Abstract
Opioid analgesics administered by patient-controlled analgesia (PCA) are frequently used for pain relief in children and adults with sickle cell disease (SCD) hospitalized for persistent vaso-occlusive pain, but optimum opioid dosing is not known. To better define PCA dosing recommendations, a multi-center phase III clinical trial was conducted comparing two alternative opioid PCA dosing strategies (HDLI-higher demand dose with low constant infusion or LDHI- lower demand dose and higher constant infusion) in 38 subjects who completed randomization prior to trial closure. Total opioid utilization (morphine equivalents, mg/kg) in 22 adults was 11.6 ± 2.6 and 4.7 ± 0.9 in the HDLI and in the LDHI arms, respectively, and in 12 children it was 3.7 ± 1.0 and 5.8 ± 2.2, respectively. Opioid-related symptoms were mild and similar in both PCA arms (mean daily opioid symptom intensity score: HDLI 0.9 ± 0.1, LDHI 0.9 ± 0.2). The slow enrollment and early study termination limited conclusions regarding superiority of either treatment regimen. This study adds to our understanding of opioid PCA usage in SCD. Future clinical trial protocol designs for opioid PCA may need to consider potential differences between adults and children in PCA usage.
Keywords: sickle cell disease, opioids, PCA, clinical trial
Pain from vaso-occlusion is the most frequent complication of SCD in adolescent and adult populations [1]. Significant strides have been made in our understanding of the pathophysiology of vaso-occlusion [2]. However, the clinical management of vaso-occlusive pain has remained relatively unchanged over the past 10-20 years, and relies largely on opioid analgesics. Despite the existence of several published SCD pain treatment guidelines [3, 4], there is little information about optimum analgesic dosing using patient controlled analgesia (PCA) opioid therapy, as only three small clinical studies have studied PCA dosing strategies in SCD [5],[6],[7].
Current PCA strategies for severe pain typically include an opioid given by continuous infusion in addition to the PCA demand doses, but the addition of continuous opioid infusions has often been associated with increased opioid-related adverse effects [8],[9],[10]. To provide an evidence base for the clinical use of opioid PCA for sickle cell acute pain management, it is crucial to compare different PCA dosing approaches to determine their safety and efficacy. The results presented here describe a study methodology and preliminary initial results of opioid usage in a randomized clinical trial of opioid PCA to guide future investigations of opioid analgesics for acute vaso-occlusive pain.
From January 1, 2010 to June 8, 2010, a total of 1116 patients' age ≥ 10 years were hospitalized for vaso-occlusive pain at study sites; 224 were ineligible, 915 were missed, and 38 subjects completed randomization prior to trial closure. Four subjects were withdrawn (1 parent permission withdrawal, 2 inadvertent withdrawals by PI, 1 ineligible). The complexities of care coordination of a research study within the inpatient setting contributed and staff availability and other logistic issues were major contributors to the large number of missed patients.
Average age of analyzed subjects was 24.0 ± 12.0 years (range 10-52 years), and the mean age was similar in both treatments arms for children (HDLI 13.6 ± 2.5 years, LDHI 13.2 ± 2.0 years) and for adults (HDLI 32.2 ± 11.3 years, LDHI 27.4 ± 10.6 years) (Table 1 supplemental materials). The gender distribution of analyzed subjects was 53% female, with some imbalance across treatment arms in both children and adults reflecting the small sample size. The SS hemoglobinopathy genotype was present in 66.7% of children and 81.8% of adult subjects Weight and baseline laboratory parameters were similar across treatment arms for both child and adult subjects. A reduction in pain intensity during PCA treatment was observed in both treatment arms (mean difference from baseline ± SEM: 2.6 ±3 cm HDLI vs 3.4 ±4 cm LDHI). Time to target improvement (2.5 cm) did not differ (2.7 ±0.3 days HDLI vs 2.6 ±0.5 days LDHI). The Patient Global Impression of Change Scale (PGIC) was rated as moderately better or higher by 79% of study participants and did not differ by treatment arm (p=0.32, Cochran-Armitage trend test). The duration of hospitalization for adults in the HDLI group was 7.1 ± 1.3 days, and for the LDHI group was 3.9 ± 0.3 days. Hospital durations were similar for children in both PCA groups (HDLI 4.0 ± 0.7 days, LDHI 4.6 ± 1.2 days).
The frequency of morphine and hydromorphone use were similar in both treatment arms (HDLI 47% morphine, LDHI 53% morphine), but hydromorphone usage was much more prevalent in adult subjects (82%) compared to children (18%) reflecting local site investigator clinical practices and PCA pump capabilities. The mean total cumulative opioid utilization for the duration of PCA usage in morphine equivalents in adults for the HDLI treatment arm was 11.6 ± 2.6 mg/kg, and for the LDHI treatment arm was 4.7 ± 0.9 mg/kg, and in children was HDLI 3.7 ± 1.0 mg/kg and LDHI was 5.8 ± 2.2 mg/kg. When converted to morphine equivalents there was little difference in opioid use between the hydromorphone and morphine treatment arms for both adults and children (Table 2 supplemental materials).
For children, cumulative total morphine utilization in both PCA strategies was virtually identical for the first 2 days of hospitalization, but began to increase thereafter in the LDHI treatment arm compared to the HDLI arm (Figure 1, bottom panels, Table 2A supplemental materials). Total morphine utilization in adults was different from that in children with the cumulative morphine utilization in the HDLI arm exceeding the LDHI arm on all days of hospitalization (Figure 1, bottom left panel, and Table 2B supplemental materials The observed opioid constant infusion dose usage was higher in the LDHI arm than in the HDLI arm as designed, and infusion dose usage within treatment arms was similar in both adults and children (the cumulative infused dose usage on discharge day for HDLI 1.5 ± 0.3 mg/kg (adults) and 1.1 ± 0.2 mg/kg (children), for LDHI 2.8 ± 0.4 mg/kg (adults) and LDHI 3.6 ± 1.4 mg/kg (children) (Figure 1, middle panels). However, the demand dosage utilization was strikingly different in children compared to adults, with little difference in cumulative demand dose usage between the HDLI and the LDHI treatment arms in children (HDLI 2.0 ± 0.5 mg/kg, LDHI 1.6 ± 0.7 mg/kg), but with a much larger usage difference in adults (HDLI 9.3 ± 2.1 mg/kg, and LDHI, 1.7 ± 0.5 mg/kg) (Figure 1, top panels). This difference in demand dose utilization largely explained the differences in total opioid utilization between children and adults. Daily opioid usage in both adults and children was lower on those days with lower pain intensity scores and higher on those days with higher pain intensities (Figure 2). This relationship was less prominent in adults randomized to the HDLI treatment arm, particularly on days with high (≥ 7/10) pain intensities. More detailed analyses of dose-response relationships were not possible given the small sample size.
Figure 1.
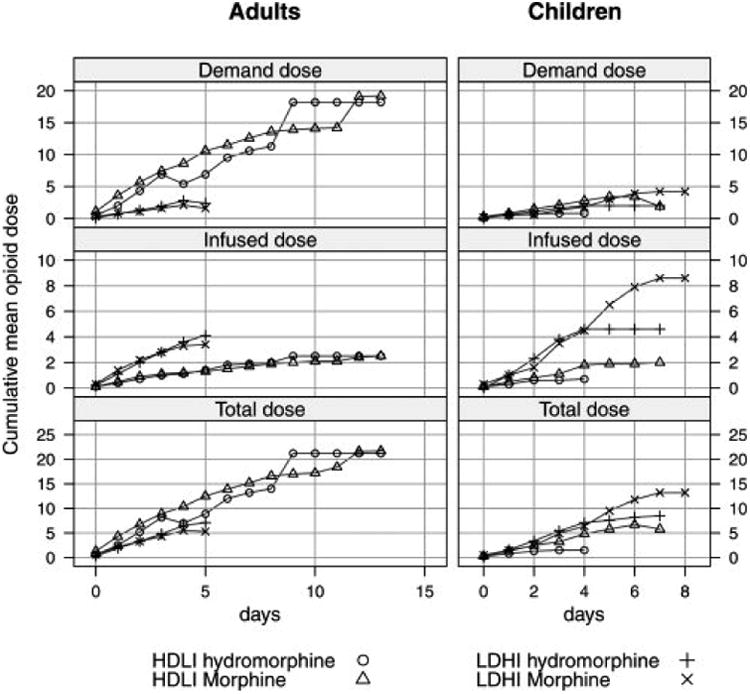
Cumulative mean opioid use by study treatment arm and PCA component.
Figure 2.
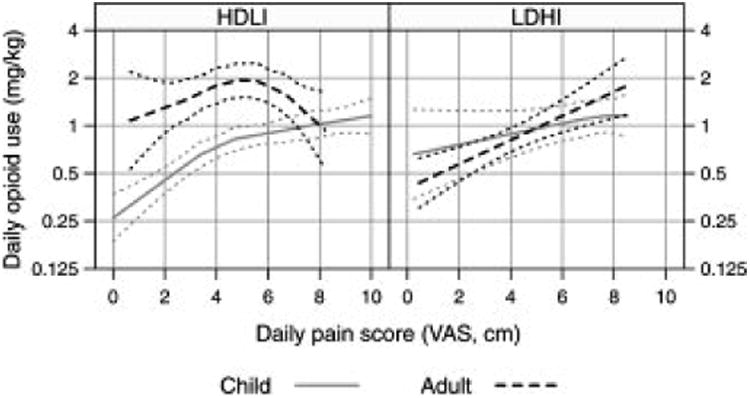
Relationships between daily opioid usage and daily VAS pain intensity.
Each of twelve opioid related symptoms was rated by intensity, duration, and bothersomeness (0-4 Likert scales) [11]. Drowsiness, lack of energy, and itching were the most prevalent opioid-related symptoms in both children and adults. Reported opioid-related symptoms were generally mild to moderate and similar in children and adults (Table 3 supplemental materials). The mean daily opioid symptom scores over the duration of PCA treatment were similar for each of the three components of the symptom score, with intensity scores for children 0.8 ± 0.2, adults 0.9 ± 0.1 (p=0.67); mean daily duration score in children 0.9 ± 0.2, adults 1.0 ± 0.1 (p=0.47); and mean daily bothersomeness score in children 1.0 ± 0.2, adults 1.0 ± 0.2 (p=0.96). Opioid-related symptoms were similar in both PCA arms with a mean daily opioid symptom intensity score: HDLI 0.9 ± 0.1, LDHI 0.9 ± 0.2; mean daily duration score HDLI 1.0 ±0.1, LDHI 0.9 ± 0.2; mean daily bothersomeness score: HDLI 1.0 ± 0.2, LDHI 0.9 ± 0.2). Mean symptom scores were relatively similar or showed a small decrease across the 3 or 5 day periods, except for increases in constipation symptoms in children and nausea and headaches in adults (Table 3 supplemental materials). Antipruritics were prescribed somewhat more often to children (67%) than adults with diphenylhydramine being the predominant medication. Anti-nausea medications were prescribed somewhat more often to adults (55%) compared to children (42%), with promethazine more frequently prescribed in adults and ondansetron in children. Most adults and children were prescribed multiple laxatives reflecting the difficulty treating constipation symptoms. While not required by the protocol, low dose naloxone infusions were used during PCA treatment as standard of care by 58% of children and 9% of adults.
Symptoms consistent with an opioid withdrawal syndrome were elicited with a telephone administered symptom scale at 3 and 14 days after discharge. Reported symptoms were somewhat more prevalent at the 3 day compared to the 14 day assessment, and were somewhat more prevalent in adults compared to children (Table 4 supplemental materials). Symptom intensity was very mild with a mean total 10 symptom score of 0.1 ± 0.1 for children and 0.3 ± 0.1 for adults, reflecting the frequent usage of long-acting oral opioids after discharge.
A number of small cohort or randomized studies [5],[7] have examined opioid PCA dosing strategies in children or adults with sickle cell disease, but several methodological issues limit the usefulness of these preliminary studies. While there were some differences in dosing strategies across studies, efficacy findings were not consistent across studies. To improve on these previous studies, we developed a comprehensive study protocol with extensive blinded assessments of opioid efficacy and safety endpoints, recognizing that balancing the degree of pain relief with the frequency of adverse effects is frequently the goal of opioid therapy for both healthcare providers and patients [12]. Detailed PCA dosing guidelines were developed to provide clinically relevant dose ranges to maximized protocol adherence. The study design did not blind the clinical team to treatment assignment to maximize subject safety. This initial subject cohort documented the feasibility of the study protocol and the PCA dosing guidelines, but demonstrated the continued challenges of recruiting SCD subjects to an in-patient interventional clinical trial. As seen in previous opioid PCA studies, the use of substantial PCA demand doses with modest continuous infusions (HDLI) in adult subjects in this study resulted in larger total cumulative opioid utilizations than a strategy relying predominantly on continuous higher opioid infusions with small demand doses (LDHI) [8]. However, similar paradigms in children showed little difference reflecting a smaller use of available demand doses [13]. As expected, both adults and children varied their opioid usage in response to pain intensity. The somewhat blunted opioid usage at high pain intensities, particularly for adults using the HDLI treatment arm, reflects the clinical difficulties in obtaining pain relief in this situation and reinforces the need to define optimum analgesic dosing strategies as these participants may have not been able to utilize a sufficient number or frequency of demand doses. Opioid-related symptoms were well managed using detailed symptom management guidelines in this cohort, and symptoms consistent with withdrawal symptoms after hospital discharge were also infrequent and relatively minor in intensity.
Conclusions from the current PCA study are limited by the small sample size. However, the results of this study demonstrate the feasibility of the study design, and provide preliminary data that will allow further optimization of study endpoints, sample size considerations, opioid dosing guidelines, and pain and symptom assessments for the design of future analgesic clinical trials in SCD. Determinations of opioid usage in future studies would be facilitated by the use of consistent policies for PCA administration and monitoring, and the use of PCA pumps that digitally record opioid dose administration. Differences in degree of utilization of opioid PCA demand dose in adults compared to children suggest the need for future studies that are adequately powered to detect and explain analgesic utilization response differences between these two age groups. Further larger studies to explore the relationships between patterns of opioid utilization, pain relief, and hospitalization duration in patients with sickle cell disease are needed to optimize clinical care for this patient population.
Methods
Protocol Development
The Sickle Cell Disease Clinical Research Network (SCDCRN) was established in 2006, to develop and conduct phase III intervention trials within 31 clinical sites. The IMPROVE PCA trial (Improving Pain Management and Outcomes with Various Strategies of Patient-Controlled Analgesia) was developed as a randomized controlled trial of two different PCA treatments, and was approved by the NHLBI Protocol Review Committee in July 2009 and by the NHLBI Data Safety Monitoring Board in August 2009. Local institutional review board (IRB) approval was obtained at all participating clinical sites and study enrollment was initiated on December 31, 2009. The study was terminated in June 2010 due to slow enrollment and insufficient time to complete the study prior to Network closure in March 2011.
Study Design
The primary study endpoint was the time to first occurrence of a clinically relevant improvement in pain intensity, defined as a 2.5 cm decrease from baseline in daily average pain intensity measured on a 10 cm VAS. This value was selected based on previous studies of SCD subjects reflecting moderately significant pain intensity improvement [14]. Secondary effectiveness endpoints included the total daily cumulative opioid dose delivered, the rate of change in daily average pain intensity scores, and the magnitude of global patient satisfaction/evaluation scores obtained at Day 3 for pediatric subjects and Day 5 for adult subjects or at day of discharge, whichever occurs first. A number of exploratory pain assessment, pain relief, and activity measures were also conducted and are reported in a separate manuscript. Secondary safety endpoints included the daily intensity opioid adverse symptoms scores, including sedation, nausea, and pruritus scores, and the magnitude of patient reported opioid-related withdrawal symptoms as assessed by scripted telephone interview at 3 and 14 days post hospital discharge.
Sample Size Calculation
Based on single institution pilot data, it is assumed that half of all subjects randomized to the Low Demand PCA arm would meet the primary endpoint criterion by 5.0 days. A total of 278 subjects (139 per arm) is needed to detect a 30% reduction attributable to the High Demand PCA strategy (i.e., half of all subjects will meet the primary endpoint in 3.5 days rather than 5.0 days) with 80% power. This calculation assumes incorporation of two inflation factors: 1) a 5% dropout rate shortly after randomization (N/0.95); and 2) a 2% inflation rate to account for interim looks at the data during trial monitoring (× 1.02). The dropout rate is assumed to be very low because if dropout occurs during the hospital stay, there is still statistical information to be derived from the days of observation on treatment prior to withdrawal from the trial, and thus such subjects are not truly full dropouts. The choice of a 30% reduction and 80% power (N=278) was based on the importance of showing a definitive reduction to guide clinical management and the available resources to complete the trial.
Study Inclusion and Exclusion Criteria
Individuals with all genotypes of sickle cell disease, ≥ age 10 years with vaso-occlusive crisis, < 12 hours of parenteral opioid therapy from time of presentation to the Emergency Department, and a 10 cm visual analogue scale (VAS) pain score ≥ 4.5, were eligible for the study. This value was chosen as it was the minimum value that encompassed the typical clinical practice of the study investigators. To reduce potential dosing issues related to opioid tolerance, subjects were excluded if they were receiving chronic moderate to high dose oral opioids such as methadone> 40 mg/day, sustained release morphine> 120 mg /day, or oxycodone> 80 mg/day. Subjects with hypoxia (pulse oximetry oxygen saturations <92%) or acute chest syndrome were excluded to avoid confounding with opioid induced respiratory depression. Because of potential alterations in opioid metabolism related to significant renal/hepatic dysfunction, subjects were excluded for ALT > 3 times institutional upper limit of normal, direct bilirubin > 0.8 mg/dl, or creatinine ≥ 1.2 mg/dl for ages >18 yrs, or ages 10-18 yrs creatinine ≥ 1.0 mg/dl).
PCA Strategies
Ratios of opioid demand dose to infusion dose were chosen at 3:1 or 1:3 (depending upon treatment assignment) to provide a reasonable difference in opioid delivery. A lockout interval of 8 minutes was used on all PCA pumps, but hourly or 4 hourly maximums were at the discretion of the treating physicians for safety considerations of individual subjects. Investigators used study provided opioid dosing tables for each morphine or hydromorphone dose range to reduce the risk of medication errors or protocol non-compliance (see materials). Dosing was weight based for patients who weighed <50 kg. The dosing range for adults (≥ 18 years) spanned a two-fold range in 7 steps while a 4-fold dosing range was used in pediatric subjects (10-17 years) to provide additional lower dosing ranges.
PCA Treatment Protocol
Patients (or their parent/legal guardian) were approached about study participation after a clinical decision had been made to admit for further vaso-occlusive pain management. Alternatively, some IRBs allowed study pre-consent in the clinic setting followed by confirmation of consent at the time of admission. Following informed consent, subjects were randomized to either a high demand dose, low infusion (HDLI) opioid PCA dosing strategy or a low demand, high infusion (LDHI) opioid PCA strategy. Either morphine or hydromorphone were used, based on physician and/or subject preference. Treatment assignment was stratified within site by opioid choice and by age group (adult versus pediatric). All subjects started study PCA treatment at doses indicated in dosing guideline tables (see supplemental materials). Long-acting opioids were discontinued at the time PCA was started. Non-opioid analgesics, such as non-steroidal anti-inflammatory drugs, were allowed and were administered as per standard practice at the respective clinical sites. If, in the judgment of the clinical investigator and inpatient clinical care team, a subject required higher opioid doses for adequate analgesia than those initially selected, options included providing a limited number of additional parenteral rescue opioid doses or increasing the opioid PCA dose to a higher level in the dosing guidelines. The protocol provided guidelines for subsequently reducing opioid PCA dosing for adequate analgesia, and for temporary opioid cessation and subsequent dose reduction for respiratory depression. The assigned PCA strategy was continued until patients were transitioned to oral analgesics, the timing of which was at the discretion of the clinical care team in collaboration with the study investigator.
Each clinical site followed their own routine nursing policies and procedures for administration of PCA, and used clinically available PCA pumps. Sites that did not have PCA pumps that could deliver two-decimal accuracy for infusion rates were not allowed to randomize individuals weighing less than 50 kg to hydromorphone, as the hydromorphone dosing tables required such accuracy. Opioid usage was calculated from clinical PCA flow sheets submitted to the study data coordinating center.
Assessments
Assessments were collected by a member of the research team blinded to treatment details. Analgesic response to PCA treatment was assessed by self report of pain using a 10 cm VAS, three times daily at 4 hour intervals between 7 am and 7 pm. This time interval was felt by investigators to be the most clinically relevant to assess pain improvement, recognizing that pain improvement during nighttime hours will be poorly recognized. At the time of the last VAS measurement of the day in the late afternoon or early evening, subjects completed an opioid-related symptom scale modified from the Memorial Symptom Assessment Scale [11]. A Global Impression of Change (7 point Likert scale from very dissatisfied to very satisfied) was obtained on day 5 for adults, day 3 for pediatric patients, or on the day of discharge, whichever occurred first. Subjects were contacted by telephone interview 3 and 14 days after discharge to ascertain the presence or absence of symptoms of opioid abstinence syndrome using the short opioid withdrawal scale [15], the use of concomitant medications, and presence of any adverse event since discharge.
The inpatient clinical care providers were not blinded to subject treatment assignment. They monitored and recorded clinical information daily until discharge including vital signs, pulse oximetry, level of consciousness, laboratory values, opioid usage, use of other medications and adverse events as per study and local practice guidelines.
Statistical Analysis
Statistical analyses were performed at the Data Coordinating Center (New England Research Institutes, Watertown, MA) with SAS® release 9.2 (SAS Institute, Cary, NC). Clinical trial endpoints were not analyzed given the small size, so the study results are reported as a feasibility study. Descriptive statistics are reported as the number and percent, the mean and standard deviation/standard error, or the median and range. Hydromophone doses were converted to morphine IV equivalents (mg) using a correction factor of 6.67. Daily total opioid use for each subject was computed as the sum of all opioid analgesic medications received in a 24 hour period (infusion, demand dose, and other bolus doses as delivered by the PCA pump, in addition to any oral, intramuscular, and transdermal opioid medications taken). The cumulative total opioid use was the sum of all opioid analgesic medications received during the hospitalization. The scores on the opioid-related symptom scale and the short opioid withdrawal symptom scale were computed as specified by the developers, and are presented as average daily means ± standard errors. Statistical graphics were produced using the R language for statistical computing, version 2.12.1 [16] with the lattice, amer, and lme4 packages [17-19]. Raw averages of the cumulative opioid trends are depicted in Figure 1. The trends in Figure 2 are computed using generalized additive mixed models with 95% bias-adjusted empirical Bayes confidence intervals that account for correlation of trends within subject.
Supplementary Material
Acknowledgments
This publication was made possible by Grant Number U10HL083721 from the National Heart, Lung, and Blood Institute, National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The SCD CRN IMPROVE Trial was registered with clinicaltrials.gov (#NCT00999245).
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
Aflac Cancer Center and Blood Disorders Service, Emory University School of Medicine and Children's Healthcare of Atlanta, Atlanta, GA: Peter A. Lane, MD, Martha Ann Felder, CRA
Children's Hospital Boston, Boston, MA: Matthew M Heeney, M.D, Debra L. Weiner, M.D., Ph.D., Natasha Soodoo, Meredith Anderson
Boston Medical Center, Boston, MA: Lillian E. C. McMahon, MD, Asif I. Qureshi, MD
Yale University School of Medicine, New Haven, CT: Farzana Pashankar, MD
Children's Hospital of Philadelphia, Philadelphia, PA St. Christopher's Hospital for Children, Philadelphia, PA: Norma B. Lerner, MD, MPH, Michele Cahill, RN, MaryLou MacDermott, CRNP, Maureen Meier, RN, CCRC
Division of Pediatric Hematology/Oncology, AI DuPont Hospital for Children, Wilmington, DE: Robin Miller, MD, Lynn Marrs, BS, RN, CCRC
Division of Pediatric Hematology/Oncology, University of Louisville, Louisville, KY: Salvatore Bertolone, MD, Ashok B. Raj, MD
Center for Sickle Cell Disease and Department of Medicine, Howard University, Washington, DC: Victor R. Gordeuk, MD, Sharmin Diaz, BSN
Georgetown-Howard Universities Center for Clinical and Translational Science and supported by the National Institutes of Health National Center for Research Resources, Grant U54 RR026076
Children's National Medical Center, Washington, DC Patrice Williams, RN, Sheronda Brown, MBA.
Cardiovascular and Pulmonary Medicine Branch, National Institutes of Health, Bethesda, MD: Marlene Peters-Lawrence, RN, RRT
Children's Hospital & Research Center, Oakland, CA: Mark Walters, MD, Marsha J. Treadwell PhD
University of Illinois at Chicago, Chicago, IL: Richard J. Labotka, MD, Sandra Gooden, RN, Daisy Pacelli, MPH, RN, Lani Krauz, RN
Children's Memorial Hospital, Chicago, IL: A. Kyle Mack, MD
Johns Hopkins University School of Medicine, Baltimore, MD: James F. Casella, MD, Jeffrey Keefer, MD, PhD, Phillip Seaman; Johns Hopkins University: Johns Hopkins Institute for Clinical and Translational Research; grant # U10 HL083721.
Herman and Walter Samuelson Children's Hospital at Sinai, Baltimore, MD: Jason Fixler, MD, Joan Marasciulo, RN
University of North Carolina at Chapel Hill, Chapel Hill, NC: Susan K. Jones, RN, Dell Strayhorn, FNP, MPH, Teresa Etscovitz; UNC Clinical and Translational Science Award,“ grant # UL1RR025747
Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA: Heather Ford
Women & Children's Hospital of Buffalo, Buffalo, NY: Steven Ambrusko, MD
University of Mississippi Medical Center, Jackson, MS: Rathi V. Iyer, MD, Mary Gail Smith, MD, Carolyn Bigelow, MD, Suvankar Majumdar, MD, Glenda Thomas, RN, Arleen Anderson, RN
Medical College of Georgia, Atlanta, GA: Abdullah Kutlar, MD, Leigh Wells, RN, MSN, Latanya Bowman, Pritam Bora
Wayne State University, Detroit, MI: Paul Swerdlow, MD
Duke University Medical Center, Durham, NC: Laura M. De Castro, MD, Courtney D. Thornburg, MD, Hai Huang
New York Methodist Hospital, Brooklyn, NY: Rita Bellevue, MD, Emmely M. Colon, Herold Duroseau, MD, Deepak Kilari, MD, Charlene Webb, LPN
Interfaith Medical Center, Brooklyn, NY: Edouard Guillaume, MD, Rafat Ahmed, MD, Miren Blackwood, Huguette Souffrant, Dorothy Williams
Baylor College of Medicine, Houston, TX: Brigitta U. Mueller, MD, MHCM
Cincinnati Children's Hospital Medical Center, Cincinnati, OH: Clinton H. Joiner, MD, PhD, Karen Kalinyak, MD
Ohio State University, Adult Sickle Cell Program Columbus, OH: Eric H. Kraut, MD, Leslie Witkoff, RN
Nationwide Children's Hospital, Columbus OH: Melissa M. Rhodes, MD, Kami Perdue, CRA
National Heart, Lung, and Blood Institute, Bethesda, MD: Harvey Luksenburg, MD, Henry Chang, MD, Liana Harvath, PhD, Myron Waclawiw, PhD, Erin Smith, Ellen M. Werner, PhD
Data and Safety Monitoring Board Members: (Chair) Ted Wun, MD, FACP, Amy Becker, MD, Lennette Benjamin, MD, Susan Claster, MD, Michael Farrell, MD, Allison A. King, MD, MPH, Jeannette Y. Lee, PhD, Robert P. McMahon, PhD, Julie A. Panepinto, MD, MSPH
References
- 1.Powars DR, Chan LS, Hiti A, et al. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 2.Conran N, Franco-Penteado CF, Costa FF. Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin. 2009;33:1–16. doi: 10.1080/03630260802625709. [DOI] [PubMed] [Google Scholar]
- 3.Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol. 2003;120:744–752. doi: 10.1046/j.1365-2141.2003.04193.x. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin LJ, Dampier CD, Jacox A, et al. Guideline for the Management of Acute and Chronic Pain in Sickle-Cell Disease. American Pain Society. 1999 [Google Scholar]
- 5.Trentadue NO, Kachoyeanos MK, Lea G. A comparison of two regimens of patient-controlled analgesia for children with sickle cell disease. J Pediatr Nurs. 1998;13:15–19. doi: 10.1016/S0882-5963(98)80064-X. [DOI] [PubMed] [Google Scholar]
- 6.van Beers EJ, van Tuijn CF, Nieuwkerk PT, et al. Patient-controlled analgesia versus continuous infusion of morphine during vaso-occlusive crisis in sickle cell disease, a randomized controlled trial. Am J Hematol. 2007;82:955–960. doi: 10.1002/ajh.20944. [DOI] [PubMed] [Google Scholar]
- 7.Jacob E, Hockenberry M, Mueller BU, et al. Analgesic Response to Morphine in Children with Sickle Cell Disease: A Pilot Study. J Pain Manag. 2008;2:179–190. [PMC free article] [PubMed] [Google Scholar]
- 8.George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6:47–54. doi: 10.5055/jom.2010.0004. [DOI] [PubMed] [Google Scholar]
- 9.Walder B, Schafer M, Henzi I, et al. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain: A quantitative systematic review. Acta Anaesthesiol Scand. 2001;45:795–804. doi: 10.1034/j.1399-6576.2001.045007795.x. [DOI] [PubMed] [Google Scholar]
- 10.McNeely JK, Trentadue NC. Comparison of patient-controlled analgesia with and without nighttime morphine infusion following lower extremity surgery in children. J Pain Symptom Manage. 1997;13:268–273. doi: 10.1016/s0885-3924(96)00324-7. [DOI] [PubMed] [Google Scholar]
- 11.Apfelbaum JL, Gan TJ, Zhao S, et al. Reliability and validity of the perioperative opioid-related symptom distress scale. Anesth Analg. 2004;99:699–709. doi: 10.1213/01.ANE.0000133143.60584.38. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Gregorian RS, Jr, Gasik A, Kwong WJ, et al. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J Pain. 2010;11:1095–1108. doi: 10.1016/j.jpain.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Doyle E, Robinson D, Morton NS. Comparison of patient-controlled analgesia with and without a background infusion after lower abdominal surgery in children. Br J Anaesth. 1993;71:670–673. doi: 10.1093/bja/71.5.670. [DOI] [PubMed] [Google Scholar]
- 14.Lopez BL, Flenders P, Davis-Moon L, et al. Clinically significant differences in the visual analog pain scale in acute vasoocclusive sickle cell crisis. Hemoglobin. 2007;31:427–432. doi: 10.1080/03630260701587810. [DOI] [PubMed] [Google Scholar]
- 15.Gossop M. The development of a Short Opiate Withdrawal Scale (SOWS) Addict Behav. 1990;15:487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- 16.R DevelopmentCore Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 17.Sarkar D. Lattice: Multivariate Data Visualization with R. New York: Springer; 2008. [Google Scholar]
- 18.Scheipl F. amer: Additive mixed models with lme4, R package version 0.6.7, 2010. http://CRAN.R-project.org/package=amer.
- 19.Bates D, Maechler M. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-37, 2010. http://CRAN.R-project.org/package=lme4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


