Abstract
This publication reports the first known use of gas chromatography-tandem mass spectrometry for the quantitation of five minor tobacco alkaloids (nornicotine, myosmine, anabasine, anatabine and isonicoteine) in various tobacco samples. A summary of the concentrations of these minor alkaloid levels in the filler from 50 popular cigarette brands were found to be 659 – 986 μg/g nornicotine, 8.64 – 17.3 μg/g myosmine, 127 – 185 μg/g anabasine, 927 – 1390 μg/g anatabine, and 23.4 – 45.5 μg/g isonicoteine. Levels of minor alkaloids found in reference cigarettes (1R5F, 2R4F, 3R4F, CM4 and CM6) as well as burley, flue-cured, oriental, reconstituted, Nicotiana rustica and Nicotiana glauca tobacco types are also reported. Quantitation of the minor tobacco alkaloids is important because the alkaloids have been shown to be precursors of carcinogenic tobacco specific N′-nitrosamines.
Keywords: Tobacco, Alkaloids, Nornicotine, Myosmine, Anabasine, Anatabine, Isonicoteine, GC-MS/MS
Introduction
More than 5 million deaths per year can be attributed to tobacco use worldwide and trends show that by 2030, more than 8 million deaths per year will be attributed to the use of tobacco.1 In the United States, one out of every 5 deaths is caused by cigarette smoking and remains the leading cause of preventable death with approximately 443,000 deaths annually.2,3 A number of structurally related alkaloids are found in tobacco, including nicotine, nornicotine, myosmine, anabasine, anatabine and isonicoteine (2,3-bypryidyl)4 (see Figure 1 for structures). While nicotine is the most abundant alkaloid, accounting for approximately 95% of alkaloid content, the minor alkaloids have been shown to exhibit biological activity in animals.5 Minor tobacco alkaloids have been characterized to a lesser extent in humans but likely play a role in the formation of carcinogenic tobacco-specific N′-nitrosamines (TSNAs).6 Alkaloid content varies widely among species.7 One species of tobacco, Nicotiana glauca (N. glauca), has relatively low nicotine but high levels of anabasine, which has been linked to accidental poisoning and fatality in a few cases.8,9
Figure 1.
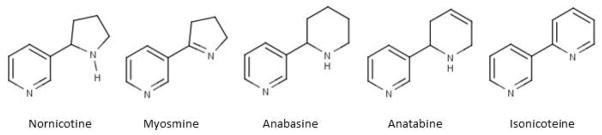
Molecular structures of minor tobacco alkaloids.
The analysis of minor alkaloids has been performed with gas chromatography (GC) coupled with a wide spectrum of detection techniques including flame ionization detection (FID), nitrogen-phosphorus detection (NPD), and mass spectrometry (MS). Other analysis approaches have included high-performance liquid chromatography-ultraviolet detection (HPLC-UV), capillary zone electrophoresis-ultraviolet detection (CZE-UV), micellar electrokinetic capillary chromatography-ultraviolet detection (MECC-UV), nitrogen chemiluminescence detection (NCD), and microemulsion electrokinetic chromatography-ultraviolet detection (MEEKC-UV).10–17 While more extravagant and/or complex approaches can be utilized, each exhibit certain limitations (i.e. standard addition construction, elaborate detection methods).13,18 Utilization of gas chromatography-tandem mass spectrometry (GC-MS/MS) in multiple reaction mode (MRM) mode allows for greater compound specificity by eliminating matrix ions arising from other compounds that share the same parent mass but lack the correct transition ion, drastically decreasing background interferences and reducing detection limits.
The aim of this research was to develop a highly specific method for quantifying the concentration of five minor alkaloids—nornicotine, myosmine, anabasine, anatabine and isonicoteine—in tobacco. This paper is the first reported use of GC-MS/MS in MRM mode to quantify minor tobacco alkaloids via GC-MS/MS. Results for tobacco from 50 top-selling cigarette brands, reference tobaccos, and various tobacco types and species are presented. This method offers a rapid, selective, and sensitive technique for measuring minor alkaloids in tobacco from smoking and smokeless products.
Experimental Section
Samples
In response to a request by the Center for Tobacco Products at the US Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC) initiated a study by which to measure the concentrations of selected chemicals (including minor alkaloids) in the 50 top-selling US cigarette brands. Cigarette samples were purchased locally from wholesale and retail outlets through The Lab Depot (Dawsonville, GA). Reference cigarettes (3R4F, 1R5F, 2R4F) were obtained from the University of Kentucky. CORESTA Monitor (CM4 and CM6) reference cigarettes were received from CORESTA (Paris, France). Pure blend cigarettes (which include 100% burley; 100% oriental; 100% flue-cured; 100% reconstituted tobacco) were prepared for CDC’s Tobacco Analysis Laboratory by Murty Pharmaceuticals and were also analyzed. A Nicotiana glauca sample was obtained from The Federal University of Paraíba (Brazil), and a Nicotiana rustica sample was graciously provided by the Great Lakes Inter-Tribal Epidemiology Center and the Wisconsin Native American Tobacco Network. Upon receipt, cigarette cartons and individual cigarette packs were logged into a custom database, assigned a unique ID, barcoded and analyzed as received.
Reagents and materials
Alkaloid standards (R,S) nornicotine, myosmine, (R,S) anabasine, (R,S) anatabine, and isonicoteine were purchased from Toronto Research Chemicals (Toronto, Ontario; Canada). Isotopically labeled internal standard, (+/−) nornicotine-2,4,5,6-D4 (pyridine-D4), was purchased from CDN Isotopes (Pointe-Claire, Quebec, Canada); DL-Nicotine (methyl-D3) was obtained from Cambridge Isotope Labs (Andover, MA). These were added to samples and used for quantification. Tomato Leaves (NIST 1573a) standard was obtained from the National Institute for Standards and Technology (NIST) (Gaithersbug, MD). The NIST 1573a matrix was used because cigarette tobacco, including nicotine-depleted products (e.g., Quest 3), contains substantial endogenous levels of minor alkaloids which interfere with quantitation. Tobacco and tomato are members of same plant family, Solanaceae,19 but tomato matrix does not contain any of the tobacco alkaloids at detectable levels, thus it serves as a suitable matrix for the measure of minor tobacco alkaloids. Also, tomato leaves are anatomically similar to tobacco leaves and commercial sources for other plant materials from the Solanaceae family are not readily available. All other chemicals were of analytical grade and were purchased through Fisher Scientific unless otherwise indicated.
Sample Preparation and Analysis Procedure
For sample preparation, a 400-mg (± 0.5 mg) sample of blank matrix or tobacco product was placed into a 15-mL amber vial and the tobacco weight recorded. Because tobacco samples can vary in consistency, a 400-mg portion is adequate to reflect the ratio of various blend components in the intact cigarette and does not require further homogenization. The sample was then spiked with 50 μL of two separate internal standard solutions, D3-Nicotine (0.85 mg/mL) and D4-Nornicotine (0.825 mg/mL) and allowed to stand for 15 min to allow absorption into the matrix. The D3-Nicotine acts as an internal standard for myosmine, anabasine, anatabine and isonicoteine; D4-Nornicotine is the internal standard for nornicotine. After the 15-min wait time, a 1-mL aliquot of 2N NaOH was added and allowed to stand at room temperature for 30 minutes after which a 10-mL aliquot of methyl tert-butyl ether was added. Vials were capped and placed on a Rugged Rotator (Glas-Col; Terre Haute, IN) to tumble at 70 revolutions/min for 1 hour. After agitating, sample extracts were expressed through a 0.45-μM syringe filter directly into individual GC vials. Per the request from FDA, individual cigarette brands were run in septuplicate (n=7) to increases statistical power.
For samples not analyzed for FDA, including reference cigarettes, and various tobacco varieties/species, triplicate samples were run and analyzed. These samples were analyzed to investigate the use of our GC-MS/MS method as a potential means of identifying tobacco types and species in smoked and smokeless tobaccos based on minor alkaloid profiles.
Instrumentation and Apparatus
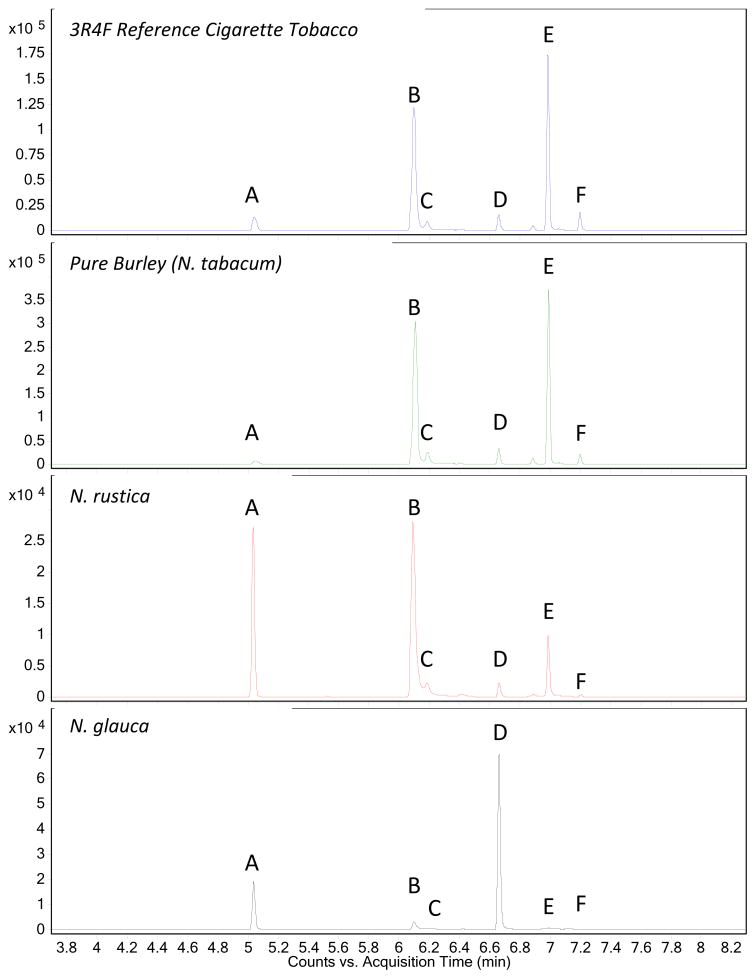
GC-MS/MS analysis was performed using an Agilent 7890 GC coupled with a 7000 Triple-Quad detector (Newark, DE) equipped with a CTC autosampler (Agilent Technologies; Andover, MA) which injects 1 μL of the extract per vial for analysis. The split/splitless injector was maintained at 250 °C with a helium flow rate of 1.0 ml/min for 7.5 min. Injections were made with a split ratio of 4:1 with a solvent delay of 3.7 min. The chromatographic separation was accomplished using a DB-1701 capillary column (30m × 0.250 μM, 0.25 μM) (J&W Scientific) with research grade (>99.9999% purity) helium used as the carrier gas (see Figure 2 for sample chromatograms). The GC ramp conditions were as follows: 35 °C, hold 0.75 min; ramp at 80 °C/min to 170 °C; ramp 2 °C/min to 178 °C; lastly ramp at 120 °C/min to 280 °C, hold 1 min. The total GC run time was 8.29 minutes and the transfer line temperature was set to 285 °C. Compounds were fragmented with electron ionization (70eV) in the ion source maintained at 230 °C. Mass measurements were made in MRM. The retention times and m/z transition values chosen for detection are in Table 1.
Figure 2.
XIC Chromatograms of a reference cigarette with blend tobacco and selected tobacco species. A: D3-Nicotine, B: Nornicotine, D4-Nornicotine, C: Myosmine, D: Anabasine, E: Anatabine, F: Isonicoteine.
Table 1.
Multiple reaction mode (MRM) transitions used for monitoring minor alkaloids and internal standards in tobacco samples.
| Compound | Retention Time (min) | Quantitation MRM Transitions, m/z (Dwell (ms); Ionization energy (V)) | Confirmation MRM Transitions, m/z (Dwell (ms); Ionization energy (V)) |
|---|---|---|---|
| Nornicotine | 6.12 | 148.0 → 106.0 (90; 1) | 148.0 → 70.0 (90; 6) |
| Myosmine | 6.20 | 146.1 → 118.1 (30; 22) | 146.1 → 91.0 (30; 35) |
| Anabasine | 6.67 | 162.1 → 106.1 (20; 20) | 162.1 → 84.0 (20; 2) |
| Anatabine | 6.98 | 160.0 → 145.1 (85; 6) | 160.0 → 82.0 (85; 7) |
| Isonicoteine | 7.21 | 156.1 → 130.0 (85; 15) | 156.1 → 101.0 (85;37) |
| D3-Nicotine (ISTD) | 5.04 | 165.1 → 136.1 (85; 25) | 165.1 → 87.1 (85; 8) |
| D4-Nornicotine (ISTD) | 6.11 | 151.2 → 109.1 (30; 22) | 121.9 → 95.1 (30; 24) |
Results
Standard curves were constructed by analysis of tomato leaf matrix spiked with known amounts of minor alkaloids. A standard stock solution was prepared by weighing each alkaloid standard and diluting with methanol to a volume of 50 mL. Table 2 shows a summary of the calibration including slope, y-intercept, linearity, and limit-of-detection (LOD). Standards used for spiking the individual calibration points were prepared by diluting known volumes of the stock solution to 10 mL with methanol to give the desired curve concentrations. Calibration samples were prepared by adding 200 μL of each calibration standard and 50 μL of each of two internal standards to approximately 400 mg of tomato leaf matrix. Curves for each analyte were plotted using 1/x weighting and all calibration curves exhibited linearity (R2) greater than 0.995. An initial LOD for each analyte was estimated from a series of standard injections (N=4). The LOD was estimated as 3 times s0, where s0 was the estimate of the standard deviation at zero analyte concentration. The value of s0 was taken as the y-intercept of a linear regression of standard deviation versus concentration as specified by Taylor et al.23
Table 2.
Summary of compound purity, limit of detections (LODs), and calibration curve range/linearity.
| Compound | CAS# | Purity (%) | LOD (μg/g) | Calibration Range (μg/g) | Slope (Average) | Intercept (Average) | Linearity, R2 (Average) |
|---|---|---|---|---|---|---|---|
| Nornicotine | 5746-86-1 | 97 | 0.08 | 1.17 – 2340 | 0.1105 | 0.0078 | 0.998 |
| Anabasine | 13078-04-1 | 98 | 0.12 | 0.83 – 1660 | 0.0429 | −0.0005 | 0.999 |
| Anatabine | 2743-90-0 | 96 | 0.12 | 1.02 – 2030 | 0.0515 | −0.0004 | 0.998 |
| Myosmine | 532-12-7 | 98 | 0.04 | 0.10 – 202 | 0.2072 | 0.0014 | 0.999 |
| Isonicoteine | 581-50-0 | 98 | 0.03 | 0.15 – 303 | 0.1315 | 0.0013 | 0.998 |
The method was validated by measuring the precision and accuracy of each analyte at three concentration levels. Precision/Accuracy data were obtained by spiking five blank matrix samples at low, medium and high concentration levels of alkaloids. A blank control was prepared by spiking five tomato matrix samples with internal standards only. Equations for the standard curves of each analyte as well as recoveries and relative standard deviation are summarized in Table 3.
Table 3.
Method precision and accuracy for minor tobacco alkaloids spiked into blank matrix at three concentrations.
| Analyte | Spike Level | Spike Concentration (μg/g) | Spike Accuracy (Recovery, %) | Spike Precision (CV, %) |
|---|---|---|---|---|
| Nornicotine | Low | 468.4 | 102.4 | 0.9 |
| Medium | 1124 | 112.4 | 3.1 | |
| High | 1991 | 101.6 | 3.3 | |
|
| ||||
| Myosmine | Low | 40.4 | 99.8 | 0.4 |
| Medium | 96.9 | 104.7 | 1.1 | |
| High | 171.6 | 100.9 | 2.0 | |
|
| ||||
| Anabasine | Low | 332.5 | 106.2 | 1.1 |
| Medium | 797.9 | 107.2 | 0.8 | |
| High | 1413 | 97.7 | 1.7 | |
|
| ||||
| Anatabine | Low | 406.3 | 106.0 | 1.1 |
| Medium | 975.1 | 108.2 | 1.8 | |
| High | 1727 | 98.9 | 1.8 | |
|
| ||||
| Isonicoteine | Low | 60.6 | 108.7 | 1.2 |
| Medium | 145.4 | 106.7 | 0.7 | |
| High | 257.4 | 96.8 | 1.6 | |
Discussion
In the measurement of compounds in complex matrices, GC-MS/MS offers increased sensitivity and greater selectivity in the elimination of background interferences than previous methods such as GC-MS, HPLC-MS and GC-NPD used to measure alkaloids. This method, which uses an alkaloid-free blank matrix, has excellent linearity (>0.995), detection limits (0.03 – 0.12 μg/g), accuracy (96.8 – 112.4%) and precision (C.V., 0.4 – 3.3%). It is also known that minor alkaloid vary among different tobacco species7 and can vary among cigarette tobaccos from different countries.13 In our research, minor alkaloid levels were measured in the tobacco filler from 50 top-selling US cigarette brands (See Table 4). In the US cigarette brands analyzed, the concentrations of anatabine (927 – 1390 μg/g), nornicotine (659 – 986 μg/g), and anabasine (127 – 185 μg/g) levels were the highest followed by isonicoteine (23.4 – 45.5 μg/g) and myosmine (8.64 – 17.3 μg/g). The levels of anatabine, nornicotine, and anabasine are important because they are precursors in the formation of N′-nitrosoanatabine (NAT), N′-nitrosonornicotine (NNN), and N′-nitrosoanabasine (NAB), respectively. Myosmine (8.64 – 17.3 μg/g) and isonicoteine (23.4 – 45.5 μg/g), which do not form TSNAs had the lowest levels of the minor alkaloids and the widest concentration variation among the brands.
Table 4.
Summary of minor alkaloid concentrations (n=7) in the 50 top-selling U.S. cigarette brands.
| Analyte | Range (μg/g) | Mean (μg/g) | Median (μg/g) |
|---|---|---|---|
| Nornicotine | 659 – 986 | 763 | 746 |
| Myosmine | 8.64 – 17.3 | 14.0 | 13.8 |
| Anabasine | 127 – 185 | 147 | 146 |
| Anatabine | 927 – 1390 | 1100 | 1090 |
| Isonicoteine | 23.4 – 45.5 | 34.1 | 33.7 |
Because mentholated products are becoming increasingly popular in the US market, an analysis of the minor alkaloid profile of mentholated products was investigated. Of the 50 top-selling brands, twelve were mentholated products. The levels of minor tobacco alkaloids in mentholated products showed no difference as compared to non-mentholated brands. The data suggests that based upon minor alkaloid profiles, the tobacco blend in mentholated cigarettes is similar to that contained in non-mentholated varieties. The comparison summary can be found in Table 5.
Table 5.
Comparison of the alkaloid profiles of menthol versus non-menthol varieties in the 50 top-selling U.S. cigarette brands (n=7).
| Analyte | Non-Menthol (38 brands)
|
Menthol (12 brands)
|
||||
|---|---|---|---|---|---|---|
| Range (μg/g) | Mean (μg/g) | Median (μg/g) | Range (μg/g) | Mean (μg/g) | Median (μg/g) | |
|
|
|
|||||
| Nornicotine | 659 – 986 | 758 | 754 | 709 – 922 | 779 | 738 |
| Myosmine | 8.64 – 17.3 | 13.9 | 13.8 | 12.8 – 17.1 | 14.4 | 13.9 |
| Anabasine | 127 – 185 | 147 | 147 | 131 – 161 | 144 | 145 |
| Anatabine | 933 – 1390 | 1100 | 1090 | 927 – 1200 | 1100 | 1100 |
| Isonicoteine | 23.4 – 45.5 | 33.8 | 33.7 | 30.0 – 45.5 | 35.0 | 33.4 |
A number of reference cigarettes and individual tobacco types were analyzed for minor tobacco alkaloids for comparison with blends present in cigarette filler (Table 6). The University of Kentucky reference cigarettes 1R5F, 2R4F and 3R4F were found to have a minor alkaloid profile similar to manufactured cigarettes. The minor tobacco alkaloid profiles of the other reference blends are also unsurprisingly similar due to the fact that the cigarettes are manufactured to exact specifications. Minor differences in alkaloid profile are most likely due to the batch variability among the tobacco itself. The CM4 and CM6 CORESTA reference cigarettes showed lower levels of nornicotine, myosmine and isonicoteine than manufactured cigarette brands.
Table 6.
Concentrations of minor alkaloid compounds found in various tobacco types and reference cigarettes (n=3).
| Tobacco Sample | Nornicotine | Myosmine | Anabasine | Anatabine | Isonicoteine1 |
|---|---|---|---|---|---|
|
| |||||
| Mean (μg/g) | |||||
| Reference cigarettes (Nicotiana tabacum) | |||||
| 1R5F Reference Cigarette | 841 | 14.4 | 172 | 1200 | 33.1 |
| 2R4F Reference Cigarette | 757 | 13.8 | 140 | 1000 | 24.9 |
| 3R4F Reference Cigarette | 682 | 11.3 | 140 | 1010 | 28.9 |
| CM4 Reference Cigarette | 505 | 5.96 | 146 | 1200 | 13.8 |
| CM6 Reference Cigarette | 566 | 8.59 | 156 | 1310 | 14.4 |
| Pure Tobacco Samples | |||||
| Nicotiana tabacum | |||||
| Burley (100%) | 2220 | 31.3 | 268 | 2210 | 36.2 |
| Flue-Cured (100%) | 678 | 19.2 | 295 | 2110 | 32.1 |
| Oriental (100%) | 591 | 8.65 | 34.2 | 218 | 3.87 |
| Reconstituted Sheet (100%) | 378 | 7.18 | 49.5 | 360 | 12.5 |
| Other Tobacco Species | |||||
| Nicotiana rustica2 | 224 | 6.12 | 76.8 | 196 | 1.80 |
| Nicotiana glauca2 | 89.4 | 1.63 | 2380 | 21.7 | 1.05 |
Isonicoteine is also known as 2,3′-bipyridyl.
No subvarieties of Nicotiana rustica or Nicotiana glauca were available.
Samples of burley, flue-cured, oriental and reconstituted tobaccos, the primary components of popular cigarette blends, were also analyzed for minor alkaloid profile. Manufacturers will adjust a blend by including different amounts of each tobacco type to achieve a signature taste. Flue-cured tobacco, which is the major component of most blended cigarettes, showed elevated levels of anatabine (2110 μg/g) and anabasine (295 μg/g) when compared to reference tobacco blends 1R5F, 2R4F and 3R4F (1000 – 1200 μg/g anatabine and 140 – 172 μg/g anabasine). Burley tobacco, also common in most US cigarette blends, showed elevated levels of all alkaloids except isonicoteine. The nornicotine level (2220 μg/g) of burley was roughly three times greater than the reference cigarettes analyzed. Oriental tobacco and reconstituted tobacco contained lower levels of the minor alkaloids.
The reference cigarettes 1R5F, 2R4F and 3R4F all exhibited similar minor alkaloid profiles. The results are unremarkable due to the fact that the University of Kentucky manufactures the cigarettes to contain an exact blend containing certain amounts of burley, flue-cured, oriental and reconstituted tobacco. Minor differences in the alkaloid profiles of the reference blends is most likely due to variability among the crop of tobacco harvested to manufacture the particular cigarette. CM4 and CM6 reference cigarettes are known to contain 100% Flue-cured tobacco free of stems. The data obtained showed a difference in the alkaloid profile between 100% Flue-cured tobacco and the CM4 and CM6 reference cigarettes. The difference in alkaloid profile may be attributed to variability among tobacco crops or inclusion of a greater amount of outer leaf lamina (which contains the highest amounts of alkaloids)25 in our batch of 100% flue-cured tobacco. Additional burley samples from other geographical regions are necessary in order to make a more complete assessment.
Some international smokeless tobacco products contain Nicotiana rustica (N. rustica) (sacred tobacco) or Nicotiana glauca (N. glauca) (Brazilian tree tobacco); for that reason those species were also analyzed. N. rustica is known for its high level of nicotine22, while the minor alkaloids are expressed in low concentrations. Products containing N. rustica include khaini and khiwam from India.24 N. glauca has been shown to exhibit extremely high levels of anabasine, which is toxic and has led to several deaths due to accidental injestion.8,9 The data shows anabasine levels for N. glauca to be approximately 2400 μg/g, or roughly 16 times higher than levels found in a typical cigarette (146.6 μg/g). It should be noted that N. glauca is not used in the manufacturing of U.S. cigarettes but has been found in gul and toombak products found in the Middle-East.24
A summary of the data is found in Table 6. The minor alkaloid levels presented are meant to be used for reference purposes are not meant to be an absolute characterization tool. Additional data from a wider range of tobacco batches is necessary in order to expand the scope of these observations.
Conclusions
This publication represents the first known method using GC-MS/MS for the quantitation of the minor tobacco alkaloids in tobacco. This chemically-selective method simultaneously measures nornicotine, myosmine, anabasine, anatabine and isonicoteine with excellent precision, accuracy and curve linearity for each analyte. The method was utilized for the analysis of the 50 top-selling U.S. cigarette brands, which had levels of minor alkaloids that were very similar. The blend similarity also extended into the mentholated versus non-mentholated products. Minor tobacco alkaloid levels were measured in common reference tobacco as well as single blend components. Minor tobacco alkaloid profiles of a tobacco product may provide insight into the tobacco type or species used in a tobacco product, such as global smokeless tobacco products that contain other tobacco species (N. rustica, N. glauca) having very distinct minor alkaloid profiles. Analysis of a larger sample set of tobaccos is needed for unambiguous identification among more similar tobaccos. Most notably, this method provides a very selective method of quantifying minor alkaloids, which are chemical precursors in carcinogenic TSNA formation.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclaimer: This information is distributed solely for the purpose of pre dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by [the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry]. It does not represent and should not be construed to represent any agency determination or policy.
References
- 1.WHO Report on the Global Tobacco Epidemic. 2009 [Google Scholar]
- 2.Centers for Disease Control and Prevention. MMWR Morb Mort Wkly Rep. 2008;51:300–303. [Google Scholar]
- 3.The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 4.Gorrod JW, Wahren J, editors. Nicotine and Related Alkaloids. Chapman and Hall; London, England: 1993. [Google Scholar]
- 5.Clark MS, Rand MJ, Vanov S. Arch Int Pharmacodyn Ther. 1965;156:363–379. [PubMed] [Google Scholar]
- 6.Hoffman D, Hecht SS. Cancer Res. 1985;45:934–944. [PubMed] [Google Scholar]
- 7.Sisson VA, Severson RF. Beitrage Tabakforsch Int. 1990;14:327–339. [Google Scholar]
- 8.Steenkamp PA, van Heerden FR, van Wyk BE. Forensic Sci Int. 2002;127:208–217. doi: 10.1016/s0379-0738(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 9.Furer V, Hersch M, Silvetzki N, Breuer GS, Zevin S. J Med Toxicol. 2011;7:47–51. doi: 10.1007/s13181-010-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng LQ, Ding L, Tong HW, Yong GP, Zhou XZ, Liu SM. Chromatographia. 2005;62:63–68. [Google Scholar]
- 11.Yang SS, Smetena I. Chromatographia. 1995;40:375–378. [Google Scholar]
- 12.Yang SS, Smetena I. Chromatographia. 1998;47:433–448. [Google Scholar]
- 13.Wu W, Ashley DL, Watson CH. Anal Chem. 2002;74:4878–4884. doi: 10.1021/ac020291p. [DOI] [PubMed] [Google Scholar]
- 14.McCalley DV. J Chromatogr. 1993;636:213–220. [Google Scholar]
- 15.Yang SS, Smetena I, Goldsmith AI. J Chromatogr A. 1996;746:131–136. doi: 10.1016/0021-9673(96)00314-7. [DOI] [PubMed] [Google Scholar]
- 16.Huang HY, Hsieh SH. J Chromatogr A. 2007;1164:313–319. doi: 10.1016/j.chroma.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 17.Cai K, Xiang Z, Zhang J, Zhou S, Feng Y, Geng Z. Anal Methods. 2012;4:2095–2100. [Google Scholar]
- 18.Wu C, Siems WF, Hill HH, Jr, Hannan RM. J Chromatogr A. 1998;811:157–161. [Google Scholar]
- 19.Encyclopædia Britannica. Solanaceae. [Accessed October 17, 2012];Encyclopædia Britannica Online. 2012 cited 2012 Jul 20 http://www.britannica.com/EBchecked/topic/552838/Solanaceae.
- 20.Piade JJ, Hoffman DJ. J Liquid Chromatogr. 1980;3:1505–1515. [Google Scholar]
- 21.Djordjevic MV, Sigountos CW, Hoffmann D, et al. Int J Cancer. 1991;47:348–51. doi: 10.1002/ijc.2910470306. [DOI] [PubMed] [Google Scholar]
- 22.Weybrew JA, Mann TJ. Tobacco Sci. 1963;7:28–36. [Google Scholar]
- 23.Taylor JK. Quality Assurance of Chemical Measurements. Lewis Publishers; Chelsea, Michigan: 1987. [Google Scholar]
- 24.Smokeless Tobacco Fact Sheets; 3rd International Conference on Smokeless Tobacco; Stockholm, Sweden. September 22–25, 2002. [Google Scholar]
- 25.Burton HR, Dye NK, Bush LP. J Agric Food Chem. 1992;40:1050–1055. [Google Scholar]