Abstract
The major turnip (Brassica rapa) pollen allergen, belongs to a family of calcium-binding proteins (i.e., two EF-hand proteins), which occur as highly cross-reactive allergens in pollen of weeds, grasses and trees. In this study, the IgE binding capacity and allergenic activity of three recombinant allergen variants containing mutations in their calcium-binding sites were analyzed in sensitized patients with the aim to identify the most suitable hypoallergenic molecule for specific immunotherapy.
Analysis of the wildtype allergen and the mutants regarding IgE reactivity and activation of basophils in allergic patients indicated that the allergen derivative mutated in both calcium-binding domains had the lowest allergenic activity. Gel filtration and circular dichroism experiments showed that both, the wildtype and the double mutant, occurred as dimers in solution and assumed alpha-helical fold, respectively. However, both fold and thermal stability were considerably reduced in the double mutant. The use of bioinformatic tools for evaluation of the solvent accessibility and charge distribution suggested that the reduced IgE reactivity and different structural properties of the double mutant may be due to a loss of negatively charged amino acids on the surface. Interestingly, immunization of rabbits showed that only the double mutant but not the wildtype allergen induced IgG antibodies which recognized the allergen and blocked binding of allergic patients IgE.
Due to the extensive structural similarity and cross-reactivity between calcium-binding pollen allergens the hypoallergenic double mutant may be useful not only for immunotherapy of turnip pollen allergy, but also for the treatment of allergies to other two EF-hand pollen allergens.
Keywords: Allergen, Allergy, Brassica, Calcium-binding allergen, Cross-reactivity, Hypoallergenic mutants, Immunotherapy
Introduction
The genus Brassica comprises a diverse group of more than 100 species including important crop plants, grown as vegetables, as sources of vegetable oil, as spices and increasingly also as sources of biodiesel. With the expansion of cultivation of Brassica crops their potential as allergen sources has become evident (Singh et al. 1995; Hemmer et al. 1997; Focke et al. 1998). Overall the prevalence of IgE-mediated allergy to Brassica species in pollen allergic patients seems to be low (7%, Hemmer et al. 1997), but among personal occupationally exposed to Brassica (e.g. plant breeders) the prevalence of sensitization was found to rise up to 44% (Hermanides et al. 2006).
The best characterized Brassica pollen allergens (Toriyama et al. 1995; Rozwadowski et al. 1999; Okada et al. 1999, 2000) belong to a protein family of highly conserved, cross-reactive calcium-binding allergens, which are not only contained in pollen of the genus Brassica, but also in pollen of taxonomically unrelated plants like trees, grasses and weeds (Valenta et al. 1998; Radauer and Breiteneder 2006). These allergens are small water-soluble proteins with a molecular weight of 8–9 kDa, which are characterized by the presence of two highly conserved Ca2+-binding domains, termed EF-hands. Each of these calcium binding motifs consists of two alpha-helices connected by a loop which coordinates the calcium ions (Kawasaki and Kretsinger 1994).
Even though these calcium-binding allergens are recognized only by relatively few (i.e., 10–20%) pollen-sensitized patients (Rossi et al. 2001; Mari 2003) their importance has been demonstrated by their potential to elicit strong allergic reactions (Niederberger et al. 1999) and their extensive cross-reactivity causing a phenotype of apparent polysensitization in allergic patients (Twardosz-Kropfmüller et al., 2010).
With the aim to develop vaccine candidates for immunotherapy we studied three Brassica two EF-hand allergen mutants regarding their IgE binding capacities and allergenic activities in patients sensitized to calcium-binding allergens and identified the mutant most suitable for specific immunotherapy. In addition a detailed characterization of the physicochemical and structural properties of the wildtype allergen and the double mutant and their immunogenicity was performed.
Materials and methods
Characterization of patients
Sera and blood samples from seven patients with a positive case history of IgE-mediated allergy to pollen from various unrelated plant species, IgE reactivity to commercially available extracts of rape (Brassica napus), timothy grass (Phleum pratense), birch (Betula verrucosa) and mugwort (Artemisia vulgaris) pollen, as determined by ImmunoCAP measurements (Phadia, Uppsala, Sweden), were analyzed in this study for initial IgE binding studies and basophil histamine release experiments after informed consent was obtained. For control purposes, blood samples from a non-allergic individual were analyzed. Demographic, clinical and serological data of these individuals are given in Table 1. Blood samples from additional 5 patients (A1–A5) containing IgE antibodies against two EF-hand pollen allergens and three non-allergic individuals (N1–N3) were used for comparing the IgE reactivity of the N- and C-terminally his-tagged wildtype and double mutant and for cellular experiments.
Table 1.
Clinical and serological characterization of individuals.
| Individual | Age (y) | Sex | Allergen sources |
Symptoms | Therapy | Total serum IgE (kU/L) |
Specific IgE (kUA/L) to |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Rape Brassica napa |
Timothy grass Phleum pratense |
Birch Betula verrucosa |
Mugwort Artemisia vulgaris |
|||||||
| 1 | 27 | m | g, t, w, m | rc, ad | ah | 141 | 0.78 | 27.1 | 7.4 | 4.0 |
| 2 | 26 | m | g, t, w | rc | ah | 140 | 2.32 | 25.9 | 5.2 | 4.4 |
| 3 | 34 | f | g, t, w, a | rc, u | IT | 168 | 3.88 | 66.6 | 10.8 | 14.9 |
| 4 | 45 | m | g, t, w, m, a | rc, as | c | 401 | 27.80 | 52.8 | 29.3 | 9.2 |
| 5 | 28 | m | g, t, w, f | rc, as | ah | 144 | 3.66 | 39.3 | 22.1 | 3.1 |
| 6 | 28 | m | g, t, w, a | rc, as, u | β, IT | 543 | 6.30 | >100.0 | 33.3 | 5.7 |
| 7 | 23 | m | g, t, w, a, f | rc, ad, as | ah, IT | 315 | 6.18 | >100.0 | 38.1 | 3.2 |
| 8a | 25 | f | – | – | – | <100 | <0.3 | <0.3 | <0.3 | <0.3 |
g, grasses; t, trees; w, weeds; m, mites; a, animals; f, food; rc, rhinoconjunctivitis; ad, atopic dermatitis; u, urticaria; as, asthma; ah, antihistamines; IT, immunotherapy with grass pollen extract; c, corticosteroids; β, beta-sympathomimetica.
Non-atopic individual.
Expression and purification of recombinant proteins
Mutations were introduced into the cDNA of the allergen, which was originally designated Bra r 1 (DDBJ/EMBL/GenBank accession number D63153; Toriyama et al. 1995) and now has been renamed according to the Allergen Nomenclature subcommittee Bra r 5.0101 in the first (mu1), in the second (mu2) and in both calcium-binding sites (muW) as described (Okada et al. 1998; Fig. 1) using a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The wildtype allergen and the mutated proteins mu1, mu2 and muW (double mutant) were expressed with a N-terminal hexa-histidine tag in Escherichia coli M15 and purified by Ni2+-affinity chromatography (QIAGEN GmbH, Hilden, Germany). For large scale expression in E. coli, genes coding for the wildtype allergen and the double mutant with a codon-usage optimized for E. coli expression were synthesized (GenScript, Piscataway, USA) and inserted into the NdeI/EcoRI sites of plasmid pET-27b (Novagen, Darmstadt, Germany). The genes contained sequences coding for a C-terminal hexa-histidine tag. Their DNA sequences were confirmed by restriction analysis and sequencing of both DNA strands. E. coli BL21(DE3) (Stratagene, La Jolla, CA) were transformed with the plasmid constructs and grown in LB medium containing 30 μg/mL kanamycin at 37 °C under continuous shaking until an OD600nm of 0.6 was reached and protein expression was induced by addition of isopropyl-β-thiogalactopyranoside (Calbiochem, Merck, Darmstadt, Germany) to a final concentration of 0.5 mM for another 4 h. After harvesting of cells by centrifugation, recombinant proteins were isolated by Nickel affinity chromatography under denaturing conditions according to the manufactures protocol (QIAGEN). Purified proteins were soluble in PBS, their concentration was determined by Micro-BCA analysis (Pierce, Rockford, IL) and their purity was determined by SDS polyacrylamide gels (SDS-PAGE) and Coomassie blue staining under reducing and non-reducing conditions (Laemmli 1970).
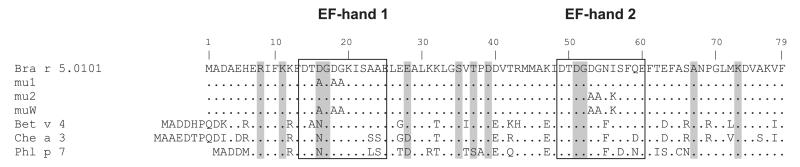
Fig. 1.
Protein sequence alignment of Bra r 5.0101 and the Bra r 5.0101 mutants (mu1, mu2, muW) with two EF-hand pollen allergens from birch (Bet v 4), from white goosefoot (Che a 3) and from timothy grass (Phl p 7). The two calcium binding sites are marked by boxes. Dots represent amino acids identical with Bra r 5.0101 and gray boxes mark putative surface exposed cross-reactive amino acids mapped to the surface of Che a 3 (Verdino et al., 2008).
Recombinant Aln g 4 and Phl p 7 were expressed in E. coli BL21(DE3) and purified by DEAE anion exchange chromatography (DEAE, Sepharose Fast flow column; GE Healthcare) (Hayek et al. 1998; Niederberger et al. 1999). Protein concentrations were determined with a Micro BCA kit (Pierce) and the purity of the proteins was evaluated by Coomassie brilliant blue staining of SDS-PAGE.
Gel filtration experiments and circular dichroism analysis
Gel filtration experiments were performed with the purified wildtype allergen and double mutant as described (Campana et al. 2011). Briefly, 150 μL aliquots of the proteins (wildtype: c = 2.5 mg/mL; muW: c = 1.5 mg/mL) were loaded on a Superdex 200 10/300 GL column (GE Healthcare, Uppsala, Sweden) at 4 °C, equilibrated with 15 mM phosphate buffer pH 7.5 containing 150 mM KCl. The flow rate was 0.6 mL/min and fractions of 0.5 mL were collected. The apparent molecular masses (MMs) of the elution peaks were calculated based on the gel filtration of standard proteins performed under identical conditions (BioRad: thyroglobulin, 670 kDa; bovine gamma globulin, 158 kDa; chicken ovalbumin, 44 kDa; equine myoglobin, 17 kDa; vitamin B12, 1.35 kDa).
Circular dichroism (CD) spectra of the purified wildtype and double mutant were recorded on a Jasco J-810 spectropolarimeter (Jasko, Tokyo, Japan) in PBS at a protein concentration of 0.1 mg/mL as described (Niederberger et al. 1999). Results are shown as mean residue ellipticities [θ] at given wavelengths and the secondary structure contents of the samples were analyzed with the secondary structure estimation programs CDSSTR (Whitmore and Wallace 2004). Thermal denaturation and refolding experiments were done by gradually increasing the temperature (10° steps) from 25 °C to 95 °C with a heat rate of 1 °C/min and cooling back to 25 °C.
Determination of IgE reactivity of rBra r 5.0101, rAln g 4 and r Phl p 7 by ELISA
ELISA plates (Nunc Maxisorb, Roskilde, Denmark) were coated with rBra r 5.0101, rAln g 4 or rPhl p 7 (2 μg/mL in 0.1 M sodium bicarbonate, pH 9.6) and blocked with 1% (w/v) BSA in Tris-buffered saline containing 0.05% (v/v) Tween 20 (TBST). Plates were incubated with sera diluted 1:5 in TBST, 0.5% (w/v) BSA and bound IgE antibodies were detected with an alkaline phosphatase-coupled mouse monoclonal anti-human IgE antibody (Pharmingen, San Diego, CA) diluted 1:1000 in TBST, 0.5% (w/v) BSA. The color reaction was developed by incubation with alkaline phosphatase substrate (Sigma–Aldrich, St. Louis, MO). The optical density (OD) was measured in an ELISA reader (Dynatech, Denkdorf, Germany) at 405 nm. The determinations were performed in duplicates with a variation of less than 10% and the results are displayed as mean OD (Table 3).
Table 3.
IgE reactivity to rBra r 5.0101, rAln g 4 and rPhl p 7as determined by ELISA.a
| Individual | IgE reactivity to |
||
|---|---|---|---|
| rBra r 5.0101 | rAln g 4 | rPhl p 7 | |
| 1 | 0.492 | 0.493 | 0.359 |
| 2 | 0.947 | 0.889 | 1.040 |
| 3 | 1.129 | 1.060 | 0.783 |
| 4 | 2.066 | 2.304 | 1.979 |
| 5 | 0.514 | 0.528 | 0.463 |
| 6 | 2.074 | 2.220 | 1.151 |
| 7 | 0.723 | 0.641 | 0.702 |
| 8 | 0.006 | 0.006 | 0.005 |
ELISA plate bound rBra r 5.0101, rAln g 4 and rPhl p 7 were exposed to sera from seven pollen-allergic patients (1–7) and from a non-atopic individual (8). OD values, corresponding to the amount of bound IgE, are displayed.
Non-denaturing, RAST-based IgE binding assays
Aliquots of 1 μg of the wildtype allergen of the mutants (mu1, mu2, muW) and, for control purposes, of human serum albumin (HSA), were dotted on nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The membranes were exposed to patients’ sera and, as a control, to the serum of a non-allergic individual diluted 1:10 in 0.5% (w/v) BSA, 50 mM Na phosphate (pH 7.5), 0.5% (v/v) Tween 20, 0.05% (w/v) NaN3. Bound IgE antibodies were detected with 125I-labeled anti-human IgE antibodies (Phadia) diluted 1:15 and visualized by autoradiography using KODAK X-OMAT films and intensifying screens (Kodak, Heidelberg, Germany) (Ball et al. 2009).
Basophil activation and T cell proliferation experiments
For histamine release assays, granulocytes of the seven allergic patients and of the non-atopic individual were isolated from heparinized blood samples by dextran sedimentation (Valent et al. 1989). Cells were incubated with increasing concentrations (0.0001 μg/mL, 0.001 μg/mL, 0.01 μg/mL, 0.1 μg/mL, 1 μg/mL, 10 μg/mL) of the wildtype allergen, the allergen mutants (mu1, mu2, muW), rPhl p 7 and rAln g 4. Histamine released into the supernatant was determined by a radioimmunoassay (Immunotech, Marseille, France). Results are expressed as mean values of triplicate determinations ± SD and represent the percent age of total histamine after freeze–thawing of the cells.
For CD203c activation assays, heparinized peripheral blood samples from patients were incubated with serial dilutions (0.001–1 μg/mL) of wildtype allergen or rmuW for 15 min at 37 °C. A monoclonal anti-IgE antibody E-124.2.8 (1 μg/mL; Immunotech, Marseille, France) and PBS were used as controls. Up-regulation of CD203c was determined and calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIcontrol) cells and is expressed as stimulation index (MFIstim: MFIcontrol) (Hauswirth et al. 2002). Experiments were performed in triplicates and are shown as means with SDs.
T cell proliferation experiments with PBMCs from sensitized patients were performed as described (Marth et al. 2013).
Sequence comparisons, structural analysis and modeling
The percentages of amino acid sequence identities of two EF-hand pollen allergens were determined using the BLASTp program (http://www.ncbi.nlm.nih.gov/blast/) and proteins were then grouped according to their sequence identities with Bra r 5.0101 manually in a table. Structural models of Bra r 5.0101 wildtype protein and the double mutant muW were obtained from the amino acid sequences by homology-based fitting to the template model of the Bet v 4 NMR structure (Neudecker et al. 2004). The structure of Bet v 4 was used as a template, because this protein displays the highest sequence homology to Bra r 5.0101. Homology modeling was performed using the Swiss-PdbViewer software for sequence and structure alignment and the Swiss-Model Server for obtaining energy-minimized homology models (Swiss-PdbViewer/-Model Server environment; Guex and Peitsch 1997).
For calculation of solvent excluded molecular surfaces the program MSMS (Sanner et al. 1996) was used with 1.5Å probe radius and a surface vertex density of 1.1Å−2. The electrostatic potential of the models was calculated with the program APBS (Baker et al. 2001), using a non-linear Poisson–Boltzmann equation and dielectric constants of 2.0 and 78.5 for protein and solvent, respectively. For projection of the relative solvent accessibility and the electrostatic potential onto the molecular surfaces the software tool SPADE (Dall’Antonia et al. 2011) was used, applying the GETAREA/FANTOM formalism (Fraczkiewicz and Braun 1998) for the calculation of accessibility values.
Immunization of rabbits, IgG reactivity of rabbit antibodies and inhibition of allergic patients allergen-specific IgE binding by rabbit IgG antibodies
Rabbits were immunized either with 200 μg of wildtype allergen or 200 μg muW per injection using Freunds (complete and incomplete) adjuvans or aluminum hydroxide (Charles River, Kisslegg, Germany). Serum samples were obtained before immunization and in 3-week intervals after immunization.
Rabbit IgG responses against the wildtype allergen were analyzed 6 weeks after immunization by ELISA with serial dilutions of the rabbit antisera, or the corresponding preimmune sera (1:1000, 1:5000, 1:10,000, and 1:50,000) as described (Twaroch et al. 2011). The ability of rabbit IgG antibodies to inhibit allergic patients IgE binding to the wildtype allergen was studied by ELISA competition experiments as described (Twaroch et al. 2011) using 1:5 diluted patients’ sera and 1:20 diluted rabbit antisera. The percentage reduction of IgE binding achieved by means of preincubation with rabbit antisera was calculated in the following way: 100 − (ODi/ODp) × 100, where ODi and ODp represent optical density values after preincubation with the rabbit immune serum or pre-immune serum, respectively.
Results
Structural similarity and IgE cross-reactivity of Bra r 5.0101 with ‘two EF-hand’ calcium-binding pollen allergens from unrelated plants
Table 2 illustrates the high percentage of amino acid sequence homology among the calcium-binding pollen allergens. Sequence identities to Bra r 5.0101 range from 61% to 77%, and highest identities of 77% occur between the Brassica pollen allergen, and the Betulaceae pollen allergens, Bet v 4 and Aln g 4. Higher sequence identities are found among allergens from plants belonging to the same families (e.g. 90% between the Betulaceae pollen allergens Bet v 4 and Aln g 4; 92% between the Oleaceae pollen allergens Ole e 3 and Syr v 3; 93% between the grass pollen allergens Phl p 7 and Cyn d 7). Fig. 1 shows an amino acid sequence alignment of Bra r 5.0101 and the mutants with calcium-binding allergens whose three-dimensional structure has been determined (Bet v 4 from birch pollen, Neudecker et al. 2003; Che a 3 from lambsquarter pollen, Verdino et al. 2008 and Phl p 7 from timothy grass pollen, Verdino et al. 2002). Twelve surface-exposed amino acids possibly involved in IgE cross-reactivity as defined by Verdino et al. 2008 are marked with gray boxes.
Table 2.
Percentage of amino acid sequence identities among two EF-hand pollen allergens.
| Bra r 5.0101 | Bet v 4 | Aln g 4 | Ole e 3 | Che a 3 | Syr v 3 | Phl p 7 | Cyn d 7 | Nic t 1 | Nic t 2 | Art v 5 | Amb a 9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P69197a | CAA73147 | O81701 | O81092 | Q84V36 | P58171 | O82040 | P94092 | Q8VWY6 | Q8VWY7 | AAX85389 | AAX77684 | |
| Bra r 5.0101 | 100% | 77% | 77% | 73% | 73% | 71% | 69% | 68% | 69% | 65% | 64% | 61% |
| Bet v 4 | 100% | 90% | 82% | 83% | 79% | 67% | 69% | 77% | 79% | 67% | 72% | |
| Aln g 4 | 100% | 84% | 84% | 78% | 68% | 67% | 74% | 76% | 64% | 66% | ||
| Ole e 3 | 100% | 82% | 92% | 67% | 65% | 79% | 77% | 65% | 72% | |||
| Che a 3 | 100% | 79% | 76% | 73% | 78% | 74% | 64% | 69% | ||||
| Syr v 3 | 100% | 70% | 65% | 78% | 74% | 65% | 72% | |||||
| Phl p 7 | 100% | 93% | 68% | 64% | 63% | 63% | ||||||
| Cyn d 7 | 100% | 66% | 61% | 61% | 58% | |||||||
| Nic t 1 | 100% | 77% | 69% | 71% | ||||||||
| Nic t 2 | 100% | 64% | 64% | |||||||||
| Art v 5 | 100% | 82% | ||||||||||
| Amb a 9 | 100% |
Accession number, allergens: Bra r 5.0101 (turnip); Bet v 4 (birch pollen); Aln g 4 (alder pollen); Ole e 3 (olive pollen); Che a 3 (white goosefoot); Syr v 3 (common lilac); Phl p 7 (timothy grass); Cyn d 7 (Bermuda grass); Nic t 1, Nic t 2 (common tobacco); Art v 5 (mugwort); Amb a 9 (ragweed).
We investigated by ELISA in seven patients allergic to two EF-hand allergens (Table 1), the IgE reactivity to rBra r 5.0101 wildtype protein, to rPhl p 7 and to rAln g 4 (Table 3). In accordance with the high sequence homology between the allergens (Fig. 1 and Table 2) each of the patients displayed comparable IgE reactivity to each of the three EF-hand pollen allergens.
Mutants of the major Brassica pollen allergen exhibit reduced IgE binding capacity
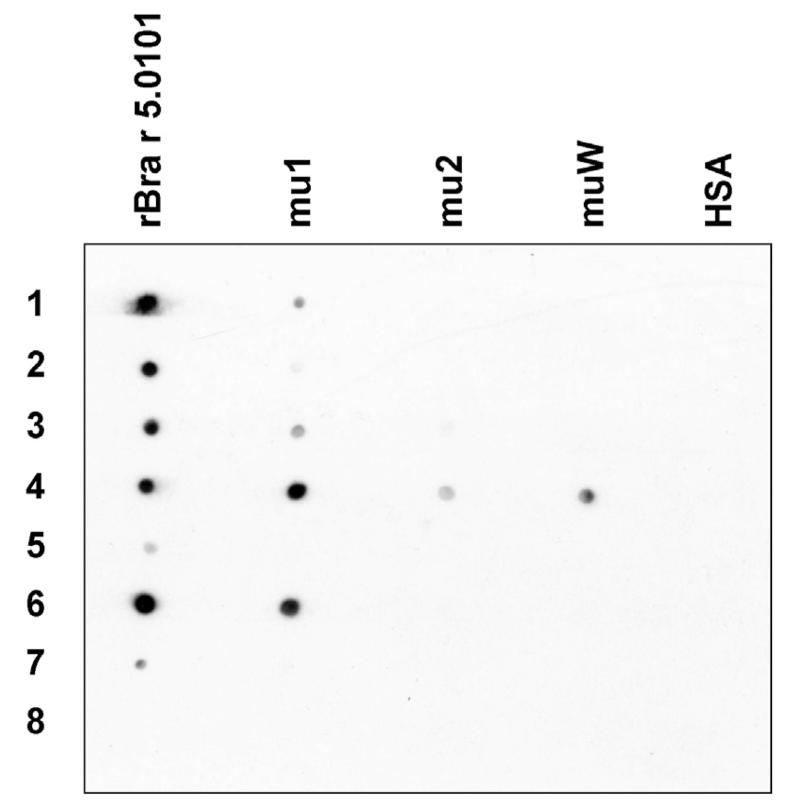
In order to identify the Bra r 5.0101 mutant most suitable for allergy vaccination we then investigated the IgE binding capacity of the three mutants by dot blot analysis using sera from seven sensitized patients (Table 1). Results (Fig. 2) showed that alterations in the first calcium-binding domain (mu1) reduced the IgE reactivity for five of the seven sera (patients 1, 2, 3, 5 and 7). The mutation of the second calcium-binding domain (mu2) reduced the IgE reactivity for each of the seven sera and led to a complete loss of IgE reactivity in five of them (patients 1, 2, 5, 6, and 7). Likewise, a reduction of IgE binding capacity was observed for muW, the mutant with two mutated EF-hand domains. A complete loss of IgE binding capacity was seen for six sera (Fig. 2). Interestingly, IgE antibodies of serum 4 showed residual reactivity to muW which was even more intensive than for mu2.
Fig. 2.
Reduced IgE reactivity of rBra r 5.0101 mutants. Nitrocellulose-dotted rBra r 5.0101, Bra r 5.0101 mutants (mu1, mu2, muW) and, for control purposes, HSA were exposed to serum IgE from the seven sensitized allergic patients (1–7) and a non-allergic individual (8).
muW shows the strongest reduction of allergenic activity of the mutants
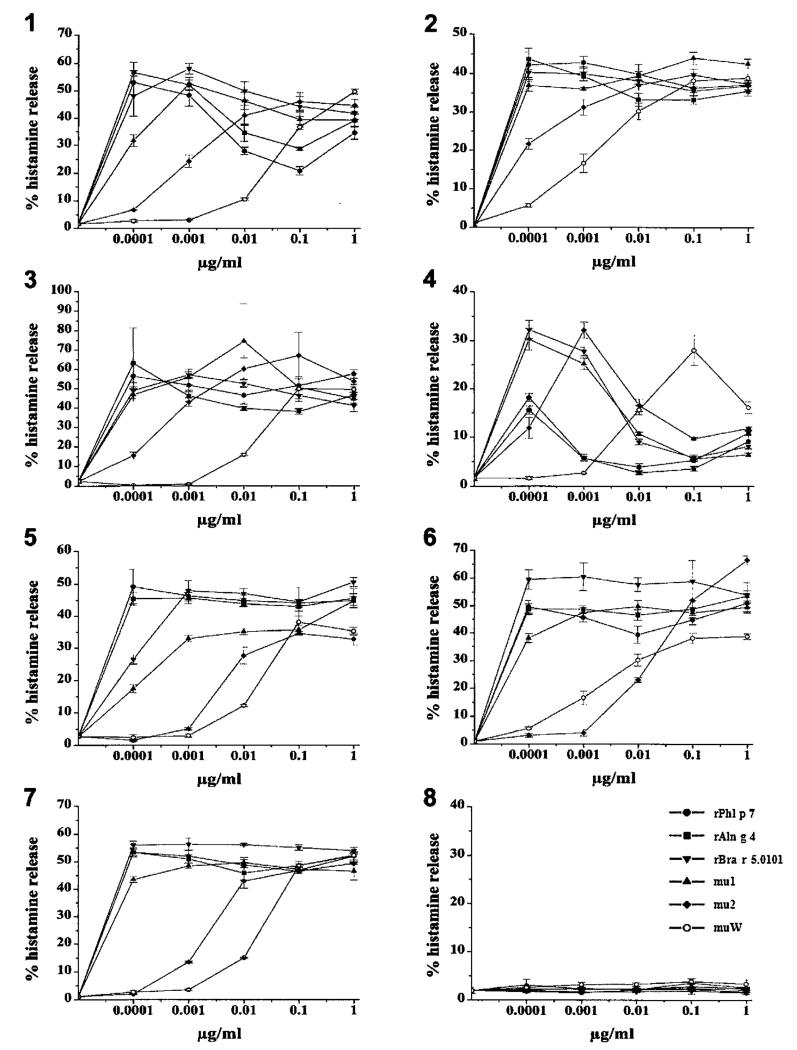
Next, we investigated the biological activity of the Bra r 5.0101 mutants using the basophil histamine release test. Granulocytes from the seven sensitized patients and from the non-allergic individual were exposed to wildtype, to the mutants (mu1, mu2, muW), to rPhl p 7 and to rAln g 4 at various concentrations ranging from 10−4 to 1 μg/mL (Fig. 3). The three recombinant wildtype allergens, rBra r 5.0101, rPhl p 7 and rAln g 4 induced a strong histamine release in basophils from all patients already at the lowest protein concentrations tested (10−4 μg/mL). The histamine release induced by mutant mu1 was in most patients similar to that of the wild-type allergen or slightly reduced. Mutant mu2 exhibited a strong reduction of allergenic activity in all patients investigated. However, patient-dependent differences were observed: in patients 5 and 6 no histamine release was induced up to concentrations of 10−2 μg/mL, whereas in patients 2 and 4 histamine release was already triggered at concentrations of 10−4 μg/mL. The double mutant, muW, clearly exhibited the strongest reduction of allergenic activity for each of the tested patients of at least 1000-fold compared to the wildtype allergen. Interestingly, although patient 4 still had displayed residual IgE reactivity to muW, the allergenic activity of muW was still 1000-fold less than that of the wildtype allergen (Fig. 3).
Fig. 3.
Reduced allergenic activity of the rBra r 5.0101 mutants. Induction of basophil histamine release with rBra r 5.0101, rBra r 5.0101 mutants (mu1, mu2, muW), rPhl p 7 and rAln g 4. Granulocytes from the seven pollen-allergic patients (1–7) and the non-allergic individual (8) were incubated with various concentrations (x-axis) of the purified recombinant proteins. Histamine release is expressed as percentage of total histamine on the y-axis (triplicates ± SD).
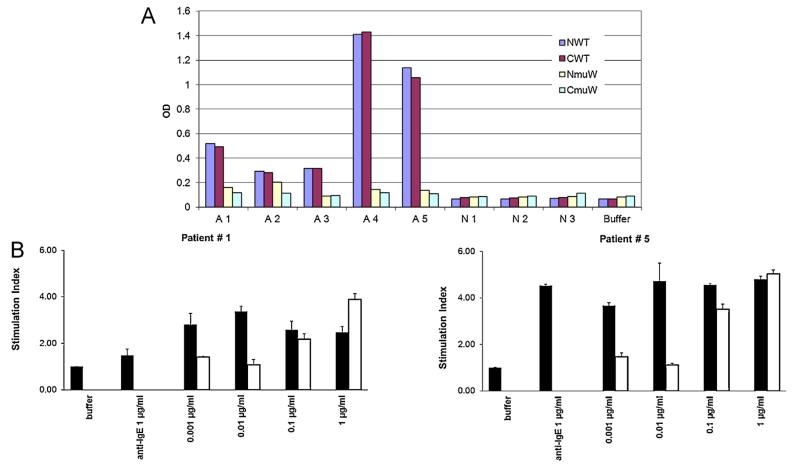
In order to obtain sufficient amount of the wildtype and mutant protein, we prepared codon-optimized constructs, purified these proteins to homogeneity and compared them with the original constructs regarding IgE-binding and allergenic activity. Fig. 4A shows that the N- as well as C-terminally his-tagged recombinant wildtype allergens show comparable IgE reactivity and that the corresponding mutants also show a comparable reduction of IgE reactivity. The C-terminally his-tagged recombinant double mutant also showed a consistent and more than 100-fold reduced allergenic activity when analyzed for up-regulation of CD203c expression in allergic patients’ basophils (Fig. 4B).
Fig. 4.
Reduced IgE reactivity and allergenic activity of the double mutant. (A) IgE reactivity of N- and C-terminally hexa-histidine-tagged wildtype allergens (NWT, CWT) and double mutants (NmuW, CmuW). Shown are specific IgE levels (y-axes: OD levels) for five sensitized patients (A1–A5), three non-allergic individuals (N1–N3) and buffer alone. (B) Reduction of allergenic activity of the double mutant (white bars) compared to the wildtype allergen (black bars). Shown is the up-regulation of CD203c expression (y-axes: stimulation indices) induced in basophils from two allergic patients (patient #1, #5) by incubation with different concentrations of the proteins (x-axes), anti-IgE or buffer alone.
When we analyzed two sensitized patients for T cell proliferation, proliferations of 2245 ± 776 cpm and 32,564 ± 3608 cpm were obtained with the wildtype allergen, compared to 1671 ± 1053 cpm and 8788 ± 1223 cpm obtained with medium alone. In both patients the double mutant induced T cell proliferations comparable to the wildtype protein (i.e., 2139 ± 408 cpm and 34,803 ± 5025 cpm).
Wildtype allergen and double mutant occur as dimers in solution and the double mutant has reduced fold
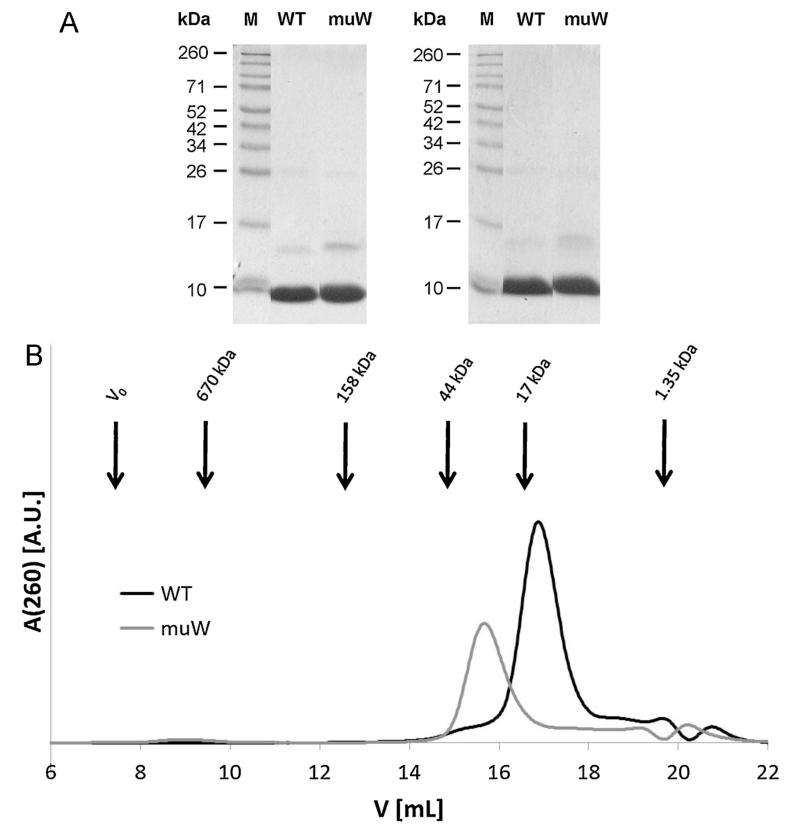
For a detailed biochemical and structural characterization purified C-terminally his-tagged recombinant wildtype and double mutant were first analyzed by SDS-PAGE under reducing and non-reducing conditions (Fig. 5A). Under both conditions purified wildtype and double mutant migrated as prominent bands corresponding to the monomeric form at approximately 8–9 kDa and, as a second weaker band, migrating at approximately 15–16 kDa which may represent their dimeric forms.
Fig. 5.
Purity and solution behavior of the wildtype allergen and double mutant. (A) Coomassie-stained SDS-PAGE showing purified wildtype allergen (WT) and double mutant (muW) under reducing (left) and non-reducing (right) conditions. Lane M represents a molecular weight marker. (B) Elution of purified WT (black) and muW (gray) as determined by gel filtration. Arrows indicate molecular weight standards, elution volumes are shown in mL (x-axes) and peak intensities are given as arbitrary units (y-axes).
In the gel filtration experiments, the wildtype eluted at 16.8 mL corresponding to a molecular mass (MM) of 16 kDa indicating that it migrates as dimer, whereas the elution peak of the mutated protein muW was shifted to 15.5 mL, corresponding to a MM of 30.1 kDa (Fig. 5B). A similar shift toward an apparent higher MM was observed for the homologous timothy grass pollen allergen rPhl p 7 in its Ca2+-depleted form (Verdino et al. 2002) indicating that the mutant protein has an increased hydrodynamic radius due to partial unfolding, but still migrates as a dimer.
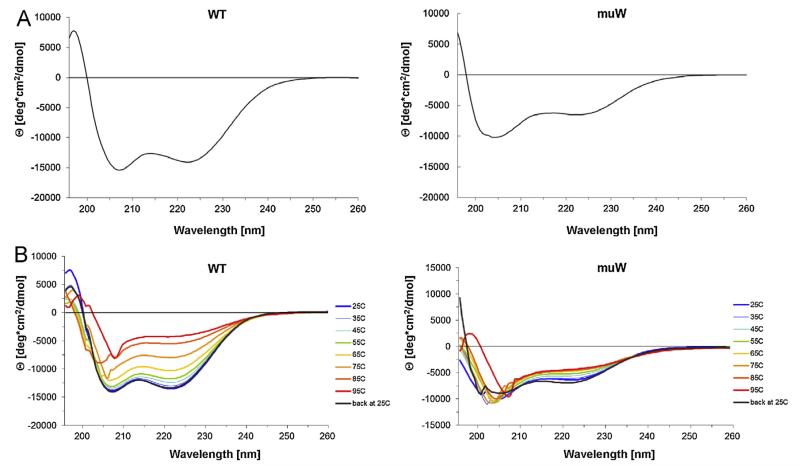
This assumption was supported by the CD experiments (Fig. 6A and B). The wildtype allergen showed a CD spectrum indicative of an alpha helical protein with minima at approximately 225 nm and 208 nm (Fig. 6A, left). Likewise, the double mutant showed a CD spectrum indicative of the presence of a large proportion of alpha helical structure in the protein but the spectrum showed a shift of minimum at 208 nm toward 205 nm indicating an increase of unfolded protein (Fig. 6A, right). The CDSSTR analysis showed the wildtype allergen contained 55% alpha helix, 18% beta sheet, 7% beta turn and 19% unordered structure. For the double mutant 30% alpha helix, 10% beta sheet, 16% beta turn and 44% unordered structure was calculated. The heat denaturation experiments showed that the wildtype allergen preserved its alpha helical structure almost up to 75 °C and after re-cooling, regained its original fold completely (Fig. 6B, left). By contrast, the double mutant quickly increased its unfolded conditions upon heating and did not regain its structure (Fig. 6B, right).
Fig. 6.
Fold and thermal stability of the wildtype allergen and double mutant by CD measurements. (A) CD spectra of the wildtype allergen (WT: left) and the double mutant (muW: right) at room temperature. (B) Thermal denaturation and refolding of the wildtype allergen (WT: left) and the double mutant (muW: right) at different temperatures. Molecular ellipticities (y-axes) are displayed for different wavelengths (x-axes).
Sequence- and structure-based surface feature calculations show that the double mutant muW differs from the wildtype regarding surfaced exposed amino acids and electrostatic potential
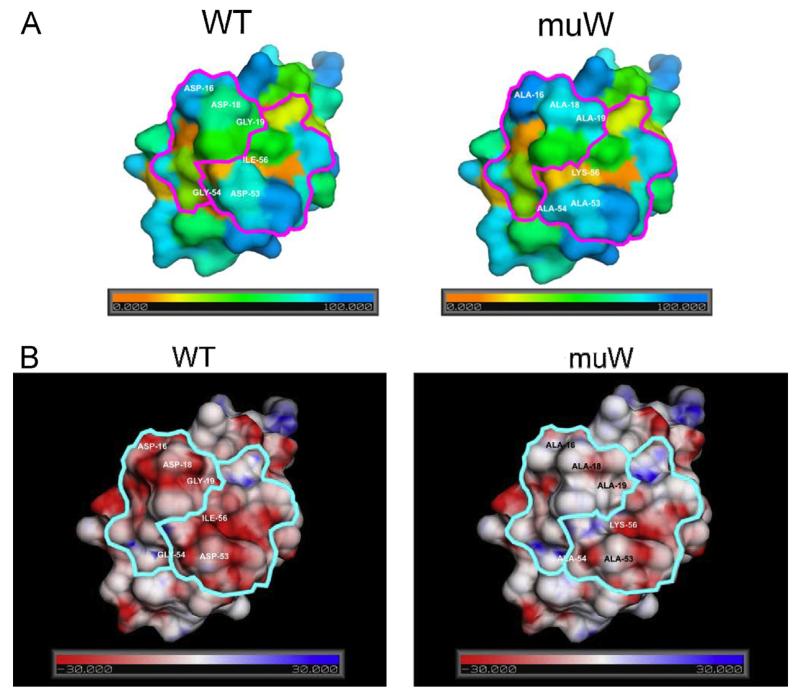
We then performed surface feature calculations analyzing the solvent accessibility and charge distribution of the surfaces of the wildtype, Bra r 5.0101 and muW. In order to analyze the theoretical effect of amino acid differences between the two proteins on the solvent accessibility and charge distribution, models of wild-type and muW were generated based on the NMR structure of Bet v 4. There was a modest difference regarding relative accessibility of surface amino acid residues between the wildtype allergen and muW (Fig. 7A). A significant difference in the electrostatic potential of the wildtype and muW could be observed (Fig. 7B): in the double mutant the surface exposed areas of both calcium binding loops, in particular the N-terminal loop, were less negatively charged than in wildtype. This finding indicates that the mutated amino acids have fewer effects on the fold of the protein but rather affect surface-exposed amino acids involved in IgE reactivity. In fact, this is in agreement with the CD analysis which shows that the double mutant was not completely unfolded, but preserved alpha helical structure.
Fig. 7.
(A) Relative solvent accessibility of surface amino acid residues of Bra r 5.0101 and muW. The color scheme ranges from red via green to blue for 0–100% relative solvent accessibility. (B) Electrostatic potential of the Bra r 5.0101 and muW surfaces. Comparison of the model surfaces of Bra r 5.0101 (left) and muW (right) are given. The color scheme ranges from red via white to blue for −30 to +30 kT/e. The calcium binding loops are encircled and the modified amino acids (Bra r 5.0101: Asp 16, Asp 18, Gly 19, Asp 53, Gly 54 and Ile 56; and muW: Ala 16, Ala 18, Ala 19, Ala 53, Ala 54, Lys 56) are marked. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Only the double mutant but not the wildtype induces upon immunization allergen-specific IgG, which inhibits allergic patients’ IgE binding
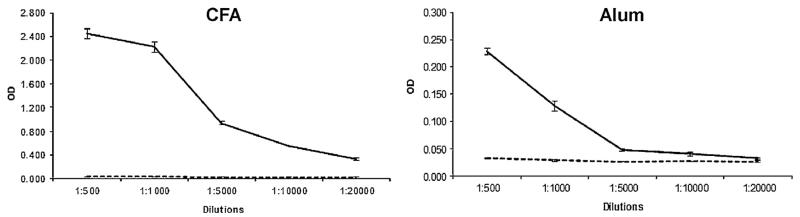
When we immunized rabbits with wildtype and double mutant using two different adjuvans formulations, i.e., CFA and aluminum hydroxide, we consistently found that wildtype protein did not induce relevant IgG antibody responses (Fig. 8). Interestingly, the double mutant induced robust IgG antibody responses against the wildtype allergen with higher titers in the CFA-immunized rabbit compared to the Alum-immunized rabbit (Fig. 8). More importantly, we could show that rabbit IgG antibodies induced with the double mutant inhibited allergic patients IgE binding to the wild-type. Rabbit anti-double mutant IgG obtained by CFA immunization inhibited IgE binding of three patients to the wildtype allergen between 43 and 89% (mean inhibition 73.6%). Rabbit anti-double mutant IgG induced with Alum inhibited IgE binding between 42 and 72% (mean inhibition 57%) (data not shown).
Fig. 8.
Immunogenicity of wildtype allergen and double mutant. IgG reactivity (OD levels corresponding to IgG levels: y-axes) of different dilutions (x-axes) of sera from rabbits which have been immunized with wildtype allergen (WT: broken lines) or double mutant (muW: solid lines) using complete Freunds adjuvans (CFA: left) or aluminum hydroxide (Alum: right).
Discussion
In the present study we investigated three mutants, mu1, mu2 and muW, of the two EF-hand calcium binding allergen Bra r 5.0101 from Brassica rapa in order to identify the derivative with the lowest allergenic activity for reducing side effects during immunotherapy. Testing for IgE reactivity showed that mu2 and muW exhibited a strongly reduced IgE reactivity. However, when we then studied the mutants for their ability to induce degranulation of basophils from sensitized patients, it turned out that muW showed by far the lowest allergenic activity (Figs. 2 and 3). In fact, muW exhibited a strongly reduced (more than 100-fold) allergenic activity as compared to the wildtype allergen. This finding indicates that allergic patients should tolerate much higher doses of muW as compared to the wildtype allergen. It should thus be possible to start treatment with higher doses and to achieve clinical effective treatment with a lower number of injections because the clinically effective maintenance dose can be reached quicker. In fact, the latter has been demonstrated for hypoallergenic derivatives of the major birch pollen allergen, Bet v 1 (Valenta et al. 2011). Another advantage of the vaccine would be the avoidance of IgE-mediated side effects which are the most dangerous side effects occurring during treatment.
Considering the fact that there is extensive sequence similarity between Bra r 5.0101 and calcium-binding allergens from other plant species, especially to those from tree pollen and extensive IgE cross-reactivity among members of the two EF-hand pollen allergens (Tinghino et al. 2002), it can be envisaged that the hypoallergenic muW variant might represent a useful tool also for treatment of patients allergic to the related calcium-binding proteins.
The double mutant muW displayed a consistent and highly reduced allergenic activity in all analyzed allergic patients. As already previously observed for a hypoallergenic trimeric form of the major birch pollen allergen, Bet v 1 (Vrtala et al. 2001; Campana et al. 2011), we found also in our study that the analysis of IgE reactivity of an allergen derivative alone is not sufficient to identify variants with reduced allergenic activity. Basophil activation tests or skin prick tests in allergic patients are necessary to determine whether the allergenic activity of a “hypoallergen” is sufficiently reduced. Although muW showed some residual IgE reactivity in patient 4, its allergenic activity was at least 100-fold reduced in comparison to the wildtype allergen Bra r 1.
The generation of the double mutant was based on studies demonstrating that calcium depletion of EF-hand allergens by addition of chelating agents (e.g. EGTA) results in a conformational change of the tertiary structure of the proteins, which leads to a reduction of the IgE binding capacity of these allergens (Seiberler et al. 1994; Valenta et al. 1998; Engel et al. 1997). Based on these findings several hypoallergenic derivatives of other calcium-binding pollen allergens (i.e., Bet v 4 and Phl p 7) have been produced which showed a varying reduction of IgE reactivity which was not as consistent as that observed for muW (Engel et al. 1997; Westritschnig et al. 2004).
Previous studies had suggested that relevant IgE binding epitopes might be predominantly located in the second calcium binding domain the calcium-binding allergens (Suphioglu et al. 1997; Okada et al. 1998). Whether this second calcium-binding domain represents a linear (continuous) IgE epitope itself or whether this domain is part of a conformational (discontinuous) epitope on the folded protein is still not known. Here we tested the latter hypothesis and mutated each of the two calcium binding domains as well as both. Mutations in the first or second calcium-binding domain alone were not sufficient for achieving a profound and consistent reduction of allergenic activity. Only the exchange of six amino acids in both calcium binding regions (D16, D18, G19 in the first EF-hand, and D53, G54, I56 in the second EF-hand domain, Fig. 1) resulted in a strong reduction of allergenic activity which interestingly was associated with a depletion of protein bound calcium (Okada et al. 1998). It was therefore tempting to speculate that the successful generation of the hypoallergenic double mutant muW was based on the conformational change of the allergen as well as the disruption of a potential linear epitope within the second calcium binding domain. However, the CD spectra of the wildtype allergen and double mutant showed that the latter still retained a considerable amount of alpha helical fold and was not completely denatured. Due to the partial unfolding which was connected with a reduced thermal stability, we found that the double mutant increased its hydrodynamic radius and thus showed a different elution profile in the gel filtration experiments. Nevertheless, it occurred similar as the wildtype allergen mainly as dimer in solution, which was also found for the homologous Phl p 7 allergen (Verdino et al. 2002). When we analyzed the biophysical changes obtained by mutation using a structure-based surface analysis tool to compare the homology models of wildtype and muW, we found that the solvent accessibility of the amino acids was partially changed. In addition, the electrostatic potential of the mutant muW differed markedly from that of Bra r 5.0101 because both mutated calcium binding loops displayed a less negative charge, which changed the electrostatic potential of the surface. In fact, it has been suggested previously that electrostatic properties of proteins can influence the specificity of antigen–antibody binding (Sinha and Smith-Gill 2002; Sinha et al. 2002). In our case, the consequence of the less negative potential of the muW surface and the partial conformational change of the whole molecule may have resulted in a reduction of IgE binding.
Besides the strong and consistent reduction of allergenic activity of the double mutant we made another important observation. In fact, we found that only the double mutant but not the wildtype allergen induced upon immunization allergen-specific IgG antibodies capable of inhibiting allergic patients IgE binding to the allergen. Several studies highlight the importance of the induction of blocking IgG antibodies for the successful outcome of allergen-specific immunotherapy (Larché et al. 2006). We therefore think that the high immunogenicity of the double mutant is another important advantage of this protein for immunotherapy.
In summary, we have identified a double mutant of the major Brassica allergen Bra r 5.0101, muW, as a promising candidate for immunotherapy of patients allergic to calcium-binding pollen allergens.
Acknowledgements
This study was supported by grants F4605, F4609 and F4611 of the Austrian Science Fund, and by research grants of the Christian Doppler Association and Biomay, Vienna, Austria.
References
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Linhart B, Sonneck K, Blatt K, Herrmann H, Valent P, Stoecklinger A, Lupinek C, Thalhamer J, Fedorov AA, Almo SC, Valenta R. Reducing allergenicity by altering allergen fold: a mosaic protein of Phl p 1 for allergy vaccination. Allergy. 2009;64:569–580. doi: 10.1111/j.1398-9995.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Campana R, Vrtala S, Maderegger B, Dall’Antonia Y, Zafred D, Blatt K, Herrmann H, Focke-Tejkl M, Swoboda I, Scheiblhofer S, Gieras A, Neubauer A, Keller W, Valent P, Thalhamer J, Spitzauer S, Valenta R. Altered IgE epitope presentation: a model for hypoallergenic activity revealed for Bet v 1 trimer. Mol. Immunol. 2011;48(4):431–441. doi: 10.1016/j.molimm.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Antonia F, Gieras A, Devanaboyina SC, Valenta R, Keller W. Prediction of IgE-binding epitopes by means of allergen surface comparison and correlation to cross-reactivity. J. Allergy Clin. Immunol. 2011;128:872–879. doi: 10.1016/j.jaci.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Engel E, Richter K, Obermeyer G, Briza P, Kungl AJ, Simon B, Auer M, Ebner C, Rheinberger HJ, Breitenbach M, Ferreira F. Immunological and biological properties of Bet v 4, a novel birch pollen allergen with two EF-hand calcium binding domains. J. Biol. Chem. 1997;272:28630–28637. doi: 10.1074/jbc.272.45.28630. [DOI] [PubMed] [Google Scholar]
- Focke M, Hemmer W, Hayek B, Götz M, Jarisch R. Identification of allergens in oilseed rape (Brassica napus) pollen. Int. Arch. Allergy Immunol. 1998;117:105–112. doi: 10.1159/000023996. [DOI] [PubMed] [Google Scholar]
- Fraczkiewicz R, Braun W. Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J. Comput. Chem. 1998;19:319–333. [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, Bühring HJ, Valenta R, Valent P. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J. Allergy Clin. Immunol. 2002;110:102–109. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- Hayek B, Vangelista L, Pastore A, Sperr WR, Valent P, Vrtala S, Niederberger V, Twardosz A, Kraft D, Valenta R. Molecular and immunologic characterization of a highly cross-reactive two EF-hand calcium-binding alder pollen allergen, Aln g 4: structural basis for calcium-modulated IgE recognition. J. Immunol. 1998;161:7031–7039. [PubMed] [Google Scholar]
- Hemmer W, Focke M, Wantke F, Jäger S, Götz M, Jarisch R. Oilseed rape pollen is a potentially relevant allergen. Clin. Exp. Allergy. 1997;27:156–161. [PubMed] [Google Scholar]
- Hermanides HK, Laheÿ-de Boer AM, Zuidmeer L, Guikers C, van Ree R, Knulst AC. Brassica oleracea pollen, a new source of occupational allergens. Allergy. 2006;61:498–502. doi: 10.1111/j.1398-9995.2006.01055.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Kretsinger R. Calcium binding proteins 1: EF-hands. Protein Profile. 1994;1:343–517. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Mari A. Skin test with a timothy grass (Phleum pratense) pollen extract vs. IgE to a timothy extract vs. IgE to rPhl pl 1, rPhl p 2, nPhl p 4, rPhl p 5, rPhl p 6, rPhl p 7, rPhl p 11 and rPhl p 12: epidemiological and diagnostic data. Clin. Exp. Allergy. 2003;33:43–51. doi: 10.1046/j.1365-2222.2003.01569.x. [DOI] [PubMed] [Google Scholar]
- Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, Gieras A, Swoboda I, Zafred D, Keller W, Valent P, Durham SR, Valenta R. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J. Immunol. 2013;190:3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudecker P, Nerkamp J, Eisenmann A, Nourse A, Lauber T, Schweimer K, Lehmann K, Schwarzinger S, Ferreira F, Rösch P. Solution structure, dynamics, and hydrodynamics of the calcium-bound cross-reactive birch pollen allergen Bet v 4 reveal a canonical monomeric two EF-hand assembly with a regulatory function. J. Mol. Biol. 2004;336:1141–1157. doi: 10.1016/j.jmb.2003.12.070. [DOI] [PubMed] [Google Scholar]
- Niederberger V, Hayek B, Vrtala S, Laffer S, Twardosz A, Vangelista L, Sperr WR, Valent P, Rumpold H, Kraft D, Ehrenberger K, Valenta R, Spitzauer S. Calcium dependent immunoglobulin E recognition of the apo- and calcium-bound form of a cross-reactive two EF-hand timothy grass pollen allergen, Phl p 7. FASEB J. 1999;13:843–856. doi: 10.1096/fasebj.13.8.843. [DOI] [PubMed] [Google Scholar]
- Okada T, Swoboda I, Bhalla PL, Toriyama K, Singh MB. Engineering of hypoallergenic mutants of the Brassica pollen allergen, Bra r I, for immunotherapy. FEBS Lett. 1998;434:255–260. doi: 10.1016/s0014-5793(98)00992-2. [DOI] [PubMed] [Google Scholar]
- Okada T, Zhang Z, Russell SD, Toriyama K. Localization of the Ca(2+)-binding protein, Bra r 1, in anthers and pollen tubes. Plant Cell Physiol. 1999;40:1243–1252. doi: 10.1093/oxfordjournals.pcp.a029512. [DOI] [PubMed] [Google Scholar]
- Okada T, Sasaki Y, Ohta R, Onozuka N, Toriyama K. Expression of Bra r 1 gene in transgenic tobacco and Bra r 1 promoter activity in pollen of various plant species. Plant Cell Physiol. 2000;41:757–766. doi: 10.1093/pcp/41.6.757. [DOI] [PubMed] [Google Scholar]
- Radauer C, Breiteneder H. Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. J. Allergy Clin. Immunol. 2006;117(1):141–147. doi: 10.1016/j.jaci.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Rossi RE, Monasterolo G, Monasterolo S. Measurement of IgE antibodies against purified grass pollen allergens (Phl p 1, 2, 3, 4, 5, 6, 7, 11, and 12) in sera of patients allergic to grass pollen. Allergy. 2001;56:1180–1185. doi: 10.1034/j.1398-9995.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- Rozwadowski K, Zhao R, Jackman L, Huebert T, Burkhart WE, Hemmingsen SM, Greenwood J, Rothstein SJ. Characterization and immunolocalization of a cytosolic calcium-binding protein from Brassica napus and Arabidopsis pollen. Plant Physiol. 1999;120:787–798. doi: 10.1104/pp.120.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanner MF, Olson AJ, Spehner JC. Reduced surface: an efficient way to compute molecular surfaces. Biopolymers. 1996;38(3):305–320. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C305::AID-BIP4%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Seiberler S, Scheiner O, Kraft D, Lonsdale D, Valenta R. Characterization of a birch pollen allergen, Bet V III, representing a novel class of Ca2+ binding proteins: specific expression in mature pollen and dependence of patients’ IgE binding on protein-bound Ca2+ EMBO J. 1994;13:3481–3486. doi: 10.1002/j.1460-2075.1994.tb06654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BP, Verma J, Rai D, Sridhara S, Gaur SN, Gangal SV. Immuno-biochemical characterization of Brassica campestris pollen allergen. Int. Arch. Allergy Immunol. 1995;108:43–48. doi: 10.1159/000237116. [DOI] [PubMed] [Google Scholar]
- Sinha N, Mohan S, Lipschultz CA, Smith-Gill SJ. Differences in electrostatic properties at antibody–antigen binding sites: implications for specificity and cross-reactivity. Biophys. J. 2002;83:2946–2968. doi: 10.1016/S0006-3495(02)75302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Smith-Gill SJ. Electrostatics in protein binding and function. Curr. Protein Pept. Sci. 2002;3:601–614. doi: 10.2174/1389203023380431. [DOI] [PubMed] [Google Scholar]
- Suphioglu C, Ferreira F, Knoxx RB. Molecular cloning and immunological characterization of Cyn d 7, a novel calcium-binding allergen from Bermuda grass pollen. FEBS Lett. 1997;402:167–172. doi: 10.1016/s0014-5793(96)01520-7. [DOI] [PubMed] [Google Scholar]
- Tinghino R, Twardosz A, Barletta B, Puggioni EM, Iacovacci P, Butteroni C, Afferini C, Mari A, Hayek B, Di Felice G, Focke M, Westritschnig K, Valenta R, Pini C. Molecular, structural, and immunologic relationships between different families of recombinant calcium-binding pollen allergens. J. Allergy Clin. Immunol. 2002;109:314–320. doi: 10.1067/mai.2002.121528. [DOI] [PubMed] [Google Scholar]
- Toriyama K, Okada T, Watanabe M, Ide T, Ashida T, Xu H, Singh MB. A cDNA clone encoding an IgE-binding protein from Brassica anther has significant sequence similarity to Ca(2+)-binding proteins. Plant Mol. Biol. 1995;29:1157–1165. doi: 10.1007/BF00020459. [DOI] [PubMed] [Google Scholar]
- Twardosz-Kropfmüller A, Singh MB, Niederberger V, Horak F, Spitzauer S, Kraft D, Valenta R, Swoboda I. Allergy diagnosis with recombinant pollen marker-allergens. Allergy. 2010;65:296–303. doi: 10.1111/j.1398-9995.2009.02202.x. [DOI] [PubMed] [Google Scholar]
- Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, Ferrara R, Quirce S, Spitzauer S, Swoboda I, Valenta R. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J. Allergy Clin. Immunol. 2011;128:178–184. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Valent P, Besemer J, Muhm M, Majdic O, Lechner K, Bettelheim P. Inter-leukin 3 activates human blood basophils via high-affinity binding sites. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5542–5546. doi: 10.1073/pnas.86.14.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta R, Hayek B, Seiberler S, Bugajska-Schretter A, Niederberger V, Twardosz A, Natter S, Vangelista L, Pastore A, Spitzauer S, Kraft D. Calcium-binding allergens: from plants to man. Int. Arch. Allergy Immunol. 1998;117:160–166. doi: 10.1159/000024005. [DOI] [PubMed] [Google Scholar]
- Valenta R, Niespodziana K, Focke-Tejkl M, Marth K, Huber H, Neubauer A, Niederberger V. Recombinant allergens: what does the future hold? J. Allergy Clin. Immunol. 2011;127:860–864. doi: 10.1016/j.jaci.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Verdino P, Westritschnig K, Valenta R, Keller W. The cross-reactive calcium binding pollen allergen, Phl p 7, reveals a novel dimer assembly. EMBO J. 2002;21:5007–5016. doi: 10.1093/emboj/cdf526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdino P, Barderas R, Villalba M, Westritschnig K, Valenta R, Rodriguez R, Keller W. Three-dimensional structure of the cross-reactive pollen allergen Che a 3: visualizing cross-reactivity on the molecular surfaces of weed, grass, and tree pollen allergens. J. Immunol. 2008;180:2313–2321. doi: 10.4049/jimmunol.180.4.2313. [DOI] [PubMed] [Google Scholar]
- Vrtala S, Hirtenlehner K, Susani M, Akdis M, Kussebi F, Akdis CA, Blaser K, Hufnagl P, Binder BR, Politou A, Pastore A, Vangelista L, Sperr WR, Semper H, Valent P, Ebner C, Kraft D, Valenta R. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001;15(11):2045–2047. doi: 10.1096/fj.00-0767fje. [DOI] [PubMed] [Google Scholar]
- Westritschnig K, Focke M, Verdino P, Goessler W, Keller W, Twardosz A, Mari A, Horak F, Wiedermann U, Hartl A, Thalhamer J, Sperr WR, Valent P, Valenta R. Generation of an allergy vaccine by disruption of the three-dimensional structure of the cross-reactive calcium-binding allergen, Phl p 7. J. Immunol. 2004;172:5684–5692. doi: 10.4049/jimmunol.172.9.5684. [DOI] [PubMed] [Google Scholar]
- Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32:W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]