Abstract
Immunoglobulin E-mediated allergies affect more than 25% of the population. Allergen exposure induces a variety of symptoms in allergic patients, which include rhinitis, conjunctivitis, asthma, dermatitis, food allergy and life-threatening systemic anaphylaxis. At present, allergen-specific immunotherapy (SIT), which is based on the administration of the disease-causing allergens, is the only disease-modifying treatment for allergy. Current therapeutic allergy vaccines are still prepared from relatively poorly defined allergen extracts. However, with the availability of the structures of the most common allergen molecules, it has become possible to produce well-defined recombinant and synthetic allergy vaccines that allow specific targeting of the mechanisms of allergic disease. Here we provide a summary of the development and mechanisms of SIT, and then review new forms of therapeutic vaccines that are based on recombinant and synthetic molecules. Finally, we discuss possible allergen-specific strategies for prevention of allergic disease.
Keywords: allergen-specific immunotherapy, immunoglobulin E, mechanisms, prophylaxis, recombinant allergens, vaccines
Introduction
Immunoglobulin E (IgE)-mediated allergy is the most common immunological hypersensitivity disease [1]. The prevalence has been continuously rising over the last decades, and currently, more than 25% of the population are affected [2, 3]. Local symptoms of allergy are observed in the skin and respiratory and gastrointestinal tracts, and systemic manifestations include life-threatening anaphylactic shock [4]. If allergy is not properly diagnosed and treated, it tends to progress to severe and chronic disabling disease; for example, a clinically silent state of IgE sensitization without symptoms can progress to symptomatic allergy, and mild forms of allergic rhinitis can develop into severe forms of asthma [5, 6].
In individuals with a genetic predisposition towards allergy (i.e. atopic individuals), postnatal exposure to harmless environmental antigens (i.e. allergens) induces the production of allergen-specific IgE antibodies, a process that is termed allergic sensitization [7]. Allergens can be derived from various allergen sources (e.g. pollen, house dust mites, pets, moulds, food and insects) and are mainly proteins or glyco-proteins [8]. Recurrent allergen exposure boosts the production of allergen-specific IgE antibodies that bind to their receptors on immune cells that are essential players in allergic inflammation [9, 10]. Cross-linking of IgE antibodies that are bound to the high-affinity receptors for IgE (i.e. FcεRI) on mast cells and basophils by invading allergens gives rise to degranulation within a few minutes and release of inflammatory mediators, proteases and pro-inflammatory cytokines [11, 12]. This immediate allergic inflammatory response is the cause for the majority of allergic symptoms. The activation of T cells and the consequent production of pro-inflammatory cytokines can lead to chronic allergic inflammation and late-phase reactions. The chronic allergic inflammatory response requires the presentation of allergens by antigen-presenting cells and may be strongly enhanced by IgE-facilitated allergen presentation [13].
Pharmacological treatment in allergy mainly focuses on the mitigation of allergic inflammation and thus only represents a symptomatic form of therapy that does not modify the allergen-specific immune response. By contrast, allergen-specific immunotherapy (SIT) modifies the allergen-specific immune response and the course of disease and has been shown to have long-lasting effects [14].
Milestones in the development of SIT
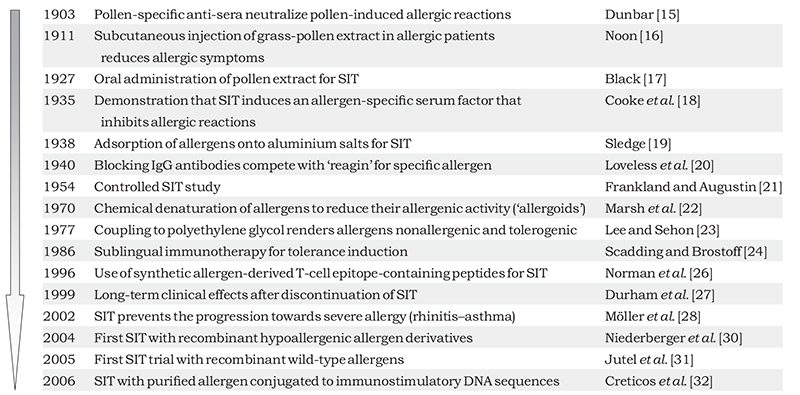
Allergen-specific immunotherapy is based on the repeated administration of disease-causing allergens with the aim of modifying the allergen-specific immune response in patients so that higher doses of the allergen can be tolerated. Originally, allergy was considered not as an immunologically mediated hyper-sensitivity disease but rather as a reaction against a toxin. Based on this idea, in 1903, Dunbar immunized animals with ‘pollen toxins’ to generate antisera that he found could neutralize the suspected toxic effects in patients [15]. These finding may be considered as an early indication that SIT represents a vaccine. Details of further milestones in the development of SIT are shown in Table 1.
Table 1. Overview of milestones in the development of SIT.
|
The experiments of Dunbar inspired Noon [16] to vaccinate patients allergic to grass pollen with this toxin. It was found that this treatment reduced allergic symptoms and the sensitivity to grass pollen in the vaccinated patients, and thus, Noon [16] had conducted the first SIT in allergic patients. In 1927, Black [17] reported the first attempt to use oral immunotherapy as a possible alternative to injection immunotherapy. A few years later, in 1935, Cooke et al. [18] published a seminal paper: using passive serum transfer, they showed that SIT induced an allergen specific serum factor that prevented allergen-induced skin sensitization. Injection of aqueous allergen extracts caused frequent systemic and often severe side effects. The finding that allergens remained at the injection site as a result of adsorption of allergen extracts onto aluminium hydroxide, thus reducing systemic side effects, was a major improvement for the safety of SIT [19]. In 1940, Loveless [20] identified the allergen-specific serum factor described by Cooke and colleagues as allergen-specific IgG-blocking antibodies that unlike the disease-causing allergen-specific IgE were stable at 56 °C. Frankland and Augustin [21] reported results from a controlled SIT trial using crude allergen extracts and purified allergenic proteins, thus introducing the principles of controlled clinical trials into clinical SIT research. To reduce side effects in the course of SIT, both Marsh et al. and Lee and Sehon developed procedures for the chemical modification of allergen extracts and obtained modified allergen extracts with low allergenic activity [22, 23]. In 1986, Scadding and Brostoff [24] demonstrated that sublingual immunotherapy was a possible alternative to injection SIT for tolerance induction in allergic patients. An important advance for diagnosis of allergy and SIT was the elucidation of allergen structures and sequences by molecular cloning techniques and the production of recombinant allergens from the late 1980s [reviewed in 25]. Allergen sequences became available, avoiding the need for cumbersome purification of allergen components from natural allergen extracts. A new phase in the development of SIT began with the ability to produce synthetic peptides, pure recombinant allergens and hypoallergenic allergen derivatives for SIT [25]. With the aim of inducing T-cell tolerance, allergen-derived T-cell epitope-containing synthetic peptides were administered to allergic patients in immunotherapy trials approximately 10 years later [26]. Two clinically important findings, the long-term effects of immunotherapy after discontinuation of treatment and the prevention of disease progression, especially from rhinitis to asthma in children, were published in 1999 and 2002, respectively [27, 28]. The study by Durham et al. has been a milestone with respect to long-term clinical efficacy of SIT. They reported that vaccination with grass-pollen allergens for 3–4 years induced prolonged clinical remission accompanied by a persistent alteration in immunological reactivity. This finding raised the question of whether SIT should be considered earlier in the course of allergic disease to prevent progression [27]. In the Preventive Allergy Treatment (PAT) study, children with seasonal allergic rhinoconjunctivitis were randomly assigned either to receive SIT for 3 years or to an open control group. The results of the study demonstrated that a 3-year course of SIT in children with allergic rhinoconjunctivitis significantly reduces the risk of developing clinical asthma and improves bronchial hyper-reactivity [28]. These findings were confirmed in the 10-year follow-up of the PAT study [29].
The results from the first SIT trials with purified recombinant hypoallergenic birch pollen allergen molecules and recombinant grass-pollen allergens were published in 2004 and 2005, respectively [30, 31]. These studies were important because they highlighted the transition from SIT with ill-defined allergen extracts towards SIT with pure allergen components. In 2006 it was reported that SIT with purified natural ragweed allergen conjugated to immunostimulatory CpG sequences may offer another possibility to reduce side effects and activate the innate immune system [32]. Today many unanswered questions remain [33] but following experimental research into defined allergen molecules, epitopes and modified allergens, clinical trials with these molecules are now being performed. It is hoped that this development may lead to highly effective, convenient forms of SIT with few side effects that will change current treatment of allergy fundamentally from only symptom-reducing pharmacotherapy to disease-modifying, patient-tailored treatment [34, 35].
Mechanisms of SIT
The availability of pure recombinant allergens and allergen-derived peptides, epitopes and structures has also allowed the mechanisms of SIT to be re-investigated [reviewed in 25]. The elegant experiments by Cooke and colleagues and the follow-up experiments by Loveless demonstrated that SIT induces allergen-specific IgG antibodies in allergic patients; these antibodies inhibit the binding of IgE to the allergen, IgE-mediated mast cell and basophil degranulation and hence immediate allergic inflammation [18, 20]. Studies using recombinant allergens and defined allergen epitopes for analysis, as well as SIT trials performed with purified recombinant allergens and recombinant hypoallergenic allergen derivatives, have confirmed that a major mechanism of SIT is the induction of allergen-specific IgG-blocking antibodies [14, 25, 36, 37]. Moreover, it has been demonstrated that allergen-specific blocking IgG can also inhibit IgE-facilitated allergen presentation by antigen-presenting cells to T cells and thus suppress allergen-induced T-cell activation [13, 38, 39]. Of interest, it has been demonstrated in several studies that allergen-specific IgE production is boosted to a lesser degree in patients who develop allergen-specific IgG antibodies, compared with patients who are naturally exposed to allergens. It may therefore be assumed that the maintenance of allergen-specific IgG levels in patients by SIT can also reduce allergen-specific IgE production in the long term [30, 32, 40].
It has also been found that SIT can alter the balance from allergen-specific T helper (Th)2 to allergen-specific Th1 immunity and that it may induce the secretion of immunoregulatory cytokines, such as interleukin (IL)-10, and T regulatory cell responses [14, 41]. Furthermore, SIT has been shown to affect other inflammatory cells such as mast cells, basophils, eosinophils and antigen-presenting cells [14]. Thus SIT has a profound immunoregulatory effect that may also explain why it can modify the course of allergic disease and why it may have long-lasting effects even after discontinuation of treatment. It is important to note that SIT is a highly allergen-specific form of treatment that only affects allergy caused by the allergens included in the vaccine, and eventually cross-reactive but not immunologically unrelated allergens [42].
Molecular and new approaches for SIT
Box 1 provides an overview of molecular and other new approaches for SIT.
Box 1. Overview of new approaches for SIT.
| A. New vaccines | |
| Recombinant proteins | |
| Recombinant wild-type allergens | |
| Allergens that are made by recombinant expression to mimick the properties of the naturally occurring allergens with regard to fold and presence of IgE and T cell epitopes | [25,31,35,58-63] |
| Recombinant hypoallergens | |
| Recombinant allergen variants which have been made to reduce IgE-mediated side effects. Characterized mainly by reduced IgE reactivity but contain allergen-specific T cell epitopes similar to the natural allergens | [7,25,30,35,50-56] |
| Carrier-bound B cell epitope-containing peptides | |
| Recombinant fusion proteins consisting of an allergen-unrelated carrier protein and hypoallergenic allergen peptides. Allergen-derived peptides are derived from the IgE-binding sites of the allergen, contain no or reduced allergen-specific T cell epitopes and exhibit no or strongly reduced IgE reactivity. Carrier proteins may be derived from viruses or other immunogens | [25,35,95,97-104] |
| Synthetic peptides | |
| T cell epitope-containing peptides | |
| Peptides obtained by synthetic chemistry which incorporate allergen-specific T cell epitopes and do not react with IgE antibodies | [25,26,35,43-49] |
| Coupled allergens | |
| CpG-coupled allergens | |
| Allergens that are chemically coupled to immunostimulatory DNA sequences | [32] |
| Virus-like particle-coupled allergens | |
| Allergens that are chemically coupled to virus-like particles | [65, 66] |
| Genetic vaccines | |
| DNA vaccines – Vaccination with allergen-encoding DNA | [88-93] |
| RNA vaccines – Vaccination with allergen-encoding RNA | [94] |
| B. Alternative routes | |
| Sublingual – Sublingual administration of allergen-containing drops or tablets | [17, 24, 74-79] |
| Oral – Oral administration of allergens (i.e. allergy vaccines that are swallowed) | [68-70] |
| Intralymphatic – Injection of allergy vaccines into the lymph node (instead of subcutaneous injection) | [81-83] |
| Epicutaneous – Epicutaneous administration of allergens using patch application | [84-87] |
| C. Virus-like particles | |
| Virus-like particles | [125] |
| D. Cell-based approaches | |
| Allergen-expressing stem cells | [122-124] |
| Engineered T regulatory cells | [126] |
| Engineered Th1 cells | [128] |
| E. Passive immunization | |
| Passive immunization with allergen-specific antibodies | [120] |
T-cell epitope-containing peptides
One approach to target allergen-specific T cells in SIT has been the use of allergen-derived synthetic peptides containing T-cell epitopes. These peptides comprise linear sequences representing small allergen fragments that bind to the receptor of allergen specific T cells and show no reactivity with IgE antibodies. The T-cell epitope-containing peptides are thus characterized by a markedly reduced ability to cross-link allergen-specific IgE, which results in reduction in IgE-mediated adverse side effects. SIT has mainly been performed with peptides from the major cat allergen Fel d 1 and venom allergens [26, 43-47]. The first clinical trials demonstrated no relevant clinical improvement and many patients reported late-phase adverse events that were most probably T cell mediated [43]. Further developments (e.g. shorter peptides and lower doses) showed promising results and clinical trials are currently ongoing [46, 48]. The treatment is thought to induce T-cell tolerance through regulatory T cells that secrete the immune regulatory cytokine IL-10 [49]. Possible drawbacks of T-cell-based epitope vaccines are the diverse T-cell epitope repertoire rendering treatment with just one or a few peptides difficult, the high rate of systemic side effects and the failure to induce allergen specific blocking IgG.
Recombinant hypoallergens
To overcome the IgE-mediated side effects observed with allergen extracts, recombinant hypoallergenic allergens were developed. Recombinant hypoallergens are made by recombinant expression in various organisms, mainly Escherichia coli. They are characterized by a strongly reduced IgE reactivity that is obtained by a variety of molecular biological manipulations such as the introduction of mutations into the allergen sequence, production of larger nonallergenic fragments, reassembly of sequences, oligomerization and deletion of sequences [for review see 7, 25, 35]. Most of allergen-specific T-cell epitopes are preserved in the hypoallergens as the manipulations mainly affect the IgE-binding sites and leave T-cell epitopes intact. The first SIT trial with recombinant hypoallergens was already initiated about 10 years ago. This was a double-blind placebo-controlled trial in which patients with an allergy to birch pollen were treated with hypoallergenic derivatives of the major birch pollen allergen Bet v 1 adsorbed onto aluminium hydroxide [30, 50-53]. The advantage of recombinant hypoallergenic molecules is their strongly reduced allergenic activity allowing administration of higher doses than the natural allergen. However, they may still induce T-cell-mediated side effects [53]. Vaccination with recombinant hypoallergenic Bet v 1 derivatives showed clinical efficacy with no IgE-mediated side effects or clinically relevant de novo sensitization [30, 52]. The therapy-induced allergen-specific IgG antibodies inhibited allergic patients’ IgE binding to Bet v 1 and were associated with reduced nasal sensitivity to birch pollen [50, 53]. Furthermore, they reduced allergen-induced boosts of IgE production and IgE-facilitated allergen presentation to T cells [30, 53, 54]. A recombinant hypoallergenic version of Bet v 1 has been successfully evaluated in SIT trials up to phase III [34, 55]. The beneficial effects of SIT with recombinant hypoallergenic allergen derivatives seem to be mainly mediated by the induction of allergen specific IgG that inhibit binding of IgE to the allergen, allergen-induced effector cell degranulation, IgE-facilitated allergen presentation and boosts of IgE production (for review see [56]). Because recombinant hypoallergens contain allergen-specific T-cell epitopes, they may be used to induce tolerance in T cells and may also be useful for prophylaxis of allergy [7, 57].
Recombinant wild-type allergens for SIT
Following the first trial with recombinant hypoallergens of Bet v 1 [30], clinical immunotherapy trials have been performed with recombinant wild-type allergens from grass and birch pollens [31, 58]. Recombinant wild-type allergens are defined as recombinant allergens that mimick the fold and IgE and T-cell reactivity of the corresponding natural allergens. Accordingly they can induce similar types of side effects as natural allergens but have the advantage that they can be produced with defined quality and quantity in reproducible production processes and thus allow the formulation of vaccine batches with consistent properties and potencies. Furthermore, they can be produced as hybrid molecules incorporating the epitopes of several allergen molecules, which facilitates vaccine production and increases immunogenicity [59-61]. That recombinant allergen-based vaccines can replace allergen extract-based vaccines has been shown in a study of subcutaneous immunotherapy (SCIT) comparing wild-type recombinant Bet v 1 with standard birch pollen vaccine and natural purified birch pollen allergen (nBet v 1) [58]. All actively treated groups demonstrated clinical improvement accompanied by marked increases in Bet v 1-specific IgG levels. These levels were higher in the recombinant Bet v 1 (rBet v 1)-treated group than in the standard birch pollen vaccine- and nBet v 1-treated groups. Thus the rBet v 1-based vaccine was shown to be safe and effective in treating birch pollen allergy [58]. SIT with recombinant major grass-pollen allergens has also been evaluated in a clinical trial [31]. In line with previous studies, induction of allergen-specific IgG antibodies against natural grass-pollen allergens and clinical efficacy were observed. In the future, it is likely that recombinant allergen-based vaccines will also be generated for other allergen sources such as venom and food allergens. The great advantage of recombinant allergen-based vaccines is that patients are treated with well-defined molecules that fulfil current quality standards for vaccine production. There are also ongoing studies investigating the use of recombinant wild-type allergens for sublingual immunotherapy (SLIT); however, currently this treatment is performed with ill-defined natural allergen extracts [62, 63]. Studies are also focusing on the mechanisms underlying SLIT, which at present remain unclear [64].
CpG-conjugated and other coupled allergens
Another approach in the development of SIT has been to combine allergens with immunomodulatory components. One such component is immunostimulatory DNA sequences containing CpG motifs that activate the innate immune system through toll-like receptors (TLRs). CpG motifs are thought to interact with TLR-9 and to inhibit Th2 immune responses. A small placebo-controlled SIT study, in which the major ragweed pollen allergen Amb a 1 was combined with CpG, has been performed [32]. The vaccination induced allergen-specific blocking IgG antibodies, and reduced the seasonal boost in IgE production. Furthermore, compared with the placebo group, the active treatment group had lower seasonal symptom scores. However, a recently initiated clinical trial was discontinued as no significant differences were found between the actively and placebo-treated patients.
Another approach to using coupled allergens has been tested for peptides of the major house dust mite allergen Der p 1, which were conjugated to virus-like particles from the bacteriophage Qbeta. Immunization of healthy subjects by subcutaneous administration of the conjugate induced Der p 1-specific IgG antibodies [65]. It was also shown that the coupling to virus-like particles reduced the allergenic activity of the major cat allergen Fel d 1 [66]. Using Fel d 1 as a model allergen in a murine model of cat allergy, it was also demonstrated that covalent coupling of vitamin D3 to Fel d 1 improved the effects of SCIT [67].
One problem with the approach of chemical coupling of allergens or allergen peptides is that it may be difficult to establish reproducible production processes following good manufacturing practice and that these processes may need to be adapted individually for each allergen.
New routes for SIT
Since the first clinical trial was published in 1911, SIT has been performed successfully as SCIT. However, other routes, in particular mucosal routes, have also been tested. As discussed previously, by the late 1920s Black had demonstrated oral administration of allergen extracts; Scadding and Brostoff later reported sublingual allergen administration [17, 24]. There were several reasons for the search for alternative routes for SIT such increasing the convenience of administration and eventually allowing self-administration, reducing side effects and targeting different immune mechanisms (e.g. mucosal tolerance). However, oral immunotherapy has not become common practice because it was found to be much less effective than SCIT. Currently, the possibility of oral immunotherapy for the treatment of various forms of food allergy such as cow’s milk and peanut allergy is being investigated [68-70]. Yet these studies are limited by the use of crude natural allergen extracts and a recent meta-analysis of studies in the area of cow’s milk allergy noted several disadvantages of the treatment such as severe side effects. Furthermore, the underlying mechanisms are less clear than for SCIT and evaluating the effects objectively can be difficult [70]. There have currently been attempts to use defined recombinant hypoallergenic food allergens for SCIT [71]. In this context it would be interesting to use such defined molecules in parallel for oral immunotherapy to compare the two types of SIT with regard to underlying mechanisms and effects. Novel approaches based on transgenic allergen-expressing food or lactic acid bacteria have also been tested in animal models [72, 73]. These approaches would need to be tested in allergic patients for safety, because they carry the risk that allergens transported by these vehicles through the gut may escape digestion and subsequently induce systemic anaphylactic reactions. The latter risk may be overcome by oral adminstration of hypoallergenic allergen derivatives instead of allergenic wild-type allergens.
At present there are several allergen extract-based preparations on the market for SLIT, but the immunological mechanisms of SLIT are still unclear. SLIT is based on the sublingual administration of allergens in the form of drops or tablets. This treatment seems to have effects on antigen-presenting cells and T cells but induces unfavourable increases in the level of allergen-specific IgE and only low levels of allergen-specific blocking IgG [74, 75]. Furthermore, it has been noted that many SLIT trials have not been performed according to international recommendations for SIT studies and that less than 30% of the studies have shown unequivocal clinical efficacy [76]. Few studies have directly compared SCIT and SLIT; in such studies the efficacy of SLIT was found to be much lower than that of SCIT [77, 78]. SLIT generally requires daily self-medication bearing thus the risk of unattended side effects that are less common than with SCIT; but even severe systemic side effects have been reported for SLIT [79].
Intralymphatic SIT (ILIT) has also been investigated as a possible route for therapy. ILIT was originally tested in the 1970s for cancer immunotherapy [80]. Through injection into a lymph node, it is hoped that ILIT will enhance the development of protective immunity [81]. Clinical trials conducted to date have demonstrated that ILIT may be clinically effective after only a few injections and induces allergen-specific IgG as with SCIT [82, 83]. In an attempt to further improve ILIT, a recombinant allergen with a modular transporter antigen molecule was used and this was shown to also stimulate regulatory T-cell responses [83]. Whether ILIT is more effective than SCIT still needs to be demonstrated, and a limitation of ILIT is that it requires the technique of intralymphatic injection.
Finally, there have been several recent attempts to administer allergens via the skin. Epicutaneous SIT is based on administration of allergens using patches that are mounted onto the skin. Several technologies for patch administration are currently being tested and data from animal studies and clinical trials are already available [84-87]. Whilst there is some evidence to support a clinical effect, further data regarding the immunological mechanisms and objective clinical parameters are needed. In addition, whether epicutaneous SIT induces allergen-specific IgG has not been investigated.
Genetic immunization
Two studies performed in murine allergy models were the first to indicate that vaccination with allergen-encoding DNA may represent a new approach for SIT [88,89]. It has been demonstrated that genetic immunization induces allergen-specific Th1 immune responses that may prevent allergic sensitization and eventually ongoing allergic immune responses [90, 91]. The advantage of using allergen-encoding DNA instead of allergen for vaccination is, however, out-weighed by the concern that genetic immunization may induce an uncontrolled production of allergens in treated subjects and thus may cause side effects [92]. To overcome this problem, genetic vaccination with DNA coding for hypoallergens has been considered as an alternative approach [93]. Furthermore, RNA immunization that gives rise to only transient allergen production has been considered as a possible alternative [94]. The latter approach may also be useful for prophylactic treatment.
Carrier-bound B cell epitope-containing peptides
The concept of carrier-bound B cell epitope-containing peptides is a further development of recombinant hypoallergenic allergen derivatives that should eliminate late, T-cell-mediated side effects [95]. Carrier-bound allergen peptide vaccines are composed of an allergen-unrelated carrier protein which, according to the peptide carrier principle described by Siskind et al., [96] provides T-cell help for the production of antibodies against the peptides covalently linked with the carrier. Because carrier molecules without allergen-specific T-cell epitopes can be chosen, it is possible to reduce the presence of allergen-specific T-cell epitopes in the vaccine. This may be a major advantage compared with treatment with T-cell epitope containing peptides, recombinant wild-type allergens or recombinant hypoallergens, which contain allergen-specific T-cell epitopes and therefore can lead to activation of allergen-specific T cells and thus T-cell-mediated side effects. The allergen-derived peptides are selected from the IgE-binding areas on allergen surfaces to induce allergen-specific blocking IgG antibodies against the IgE-binding sites [97, 98]. As IgE antibodies preferentially recognize conformational IgE epitopes, it possible to identify peptides that are part of the IgE-binding sites but do not react with IgE antibodies and hence do not induce IgE-mediated allergic reactions. Vaccines based on carrier-bound allergen peptides should therefore allow the elimination of both IgE- and T-cell-mediated side effects, whereas they induce robust allergen-specific IgG antibodies which block IgE binding to the allergen as well as IgE-mediated allergic reactions.
The first proof of principle studies using allergen peptides chemically coupled to a carrier molecule were performed in in vitro and in animal experiments [97, 98]. Carrier-bound allergen peptide vaccines have been made as recombinant fusion proteins which can be produced in E. coli in large-scale and under-defined conditions suitable for state of the art vaccine production [99, 100]. The use of viral-derived carrier proteins may offer the additional advantage of vaccines that induce protective IgG antibodies both against the allergen and against infectious diseases [101]. Hypoallergenic allergen-derived peptides suitable for the production of carrier-bound peptide vaccines have been identified for several important respiratory allergens, and it thus appears that the technology is generally applicable to many important allergen sources [102-104]. Because of the hypoallergenic nature of the carrier-bound peptide vaccines, it is hoped that SCIT can be performed with only a few injections, approximately four per year, without need for cumbersome up-dosing schemes requiring multiple treatments. A grass-pollen allergy vaccine based on carrier-bound peptides of the four major grass-pollen allergens (Phl p 1, Phl p 2, Phl p 5 and Phl p 6) has been successfully evaluated in a safety skin test study in allergic patients; in addition, a phase II study of SCIT has been completed and a multicentre phase IIb study is starting (see http://clinicaltrials.gov: Clinical trial numbers: NCT01350635; NCT01445002; NCT 01538979, respectively).
Prophylactic SIT approaches
SIT has not yet been used as a prophylactic vaccine to prevent the development of allergic sensitization. It is clear that prophylactic treatment would be a major step forward because it would not be limited to the treatment of allergic patients but would also prevent allergies and hence stop the currently exploding allergy epidemic. However, the application of SIT for the prevention of allergic sensitization requires the availability of suitable technologies.
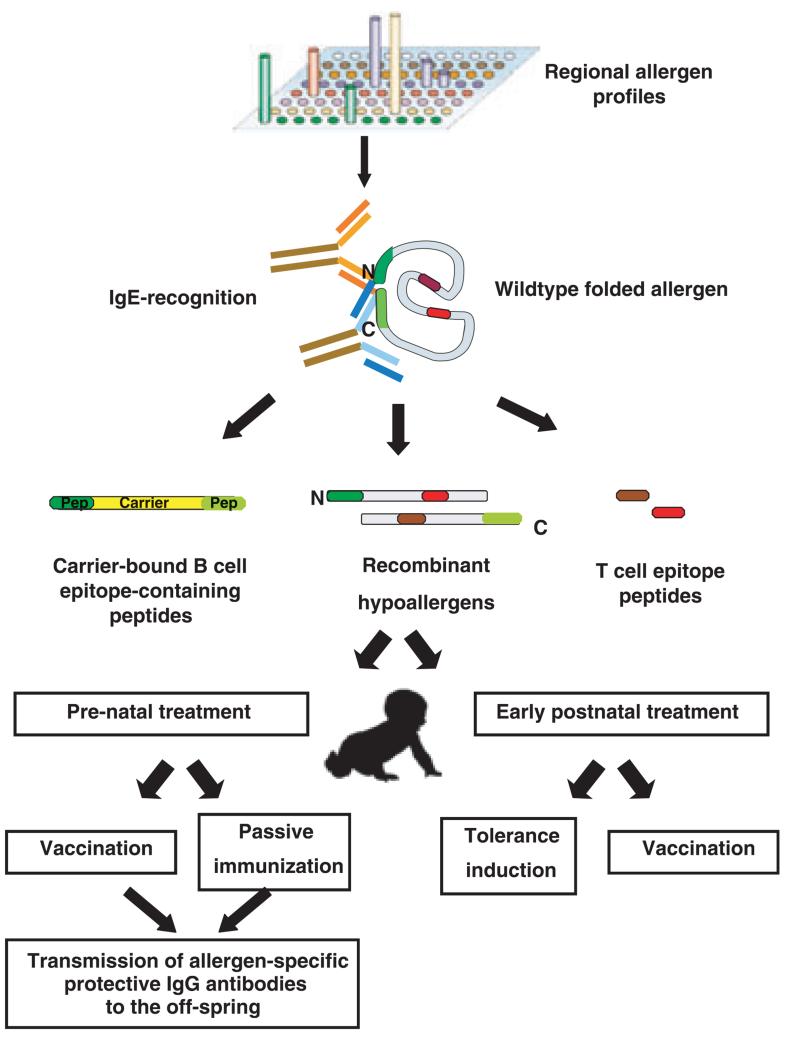
First, it is important to determine which allergens should be included for prophylactic treatment. At present we have suitable diagnostic tests that allow population-wide testing of sensitization against more than 100 individual allergen molecules to establish and monitor regional allergen profiles (Fig. 1, top) [105, 106]. These tests are based on micro-arrayed allergen molecules that allow IgE reactivities to be assessed with small serum volumes against a multitude of allergens using chip technology (Fig. 1; top). Allergen component-based testing has already provided interesting insights into regional differences in allergen recognition [107, 108] and relatively complete molecular sensitization maps are likely to become available soon through extensive population testing with chip technology. Based on these results it should be possible to identify the allergen components for which prophylactic strategies need to be developed.
Fig. 1. Strategies for prophylactic SIT.
Besides the definition of the relevant allergen molecules, the currently used crude allergen extracts, which comprise ill-defined mixtures of allergenic and nonallergenic components, present another major technical barrier to the further development of SIT [109-111]. Furthermore, natural allergen extracts exhibit high allergenic activity that often causes side effects and it has been reported that SIT with allergen extracts can induce IgE sensitization to new allergens [112]. If natural allergen extracts are used for prophylactic treatment, there is a considerable risk that IgE-mediated sensitization will be induced because the allergens that are present in natural extracts are in native conformation, have high allergenicity (i.e. potential to induce allergic sensitization) and hence may induce IgE responses against naturally occurring allergens. Therefore, to reduce the risk of sensitization against naturally occurring allergens during prophylactic treatment, modified allergen derivatives should be preferred for vaccination. In addition, approaches may be chosen to prevent allergen-specific immune response such as allergen-specific tolerance induction for early postnatal treatment in those subjects who have not yet been sensitized (Fig. 1).
Several types of allergen modifications are currently available for specifically targeting the immune system [25]. Allergen-derived T-cell epitope-containing peptides can be synthesized for each allergen for which the sequence is available. These peptides do not induce allergen-specific IgE or IgG antibody responses but allow targeting of allergen specific T cells via the T-cell receptor and therefore can be used to induce selective T-cell tolerance [49] (Fig. 1; middle part, right). Recombinant hypoallergenic allergen derivatives harbour most of the allergen-specific T-cell epitopes within one or a few molecules and hence can also be used for induction of T-cell tolerance [7]. Furthermore, they can be used for vaccination because they induce allergen specific IgG antibodies and low allergen-specific IgE responses (Fig. 1; middle part, middle). Carrier-bound B cell epitope-containing peptides lack most of the allergen-specific T-cell epitopes and therefore are less useful for induction of T-cell tolerance [95]. However, they induce robust allergen-specific IgG responses without activating allergen-specific T cells, and their capacity for inducing IgE responses against natural allergens is lower than that of recombinant hypoallergens. These derivatives should therefore be well suited for vaccination approaches because of a very low risk of inducing allergic sensitization.
Another important aspect for prophylaxis is the determination of the time window of allergic sensitization so that preventive interventions can be performed before sensitization has occurred.
From the analysis of birth cohorts that have been assessed for the development of allergic sensitization, we are beginning to gain an understanding of the development of allergic sensitization. Of interest, it has been shown that allergy to food allergens precedes allergy to respiratory allergens [113]. Nevertheless, population studies have shown a relationship between the month of birth and the development of seasonal pollen allergies [114], suggesting that allergic sensitization to respiratory allergens also occurs in the first months of life. It is therefore possible that allergic sensitization mainly occurs shortly after birth. The precise analysis of the period during which new sensitization occurs is currently one of the research topics of the EU-funded research programme MeDALL (http://medall-fp7.eu/ [115]) in which the development of allergic sensitization against more than 170 micro-arrayed allergen molecules will be analysed in serum samples from several birth cohorts using chip technology. The results of a recent analysis of samples from adult allergic patients and nonallergic subjects over a period of 10 years using chips containing almost 100 different micro-arrayed allergen molecules indicate that de novo allergic sensitization to new allergen molecules in allergic adults does not occur during the natural course of allergy (C. Lupinek, K. Marth, R. Valenta, unpublished observations).
There are basically two scenarios for prophylactic intervention depending on the time window during which allergic sensitization can occur. If confined to a very short period immediately after birth, prenatal interventions and/or very early postnatal interventions may be considered. Experimental data from animal models have elegantly demonstrated that the transmission of allergen-specific blocking IgG antibodies via the placenta and breast milk can suppress allergic sensitization [116, 117]. It should therefore be possible to induce allergen-specific IgG responses in mothers, through SIT with hypoallergens or carrier-bound B cell epitope-containing peptides, which are then transmitted to the child. Indeed it has been shown that SIT-induced IgG is transmitted through the placenta and there is evidence that SIT performed in pregnant mothers can prevent allergy in the child [118, 119]. Alternatively, passive immunization through administering allergen-specific IgG to mothers, which could be transmitted via the placenta or breast milk to the child, may be considered [120]. Another possibility is to add allergen specific IgG to the child’s diet in the early postnatal period.
Several possibilities may be considered for early postnatal treatment. First, early tolerance induction using T-cell epitope-containing allergen peptides or recombinant hypoallergens may be given via mucosal routes (e.g. oral tolerance) or by injection [121]. Second, peptides and/or hypoallergens could be presented on the surface of haematopoetic stem cells to induce tolerance as has been shown in experimental animal models [122-124]. Third, viral-like particle-or experimental cell-based forms of prophylaxis may be considered [125-128]. These approaches would focus on the robust induction of early T-cell tolerance so that no allergic immune response could develop. An alternative strategy would be the early postnatal induction of allergen-specific IgG by vaccination with hypoallergenic allergen derivatives or carrier-bound B cell epitope-containing peptides with the goal to prevent allergic sensitization. Early immunomodulation may also be achieved by genetic immunization using for example preventive RNA vaccination [94].
These mentioned strategies have already been tested in experimental animal models and may now be evaluated in first clinical studies in humans. An initial important step will be to demonstrate in nonallergic adults that the interventions do not induce allergic sensitization or other harmful reactions. Once safety and lack of allergenicity have been assessed, trials in subjects at risk of developing allergic sensitization, such as children of highly atopic parents, may be considered. Another way to advance prenatal vaccination may be to continue SIT with therapeutic vaccines with an extremely high safety profile (i.e. lack of side effects) during pregnancy and perform controlled studies to analyse whether such treatment can prevent allergic sensitization in the offspring. It is clear that many difficulties still need to be overcome in developing prophylactic SIT strategies, and the design of clinical studies exploring the risks, feasibility and benefits of such approaches will be affected by technical as well as ethical issues. However, we are now at a stage where we have the knowledge to manipulate the immune system and techniques to generate the new vaccines so that we can start to explore the possibilities for prophylactic SIT in an effort to stop the allergy pandemic.
Acknowledgements
Supported by the SFB programme F46 of the Austrian Science Fund (FWF), the Swedish Research Council, the Stockholm County Council, the Swedish Asthma and Allergy Association’s Research Foundation, the King Gustaf V 80th Birthday Foundation, the Swedish Heart-Lung Foundation, the Hesselman Foundation, the Konsul Th C Bergh Foundation, the Centre for Allergy Research, the Center for Inflammatory Diseases, the Swedish Cancer and Allergy Foundation and Karolinska Institutet.
Footnotes
Conflict of interest statement
Rudolf Valenta is a consultant for Biomay, Vienna, Austria and has received research grants from Phadia/Thermofisher, Uppsala, Sweden and Biomay. The other authors have no conflicts of interest to declare.
References
- 1.Kay AB. Allergy and Allergic Diseases. Blackwell Publishing Ltd; Oxford: 2008. [Google Scholar]
- 2.Wuthrich B, Schindler C, Leuenberger P, Ackermann-Liebrich U. Prevalence of atopy and pollinosis in the adult population of Switzerland (SAPALDIA study). Swiss Study on Air Pollution and Lung Diseases in Adults. Int Arch Allergy Immunol. 1995;106:149–56. doi: 10.1159/000236836. [DOI] [PubMed] [Google Scholar]
- 3.Floistrup H, Swartz J, Bergstrom A, et al. Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol. 2006;117:59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Anto JM, Demoly P, et al. Severe chronic allergic (and related) diseases: a uniform approach – a MeDALL – GA(2)-LEN – ARIA position paper. Int Arch Allergy Immunol. 2012;158:216–31. doi: 10.1159/000332924. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Anto JM, Bachert C, et al. A GA2LEN project Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitization to allergens. Allergy. 2006;61:671–80. doi: 10.1111/j.1398-9995.2006.01048.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Cauwenberge P, Watelet JB, Van Zele T, et al. Does rhinitis lead to asthma? Rhinology. 2007;45:112–21. [PubMed] [Google Scholar]
- 7.Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002;2:446–53. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- 8.Valenta R. Biochemistry of allergens and recombinant allergens. In: Kay AB, editor. Allery and Allergic Diseases. Blackwell Publishing Ltd; Oxford: 2008. pp. 895–912. [Google Scholar]
- 9.Henderson LL, Larson JB, Gleich GJ. Maximal rise in IgE antibody following ragweed pollination season. J Allergy Clin Immunol. 1975;55:10–5. doi: 10.1016/s0091-6749(75)80003-0. [DOI] [PubMed] [Google Scholar]
- 10.Niederberger V, Ring J, Rakoski J, et al. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int Arch Allergy Immunol. 2007;142:133–44. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 12.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–23. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–29. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- 14.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 15.Dunbar WP. Zur Ursache und spezifischen Heilung des Heufiebers. Dtsch Med Wochenschr. 1903;9:24–8. [Google Scholar]
- 16.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–3. [Google Scholar]
- 17.Black JH. The oral administration of pollen. J Lab Clin Med. 1927;12:1156. [Google Scholar]
- 18.Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever) J Exp Med. 1935;62:733–50. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sledge RF. Treatment of hay-fever with alum-preticipated pollen. U S Nav Med Bull. 1938;38:18. [Google Scholar]
- 20.Loveless MH. Immunological studies of pollinosis: I. The presence of two antibodies related to the same pollen-antigen in the serum of treated hay-fever patients. J Immunol. 1940;38:25–50. [Google Scholar]
- 21.Frankland AW, Augustin R. Prophylaxis of summer hay-fever and asthma: a controlled trial comparing crude grass-pollen extracts with the isolated main protein component. Lancet. 1954;266:1055–7. doi: 10.1016/s0140-6736(54)91620-7. [DOI] [PubMed] [Google Scholar]
- 22.Marsh DG, Lichtenstein LM, Campbell DH. Studies on “allergoids” prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970;18:705–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WY, Sehon AH. Abrogation of reaginic antibodies with modified allergens. Nature. 1977;267:618–9. doi: 10.1038/267618a0. [DOI] [PubMed] [Google Scholar]
- 24.Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin Allergy. 1986;16:483–91. doi: 10.1111/j.1365-2222.1986.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 25.Valenta R, Ferreira F, Focke-Tejkl M, et al. From allergen genes to allergy vaccines. AnnuRev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 26.Norman PS, Ohman JL, Jr, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–8. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 27.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 28.Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 30.Niederberger V, Horak F, Vrtala S, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 33.Calderon M, Cardona V, Demoly P. One hundred years of allergen immunotherapy European Academy of Allergy and Clinical Immunology celebration: review of unanswered questions. Allergy. 2012;67:462–76. doi: 10.1111/j.1398-9995.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 34.Valenta R, Niespodziana K, Focke-Tejkl M, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127:860–4. doi: 10.1016/j.jaci.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–83. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 36.Gadermaier E, Flicker S, Aberer W, et al. Analysis of the antibody responses induced by subcutaneous injection immunotherapy with birch and Fagales pollen extracts adsorbed onto aluminum hydroxide. Int Arch Allergy Immunol. 2010;151:17–27. doi: 10.1159/000232567. [DOI] [PubMed] [Google Scholar]
- 37.Gadermaier E, Staikuniene J, Scheiblhofer S, et al. Recombinant allergen-based monitoring of antibody responses during injection grass pollen immunotherapy and after 5 years of discontinuation. Allergy. 2011;66:1174–82. doi: 10.1111/j.1398-9995.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- 38.van Neerven RJ, Wikborg T, Lund G, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4 + T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- 39.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–22. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 40.Mothes N, Heinzkill M, Drachenberg KJ, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 41.Maggi E, Vultaggio A, Matucci A. T-cell responses during allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12:1–6. doi: 10.1097/ACI.0b013e32834ecc9a. [DOI] [PubMed] [Google Scholar]
- 42.Dreborg S, Lee TH, Kay AB, Durham SR. Immunotherapy is allergen-specific: a double-blind trial of mite or timothy extract in mite and grass dual-allergic patients. Int Arch Allergy Immunol. 2011;158:63–70. doi: 10.1159/000330649. [DOI] [PubMed] [Google Scholar]
- 43.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect onskin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–45. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 44.Muller U, Akdis CA, Fricker M, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy ClinImmunol. 1998;101:747–54. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 45.Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 47.Fellrath JM, Kettner A, Dufour N, et al. Allergen-specific T-cell tolerance induction with allergen-derived long synthetic peptides: results of a phase I trial. J Allergy Clin Immunol. 2003;111:854–61. doi: 10.1067/mai.2003.1337. [DOI] [PubMed] [Google Scholar]
- 48.Worm M, Lee HH, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. e1–14. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 49.Larche M. T cell epitope-based allergy vaccines. Curr Top Micro-biol Immunol. 2011;352:107–19. doi: 10.1007/82_2011_131. [DOI] [PubMed] [Google Scholar]
- 50.Reisinger J, Horak F, Pauli G, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy ClinImmunol. 2005;116:347–54. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Gafvelin G, Thunberg S, Kronqvist M, et al. Cytokine and antibody responses in birch-pollen-allergic patients treated with genetically modified derivatives of the major birch pollen allergen Bet v 1. Int Arch Allergy Immunol. 2005;138:59–66. doi: 10.1159/000087358. [DOI] [PubMed] [Google Scholar]
- 52.Pree I, Reisinger J, Focke M, et al. Analysis of epitope-specific immune responses induced by vaccination with structurally folded and unfolded recombinant Bet v 1 allergen derivatives in man. J Immunol. 2007;179:5309–16. doi: 10.4049/jimmunol.179.8.5309. [DOI] [PubMed] [Google Scholar]
- 53.Purohit A, Niederberger V, Kronqvist M, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–25. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 54.Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010;40:1346–52. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- 55.Kahlert H, Suck R, Weber B, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergenspecific immunotherapy. Int Arch Allergy Immunol. 2008;145:193–206. doi: 10.1159/000109288. [DOI] [PubMed] [Google Scholar]
- 56.Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2011;30:4328–35. doi: 10.1016/j.vaccine.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiedermann U, Herz U, Baier K, et al. Intranasal treatment with a recombinant hypoallergenic derivative of the major birch pollen allergen Bet v 1 prevents allergic sensitization and airway inflammation in mice. Int Arch Allergy Immunol. 2001;126:68–77. doi: 10.1159/000049496. [DOI] [PubMed] [Google Scholar]
- 58.Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy ClinImmunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Linhart B, Jahn-Schmid B, Verdino P, et al. Combination vaccines for the treatment of grass pollen allergy consisting of genetically engineered hybrid molecules with increased immunogenicity. FASEB J. 2002;16:1301–3. doi: 10.1096/fj.01-1012fje. [DOI] [PubMed] [Google Scholar]
- 60.Linhart B, Valenta R. Vaccine engineering improved by hybrid technology. Int Arch Allergy Immunol. 2004;134:324–31. doi: 10.1159/000079535. [DOI] [PubMed] [Google Scholar]
- 61.Linhart B, Hartl A, Jahn-Schmid B, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115:1010–6. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 62.Larenas-Linnemann D. Oralair Birch, a recombinant major birch pollen allergen tablet for sublingual immunotherapy of allergic rhinitis caused by birch pollen. Curr Opin Investig Drugs. 2010;11:586–96. [PubMed] [Google Scholar]
- 63.Bordas-Le Floch V, Bussieres L, Airouche S, et al. Expression and characterization of natural-like recombinant Der p 2 for sublingual immunotherapy. Int Arch Allergy Immunol. 2012;158:157–67. doi: 10.1159/000331143. [DOI] [PubMed] [Google Scholar]
- 64.Allam JP, Wurtzen PA, Reinartz M, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–45. e1. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 65.Kundig TM, Senti G, Schnetzler G, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006;117:1470–6. doi: 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 66.Schmitz N, Dietmeier K, Bauer M, et al. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206:1941–55. doi: 10.1084/jem.20090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grundstrom J, Neimert-Andersson T, Kemi C, et al. Covalent coupling of vitamin D3 to the major cat allergen Fel d 1 improves the effects of allergen-specific immunotherapy in a mouse model for cat allergy. Int Arch Allergy Immunol. 2012;157:136–46. doi: 10.1159/000327546. [DOI] [PubMed] [Google Scholar]
- 68.Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rancitelli P, Hofmann A, Burks AW. Vaccine approaches for food allergy. Curr Top Microbiol Immunol. 2011;352:55–69. doi: 10.1007/82_2011_126. [DOI] [PubMed] [Google Scholar]
- 70.Brozek JL, Terracciano L, Hsu J, et al. Oral immunotherapy for IgE-mediated cow’s milk allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2012;42:363–74. doi: 10.1111/j.1365-2222.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 71.Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK, et al. FAST: towards safe and effective subcutaneous immunotherapy of persistent life-threatening food allergies. Clin Transl Allergy. 2012;2:5. doi: 10.1186/2045-7022-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hiroi T, Kaminuma O, Takaiwa F. Vaccination with transgenic rice seed expressing mite allergen: a new option for asthma sufferers? Expert Rev Vaccines. 2011;10:1249–51. doi: 10.1586/erv.11.102. [DOI] [PubMed] [Google Scholar]
- 73.Daniel C, Repa A, Wild C, et al. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy. 2006;61:812–9. doi: 10.1111/j.1398-9995.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 74.Novak N, Bieber T, Allam JP. Immunological mechanisms of sublingual allergen-specific immunotherapy. Allergy. 2011;66:733–9. doi: 10.1111/j.1398-9995.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 75.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–9. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 76.Malling HJ. Sublingual immunotherapy: efficacy – methodology and outcome of clinical trials. Allergy. 2006;61(Suppl 81):24–8. doi: 10.1111/j.1398-9995.2006.01158.x. [DOI] [PubMed] [Google Scholar]
- 77.Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol. 2012;157:288–98. doi: 10.1159/000327566. [DOI] [PubMed] [Google Scholar]
- 78.Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT) Allergy. 2011;66:740–52. doi: 10.1111/j.1398-9995.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 79.Calderon MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67:302–11. doi: 10.1111/j.1398-9995.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 80.Juillard GJ, Boyer PJ, Yamashiro CH. A phase I study of active specific intralymphatic immunotherapy (ASILI) Cancer. 1978;41:2215–25. doi: 10.1002/1097-0142(197806)41:6<2215::aid-cncr2820410622>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 81.Senti G, Johansen P, Kundig TM. Intralymphatic immunotherapy: from the rationale to human applications. Curr Top Microbiol Immunol. 2011;352:71–84. doi: 10.1007/82_2011_133. [DOI] [PubMed] [Google Scholar]
- 82.Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105:17908–12. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–6. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 84.Senti G, Graf N, Haug S, et al. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;124:997–1002. doi: 10.1016/j.jaci.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Dioszeghy V, Mondoulet L, Dhelft V, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response insensitized mice. J Immunol. 2011;186:5629–37. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 86.Mondoulet L, Dioszeghy V, Vanoirbeek JA, Nemery B, Dupont C, Benhamou PH. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol. 2011;154:299–309. doi: 10.1159/000321822. [DOI] [PubMed] [Google Scholar]
- 87.Senti G, von Moos S, Tay F, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–35. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 88.Raz E, Tighe H, Sato Y, et al. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci U S A. 1996;93:5141–5. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu CH, Chua KY, Tao MH, et al. Immunoprophylaxis of allergen-induced immunoglobulin E synthesis and airway hyperresponsiveness in vivo by genetic immunization. Nat Med. 1996;2:540–4. doi: 10.1038/nm0596-540. [DOI] [PubMed] [Google Scholar]
- 90.Hartl A, Hochreiter R, Stepanoska T, Ferreira F, Thalhamer J. Characterization of the protective and therapeutic efficiency of a DNA vaccine encoding the major birch pollen allergen Bet v 1a. Allergy. 2004;59:65–73. doi: 10.1046/j.1398-9995.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 91.Huang CF, Chu CH, Wu CC, Chang ZN, Chue FL, Peng HJ. Induction of specific Th1 responses and suppression of IgE antibody formation by vaccination with plasmid DNA encoding Cyn d 1. Int Arch Allergy Immunol. 2012;158:142–50. doi: 10.1159/000331140. [DOI] [PubMed] [Google Scholar]
- 92.Slater JE, Paupore E, Zhang YT, Colberg-Poley AM. The latex allergen Hev b 5 transcript is widely distributed after subcutaneous injection in BALB/c mice of its DNA vaccine. J Allergy Clin Immunol. 1998;102:469–75. doi: 10.1016/s0091-6749(98)70137-x. [DOI] [PubMed] [Google Scholar]
- 93.Hochreiter R, Stepanoska T, Ferreira F, et al. Prevention of allergen-specific IgE production and suppression of an established Th2-type response by immunization with DNA encoding hypoallergenic allergen derivatives of Bet v 1, the major birch-pollen allergen. Eur J Immunol. 2003;33:1667–76. doi: 10.1002/eji.200323377. [DOI] [PubMed] [Google Scholar]
- 94.Roesler E, Weiss R, Weinberger EE, et al. Immunize and disappear-safety-optimized mRNA vaccination with a panel of 29 allergens. J Allergy Clin Immunol. 2009;124:1070–7. e1–11. doi: 10.1016/j.jaci.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 95.Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–97. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 96.Siskind GW, Paul WE, Benacerraf B. Studies on the effect of the carrier molecule on antihapten antibody synthesis. I. Effect of carrier on the nature of the antibody synthesized. J Exp Med. 1966;123:673–88. doi: 10.1084/jem.123.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Focke M, Mahler V, Ball T, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 98.Focke M, Linhart B, Hartl A, et al. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–33. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 99.Edlmayr J, Niespodziana K, Linhart B, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 100.Niespodziana K, Focke-Tejkl M, Linhart B, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–70. e6. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edlmayr J, Niespodziana K, Focke-Tejkl M, Linhart B, Valenta R. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol. 2011;352:121–40. doi: 10.1007/82_2011_130. [DOI] [PubMed] [Google Scholar]
- 102.Twaroch TE, Focke M, Civaj V, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–84. e7. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Chen KW, Focke-Tejkl M, Blatt K, et al. Carrier-bound nonallergenic Der p 2 peptides induce IgG antibodies blocking allergen-induced basophil activation in allergic patients: experimental Allergy and Immunology. Allergy. 2012;67:609–21. doi: 10.1111/j.1398-9995.2012.02794.x. doi: 10.1111/j.1398-9995.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Twaroch TE, Focke M, Fleischmann K, et al. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternate allergy. Clin Exp Allergy. 2012 doi: 10.1111/j.1365-2222.2012.03996.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hiller R, Laffer S, Harwanegg C, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–6. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 106.Harwanegg C, Laffer S, Hiller R, et al. Microarrayed recombinant allergens for diagnosis of allergy. Clin Exp Allergy. 2003;33:7–13. doi: 10.1046/j.1365-2222.2003.01550.x. [DOI] [PubMed] [Google Scholar]
- 107.Moverare R, Westritschnig K, Svensson M, et al. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol. 2002;128:325–35. doi: 10.1159/000063855. [DOI] [PubMed] [Google Scholar]
- 108.Westritschnig K, Sibanda E, Thomas W, et al. Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy. 2003;33:22–7. doi: 10.1046/j.1365-2222.2003.01540.x. [DOI] [PubMed] [Google Scholar]
- 109.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–8. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 110.Brunetto B, Tinghino R, Braschi MC, Antonicelli L, Pini C, Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65:184–90. doi: 10.1111/j.1398-9995.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 111.Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–63. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- 112.Moverare R, Elfman L, Vesterinen E, Metso T, Haahtela T. Development of new IgE specificities to allergenic components in birch pollen extract during specific immunotherapy studied with immunoblotting and Pharmacia CAP System. Allergy. 2002;57:423–30. doi: 10.1034/j.1398-9995.2002.13248.x. [DOI] [PubMed] [Google Scholar]
- 113.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 114.Graf N, Johansen P, Schindler C, et al. Analysis of the relationship between pollinosis and date of birth in Switzerland. Int Arch Allergy Immunol. 2007;143:269–75. doi: 10.1159/000100572. [DOI] [PubMed] [Google Scholar]
- 115.Anto JM, Pinart M, Akdis M, et al. Understanding the complexity of IgE-related phenotypes from childhood to young adulthood: a Mechanisms of the Development of Allergy (MeDALL) Seminar. J Allergy Clin Immunol. 2012;129:943–54. e4. doi: 10.1016/j.jaci.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 116.Uthoff H, Spenner A, Reckelkamm W, et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J Immunol. 2003;171:3485–92. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- 117.Hansen JS, Nygaard UC, Lyle R, Lovik M. Early Life Interventions to Prevent Allergy in the Offspring: the Role of Maternal Immunization and Postnatal Mucosal Allergen Exposure. Int Arch Allergy Immunol. 2012;158:261–75. doi: 10.1159/000332963. [DOI] [PubMed] [Google Scholar]
- 118.Flicker S, Marth K, Kofler H, Valenta R. Placental transfer of allergen-specific IgG but not IgE from a specific immunotherapy-treated mother. J Allergy Clin Immunol. 2009;124:1358–60. e1. doi: 10.1016/j.jaci.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 119.Glovsky MM, Ghekiere L, Rejzek E. Effect of maternal immunotherapy on immediate skin test reactivity, specific rye I IgG and IgE antibody, and total IgE of the children. Ann Allergy. 1991;67:21–4. [PubMed] [Google Scholar]
- 120.Flicker S, Gadermaier E, Madritsch C, Valenta R. Passive immunization with allergen-specific antibodies. Curr Top Microbiol Immunol. 2011;352:141–59. doi: 10.1007/82_2011_143. [DOI] [PubMed] [Google Scholar]
- 121.Pecquet S, Pfeifer A, Gauldie S, Fritsche R. Immunoglobulin E suppression and cytokine modulation in mice orally tolerized to beta-lactoglobulin. Immunology. 1999;96:278–85. doi: 10.1046/j.1365-2567.1999.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baranyi U, Linhart B, Pilat N, et al. Tolerization of a type I allergic immune response through transplantation of genetically modified hematopoietic stem cells. J Immunol. 2008;180:8168–75. doi: 10.4049/jimmunol.180.12.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baranyi U, Gattringer M, Valenta R, Wekerle T. Cell-based therapy in allergy. Curr Top Microbiol Immunol. 2011;352:161–79. doi: 10.1007/82_2011_127. [DOI] [PubMed] [Google Scholar]
- 124.Baranyi U, Gattringer M, Boehm A, et al. Expression of a major plant allergen as membrane-anchored and secreted protein in human cells with preserved T cell and B cell epitopes. Int Arch Allergy Immunol. 2011;156:259–66. doi: 10.1159/000323733. [DOI] [PubMed] [Google Scholar]
- 125.Leb VM, Jahn-Schmid B, Kueng HJ, et al. Modulation of allergen-specific T-lymphocyte function by virus-like particles decorated with HLA class II molecules. J Allergy Clin Immunol. 2009;124:121–8. doi: 10.1016/j.jaci.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 126.Schmetterer KG, Haiderer D, Leb-Reichl VM, et al. Bet v 1-specific T-cell receptor/forkhead box protein 3 transgenic T cells suppress Bet v 1-specific T-cell effector function in an activation-dependent manner. J Allergy Clin Immunol. 2011;127:238–45. e1–3. doi: 10.1016/j.jaci.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 127.Jahn-Schmid B, Pickl WF, Bohle B. Interaction of allergens, major histocompatibility complex molecules, and T cell receptors: a ‘menage a trois’ that opens new avenues for therapeutic intervention in type I allergy. Int Arch Allergy Immunol. 2011;156:27–42. doi: 10.1159/000321904. [DOI] [PubMed] [Google Scholar]
- 128.Neunkirchner A, Leb-Reichl VM, Schmetterer KG, et al. Human TCR transgenic Bet v 1-specific Th1 cells suppress the effector function of Bet v 1-specific Th2 cells. J Immunol. 2011;187:4077–87. doi: 10.4049/jimmunol.1003220. [DOI] [PubMed] [Google Scholar]