Abstract
Objectives
To develop a low-energy electrotherapy that terminates ventricular tachycardia (VT) when anti-tachycardia pacing (ATP) fails.
Background
High-energy ICD shocks are associated with device failure, significant morbidity and increased mortality. A low-energy alternative to ICD shocks is desirable.
Methods
Myocardial infarction (MI) was created in 25 dogs. Sustained, monomorphic VT was induced by programmed stimulation. Defibrillation electrodes were placed in the RV apex, and coronary sinus (CS) and LV epicardium (LVP). If ATP failed to terminate sustained VT, the defibrillation thresholds (DFTs) of standard versus experimental electrotherapies were measured.
Results
Sustained VT ranged from 276–438 bpm (mean 339 bpm). The RV-CS shock vector had lower impedance than RV-LVP (54.4±18.1 Ω versus 109.8±16.9, Ω p<0.001). A single shock required between 0.3±0.2 J to 5.9±2.5 J (mean 2.64±3.22 J; p=0.008) to terminate VT, and varied depending upon the phase of the VT cycle at which it was delivered. In contrast, multiple shocks delivered within 1 VT cycle length were not phase-dependent and achieved lower DFT compared to a single shock (0.13±0.09 J for 3 shocks, 0.08±0.04 J for 5 shocks, 0.09±0.07 J for 7 shocks; p<0.001). Finally, a multi-stage electrotherapy (MSE) achieved significantly lower DFT compared to a single biphasic shock (0.03±0.05 J versus 2.37±1.20 J, respectively, p<0.001). At a peak shock amplitude of 20 V, MSE achieved 91.3% of terminations versus 10.5% for a biphasic shock (p<0.001).
Conclusions
MSE achieved a major reduction in DFT compared to a single biphasic shock for ATP-refractory monomorphic VT, and represents a novel electrotherapy to reduce high-energy ICD shocks.
Keywords: Ventricular tachycardia, defibrillation, ICD, myocardial infarction, multi-stage electrotherapy
Introduction
Randomized, prospective clinical trials have demonstrated that implantable cardioverter-defibrillators (ICDs) decrease mortality in patients with CAD or prior myocardial infarction (MI) who are at increased risk of VT or ventricular fibrillation (VF)(1,2). More than 80% of victims of sudden cardiac death suffer from coronary artery disease (CAD)(3), with the most common arrhythmia being sustained monomorphic ventricular tachycardia (VT)(4). A high-energy biphasic shock is the only existing electrotherapy when anti-tachycardia pacing (ATP) fails to terminate VT. However, ICD shocks have been shown to damage the myocardium and reduce the quality of life (5,6). Moreover, patients receiving high-energy shocks have a 2–3 fold increase in mortality (7,8) compared to those who do not receive shocks. While it is unclear if it is the shocks themselves or a change in underlying cardiac status that actually caused higher mortality, even inappropriate shocks (9) are associated with a higher mortality, e.g., inappropriate shocks are often due to atrial fibrillation, which itself can result in a higher mortality in patients with poor left ventricular function. Despite these disadvantages, alternative low-energy electrotherapies have not been introduced into ICD technology since the incorporation of ATP. The purpose of this study was to develop a novel electrotherapy that terminates VT with lower energy than a biphasic shock for ATP-refractory monomorphic VT.
Methods
Surgical Procedures
All animal procedures were carried out in accordance with the Position of the American Heart Association on the use of research animals (updated in 1985) and were approved by the Animal Studies Committee at Washington University. Myocardial infarction (MI) was created after anesthetization of mongrel dogs (n=25) of either sex weighing 20–25 kg by surgical ligation of the left anterior descending (LAD) coronary artery for 2 hours, as described previously (10). Four days after MI, animals were re-anesthetized, a median sternotomy was performed, and cardiopulmonary bypass was initiated after the administration of heparin.
Electrode Configuration
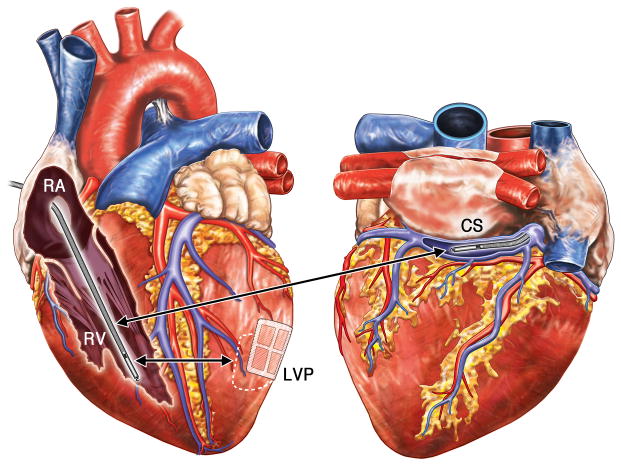
In the 24 dogs that survived MI (one dog died suddenly one day after LAD ligation), a 15 cm2 custom epicardial LV patch (LVP) was placed over the posterolateral LV, and an 8 Fr (French (Fr)) standard defibrillation/bipolar pacing lead (6935, Medtronic, Minneapolis, MN) was implanted in the RV apex (Figure 1). In five animals, an additional 8 Fr standard defibrillation lead (6937A, Medtronic, Minneapolis, MN) was placed into the coronary sinus (CS, Figure 1). Pacing was delivered from the RV bipole. Shock therapies were delivered across the RV-LVP or RV-CS vectors; all shocks were RV anodal. A bipolar button electrode was sewn to the RV epicardium for ventricular sensing.
Figure 1. Anatomic Position of Defibrillation Electrodes.
Schematic of a canine heart with the locations of electrodes used for application of electrotherapies. Anteroposterior (left panel) and posteroanterior (right panel) views showing locations of the defibrillations leads (red lettering) and shock vectors (red arrows). Shocks were delivered from the RV defibrillation coil (RV) to the CS defibrillation coil (CS), or from the RV coil to epicardial LV defibrillation Patch (LVP). RA, right atrium; LA, left atrium; CS; coronary sinus; PT, pulmonary trunk; RV, right ventricle; LV, left ventricle.
Defibrillation Protocol
Four days after MI, loading (2 mg/kg) and maintenance (0.05 mg/kg/min) infusions of flecainide acetate (Sigma-Aldrich, St. Louis, MO) were administered intravenously. VT was induced by rapid RV pacing protocols (programmed electrical stimulation). Sustained, monomorphic VT was defined as a fast but organized ventricular rhythm <500 bpm, lasting >30 s. VF was defined as an arrhythmia >500 bpm, and, if induced, terminated using an external defibrillator. The heart was allowed to recover for 5–10 min after any external defibrillation before re-induction of VT.
After monomorphic VT was induced, anti-tachycardia pacing (ATP) was attempted. ATP consisted of eight pacing stimuli, each of 2 ms duration delivered at a rate of 88% of the VT cycle length (CL), delivered from the RV bipole at an amplitude four times the ventricular pacing capture threshold current. If ATP failed to terminate VT, defibrillation thresholds (DFTs) of various electrotherapies (defined below) were measured. Defibrillation threshold (DFT), in volts, was defined as the peak shock amplitude that terminated VT. DFT, in Joules (J) was calculated by multiplying the energy of a single shock by the number of shocks applied (for multiple shock- and multi-stage- electrotherapies).
Experimental and standard electrotherapies were delivered using a randomized protocol with respect to sequence to avoid time bias, using a voltage-regulated, step-up protocol. The amplitude of the peak shock voltage was increased after each unsuccessful termination, beginning at twice the minimum voltage required to capture the ventricle with a 10ms monophasic shock, until VT was terminated. Re-induction of VT was attempted after a 5 min recovery period, according to the protocol. Electrotherapies were delivered from computer-controlled regulated power supplies (BOP 100–4M; Kepco, Flushing, NY). Impedances were calculated using a current probe (A622; Tektronix, Inc., Beaverton, OR).
Electrotherapies tested
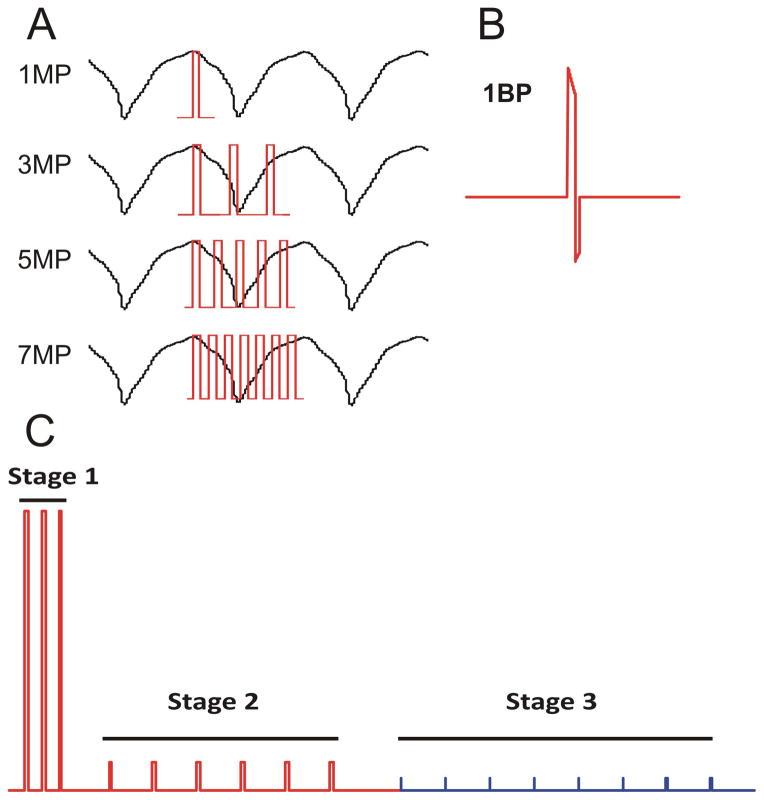
The electrotherapies tested are shown in Figure 2. One (1MP), three (3MP), five (5MP) or seven (7MP) monophasic shocks were delivered within one VT CL (Figure 2A); each individual shock was 10 ms in duration. A single biphasic shock (1BP, 6 ms and 4 ms durations of the first and second phases, respectively; 2:1 ratio of the leading edge voltages of the two phases is shown in Figure 2B. Multi-Stage Electrotherapy (MSE), shown in Figure 2C, consisted of 3 monophasic shocks delivered within 1 VT CL (Stage 1), followed by 6 MP shocks delivered with an interval of 88% of the VT CL at twice the ventricular shock capture voltage (Stage 2), followed by ATP (Stage 3); individual stages were separated by 100 ms delays.
Figure 2. Defibrillation Waveforms and Low Energy Electrotherapies.
Schematic diagrams of the electrotherapies applied. Pulse widths of individual shocks were 10 ms. A: One (1MP), three (3MP), five (5MP) or seven (7MP) monophasic shocks delivered within one VT cycle length. B: A single biphasic shock (1BP) with pulse widths of 6 ms for the first (positive) phase and 4 ms for the second (negative) phase. C: Multi-Stage Electrotherapy (MSE). Stage 1 consisted of three monophasic shocks delivered in one VT cycle length, followed by a 100 ms delay. Stage 2 consisted of six lower voltage monophasic shocks delivered at a rate of 88 % of the VT CL, followed by a 100 ms delay. Stage 3 consisted of eight pacing stimuli each of 2 ms duration delivered from the endocardial RV lead (tip to ring) at a rate of 88 % of the VT CL. All shocks in MSE (stages 1 and 2) and all biphasic shocks were delivered from the RV coil to CS coil. MP, monophasic; BP, biphasic; CL, cycle length.
Statistical Analysis
The defibrillation protocol randomized the sequence of electrotherapies to prevent the confounding effects of treatments with respect to time. Recovery periods were observed after each termination to prevent carry-over effects. DFTs for comparisons of electrotherapies tested were analyzed using a linear mixed random effects model with animal ID as a random effect and treatment as a fixed effect. Energy and voltage DFT values were log transformed to stabilize the variance. Estimates were calculated with the MIXED procedure in SAS (Version 9.3, SAS Institute, Cary, NC). Paired Student’s t-test was used to compare impedances of the two shock vectors tested, and performed in Prism 5.0c (GraphPad Software, La Jolla, CA). Results are reported as mean ± standard deviation. A p value of ≤ 0.05 was considered significant.
Results
Characteristics of Monomorphic VT in Canine Hearts with 4-day Infarct
A total of 190 episodes of monomorphic, sustained VT (lasting >30 sec), were induced in 16 of the 24 dogs (66.7%) that survived MI; in eight dogs, only VF or polymorphic VT could be induced. VT ranged from 276–438 bpm (mean 339 bpm). The post-MI surface ECG during sinus rhythm revealed residual ST-segment elevation and a ventricular premature depolarization. ATP successfully terminated VT in 17 of 170 trials, a success rate of 10.0%.
Phase Dependence of a Single Shock
The energy required for a single shock to terminate VT varied significantly depending upon the phase of the VT cycle at which it was delivered (DFT phase-dependence) in this in vivo model. The DFT of a single 10 ms monophasic shock varied significantly when delivered at 0%, 20%, 40%, 60% or 80% of the VT CL. Shocks were delivered between the RV coil and LV epicardial patch (LVP). Application of a single shock revealed that the DFT dramatically changed based upon the phase of application. Phase-dependent DFT for a single shock was seen in all dogs tested (N=5). Across all dogs, the mean DFT of the optimal phase in each animal compared to the mean DFT of the least effective phase was 0.3 ± 0.2 J versus 5.9 ± 2.5 J, respectively (p = 0.008). Notably, the optimal phase varied between animals, and could not be determined a priori.
DFTs of Single versus Multiple Shocks
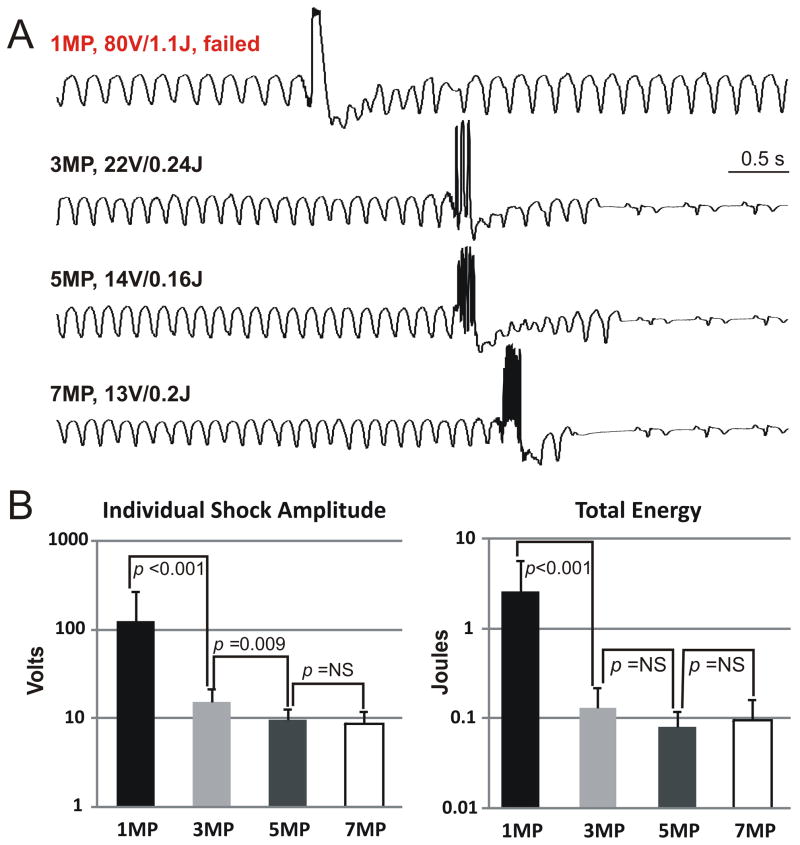
We hypothesized that multiple low-energy shocks applied evenly within one VT CL would not have phase-dependence, thus enabling a reduction in the DFT compared to a single shock. After induction of sustained VT, ATP was attempted. If ATP failed, one, three, five or seven MP shocks (1MP, 3MP, 5MP or 7MP, respectively) were applied within one VT CL (Figure 3A). A single (1MP) 1.1 J MP shock failed to terminate VT (upper tracing), while 3MP, 5MP, and 7MP shocks (of 0.24 J, 0.16 J, and 0.2 J, respectively) terminated VT. Mean DFTs across all dogs tested (N=6) are summarized in Figure 3B. In all animals tested, multiple shocks significantly lowered the DFT compared to a single shock, and did not exhibit phase-dependence.
Figure 3. DFTs of Single and Multiple Monophasic Shock Electrotherapies.
A: Surface ECG tracings during applications of single- and multiple- monophasic shock electrotherapies. An 80V (1.1 J) single monophasic shock (1MP) failed to terminate VT (top panel). In the same animal, 3 monophasic shocks (3MP; second panel), 5 monophasic shocks (5MP; third panel) and 7 monophasic shocks (7MP; lower panel) delivered within one VT cycle terminated VT successfully with 22V (0.24 J), 14V (0.16 J), and 13V (0.2 J), respectively. B: Mean DFTs (logarithmic scale) of 1MP, 3MP, 5MP, and 7MP with respect to peak voltage (left panel) and total energy (right panel) over N=6 dogs tested is shown. NS, not significant.
Multi-Stage Electrotherapy
Previous electrotherapies were applied from endocardium to epicardium (RV-LVP vector; Figure 1). To move towards the goal of developing a lead system that is practical in human yet enables low DFT, we tested whether shocks applied entirely within the heart (RV-CS vector; Figure 1) would achieve low impedance. The mean impedance of shocks delivered between the RV-CS vector was significantly lower than the RV-LVP vector (54.4 ± 18.1 Ohms versus 109.8 ± 16.9 Ohms, respectively, p < 0.001). These results show that low impedance can be achieved using transvenous electrodes rather than an epicardial patch electrode.
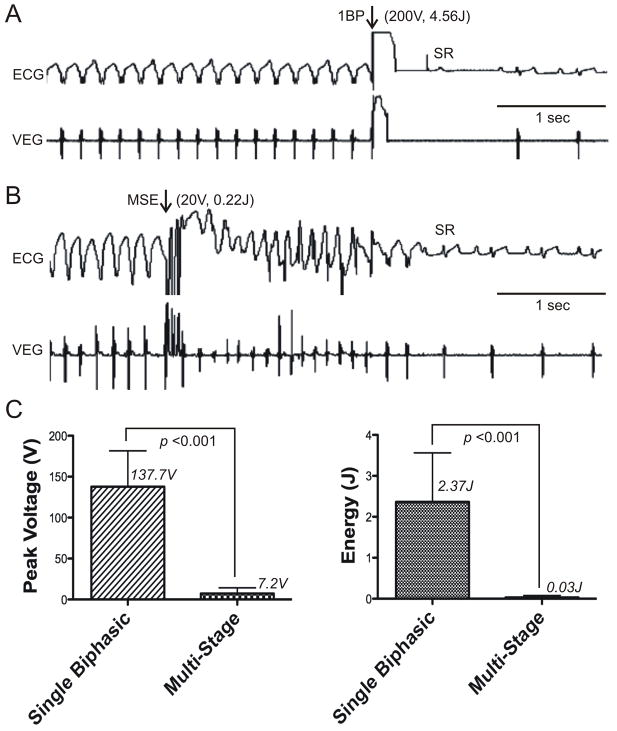
To further reduce the DFT, we expanded multiple shocks to a Multi-Stage Electrotherapy (MSE; Figure 2C). Using the RV-CS vector, MSE was compared to the existing clinical standard, a single biphasic shock. After induction of sustained monomorphic VT, ATP was attempted, and if failed, electrotherapies were delivered according to a randomized protocol. Sample terminations using a single biphasic shock and MSE are shown in Figure 4A, where the DFT of a single BP shock was 200 V (4.56 J) compared to 20 V (0.22 J) for MSE. Pooled results (N=5 dogs) are shown in Figure 4B. The mean DFT of MSE was significantly lower than that of a single biphasic shock in terms of total energy (0.03 ± 0.05 J versus 2.37 ± 1.20 J, respectively, p < 0.001) and peak shock voltage (7.2 ± 6.9 V versus 137.7 ± 43.8 V, respectively, p < 0.001). At a peak shock amplitude of 20 V, MSE achieved 91.3% of terminations versus 10.5% for a biphasic shock (p<0.001). Importantly, these results demonstrate that low DFT can be achieved using transvenously implanted leads, and does not require an epicardial electrode.
Figure 4. Sample Terminations and Mean Defibrillation Thresholds of a Single Biphasic Shock versus Multi-Stage Electrotherapy.
A: Surface ECG and ventricular electrogram (VEG) of a termination of monomorphic VT by a single biphasic shock with peak leading edge voltage of 200 V (4.56 J). Arrows indicate the time of electrotherapy application. B: Surface ECG and VEG of a terminations of monomorphic VT by Multi-Stage Electrotherapy (MSE) with peak voltage of 20 V (0.22 J). C: Mean DFTs of a single biphasic shock (Single Biphasic) and MSE (Multi-Stage) are shown with respect to peak voltage (left panel) and total energy (right panel). 1BP, single biphasic shock; MSE, multi-stage electrotherapy; SR: sinus rhythm.
Discussion
Our study showed that the DFT of a single shock to terminate post-MI monomorphic VT in vivo was phase-dependent, and that multiple low-energy shocks delivered within a single VT CL eliminated phase-dependence. Expanding upon this concept, MSE dramatically reduced the DFT compared to a single biphasic shock for ATP-refractory monomorphic VT. Importantly, we showed that MSE (and a single biphasic shock) could be delivered endocardially using commercially available transvenous defibrillation leads placed in RV and CS.
In this model, the success rate of ATP was 10%. This contrasts with human trials in which ATP terminated 78% to 94% of monomorphic VT <200 bpm (11–13). In humans, the efficacy of ATP has been shown to be lower with increasing rate of VT, terminating 84% of VT episodes ranging from 188–214 bpm and only 69% of episodes ranging from 214–250 bpm (14). Other studies have shown the success rate for fast VT to range from 47% to 79% (15–18). The average rate of VT in our model was 339 bpm, significantly faster than those reported in human ATP trials, and likely explains why ATP was relatively unsuccessful in this model. Importantly, the low-energy MSE described in this report is not envisioned as an alternative to ATP. Rather, it is intended to complement ATP and provide an alternative to potentially damaging, high-energy biphasic shocks when ATP fails.
Our study agrees with in vitro studies in post-MI rabbit hearts showing the phase-dependence of single monophasic and biphasic shocks (19,20). We found a 20-fold energy difference between single optimally- and poorly-timed shocks, similar to those reported in in vitro studies. Notably, in both the current in vivo canine study and prior in vitro rabbit studies, and the optimal phase for single shock application could not be predicted a priori.
Lowering the DFT by repetitive shocks was first shown by Gurvich in 1945 (21). More recently, repetitive sub-threshold (below the ventricular capture threshold) pacing was shown to terminate VT in Guinea pig hearts when applied to the endocardium (22). Previous studies in in vitro rabbit heart preparations showed that multiple shocks extinguished the reentrant circuit by maintaining an area of myocardium refractory to activation, into which the reentrant wave front collides (19). It is likely that the mechanism of defibrillation in the canine MI model of VT is similar.
The current standard ICD electrotherapy for ATP-refractory monomorphic VT is a high-energy biphasic shock. A low-energy electrotherapy would be desirable to replace the shock if it were effective. The mean DFT of the MSE in this report was nearly 80-fold lower than that of a biphasic shock, in energy. Moreover, at low voltage (20 V), MSE achieved significantly more terminations than a biphasic shock, making it a suitable low-energy electrotherapy to reduce the need for full output ICD shocks.
Nearly all ICD shocks are delivered from the RV coil to an active can/SVC coil configuration, delivering current not only through the heart itself, but also through the chest wall musculature and sensory nerves, thereby dissipating energy over structures outside of the heart and causing pain. Implanting commercially available defibrillation leads in the CS (FDA approved for defibrillation from the CS and Azygous vein) and RV enables application of a shock vector that largely confines energy to the heart itself, and was shown to be effective for delivering MSE. Notably, this configuration would still allow ICD shocks to be applied via the traditional RV to SVC/active can vector should MSE fail.
Though MSE may require design changes prior to incorporation into ICDs, such therapies require significantly lower voltage for defibrillation, which requires much less time for charging the capacitor than a full output ICD shock. Reducing the number of full output ICD shocks would likely prolong battery life and may reduce the malfunction rate of high-voltage components. Effective low-energy electrotherapy may be significantly less painful to patients, and importantly, reduce the likelihood of patients receiving a high-energy shock. Also, if high-energy shocks are themselves partially responsible for the increased mortality observed in patients who receive them, effective lower-energy electrotherapy may conceivably reduce mortality.
Conclusions
In the present study, we found that the DFT of a single MP shock to terminate VT in canine hearts with healing myocardial infarction is phase-dependent. Multiple shocks delivered within a single VT CL achieved lower DFTs than a single randomly timed shock without requiring a priori knowledge of phase-dependence. MSE, which incorporated multiple MP shocks within the first stage, further reduced the DFT.
MSE is a low-voltage, low-energy electrotherapy that terminates VT with lower DFT than a single biphasic shock, yet it is significantly more effective than ATP in this model. If successful in humans, MSE may reduce the need for high-energy shocks.
Acknowledgments
Source of Funding:
This study was supported by NIH grants R01 HL-067322, R01 HL-082729 and T32 HL-007081.
The authors thank Timo Weimar, Yoshiyuki Watanabe, Toshinobu Kazui, Sarah Gutbrod, Diane Toeniskoetter and Naomi Still for technical assistance.
ABBREVIATIONS
- RV
right ventricle
- LV
left ventricle
- CS
coronary sinus
- VT
ventricular tachycardia
- VF
ventricular fibrillation
- ICD
internal cardioverter-defibrillator
- DFT
defibrillation threshold
- MSE
multi-stage electrotherapy
- CAD
coronary artery disease
- MI
myocardial infarction
Footnotes
Disclosures
Igor R. Efimov is a co-founder, shareholder, member of the board of directors, and chairman of the scientific advisory board of Cardialen, Inc. Wenwen Li is an employee of Cardialen, Inc. Richard Schuessler had a research grant from Cardialen. The other authors have no relationship with industry.
References
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson WG. Ventricular scars and ventricular tachycardia. Trans Am Clin Climatol Assoc. 2009;120:403–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Schron EB, Exner DV, Yao Q, et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–94. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 6.Tereshchenko LG, Faddis MN, Fetics BJ, Zelik KE, Efimov IR, Berger RD. Transient local injury current in right ventricular electrogram after implantable cardioverter-defibrillator shock predicts heart failure progression. Journal of the American College of Cardiology. 2009;54:822–8. doi: 10.1016/j.jacc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 8.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. The New England journal of medicine. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. Journal of the American College of Cardiology. 2008;51:1357–65. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 10.El-Sherif N, Scherlag BJ, Lazzara R, Hope RR. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 4. Mechanism of action of lidocaine. Circulation. 1977;56:395–402. doi: 10.1161/01.cir.56.3.395. [DOI] [PubMed] [Google Scholar]
- 11.Yee R, Klein GJ, Guiraudon GM, Jones DL, Sharma AD, Norris C. Initial clinical experience with the pacemaker-cardioverter-defibrillator. Can J Cardiol. 1990;6:147–56. [PubMed] [Google Scholar]
- 12.Luceri RM, Habal SM, David IB, Puchferran RL, Muratore C, Rabinovich R. Changing trends in therapy delivery with a third generation noncommitted implantable defibrillator: results of a large single center clinical trial. Pacing and clinical electrophysiology : PACE. 1993;16:159–64. doi: 10.1111/j.1540-8159.1993.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 13.Trappe HJ, Klein H, Fieguth HG, Kielblock B, Wenzlaff P, Lichtlen PR. Clinical efficacy and safety of the new cardioverter defibrillator systems. Pacing and clinical electrophysiology : PACE. 1993;16:153–8. doi: 10.1111/j.1540-8159.1993.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 14.Wathen MS, DeGroot PJ, Sweeney MO, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–6. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 15.Peinado R, Almendral J, Rius T, et al. Randomized, prospective comparison of four burst pacing algorithms for spontaneous ventricular tachycardia. The American journal of cardiology. 1998;82:1422–5. A8–9. doi: 10.1016/s0002-9149(98)00654-7. [DOI] [PubMed] [Google Scholar]
- 16.Schaumann A, von zur Muhlen F, Herse B, Gonska BD, Kreuzer H. Empirical versus tested antitachycardia pacing in implantable cardioverter defibrillators: a prospective study including 200 patients. Circulation. 1998;97:66–74. doi: 10.1161/01.cir.97.1.66. [DOI] [PubMed] [Google Scholar]
- 17.Wathen MS, Sweeney MO, DeGroot PJ, et al. Shock reduction using antitachycardia pacing for spontaneous rapid ventricular tachycardia in patients with coronary artery disease. Circulation. 2001;104:796–801. doi: 10.1161/hc3101.093906. [DOI] [PubMed] [Google Scholar]
- 18.Santini M, Lunati M, Defaye P, et al. Prospective multicenter randomized trial of fast ventricular tachycardia termination by prolonged versus conventional anti-tachyarrhythmia burst pacing in implantable cardioverter-defibrillator patients-Atp DeliVery for pAiNless ICD thErapy (ADVANCE-D) Trial results. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2010;27:127–35. doi: 10.1007/s10840-009-9454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Ripplinger CM, Lou Q, Efimov IR. Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm. 2009;6:1020–7. doi: 10.1016/j.hrthm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ripplinger CM, Lou Q, Li W, Hadley J, Efimov IR. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: Implications for low-voltage cardioversion. Heart Rhythm. 2008 doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurvich NL. Control of heart fibrillation by means of condensatory discharges of subthreshold power. Biull Eksp Biol Med. 1945;20:55–8. [PubMed] [Google Scholar]
- 22.Salama G, Kanai A, Efimov IR. Subthreshold stimulation of Purkinje fibers interrupts ventricular tachycardia in intact hearts. Experimental study with voltage-sensitive dyes and imaging techniques. Circ Res. 1994;74:604–19. doi: 10.1161/01.res.74.4.604. [DOI] [PubMed] [Google Scholar]