Abstract
Objectives
We examined interrelationships between chemokine C-C motif ligand 2 (CCL2) genotype and expression of inflammatory markers in the cerebrospinal fluid (CSF), plasma viral load, CD4+ cell count and neurocognitive functioning among HIV-infected adults. We hypothesized that HIV-positive carriers of the ‘risk’ CCL2 −2578G allele, caused by a single nucleotide polymorphism (SNP) at rs1024611, would have a higher concentration of CCL2 in CSF, and that CSF CCL2 would be associated with both higher concentrations of other proinflammatory markers in CSF and worse neurocognitive functioning.
Design
A cross-sectional study of 145 HIV-infected individuals enrolled in the National NeuroAIDS Tissue Consortium cohort for whom genotyping, CSF and neurocognitive data were available.
Methods
Genomic DNA was extracted from peripheral blood mononuclear cells and/or frozen tissue specimens. CSF levels of CCL2, interleukin (IL)-2, IL-6, tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), soluble tumor necrosis factor receptor 2, sIL-6Rα, sIL-2, sCD14 and B-cell activating factor were quantified. Neurocognitive functioning was measured using a comprehensive battery of neuropsychological tests.
Results
Carriers of the CCL2 −2578G allele had a significantly higher concentration of CCL2 in CSF. CSF CCL2 level was positively and significantly associated with other CSF neuroinflammatory markers and worse cognitive functioning. There was a significant association between genotype and plasma viral load, such that carriers of the CCL2 −2578G allele with high viral load expressed greater levels of CCL2 and had higher neurocognitive deficit scores than other genotype/viral load groups.
Conclusion
Individuals with the CCL2 −2578G allele had higher levels of CCL2 in CSF, which was associated with increased pro-inflammatory markers in CSF and worse neurocognitive functioning. The results highlight the potential role of intermediate phenotypes in studies of genotype and cognition.
Video abstract
Keywords: CCL2, HIV/AIDS, HIV-associated neurocognitive disorder, MCP-1, plasma viral load, rs1024611
Introduction
Despite the success of antiretroviral therapy, approximately 20–50% of HIV-infected individuals have HIV-associated neurocognitive disorder (HAND) [1,2]. HIV infection and stimulation of monocytes and lymphocytes promotes trafficking into the central nervous system (CNS) [3], triggering a neuroinflammatory response. Within the CNS, inflammation leads to activation of microglia, the resident immune cells in the brain, which induces chemokines and cytokines that drive a chemotactic gradient along the blood -brain barrier (BBB) and allows further infiltration of infected and uninfected peripheral immune cells. Chemokines and cytokines function as immunomodulatory proteins that influence HIV neuropathogenesis with both positive and negative effects that may contribute to the ongoing prevalence of HAND [4].
The chemokine C-C motif ligand 2 (CCL2), alternatively known as monocyte chemoattractant protein-1 (MCP-1), is a β-chemokine that is expressed during inflammation and that, upon activation of its receptor (CCR2), can induce chemotaxis of monocytes to inflammatory sites generated by injury and infectious events [3]. CCL2 is expressed by monocytes, macrophages, dendritic cells, neurons, astrocytes, microglia and endothelial cells, while CCR2 is expressed by monocytes, microglia, astrocytes, epithelial cells, activated T cells and dendritic cells [3,5]. CCL2 has been identified as the most potent activator of macrophages in comparison to other monocyte chemoattractants, including RANTES, macrophage inflammatory protein-1α (MIP-1α), MIP-1 β, MCP-2 β and MCP-3 [6,7]. CCL2 levels in the brain and cerebrospinal fluid (CSF) are elevated in HIV patients with encephalitis [8], AIDS patients with cytomegalovirus [9], AIDS dementia and HIV-positive patients with cerebral inflammation [10–16]. Recently, peripheral blood monocyte expression of CCR2 has been shown to predict HAND in combination ART (cART)-era HIV cohorts [17]. Elevated levels of CCL2 expression have also been observed in non HIV-positive samples. In patients with mild cognitive impairment (MCI) and Alzheimer’s disease, higher levels of CSF CCL2 correlated with lower cognitive scores [18].
The presence of elevated CCL2 in HIV-positive patients with neuroinflammation, predictive power of monocyte CCR2 for HAND and elevations of CCL2 in non HIV-positive patients with neurocognitive deficits suggests that CCL2 may have a critical role in the neuropathogenesis of HAND and other noninfectious dementing disorders. A number of studies have examined genetic variation in the CCL2 gene and identified single nucleotide polymorphisms (SNPs) to be associated with HIV-disease progression and neurocognitive functioning over time [12,19]. Individuals with an A to G polymorphism in the CCL2 enhancer region, annotated as rs1024611 (dbSNP database, originally designated as −2518G or −2578G), have higher CCL2 levels in serum, plasma and CSF [16,19,20] than individuals without the A to G polymorphism. Increased CCL2 expression from the −2578G allele has also been investigated in pathologic conditions and was found to be associated with higher incidences of tuberculosis, breast cancer and atherosclerosis, suggesting that the SNP is involved in chronic inflammatory conditions [6,21,22]. Among HIV-infected individuals, homozygosity for the −2578G allele was associated with accelerated disease progression, enhanced leukocyte recruitment to tissues and a 4.5-fold risk for HIV-associated dementia (HAD) [12]. In a study examining the −2578G allele in a cognitively impaired population, elderly patients with senile dementia due to Alzheimer’s disease, CCL2 serum levels were significantly higher in patients who carried at least one G allele, whereas the highest levels of CCL2 were present in patients carrying two G alleles [20]. The −2578G allele has also been reported to be associated with diminished performance in working memory over time in HIV-infected individuals. The HIV-positive group who did not carry the −2578G allele improved at a faster rate in working memory than the HIV-positive group who carried the −2578G allele, but not faster than the HIV-negative groups [19]. However, direct associations between the CCL2 rs1024611 SNP and HIV-disease progression have not been consistent across studies (e.g., [23] and also reviewed in [19]), suggesting that there may be intermediate mechanisms that mediate the association between CCL2 genotype, host immune responses and neurocognitive outcomes.
Although CCL2 expression in both plasma and serum has been linked to neurocognitive impairment, the purpose of the current study was to elucidate interrelationships between CCL2 genotype at the rs1024611 SNP, CCL2 levels in CSF, expression of other neuroinflammatory markers in the CSF. In addition, we considered plasma viral load, CD4+ cell count and neurocognitive performance in our analysis of HIV-infected individuals. We hypothesized that HIV-positive carriers of the CCL2 −2578G allele would exhibit high levels of CCL2 expression in CSF, and that elevated levels of CCL2 would be associated with higher concentrations of other proinflammatory markers in CSF, higher neurocognitive deficit scores, higher HIV viral load and a lower CD4+ T-cell count in blood plasma. We also hypothesized that accounting for CSF levels of CCL2 would explicate the relationship between CCL2 genotype and cognition.
Materials and methods
The cohort that was examined consisted of 145 HIV-infected individuals enrolled in the National NeuroAIDS Tissue Consortium (NNTC) cohort for whom CCL2 genotyping and CSF samples were available. The NNTC is a multicentre consortium engaged in a longitudinal study of adults with HIV/AIDS. The four participating clinical centres in the United States were The National Neurological AIDS Bank located in Los Angeles, California, USA; the Texas NeuroAIDS Research Center located in Galveston, Texas, USA; the Manhattan HIV Brain Bank located in New York, New York, USA; and the California NeuroAIDS Tissue Network located in San Diego, California, USA. Participants are administered a comprehensive battery of psychometric measurements that include tests of neuropsychological function and self-report instruments that estimate past and current substance and psychiatric illness. Neurological examinations, lumbar puncture for CSF collection and laboratory tests were conducted for plasma HIV viral load and plasma CD4+ lymphocyte count. The following were the inclusion criteria in the current study: at least 18 years of age, fluent in the English language, at least sixth grade education, able to provide informed consent. All participants had cognitive symptoms of sufficient severity to warrant a HAND diagnosis. Exclusion criteria were as follows: no history of CNS opportunistic infections (including cryptococcal meningitis, toxoplasmosis and progressive multifocal leukoencephalopathy), no history of traumatic brain injury, no history of learning disability or other developmental disorders and no other major neurologic syndromes (e.g. epilepsy, multiple sclerosis, Parkinson’s disease, brain tumour).
Genotyping
Genotyping was conducted on a subset of NNTC participants as part of a previously reported study [24]. Peripheral blood mononuclear cells (PBMCs) and/or frozen tissue samples were shipped to the University of California Los Angeles (UCLA) Biological Samples Processing Core from the four NNTC sites for DNA extraction. The Autopure LS nucleic acid purification instrument was used to extract DNA. Sample purity was determined via OD 260/280. Extracted DNA was then sent to the UCLA Genotyping Core for genotyping. Prior to genotyping, the samples were checked for concentration by Quant-iT ds DNA Assay kit (Invitrogen, Carlsbad, California, USA) and for quality by agarose gel. DNA amplification by PCR was performed on 96 and 384-well plates on GeneAmp PCR System 9700 thermal cyclers (Applied Biosystems, Foster City, California, USA). Genotypes were determined using the allelic discrimination assay on an Applied Biosystems 7900 Taqman instrument analysed with SDS2.3 software. Data then underwent error-checking and data-cleaning, including control checks, duplicates checks and checking for Hardy–Weinberg equilibrium. Each genotype was evaluated independently according to a number of quality parameters. Data from cases with genotyping success rate of less than 75% were removed. [Note that brain tissue had poorer genotyping success rate than PBMCs (74 vs. 88%, respectively)]. For the CCL2 rs1024611 SNP, haplotype analysis has shown that the rs1024611G polymorphism is associated with allelic expression imbalance of CCL2 and the allele containing −2578G is preferentially transcribed [25].Within this sample, only 5% of participants (n = 8) were homozygous for the −2578G allele (GG); therefore, consistent with previous reports [24,25], we combined GA and GG genotypes for statistical analyses to test the hypothesis that carriers of the −2578G allele (GA and GG) would express higher levels of CCL2 and greater neurocognitive deficit than noncarriers containing the −2578A allele (AA).
Soluble receptor, ligand and chemokine analyses
CSF samples were collected via lumbar puncture. Biomarker assays were performed using multiplexed (Luminex platform) immunometric assays (R & D Systems, Minneapolis, Minnesota, USA) according to manufacturer directions, using a Bio-Plex 200 Luminex instrument and Bio-Plex analysis software (Bio-Rad, Hercules, California, USA). CSF levels of CCL2, IL-2, IL-6,TNF-α, IFN-γ, soluble tumor necrosis factor receptor 2 (sTNFR2), sIL-6Rα, sIL-2, sCD14 and B-cell activating factor (BAFF) were quantified. Of note, due to budgetary constraints, we did not quantify inflammatory markers in plasma; however, studies have shown that CSF inflammatory markers are highly correlated with plasma [26,27].
Neurocognitive functioning
Global neurocognitive functioning was determined with a comprehensive battery of neuropsychological measures that included the Trail Making Test (Parts A & B), Hopkins Verbal Learning Test, Brief Visuospatial Memory Test, Paced Auditory Serial Addition Test, Wisconsin Card Sorting Test-64 Card Version, Grooved Pegboard, Letter-Number Sequencing, Digit Symbol Test, Controlled Oral Word Association Test and Symbol Search (see [24] for detail). Validated standard approaches were used in transforming raw scores into standardized T scores and deficit scores [28]. A Global Deficit Score was calculated on the basis of averaging individual test deficit scores [28,29].
Statistical analyses
Analysis of variance (ANOVA) and Pearson chi-square were used to examine demographic differences between genotype groups. ANOVA was used to test CCL2 genotype differences in CCL2 expression in CSF. Bivariate Pearson correlations were used to examine associations between CCL2, other proinflammatory markers in CSF, neurocognitive functioning and HIV viral load and CD4+ T-cell count in blood plasma. Linear regression was used to examine whether controlling for CSF levels of CCL2 would explain the relationship between CCL2 genotype and cognition.
Results
Demographic comparisons between chemokine C-C motif ligand 2 genotype groups
CCL2 genotype groups for the rs1024611 SNP were separated as follows: carriers of the −2578G allele (n = 70), includes genotypes GA (n = 62) and GG (n = 8) vs. noncarriers containing the −2578A allele, includes genotype AA (n = 75). CCL2 genotype groups were compared on demographic characteristics of age, sex, ethnicity and education. There were no significant group differences in age, F (1, 144) = 0.813, P = .37, education, F (1, 144) = 0.454, P = .501, or sex, χ2 (1, 145) = 2.75, P = 0.253 (Table 1). Although not significant, there was a statistical trend towards significance for ethnicity (i.e. non-Hispanic white vs. African-American), χ2 (1, 145) = 3.57, P = 0.06. Consistent with population-based studies (i.e. HapMAP), non-Hispanic whites were more likely to carry the ‘G’ allele (GG/GA genotypes). There were no significant group differences in current drug abuse, or in current or past drug dependence (all P > 0.05).
Table 1.
Sample characteristics (N = 145).
| CCL2 genotype | |||
|---|---|---|---|
| −2578G Mean/% (SD) | −2578A Mean/% (SD) | CCL2 risk group comparisonsa | |
| Age | 43.47 (8.82) | 45.14 (7.25) | NS |
| Education | 13.29 (2.26) | 13.28 (2.49) | NS |
| Race/Ethnicity (%) | NS | ||
| Black (African-American) | 22% | 38% | |
| Non-Hispanic white | 76% | 61% | |
| Sex (% male) | 86% | 89% | |
| Length of infection | 10.71 (4.92) | 11.43 (5.25) | NS |
| CD4+ cell count | 213.23 (304.32) | 213.21 (191.54) | NS |
| Viral load (log) | 3.62 (1.45) | 3.66 (1.35) | NS |
| Current drug abuse (%) | |||
| Alcohol | 2% | 4.1% | NS χ2 = 3.7, P = 0.28 |
| Cannabis | 7.6% | 1.4% | NS χ2 = 2.6, P = 0.45 |
| Cocaine | 3% | 1.4% | NS χ2 = 2.8, P = 0.41 |
| Opiates | 0.7% | 2.2% | NS χ2 = 2.5, P = 0.28 |
| Stimulants (other) | 0.7% | 1% | NS χ2 = 0.06, P = 0.96 |
| Current drug dependence (%) | |||
| Alcohol | 6.1% | 5.5% | NS χ2 = 2.2, P = 0.68 |
| Cannabis | 3% | 4.1% | NS χ2 = 1.2, P = 0.53 |
| Cocaine | 6.1% | 5.5% | NS χ2 = 3.5, P = 0.31 |
| Opiates | 1.5% | 4% | NS χ2 = 2.5, P = 0.28 |
| Stimulants (other) | 4.5% | 4% | NS χ2 = 1.4, P = 0.49 |
| Positive toxicology screen (%) | |||
| Amphetamine | 3.1% | 3.1% | NS χ2 = 0.08, P = 0.92 |
| Cannabis | 21.9% | 13.9% | NS χ2 = 2.0, P = 0.55 |
| Cocaine | 1% | 1.7% | NS χ2 = 6.2, P = 0.10 |
| Opiates | 4.6% | 5.6% | NS χ2 = 4.7, P = .28 |
| Barbiturates | 1.6% | 1.4% | NS χ2 = 0.92, P = .63 |
| Past drug dependence | |||
| Alcohol | 34.8% | 37% | NS χ2 = 1.1, P = 0.75 |
| Cannabis | 12.1% | 16% | NS χ2 = 1.2, P = 0.73 |
| Cocaine | 22.7% | 32.9% | NS χ2 = 1.4, P = 0.51 |
| Opiates | 7.6% | 6.8% | NS χ2 = 2.8, P = 0.24 |
| Stimulants (other) | 21.2% | 13.7% | NS χ2 = 1.4, P = 0.49 |
CCL2, chemokine C-C motif ligand 2.
Statistical tests shown if not presented in Results section; NS, nonsignificant based upon P > 0.05.
Clinical and cognitive comparisons between genotype groups
There were no significant differences between CCL2 genotype groups on HIV-disease related characteristics such as total CD4+ cell count, F (1, 144) = 0.21, P = 0.65, plasma viral load, F (1144) = 1.68, P = 0.20, length of HIV infection, F (1144) = 0.01, P = 0.91 or global deficit score, F (1, 144) = 1.64, P = 0.20 (Table 1).
Chemokine C-C motif ligand 2 genotype, cerebrospinal fluid expression of inflammatory markers and HIV-disease severity
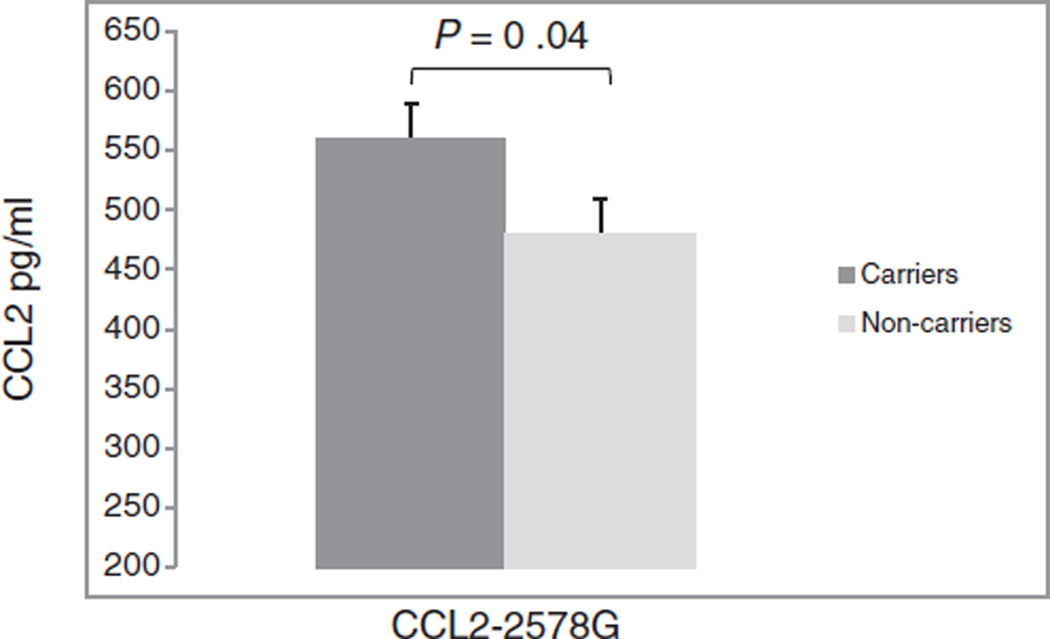
As expected, individuals who carried the CCL2 −2578G allele (GG/GA genotypes) had higher levels of CCL2 expression in CSF, F (1, 144) = 4.16, P = 0.04 (Fig. 1). No other proinflammatory markers were statistically significant between CCL2 genotype groups (all P > .05). Higher levels of CCL2 were associated with higher plasma viral load, r (145) = 0.33, P < 0.0001, lower CD4+ cell count, r (145) = −0.26, P = 0.002, and increased proinflammatory markers in CSF, including sCD14, r (145) = 0.305, P < 0.0001, sIL-6Rα, r (145) = 0.224, P = 0.007, sIL-2, r (145) = 0.245, P = 0.002, IL-6, r (145) = 0.443, P < 0.0001, BAFF, r (145) = 0.57, P < 0.0001, and sTNFR2, r (145) = 0.240, P < 0.003 (Table 2). Higher levels of CCL2 were significantly associated with global cognitive deficit score, r (145) = 0.20, P = 0.01. Higher levels of BAFF were also positively correlated with global cognitive deficit score, r (145) = 0.30, P < 0.001. Similarly, higher levels of sTNFR2 were significantly correlated with higher global deficit scores, r (145) = 0.24, P = 0.003. There were no significant relationships between plasma CD4+ cell count and viral load on cognitive deficit score (P > 0.05) (Table 2).
Fig. 1. Cerebrospinal fluid chemokine C-C motif ligand 2 levels among carriers and noncarriers of the CCL2 −2578G.
CSF samples were analysed for CCL2 expression via multiplex assay. Carriers of the −2578G allele include genotypes GG and GA, while noncarriers containing the −2578A allele include genotype AA. The mean expression level with SEM error bars is shown. P value was calculated by ANOVA.
Table 2.
Bivariate Pearson correlations between cerebrospinal fluid neuroinflammatory markers, plasma CD4+ cell count, plasma viral load and global cognitive deficit score.
| CCL2 | Plasma VL | CD4+ | sCD14 | sIL-6Rα | sIL-2 | IL-6 | IL-2 | TNF- α | IFN-γ | BAFF | sTNFR2 | GDS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCL2 | 0.36** | −0.26* | 0.31** | 0.22* | 0.25* | 0.44** | 0.09 | 0.10 | 0.03 | 0.57** | 0.24* | 0.20* | |
| Plasma VL | −0.37** | −0.06 | 0.05 | 0.15 | 0.12 | −0.12 | −0.14 | −0.03 | 0.27* | 0.07 | 0.03 | ||
| CD4+ | 0.20* | 0.12 | −0.03 | −0.15 | 0.01 | 0.09 | −0.07 | −0.13 | 0.04 | 0.004 | |||
| sCD14 | 0.67** | 0.52** | 0.23* | 0.28* | 0.31* | −0.19* | 0.56** | 0.77** | −0.008 | ||||
| sIL-6Ra | 0.44** | 0.23* | 0.28* | 0.26* | −0.06 | 0.52** | 0.70** | −0.02 | |||||
| sIL-2 | 0.26* | 0.80** | 0.87** | −0.03 | 0.64** | 0.79** | 0.12 | ||||||
| IL-6 | 0.12 | 0.13 | 0.02 | 0.45** | 0.36** | 0.04 | |||||||
| IL-2 | 0.85** | 0.21* | 0.51** | 0.65** | 0.16 | ||||||||
| TNF-α | 0.17 | 0.51** | 0.68** | 0.18 | |||||||||
| IFN-γ | −0.11 | −0.06 | −0.04 | ||||||||||
| BAFF | 0.83** | 0.26* | |||||||||||
| sTNFR2 | 0.22* | ||||||||||||
| GDS |
CCL2, chemokine C-C motif ligand 2; GDS, global deficit score; IFN, interferon; IL, interleukin; TNF, tumour necrosis factor; VL, viral load.
P < 0.01.
P < 0.001.
CCL2 genotype and CCL2 expression on cognitive deficit score
Given the multicollinearity between CCL2 genotype and CCL2 CSF levels, hierarchical linear regressions were conducted to examine the independent effects of CCL2 genotype and CSF CCL2 expression on cognition. As expected, when CCL2 genotype alone was entered into the first block, the model was not statistically significant, F (1, 144) = 1.43, P = 0.23, with an R2 value of 0.006. However, after controlling for CCL2 expression in the second model, CCL2 genotype significantly predicted cognitive deficit score, R2 change = 0.03, F (1, 144) = 3.67, P = 0.05, with an R2 value of 0.08.
Chemokine C-C motif ligand 2 genotype, cerebrospinal fluid chemokine C-C motif ligand 2 and plasma viral load on cognitive impairment
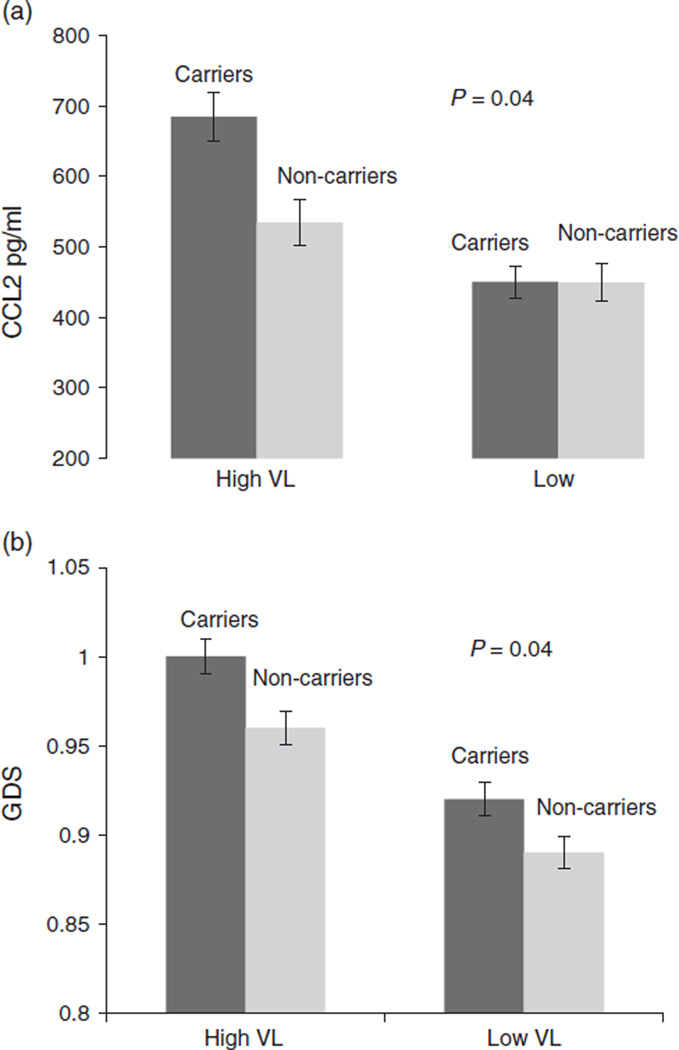
To determine whether the presence of high viral load modulated the effects of the CCL2 genotype, we examined the interactive effects of CCL2 genotype groups, carriers of the −2578G allele (GG/GA) vs. noncarriers containing the −2578A allele (AA), and plasma viral load (low ≤5000 copies/ml and high >5000 copies/ml) on expression of CSF CCL2 and neurocognitive functioning. As expected based upon the previously stated correlational results, there were statistically significant main effects for CCL2 genotype, F (1, 137) = 4.47, P = 0.03 and viral load, F (1, 137) = 19.96, P < 0.0001 on CCL2 expression. There was a statistically significant interaction between genotype and viral load F (3, 135) = 4.37, P = 0.04 (Fig. 2a), such that individuals who carried the −2578G allele (GG/GA) and had high viral load had the greatest expression of CCL2 (M = 684.96; SE = 38.91), followed by noncarriers (AA) with high viral load (M = 534.55; SE = 33.55), carriers (GG/GA) with low viral load (M = 450.43; SE = 34.49) and noncarriers (AA) with low viral load (449.50; SE = 35.93). In addition, there was a statistically significant interaction between CCL2 genotype and plasma viral load on global deficit score, F (3, 135) = 4.124, P = 0.04 (Fig. 2b). Specifically, those who carried the −2578G allele (GG/GA) and had high viral load demonstrated greater impairment (M = 1.00; SE = 0.13), followed by noncarriers (AA) with high viral load (M = 0.96; SE = .12), carriers (GG/GA) with low viral load (M = 0.92; SE = 0.10) and noncarriers (AA) with low viral load (M = 0.89; SE = 0.11).
Fig. 2. CCL2−2578G genotype and plasma viral load on chemokine C-C motif ligand 2 expression and global deficit score.
Carriers of the −2578G allele include genotypes GG and GA, while noncarriers containing the −2578A allele include genotype AA. Plasma viral load was measured and groups were separated as high (≥5000 copies/ml) and low (<5000 copies/ml). (a) CSF samples were analysed for CCL2 expression via multiplex assay. The mean expression level with SEM error bars for each group is shown. (b) Global deficit score (GDS) was calculated on the basis of averaging individual test deficit scores. The mean GDS with SEM error bars for each group is shown.
Discussion
In line with previous reports, the results of this study showed that carriers of the −2578G allele had significantly higher levels of CCL2 in CSF. In addition, higher CCL2 expression was correlated with neurocognitive deficit score, higher levels of other proinflammatory markers in CSF, higher plasma viral load and lower CD4+ lymphocyte counts. We did not observe a significant interaction between CCL2 genotype at rs1024611 and neurocognitive deficit score, suggesting that carrying the −2578G allele alone does not appear to effect cognition. Instead, the findings suggest that increased expression of CCL2, modulated by CCL2 genotype, influences neurocognitive test performance. As expected, there was a strong correlation between CCL2 genotype and CCL2 expression, which led to the investigation of whether CSF CCL2 expression was acting as a moderating variable to the effects of CCL2 genotype on cognition. This suggests that the −2578G genotype results in a more reactive immune response, and increased viral load results in higher concentrations of CCL2 than normal, resulting in neurocognitive dysfunction.
After controlling for CCL2 expression, the association between genotype and cognition emerged, indicating that CCL2 genotype has an effect on cognition, which may be moderated by CCL2 expression. Using plasma viral load to further probe the relationship between CCL2 genotype on cognition in the context of HIV infection, we found that in the presence of high viral load, the CCL2 −2578G allele was associated with greater CCL2 expression and neurocognitive deficit. Although plasma viral load was used as a surrogate for CSF viral load (cohort samples unavailable for this study), these results suggest that as HIV infection persists, carrying the −2578G allele will lead to worse cognitive outcomes (presumably due to the overexpression of CCL2).
The results also suggest that carrying the CCL2 −2578G allele and thereby expressing higher levels of CCL2 may contribute to or support a pro-inflammatory state in the CNS. We found that CSF CCL2 was associated with increased sCD14, sIL-6Rα, IL-2, IL-6, BAFF and sTNFR2. However, we are unable to determine whether increased CCL2 expression is a consequence of an already established pro-inflammatory state or if induction of CCL2 drives the pro-inflammatory immune response. Interestingly, in addition to CCL2, we found that BAFF and sTNFR2 correlated with cognitive performance; however, CCL2 was the only marker that was associated with CCL2 genotype. These results suggest that BAFF and sTNFR2 may also play an important role in neuroinflammation and cognitive impairment among HIV-infected individuals.
B-cell activating factor (BAFF; BLyS; TNFSF13B), a cytokine that is a member of the TNF superfamily, plays a critical role in mediating B-cell differentiation, activation and survival to generate efficient B-cell responses [30]. Within the CNS, BAFF is expressed by microglia and astrocytes, with recombinant BAFF inducing secretion of inflammatory markers, IL-6 and TNF-α, and IL-10, highlighting BAFF’s contribution to the inflammatory response [31]. Consistent with our results, elevated levels of BAFF in CSF from patients with inflammatory neurological diseases, including HIV, have been reported to be significantly higher than in patients with noninflammatory neurological diseases [32]. These findings highlight the importance of controlled BAFF expression for an efficient B-cell response, which is a contributing factor in HIV disease progression [33]. Increased CSF BAFF may be indicative of neuroinflammation and may be important in the persistence of HIV within the CNS.
TNFR2 (p75; TNFRSF1B) is a receptor for TNF-α, a key regulatory cytokine in the inflammatory response, and upon binding, induces a signalling cascade to promote cell survival. TNFR2 is expressed by lymphocytes (CD4+ and CD8+ T cells), microglia, oligodendrocytes, astrocytes, endothelial cells, myocytes, thymocytes and mesenchymal stem cells [34–37]. Soluble TNFR2 (sTNFR2) can be generated via shedding from the cell surface and may act as a scavenger in a protective capacity by sequestering TNF-α to reduce TNF-mediated inflammation [38,39]. TNFR2 signalling in neurologic disorders and cognitive impairment has been examined and increased levels of sTNFR2 have been reported in CSF and plasma from patients diagnosed with bipolar disorder, mild cognitive impairment and AD compared with healthy controls [40–43]. Increased staining for TNFRs has also been demonstrated in brains of individuals with HIV encephalitis and other opportunistic infections [44], and one report has described an association of plasma sTNFR2 with HIV-associated cognitive abnormalities [45]. These results suggest that increased sTNFR2 expression in CSF may serve as a marker of the neuroinflammatory response to HIV and may play an important role in HIV neuropathogenesis and neurocognitive impairment.
Our results indicate that individuals carrying the CCL2 −2578G allele expressed higher levels of CCL2 in CSF and correlational analyses demonstrated that increased CCL2 was associated with increased pro-inflammatory markers, including sCD14, sIL-6Rα, IL-2, IL-6, BAFF and sTNFR2, in addition to greater cognitive deficit. These results are in line with previous studies reporting that increased levels of CCL2 are associated with a faster rate of cognitive decline [46]. Furthermore, the CCL2-CCR2 axis was recently reported to be a critical signalling pathway in HAND, with CCR2 on CD14+CD16+ monocytes serving as a peripheral biomarker for HAND [17].
Overproduction of cytokines in the CNS may allow for HIV-infected cells to persist in the brain despite antiretroviral therapy treatment [47]. As stated previously, CCL2 expression was also correlated with plasma viral load. This is of particular clinical importance because the failure to adequately suppress viral replication may result in repeated BBB insults (via overexpression of proinflammatory markers) that further contribute to peripheral immune cell migration into the CNS. This is an area that requires further investigation and has the potential to inform therapeutic interventions.
Owing to the cross-sectional nature of the current study, we cannot determine whether the expression of CCL2 is a precursor, consequence or simply correlative to cognitive status. It is possible that elevations in CCL2 expression in CSF may signal other inflammatory processes or genetic influences that were not evaluated in the current study. For instance, HIV-1 Tat protein is produced in infected astrocytes and may be secreted and taken up by neighbouring cells, and has been identified as a potential factor in the pathophysiology of HAND [48]. Expression of the CCL2 gene has been shown to be directly transactivated by HIV-1 Tat protein in human astrocytes. Although we cannot determine from this study whether the CCL2 gene is solely driving the CCL2 gradient or whether the presence of infected cells or HIV-1 Tat are also contributing to CCL2 expression, the results suggest a link between CCL2 genotype and cognition that warrants further study. Overall, these results underscore the importance of examining intermediate phenotypes as modulating factors that may link host genotype to cognitive outcomes in HIV.
Acknowledgements
This work was supported by the National Institute of Mental Health (NIMH) grant R25 MH080661 (subaward PI: A.T.). A.T. is also currently supported by a career development award from the National Institute of Mental Health (NIMH K23 MH096551).
California HIV/AIDS Research Program grant ID06-LA-187 (Levine); Texas NeuroAIDS Research Center: U24MH100930; California NeuroAIDS Tissue Network: U24MH100928; National Neurological AIDS Bank: U24MH100929; Manhattan HIV Brain Bank: U24MH100931; Data Coordinating Center: U24MH 100925.
Footnotes
A.T., M.B., C.H. and A.J. designed the current study. A.T. and M.B. drafted the manuscript. A.T. performed statistical analysis and prepared figures. L.I.M. and O.M.M. performed the multiplex assays for CSF samples. B.B.G., S.M., E.J.S and D.J.M. supervised and managed clinical cohorts that were used in this study. All authors read, participated in editing the manuscript, providing scientific input on the study design and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–697. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND) AIDS Res Ther. 2014;11:13. doi: 10.1186/1742-6405-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conductier G, Biondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Hussain R, Ansari A, Talat N, Hasan Z, Dawood G. CCL2/MCP-I genotype-phenotype relationship in latent tuberculosis infection. PLoS One. 2011;6:e25803. doi: 10.1371/journal.pone.0025803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uguccioni M, D’Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1 alpha and MIP-1 beta on human monocytes. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 8.Cinque P, Vago L, Ceresa D, Mainini F, Terreni MR, Vagani A, et al. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Bernasconi S, Cinque P, Peri G, Sozzani S, Crociati A, Torri W, et al. Selective elevation of monocyte chemotactic protein-1 in the cerebrospinal fluid of AIDS patients with cytomegalovirus encephalitis. J Infect Dis. 1996;174:1098–1101. doi: 10.1093/infdis/174.5.1098. [DOI] [PubMed] [Google Scholar]
- 10.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummid S, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- 14.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann MH, Masanetz S, Kramer S, Erfie V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci. 2006;119(Pt 21):4520–4530. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
- 16.Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17:63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm. 2014;1:e36. doi: 10.1212/NXI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 19.Levine AJ, Reynolds S, Cox C, Miller EN, Sinsheimer JS, Becker JT, et al. The longitudinal and interactive effects of HIV status, stimulant use, host genotype upon neurocognitive functioning. J Neurovirol. 2014;20:243–257. doi: 10.1007/s13365-014-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenoglio C, Galimberti D, Lovati C, Guidi I, Gatti A, Fogliarino S, et al. MCP-1 in Alzheimer’s disease patients: A−2518G polymorphism and serum levels. Neurobiol Aging. 2004;25:1169–1173. doi: 10.1016/j.neurobiolaging.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 21.McDermott DH, Yang Q, Kathiresan S, Cupples LA, Massaro JM, Keaney JF, et al. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation. 2005;112:1113–1120. doi: 10.1161/CIRCULATIONAHA.105.543579. [DOI] [PubMed] [Google Scholar]
- 22.Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999;259:344–348. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- 23.Singh KK, Hughes MD, Chen J, Spector SA. Impact of MCP-1-2518-G allele on the HIV-1 disease of children in the United States. AIDS. 2006;20:475–478. doi: 10.1097/01.aids.0000200540.09856.58. [DOI] [PubMed] [Google Scholar]
- 24.Levine AJ, Singer EJ, Sinsheimer JS, Hinkin CH, Papp J, Dandekar S, et al. CCL3 genotype and current depression increase risk of HIV-associated dementia. Neurobehav HIV Med. 2009;1:1–7. doi: 10.2147/nbhiv.s6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham MH, Bonello GB, Castiblanco J, Le T, Sigala J, He W, Mummidi S. The rs1024611 regulatory region polymorphism is associated with CCL2 allelic expression imbalance. PLoS One. 2012;7:e49498. doi: 10.1371/journal.pone.0049498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdium S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The HNRC 500 – neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 29.Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, et al. Medication and finance management among HIV-infected adults: the impact of age and cognition. J Clin Exp Neuropsychol. 2011;33:200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24:203–215. doi: 10.1016/j.cytogfr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KS, Park JY, Jou I, Park SM. Functional implication of BAFF synthesis and release in gangliosides-stimulated microglia. J Leukoc Biol. 2009;86:349–359. doi: 10.1189/jlb.1008659. [DOI] [PubMed] [Google Scholar]
- 32.Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T, et al. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflamm. 2012;9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poudrier J, Chagnon-Choquet J, Roger M. Influence of dendritic cells on B-cell responses during HIV infection. Clin Dev Immunol. 2012;2012:592187. doi: 10.1155/2012/592187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchhave P, Zetterberg H, Blennow K, Minthon L, Janciauskiene S, Hansson O. Soluble TNF receptors are associated with Abeta metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging. 2010;31:1877–1884. doi: 10.1016/j.neurobiolaging.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Ware CF, Crowe PD, Vanarsdale TL, Andrews JL, Grayson MH, Jerzy R, et al. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J Immunol. 1991;147:4229–4238. [PubMed] [Google Scholar]
- 36.Bocker W, Docheva D, Prall WC, Egea V, Pappou E, Rossmann P, et al. IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. J Mol Med (Berl) 2008;86:1183–1192. doi: 10.1007/s00109-008-0378-3. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 38.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 39.Martin EM, Remke A, Pfeifer E, Polz J, Pietryga-Krieger A, Steffens-Weber D, et al. TNFR2 maintains adequate IL-12 production by dendritic cells in inflammatory responses by regulating endogenous TNF levels. Innate Immun. 2014;20:712–720. doi: 10.1177/1753425913506949. [DOI] [PubMed] [Google Scholar]
- 40.Doganavsargil-Baysal O, Cinemre B, Aksoy UM, Akbas H, Metin O, Fettahoglu C, et al. Levels of TNF-α soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Hum Psychopharmacol Clin Exp. 2013;28:160–167. doi: 10.1002/hup.2301. [DOI] [PubMed] [Google Scholar]
- 41.Buchhave P, Zetterberg H, Blennow K, Minthon L, Janciauskiene S, Hansson O. Soluble TNF receptors are associated with metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging. 2010;31:1877–1884. doi: 10.1016/j.neurobiolaging.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Hampel H, Prvulovic D, Wallin A, Blennow K, Li R, Shen Y. Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer’s disease. Mol Neurodegener. 2011;6:69. doi: 10.1186/1750-1326-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Hampel H, Prvulovic D, Wallin A, Blennow K, Li R, Shen Y. Combination of plasma tumor necrosis factor receptors signaling proteins, beta-amyloid and apolipoprotein E for the detection of Alzheimer’s disease. Neurosci Lett. 2013;541:99–104. doi: 10.1016/j.neulet.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Sippy BD, Hofman FM, Wallach D, Hinton DR. Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:511–521. [PubMed] [Google Scholar]
- 45.Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184:699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 46.Westin K, Buchhave P, Nielsen H, Minthon L, Janciauskiene S, Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS One. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 48.Sheng WS, Hu S, Lokensgard JR, Peterson PK. U50, 488 inhibits HIV-1 Tat-induced monocyte chemoattractant protein-1 (CCL2) production by human astrocytes. Biochem Pharmacol. 2003;65:9–14. doi: 10.1016/s0006-2952(02)01480-6. [DOI] [PubMed] [Google Scholar]