Summary
Humans exposed to Mycobacterium tuberculosis (Mtb) show variation in susceptibility to infection and differences in tuberculosis (TB) disease outcome. Toll-like receptor 9 (TLR9) is a pattern recognition receptor that mediates recognition of Mtb and modulates Mtb-specific T-cell responses. Using a case-population design, we evaluated whether single nucleotide polymorphisms (SNPs) in the TLR9 gene region are associated with susceptibility to pulmonary or meningeal TB as well as neurologic presentation and mortality in the meningeal TB group. In a discovery cohort (n = 352 cases, 382 controls), three SNPs were associated with TB (all forms, p<0.05) while three additional SNPs neared significance (0.05<p<0.1). When these six SNPs were evaluated in a validation cohort (n = 339 cases, 367 controls), one was significant (rs352142) while another neared significance (rs352143). When the cohorts were combined, rs352142 was most strongly associated with meningeal tuberculosis (dominant model; p=0.0002, OR 2.36, CI 1.43-3.87) while rs352143 was associated with pulmonary tuberculosis (recessive model; p=0.006, OR 5.3, CI 1.26-31.13). None of the SNPs were associated with mortality. This is the first demonstration of an association between a TLR9 gene region SNP and tuberculous meningitis. In addition, this extends previous findings that support associations of TLR9 SNPs with pulmonary tuberculosis.
Keywords: TLR9, Meningitis, Tuberculosis, Mycobacterium tuberculosis
1. Introduction
Mycobacterium tuberculosis (Mtb) is one of the leading infectious causes of morbidity and mortality worldwide. In 2012 there were an estimated 8.6 million incident cases of tuberculosis (TB) globally with 1.3 million deaths attributed to the disease[1]. There is substantial variation between individual susceptibility to the organism despite seemingly similar infectious exposures [2,3]. While the majority of patients with active TB present with pulmonary disease, other individuals develop extra-pulmonary disease, including TB meningitis (TBM) [1,4]. The mechanisms responsible for the varied susceptibility to infection as well as the disease phenotype after infection are not well understood.
The Toll-like receptors (TLRs) are a family of ten pattern recognition molecules that play a critical role in innate immunity. Mutations and polymorphisms within the genes involved in the TLR pathway are associated with susceptibility to infection in general [5] as well as to tuberculosis specifically [5-8]. Toll-like receptor 9 (TLR9) is located in the endosomal compartment of plasmacytoid dendritic cells and monocytes/macrophages[9]. The receptor recognizes unmethylated nucleic acid, specifically cytosine-phosphate-guanine (CpG) motifs, in bacterial and viral DNA[9]. The gene is approximately 5kb in size, contains two exons, and is located on chromosome 3p21.3. In humans, TLR9 genetic variants have been associated with HIV progression as well as more broadly with asthma, systemic lupus erythematosus (SLE), and atherosclerosis [5,10]. TLR9 has been shown to be an important regulator of the Th1-mediated cytokine response[9]. TLR9 agonists used as vaccine adjuvants in mice increase IFN-γ, TNF, and IL-2 responses and are components of multiple human candidate vaccines against Mtb [11,12].
Evidence from animal models suggests a role for TLR9 in mycobacterial infection. Tlr9−/− mice have reduced levels of IFN-γ and IL-12 and increased levels of the Th2 cytokines IL-4, IL-5, and IL-13 during lung granuloma formation in comparison to WT mice following challenge with purified protein derivative [13]. Tlr9−/− mice also had reduced IFN-γ levels in the lungs in comparison to WT mice upon exposure to aerosolized Mtb [14]. Despite the impact on the Th1 cytokine response, Tlr9 knockouts were only mildly more susceptible to Mtb infection. Combination knockouts with Tlr9 and other molecules, in particular Tlr2, have yielded inconsistent results with regards to Mtb susceptibility [14,15]. Despite the implications from animal models, the role of TLR9 in human susceptibility to tuberculosis is not completely understood.
Evidence from twin comparisons, infection in immunocompromised patients, genome wide linkage studies, and candidate gene studies suggest that host genetic factors influence tuberculosis susceptibility[3,16,17]. Recent analyses have focused on the role of the host innate immune system in influencing tuberculosis susceptibility and infection outcome[2,3]. TLR9 has been examined in TB case-control studies [18-22]. These studies suggest a possible association of TLR9 variants with susceptibility to pulmonary TB. To our knowledge, no one has examined whether TLR9 variants are associated with TB meningitis, mortality, neurologic outcomes, or the infecting Mtb strain.
In the current study, we examined whether haplotype-tagging TLR9 gene region SNPs are associated with susceptibility to pulmonary and meningeal TB in Vietnam. We also evaluated associations between these SNPs and mortality as well as other clinical presentation characteristics within the tuberculous meningitis subgroup.
2. Materials and methods
2.1 Human Subject Recruitment
Subjects were recruited as previously described [6,7,23]. Briefly, adult subjects (age >15 years) with pulmonary tuberculosis (PTB) were recruited in Ho Chi Minh City, Vietnam from a network of district tuberculosis units or Pham Ngoc Thach Hospital for Tuberculosis and Lung Diseases. Subjects had sputum smears positive for acid-fast bacilli and also met the following criteria: no history of tuberculosis treatment, no evidence of miliary or extrapulmonary tuberculosis, and negative HIV testing.
HIV-negative adults with tuberculous meningitis (TBM) were recruited from 1997 through 2008 from two hospitals in Ho Chi Minh City, Vietnam: Pham Ngoc Thach Hospital and the Hospital for Tropical Diseases. Subjects were diagnosed with TBM using the following two sets of criteria: “Definite TBM” was defined as clinical meningitis (nuchal rigidity, abnormal CSF parameters) and positive Ziehl-Neelsen stain or positive M. tuberculosis culture from the cerebrospinal fluid. “Probable TBM” was defined as clinical meningitis plus one or more of the following: chest radiograph consistent with active tuberculosis, acid-fast bacilli found in any specimen other than CSF, or clinical evidence of extrapulmonary tuberculosis. Cases were divided into a discovery and validation cohort. The discovery cohort was comprised of “Definite TBM” cases recruited from 2003-2004. The validation cohort included “Definite TBM” and “Probable TBM” recruited between 2006-2008. Severity of TBM was assessed at presentation using the British Medical Research Council TBM grade, the Glasgow Coma Scale, and the presence of a focal neurologic deficit (defined as the presence of cranial nerve palsy, monoplegia, hemiplegia, paraplegia, or quadriplegia) [24]. All patients with TBM were followed up until the end of anti-tuberculosis treatment.
Controls consisted of umbilical cord blood from newborns at Hung Vuong Hospital, Ho Chi Minh City. All subjects were unrelated and >99% were of the Vietnamese Kinh ethnicity. Written, informed consent was obtained from patients or their relatives for the cord blood samples and if the patient was unable to provide consent. All protocols were approved by human subject review committees at the Hospital for Tropical Diseases, Pham Ngoc Thach Hospital, Health Services of Ho Chi Minh City, Hung Vuong Hospital, Oxford Tropical Research Ethics Committee, and the University of Washington. Subjects from this cohort have been used in other candidate gene association studies as previously described [6,7,23,25-28].
2.2 SNP Selection
We identified haplotype tagging SNPs from the International HapMap Project (http://www.hapmap.org) and other public databases within the Genome Variation Server (http://www.ncbi.nlm.nih.gov.offcampus.lib.washington.edu/SNP/ and www.innateimmunity.net). Due to the presence of these polymorphisms in multiple populations, we derived haplotype tagging SNPs independently in the Han Chinese in Beijing (CHB), Utah residents with northern and western European ancestry (CEU), and Yoruba in Ibadan (YRI) populations. We searched a region on chromosome 3 twenty kilobases upstream and downstream of the TLR9 gene, as well as within the gene itself, for tagged SNPs using an R2 cutoff of 0.8 for linkage disequilibrium. We identified 16 SNPs for further analysis within this region, which includes one gene flanking each side of TLR9. The gene upstream of TLR9 on chromosome 3p21 is ALAS1, which encodes an enzyme that catalyzes the first step of heme biosynthesis [29]. The flanking downstream gene is TWF2, whose protein product is a regulator of actin dynamics [30].
2.3 Genomic Techniques
Genomic DNA was prepared using the QIAamp DNA blood kit (Qiagen) from peripheral blood. Polymerase chain reaction products were sequenced with Big Dye Terminator v3.0 and analyzed on an ABI PRISM 3730 capillary sequencer (Applied Biosystems). Sequence alignment and analysis was performed using the programs PHRED/PHRAP and CONSED (all at the University of Washington). Genotyping was performed using a MassARRAY technique (Sequenom) as described elsewhere[23].
2.4 Mtb genotyping
Mtb was genotyped using large sequence polymorphism typing to classify isolates into one of the three major lineages found in Vietnam: East Asian (including the Beijing sub-lineage), Indo-Oceanic or Euro-American, as previously described [31].
2.5 Statistical Analysis
We used STATA 11 software and the user-written package genass[32] for statistical analyses unless otherwise specified. SNPs displaying significant Hardy-Weinberg deviation in the control population were excluded. Of the sixteen SNPs included in the analysis, four were monomorphic within our cohort and were removed from further analysis (rs5743842, rs17052020, rs4082828, and rs5743846). One SNP (rs352167) deviated significantly from Hardy-Weinberg equilibrium in the control population and was not included. For the remaining eleven SNPs, we genotyped 352 patients with TB and 382 controls. This served as the initial (discovery) cohort, which we assessed for association with the diagnosis of “all tuberculosis” under genotypic, dominant, or recessive models of association. Because this was an exploratory analysis with a discovery sample set, we did not introduce a correction for multiple comparisons. SNPs with P≤0.05 or P>0.05 and <0.1 in the discovery sample set were analyzed further in a validation sample set (339 cased and 376 controls) for association with all TB as well as PTB and TBM. For SNPs with significant association with TB in the validation cohort, we performed a likelihood ratio (LR) test to determine which genetic model (additive, dominant, or recessive compared to genotypic) provided the best fit. We did so by performing a logistic regression for each model in STATA and then comparing estimates using the lrtest command.
Linkage disequilibrium within our dataset was assessed using the STATA command pwld. To evaluate for linkage between the SNPs that we studied and other SNPs in the region, we downloaded SNP data for the Han Chinese population (CHB and CHS) from the 1,000 genomes project (1000genomes.org, accessed 5/1/2014). We calculated pairwise linkage disequilibrium by the genotype coefficient R2 using the software Haploview version 4.2. Haplotypes were constructed with an expectation-maximization algorithm implemented using the STATA hapipf function. We used Chi-square tests, Cox proportional hazards regression and logistic regression, to evaluate associations with TB, survival and neurologic outcome in cases of TBM, respectively.
3. Results
3.1 Association of TLR9 Gene Region SNPs with Tuberculosis
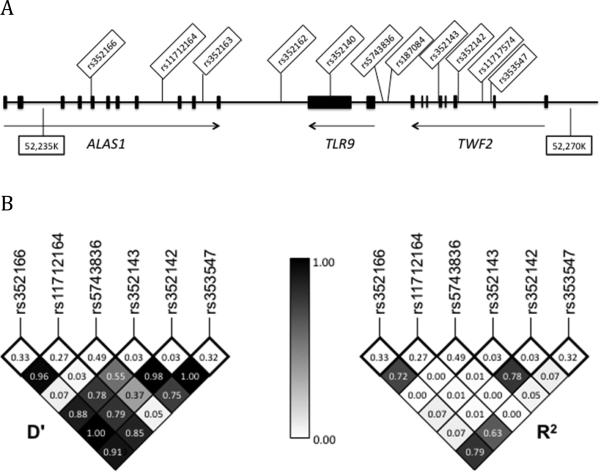
We used a case-population study design with discovery (N=352 cases and 382 controls) and validation (N=339 cases and 376 controls) cohorts to examine whether 11 polymorphisms in the TLR9 gene region were associated with susceptibility to tuberculosis in Vietnamese adults (Figure 1A). Three SNPs were associated with TB (PTB and TBM) (p<0.05) while three others showed a trend towards significance (p<0.1) in the discovery cohort (Table 1).
Figure 1.
A: Genomic locations of eleven TLR9 gene-region SNPs on chromosome 3. Exons are designated by black rectangles. B: Linkage disequilibrium plot for TLR9 gene region SNPs associated with TB. D’ and R2 values were calculated in the control population for those SNPs associated with TB in the discovery cohort with a p-value <0.10. Values are shown numerically and by shading. The minor allele frequency is shown in the box immediately below each corresponding SNP.
Table 1.
TLR9 haplotype tagging polymorphisms and their association with TB.
| Genotypic | Dominant | Recessive | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Group | 00 | 01 | 11 | H-W | X2 | p | OR | p | OR | p |
| rs352166 | Control | 173 (0.461) | 157 (0.419) | 45 (0.12) | 0.31 | ||||||
| G→A | TB | 164 (0.522) | 105 (0.334) | 45 (0.143) | 5.20 | 0.074 | 0.78 (0.57-1.07) | 0.111 | 1.22 (0.77-1.96) | 0.366 | |
| rs11712164 | Control | 190 (0.495) | 164 (0.427) | 30 (0.078) | 0.51 | ||||||
| A→T | TB | 186 (0.592) | 100 (0.318) | 28 (0.089) | 8.69 | 0.013 | 0.67 (0.49-0.92) | 0.010 | 1.16 (0.65-2.05) | 0.599 | |
| rs352163 | Control | 179 (0.477) | 151 (0.403) | 45 (0.12) | 0.14 | ||||||
| C→T | TB | 171 (0.529) | 117 (0.362) | 35 (0.108) | 1.88 | 0.390 | 0.81 (0.60-1.11) | 0.170 | 0.89 (0.54-1.46) | 0.630 | |
| rs352162 | Control | 168 (0.446) | 162 (0.430) | 47 (0.125) | 0.42 | ||||||
| T→C | TB | 162 (0.497) | 125 (0.383) | 39 (0.120) | 1.93 | 0.380 | 0.81 (0.60-1.11) | 0.174 | 0.95 (0.59-1.54) | 0.839 | |
| rs352140 | Control | 177 (0.472) | 155 (0.413) | 43 (0.115) | 0.06 | ||||||
| G→A | TB | 170 (0.531) | 117 (0.366) | 33 (0.103) | 2.43 | 0.297 | 0.79 (0.58-1.08) | 0.119 | 0.89 (0.53-1.47) | 0.627 | |
| rs5743836 | Control | 363 (0.979) | 8 (0.021) | 0 (0.0) | 0.83 | ||||||
| T→C | TB | 320 (0.994) | 2 (0.006) | 0 (0.0) | 2.83 | 0.093 | 0.29 (0.029-1.45) | 0.093 | NA | NA | |
| rs187084 | Control | 182 (0.465) | 165 (0.422) | 44 (0.113) | 0.48 | ||||||
| T→C | TB | 168 (0.493) | 136 (0.399) | 37 (0.109) | 0.55 | 0.761 | 0.90 (0.66-1.21) | 0.462 | 0.96 (0.59-1.57) | 0.862 | |
| rs352143 | Control | 355 (0.924) | 27 ( 0.070) | 2 (0.005) | 0.07 | ||||||
| A→G | TB | 303 (0.915) | 20 (0.060) | 8 (0.024) | 4.85 | 0.088 | 1.13 (0.63-2.02) | 0.655 | 4.73 (0.93-45.9) | 0.031 | |
| rs352142 | Control | 367 (0.939) | 23 (0.059) | 1(0.003) | 0.33 | ||||||
| T→G | TB | 303 (0.904) | 30 (0.090) | 2 (0.006) | 3.07 | 0.215 | 1.62 (0.90-2.93) | 0.086 | 2.34 (0.12-138.5) | 0.475 | |
| rs11717574 | Control | 374 (0.992) | 3 (0.008) | 0 (0.00) | 0.94 | ||||||
| T→C | TB | 339 (0.997) | 1 (0.003) | 0 (0.00) | 0.81 | 0.368 | 0.37 (0.01-4.61) | 0.368 | NA | NA | |
| rs353547 | Control | 177 (0.473) | 154 (0.142) | 43 (0.115) | 0.29 | ||||||
| G→A | TB | 179 (0.561) | 105 (0.329) | 35 (0.110) | 5.77 | 0.056 | 0.70 (0.51-0.96) | 0.021 | 0.95 (0.57-1.56) | 0.827 | |
SNPs are listed in chromosomal order and bolded if they met the conservative p<0.10 significance threshold for the discovery cohort.
00 = homozygous common allele. 01 = heterozygote. 11 = homozygous uncommon allele. Whole numbers represent number of samples with each genotype. Decimals in parentheses represent genotype frequency.
H-W = Hardy-Weinberg equilibrium p-value calculation for the control population. X2= chi square test. OR= odds ratio; number ranges in parentheses following odds ratios are 95% confidence intervals.
We next examined whether the SNP associations with TB were independent. We looked at patterns of linkage disequilibrium (Figure 1B) between the six SNPs, which revealed that three of the SNPs (rs11712164, rs352166, and rs353547) were in moderate to strong linkage disequilibrium (R2/D’: 0.72/0.96, 0.63/0.85, and 0.79/0.91 for rs11712164 to rs352166, rs11712166 to rs353547, and rs352166 to rs353547, respectively). Due to this level of LD, we only included rs11712164 in the validation cohort since it had the strongest association under the genotypic model. SNPs rs342142 and rs352143 also displayed strong linkage disequilibrium (R2/D’: 0.78/0.98). Given that the significant associations were found under different genetic models (genotypic and recessive model for rs352143, genotypic and dominant model for rs352142), both SNPs were carried forward in the validation cohort.
We genotyped 4 SNPs (rs11712164, rs5743836, rs352143, rs352142) in the validation cohort and found that only SNP rs352142 was significant with a p value of 0.004 in the dominant model (Table 2). In the recessive model, SNP rs352143's p value of 0.080 was near the significance threshold. We next performed a likelihood ratio test to determine which genetic model provided the best fit for the association of rs352142 and rs352143 with susceptibility to TB. We compared the genotypic model to additive, dominant, and recessive models. For rs352142, the additive and dominant models provided an equivalent fit (χ2=0.33, p=0.57 for additive and χ2=1.55, p=0.21 for dominant) while the recessive model provided a significantly worse fit (χ2=8.43, p=0.004) when compared to the genotypic model. For SNP rs352143, the additive and dominant models provided a worse fit (χ2=7.11, p=0.008 for additive and χ2=8.55, p=0.004 for dominant), while the recessive model had a similar fit (χ 2=0.63, p=0.43) when compared to the genotypic model. Together, the likelihood ratio test data suggests that rs352142 fits an additive or dominant model while rs352143 fits a recessive model.
Table 2.
Genotype frequencies and models of association with tuberculosis in the validation cohort.
| Genotypic | Dominant | Recessive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Group | 00 | 01 | 11 | χ 2 | P | OR | p | OR | p |
| rsl 1712164 | Control | 207 (0.569) | 136 (0.374) | 21 (0.058) | ||||||
| TB | 199 (0.628) | 100 (0.315) | 18 (0.057) | 2.65 | 0.266 | 0.78 (0.57-1.08) | 0.117 | 0.98 (0.48-1.98) | 0.959 | |
| rs5743836 | Control | 333 (0.988) | 4 (0.012) | 0 (0.00) | ||||||
| TB | 298 (0.990) | 3 (0.010) | 0 (0.00) | 0.05 | 0.818 | 0.83 (0.12-5.00) | 0.818 | NA | NA | |
| rs352143 | Control | 347 (0.048) | 18 (0.049) | 1 (0.003) | ||||||
| TB | 317 (0.946) | 13 (0.039) | 5 (0.015) | 3.46 | 0.177 | 1.04 (0.50-2.13) | 0.914 | 5.53 (0.61-262) | 0.080 | |
| rs352142 | Control | 347 (0.948) | 19 (0.052) | 0 (0.00) | ||||||
| TB | 267 (0.887) | 31 (0.103) | 3 (0.010) | 10.06 | 0.007 | 2.33 (1.26-4.41) | 0.004 | NA | 0.056 | |
Significant p-values (p<0.05) appear in bold.
3.2 TLR9 Gene Region SNPs, Tuberculosis Phenotype, and Mtb Lineage
We next examined whether SNPs rs352142 and rs352143 were preferentially associated with specific types of tuberculosis. In the combined discovery and validation cohorts, both SNPs were associated with susceptibility to all forms of TB (rs352142: p=0.001, OR 1.92, CI 1.27-2.94, dominant model; rs352143: p=0.006, OR 4.96, CI 1.35-27.2, recessive model; Table 3). rs352142 had a higher magnitude of association with TBM as compared to PTB in a dominant model (p=0.0002, OR 2.36, CI 1.43-3.87) while rs352143 had a slightly higher OR with PTB as compared to TBM in a recessive model (p=0.006, OR 5.3, CI 1.26-31.13). The association between both SNPs and susceptibility remained significant after adjusting for gender as a covariate (p=0.002 and 0.020 for rs352142 and rs352143, respectively). Due to the low genotype frequency, opposite genetic models of association, and levels of LD between rs352142 and rs352143, we were unable to analyze haplotypes and determine whether combinations of alleles altered the magnitude of the association. We performed a stratified analysis evaluating for susceptibility to Beijing versus non-Beijing strains for all forms of tuberculosis. Under a dominant model, rs352142 was not significantly associated with tuberculosis strain, though the relationship trended towards a greater susceptibility in non-Beijing (Indo-oceanic & Euro-american) than in Beijing strains (Χ2=3.67, p=0.056). We were unable to assess rs352143 and strain association due to the low frequency of the uncommon allele in a recessive model.
Table 3.
Association of TLR9 polymorphisms with TB clinical types for the combined discovery and validation cohorts.
| Gen | Add | Dominant | Recessive | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Group | 00 | 01 | 11 | p | p | OR | CI | p | OR | CI | p |
| rs352143 | Control | 702 (0.396) | 45 (0.060) | 3 (0.004) | ||||||||
| AllTB | 620 (0.931) | 33 (0.050) | 13 (0.020) | 0.016 | 0.21 | 1.09 | 0.70-1.69 | 0.702 | 4.96 | 1.35-27.2 | 0.006 | |
| PTB | 362 (0.943) | 14 (0.036) | 8 (0.021) | 0.006 | 0.58 | 0.89 | 0.50-1.53 | 0.657 | 5.30 | 1.26-31.1 | 0.006 | |
| TBM | 258 (0.915) | 19 (0.067) | 5 (0.018) | 0.072 | 0.09 | 1.36 | 0.78-1.32 | 0.236 | 4.49 | 0.87-29.1 | 0.025 | |
| rs352142 | Control | 714 (0.943) | 43 (0.055) | 1 (0.001) | ||||||||
| AllTB | 570 (0.896) | 61 (0.096) | 5 (0.008) | 0.003 | 0.0006 | 1.92 | 1.27-2.94 | 0.001 | 5.99 | 0.67-283.7 | 0.063 | |
| PTB | 324 (0.913) | 27 (0.076) | 4 (0.011) | 0.027 | 0.022 | 1.59 | 0.95-2.63 | 0.057 | 8.62 | 0.85-424.8 | 0.021 | |
| TBM | 246 (0.875) | 34 (0.121) | 1 (0.004) | 0.001 | 0.0003 | 2.36 | 1.43-3.87 | 0.0002 | 2.70 | 0.34-212.2 | 0.465 | |
Significant p-values (p<0.05) appear in bold. Gen = genotypic model, Add = additive model
3.3 TLR9 Gene Region SNPs and Tuberculous Meningitis Presentation and Outcomes
To evaluate for association with mortality from TBM, we performed a Cox regression of the TLR9 SNPs rs352142 and rs352143 using the combined discovery and validation cohorts. Neither SNP showed a significant association with mortality (data not shown). Given that SNP rs352142 was associated with susceptibility to TBM, we also examined whether this SNP was associated with TBM disease severity including meningitis grade, Glasgow coma scale, and focal neurologic deficit at the time of clinical presentation. rs352142 was associated with a lower Glasgow coma scale score (p=0.032, OR 0.56, CI 0.33-0.95), indicating a lower level of consciousness in TBM patients in the presence of the SNP. Rs352142 was not associated with other neurological findings (data not shown).
4. Discussion
This study identifies a SNP within the TLR9 gene region that is associated with susceptibility to tuberculous meningitis. To our knowledge, this is the first description of a TLR9 association with this severe form of tuberculosis disease. Additionally, we identified a second SNP that was associated with pulmonary tuberculosis; this SNP, rs352143, has also been associated with pulmonary TB in an independent analysis in an African-American and Caucasian but failed to show association in a Guinea-Bissau population [18].
Other candidate gene studies have examined the relationship between TLR9 SNPs and pulmonary TB. Jahantigh et al identified a synergic effect of the TLR9 SNP rs187084 with a TLR4 SNP; the combination of the two polymorphisms increased risk of pulmonary TB. Similar to our findings, rs187084 alone was not associated with TB susceptibility [33]. Yang et al found two SNPs, rs352139 and rs352140, that were associated with pulmonary TB when compared to latent TB controls but not with healthy controls[21]. SNP rs352139, located in an intronic region of the TLR9 gene, was also found to be associated with pulmonary TB in an African American and Indonesian female but not in a Guinea-Bissau, Caucasian, or Colombian population [18-20]. This SNP was not included in our study. To determine whether SNP rs352142 or rs352143 were in linkage disequilibrium with SNP rs352139, we calculated pairwise linkage disequilibrium by the genotype coefficient R2 using SNP frequency data for the Han Chinese population from 1,000 genomes. The two SNPs identified in our study were not strongly linked to rs352139 (R2 < 0.05 for either SNP, data not shown), suggesting an independent association between rs352139 and the SNPs we identified in influencing TB susceptibility. None of the aforementioned TLR9 gene region SNPs are associated with a functional change in the TLR9 protein product. Rs352139 is intronic while rs352140 is a synonymous polymorphism. Together, these data suggest that several TLR9 SNPs are associated with susceptibility to TB. However, fine mapping studies are needed to discover a causal SNP and mechanistic explanation for the findings.
Both SNPs rs352142 and rs352143 are located outside of the TLR9 coding region and within the twinfillin-2 (TWF2) gene. rs352142 is intronic while rs352143 is located in an exon and produces a silent mutation. TWF2 is a regulator of actin dynamics that plays an important developmental role in lower eukaryotes but has been shown to be dispensable in mammals, potentially due to the overlapping function of twinfillin-1 [30]. There is currently no evidence linking TWF2 with human infection or tuberculosis. The upstream flanking gene of TLR9 is aminolevulinic synthase-1 (ALAS1), whose protein product catalyzes the first and rate-limiting step in nonerythropoetic heme biosynthesis [29]. ALAS1 also has not been implicated in human infection. However, a recent study found that mice lacking heme oxygenase-1 (HO-1), which catalyzes the conversion of heme to other products such as bilverdin and carbon monoxide [34], were more susceptible to both M. avium and M. tuberculosis via a process that was independent of the adaptive immune response [35]. It is possible that ALAS1 could play an as-yet undetermined role in tuberculosis infection through its mediation of heme synthesis. Together, these previous studies suggest that TLR9 remains the most likely candidate for a causal relationship given its established role in the immune response to tuberculosis. A plausible explanation is that the SNPs that we identified are in linkage disequilibrium with another mutation that either confers a functional or a regulatory change in TLR9.
The role for TLR9 in the Th1-mediated response to MTB infection has yet to be completely described in humans, though animal studies suggest an important relationship. Compared to control mice, Tlr9−/− mice infected with Leishmania major, another intracellular pathogen, have a greater parasite burden, inhibited wound healing, and reduced lymph node IFN-γ and IL-12 production [36]. When infected with MTB, Tlr9−/− knockouts have reduced IL-12 and TNF-α production. However, TLR9-deficiency had variable impact on bacterial burden and mortality that was dose-dependent. The combined knockout Tlr9 and Tlr2 had a more substantial impact on infection outcome compared to either single knockout alone [14], suggesting the possibility of gene-gene interactions. Given the low frequency of the TLR9 SNPs in Vietnam, we were unable to test for an interaction between TLR2 and TLR9 SNPs in Vietnam.
SNP rs352142 was most strongly associated with tuberculous meningitis in our study. The mechanisms by which tuberculosis invades the CNS are not completely understood, though the widely accepted hypothesis initially proposed by Rich and McCordock in 1933 describes a two-step model in which lesions develop around bacteria seeded in the meninges during hematogenous dissemination and then rupture, releasing bacteria into the cerebrospinal fluid [37]. The hematogenous dissemination is thought to occur early in infection, prior to control by the adaptive immune response[4]. Since the TLRs are a component of the innate immune system and thus are involved in the initial response to infection, it is plausible that a TLR9 variant might influence the ability of the immune system to control hematogenous spread or the development of tuberculous granulomas (Rich foci) in an early stage of infection. We previously found variants in TLR2 and TIRAP that were associated with tuberculous meningitis [6,23]. Unfortunately the frequency of the rs352142 minor allele was too low to allow for an evaluation of a gene-gene interaction with the TLR2 variant in our cohort. With regards to the risk attributable to the TLR9 polymorphisms, the odds ratios for rs352142 and rs352413 are 1.92 and 4.96, respectively (Table 3). These odds ratios are similar or higher than those found for the SNPs in SIGRR[7], TOLLIP[25], CD43[26] and LTA4H[28] which have also been associated with PTB and/or TBM. However, the frequencies of the TLR9 SNPs are both low which diminishes the population level attributable risk.
Strengths of this study include the large size of the SNP dataset, the use of separate discovery and validation cohorts, the categorization of multiple tuberculosis infection phenotypes, and the availability of clinical data including mortality for the TBM group. One potential limitation in our study was a lack of correction for multiple comparisons. In addition, given that this was an exploratory analysis we chose to minimize type II error by accepting p<0.1 in the discovery cohort as a threshold for further evaluation. A conservative Bonferroni correction (considering 11 total comparisons after removal of those SNPs lacking polymorphisms in our cohort) would have required a p < 0.0045 to reject the null hypothesis. The use of a separate discovery and validation cohort mitigates this limitation. In the combined discovery and validation cohorts, rs352142 remained significant in its association with tuberculous meningitis after adjusting for multiple comparisons. A second limitation of our study is the use of cord blood for our control population, as some of those infants whose cord blood was included may go on to contract tuberculosis at some time in their lives. This is a concern with case-population studies in general and is also true of case-control studies for TB. Ultimately it is expected to underestimate true associations between polymorphism and disease.
In summary, this study suggests a relationship between polymorphisms localized to the gene region surrounding TLR9 and susceptibility to tuberculosis infection. A more complete understanding will require evaluation of functional variants of TLR9 and the associated molecules in its signaling cascade.
Acknowledgements
We would like to acknowledge the clinical staff from the Hospital of Tropical Diseases and Pham Ngoc Thach Hospital who initially diagnosed and studied the patients with tuberculous meningitis and pulmonary tuberculosis. We would like to thank Dr. Nguyen Thi Hieu from Hung Vuong Obstetric Hospital Vietnam, Dr. Tran Tinh Hien from the Hospital for Tropical Diseases Vietnam, and all Vietnamese individuals who participated in this study. We thank Drs Alan Aderem and Marta Janer from the Institute for Systems Biology for advice and Sarah Li for technical assistance with genotyping. From the University of Washington, we thank Glenna Peterson and Dr. Monica Campo for their assistance with sample preparation and statistical analysis. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant K24AI089794 to T. R. H.), the Burroughs Wellcome Foundation (T. R. H.), and the Wellcome Trust of Great Britain supporting the Major Overseas Program in Vietnam (grant 089276/Z/09/Z). Study sponsors had no involvement in the study design or the manuscript preparation.
Abbreviations
- Mtb
Mycobacterium tuberculosis
- PTB
Pulmonary tuberculosis
- TBM
Tuberculous meningitis
- TB
tuberculosis disease
- TLR9
Toll-like receptor 9
- ALAS1
aminolevulinic synthase-1
- TWF2
twinfillin-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013;368(8):745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 2.Berrington WR, Hawn TR. Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunol Rev. 2007;219:167–186. doi: 10.1111/j.1600-065X.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel L, El-Baghdadi J, Bousfiha AA, Casanova JL, Schurr E. Human genetics of tuberculosis: a long and winding road. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130428. doi: 10.1098/rstb.2013.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi: 10.1016/S1474-4422(13)70168-6. [DOI] [PubMed] [Google Scholar]
- 5.Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114(5):347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- 6.Thuong NT, Hawn TR, Thwaites GE, Chau TT, Lan NT, Quy HT, et al. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007;8(5):422–428. doi: 10.1038/sj.gene.6364405. [DOI] [PubMed] [Google Scholar]
- 7.Horne DJ, Randhawa AK, Chau TT, Bang ND, Yen NT, Farrar JJ, et al. Common polymorphisms in the PKP3-SIGIRR-TMEM16J gene region are associated with susceptibility to tuberculosis. J Infect Dis. 2012;205(4):586–594. doi: 10.1093/infdis/jir785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Jiang T, Yang X, Xue Y, Wang C, Liu J, et al. Toll-like receptor -1, -2, and -6 polymorphisms and pulmonary tuberculosis susceptibility: a systematic review and meta-analysis. PLoS One. 2013;8(5):e63357. doi: 10.1371/journal.pone.0063357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortaz E, Adcock IM, Tabarsi P, Masjedi MR, Mansouri D, Velayati AA, et al. Interaction of Pattern Recognition Receptors with Mycobacterium Tuberculosis. J Clin Immunol. 2014 doi: 10.1007/s10875-014-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13(5):543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Matsumoto M, Umemori K, Ozeki Y, Furugen M, Tatsuo T, et al. DNA augments antigenicity of mycobacterial DNA-binding protein 1 and confers protection against Mycobacterium tuberculosis infection in mice. J Immunol. 2005;175(1):441–449. doi: 10.4049/jimmunol.175.1.441. [DOI] [PubMed] [Google Scholar]
- 12.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181(11):7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Schaller M, Hogaboam CM, Standiford TJ, Chensue SW, Kunkel SL. TLR9 activation is a key event for the maintenance of a mycobacterial antigen-elicited pulmonary granulomatous response. Eur J Immunol. 2007;37(10):2847–2855. doi: 10.1002/eji.200737603. [DOI] [PubMed] [Google Scholar]
- 14.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202(12):1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holscher C, Reiling N, Schaible UE, Holscher A, Bathmann C, Korbel D, et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. Eur J Immunol. 2008;38(3):680–694. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- 16.Thye T, Owusu-Dabo E, Vannberg FO, van Crevel R, Curtis J, Sahiratmadja E, et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat Genet. 2012;44(3):257–259. doi: 10.1038/ng.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, et al. Risk Assessment of Tuberculosis in Immunocompromised Patients. A TBNET Study. Am J Respir Crit Care Med. 2014;190(10):1168–1176. doi: 10.1164/rccm.201405-0967OC. [DOI] [PubMed] [Google Scholar]
- 18.Velez DR, Wejse C, Stryjewski ME, Abbate E, Hulme WF, Myers JL, et al. Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet. 2010;127(1):65–73. doi: 10.1007/s00439-009-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi K, Yuliwulandari R, Yanai H, Naka I, Lien LT, Hang NT, et al. Association of TLR polymorphisms with development of tuberculosis in Indonesian females. Tissue Antigens. 2012;79(3):190–197. doi: 10.1111/j.1399-0039.2011.01821.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez D, Lefebvre C, Rioux J, Garcia LF, Barrera LF. Evaluation of Toll-like receptor and adaptor molecule polymorphisms for susceptibility to tuberculosis in a Colombian population. Int J Immunogenet. 2012;39(3):216–223. doi: 10.1111/j.1744-313X.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Li X, Cui W, Guan L, Shen F, Xu J, et al. Potential association of pulmonary tuberculosis with genetic polymorphisms of toll-like receptor 9 and interferon-gamma in a Chinese population. BMC Infect Dis. 2013;13:511. doi: 10.1186/1471-2334-13-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharti D, Kumar A, Mahla RS, Kumar S, Ingle H, Shankar H, et al. The role of TLR9 polymorphism in susceptibility to pulmonary tuberculosis. Immunogenetics. 2014;66(12):675–681. doi: 10.1007/s00251-014-0806-1. [DOI] [PubMed] [Google Scholar]
- 23.Hawn TR, Dunstan SJ, Thwaites GE, Simmons CP, Thuong NT, Lan NT, et al. A polymorphism in Toll-interleukin 1 receptor domain containing adaptor protein is associated with susceptibility to meningeal tuberculosis. J Infect Dis. 2006;194(8):1127–1134. doi: 10.1086/507907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green JA, Tran CT, Farrar JJ, Nguyen MT, Nguyen PH, Dinh SX, et al. Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS One. 2009;4(9):e7277. doi: 10.1371/journal.pone.0007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah JA, Vary JC, Chau TT, Bang ND, Yen NT, Farrar JJ, et al. Human TOLLIP regulates TLR2 and TLR4 signaling and its polymorphisms are associated with susceptibility to tuberculosis. J Immunol. 2012;189(4):1737–1746. doi: 10.4049/jimmunol.1103541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campo M, Randhawa AK, Dunstan S, Farrar J, Caws M, Bang ND, et al. Common Polymorphisms in the CD43 Gene Region Are Associated With Tuberculosis Disease and Mortality. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2014-0114OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seshadri C, Thuong NT, Yen NT, Bang ND, Chau TT, Thwaites GE, et al. A polymorphism in human CD1A is associated with susceptibility to tuberculosis. Genes Immun. 2014;15(3):195–198. doi: 10.1038/gene.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobin DM, Vary JC, Jr., Ray JP, Walsh GS, Dunstan SJ, Bang ND, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140(5):717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser DJ, Podvinec M, Kaufmann MR, Meyer UA. Drugs mediate the transcriptional activation of the 5-aminolevulinic acid synthase (ALAS1) gene via the chicken xenobiotic-sensing nuclear receptor (CXR). J Biol Chem. 2002;277(38):34717–34726. doi: 10.1074/jbc.M204699200. [DOI] [PubMed] [Google Scholar]
- 30.Nevalainen EM, Skwarek-Maruszewska A, Braun A, Moser M, Lappalainen P. Two biochemically distinct and tissue-specific twinfilin isoforms are generated from the mouse Twf2 gene by alternative promoter usage. Biochem J. 2009;417(2):593–600. doi: 10.1042/BJ20080608. [DOI] [PubMed] [Google Scholar]
- 31.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, Thuong NT, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4(3):e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shephard N, editor. GENASS: Stata module to perform genetic case-control association tests. Boston College Department of Economics; Chestnut Hill, MA: 2005. [Google Scholar]
- 33.Jahantigh D, Salimi S, Alavi-Naini R, Emamdadi A, Owaysee Osquee H, Farajian Mashhadi F. Association between TLR4 and TLR9 gene polymorphisms with development of pulmonary tuberculosis in Zahedan, southeastern Iran. ScientificWorldJournal. 2013;2013:534053. doi: 10.1155/2013/534053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 35.Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection. Infect Immun. 2013;81(7):2536–2545. doi: 10.1128/IAI.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abou Fakher FH, Rachinel N, Klimczak M, Louis J, Doyen N. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J Immunol. 2009;182(3):1386–1396. doi: 10.4049/jimmunol.182.3.1386. [DOI] [PubMed] [Google Scholar]
- 37.Rich AR, McCordock HA. The pathogenesis of tuberculous meningitis. Bull Johns Hopkins Hosp. 1933;52:5–37. [Google Scholar]