, Pushkin A, Abuladze N, Newman D, Kurtz I. Phosphorylation-induced modulation of pNBC1 function: distinct roles for the amino- and carboxy-termini. The Journal of physiology. 2003;549(Pt 3):673–82. 10.1113/jphysiol.2003.042226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perry C, Le H, Grichtchenko II. ANG II and calmodulin/CaMKII regulate surface expression and functional activity of NBCe1 via separate means. American journal of physiology Renal physiology. 2007;293(1):F68–77. 10.1152/ajprenal.00454.2006 . [DOI] [PubMed] [Google Scholar]
- 38. Ruiz OS, Qiu YY, Wang LJ, Cardoso LR, Arruda JA. Regulation of renal Na-HCO3 cotransporter: VIII. Mechanism Of stimulatory effect of respiratory acidosis. The Journal of membrane biology. 1998;162(3):201–8. . [DOI] [PubMed] [Google Scholar]
- 39. Ruiz OS, Robey RB, Qiu YY, Wang LJ, Li CJ, Ma J, et al. Regulation of the renal Na-HCO(3) cotransporter. XI. Signal transduction underlying CO(2) stimulation. The American journal of physiology. 1999;277(4 Pt 2):F580–6. . [DOI] [PubMed] [Google Scholar]
- 40. Bernardo AA, Espiritu DJ, Ruiz OS, Robey RB, Arruda JA. The role of phosphatidylinositol 3-kinase (PI3K) in CO2 stimulation of the Na+/HCO3- cotransporter (NBC). The Journal of membrane biology. 2003;191(2):141–8. 10.1007/s00232-002-1051-3 . [DOI] [PubMed] [Google Scholar]
- 41. Yamaji Y, Tsuganezawa H, Moe OW, Alpern RJ. Intracellular acidosis activates c-Src. The American journal of physiology. 1997;272(3 Pt 1):C886–93. . [DOI] [PubMed] [Google Scholar]
- 42. Lin AL, Zhu B, Zhang W, Dang H, Zhang BX, Katz MS, et al. Distinct pathways of ERK activation by the muscarinic agonists pilocarpine and carbachol in a human salivary cell line. American journal of physiology Cell physiology. 2008;294(6):C1454–64. 10.1152/ajpcell.00151.2007 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Fluorescence ratio (490/440 nm) changes during exposure to nigericin-containing solutions at pH 6.2, 6.6, 7.0, 7.4, and 7.8. (B) Dependence of fluorescence ratio on intracellular pH (n = 16).
(TIF)
Data Availability Statement
All relevant data are within the paper.