Synopsis
The NMJ (neuromuscular junction) serves as the ultimate output of the motor neurons. The NMJ is composed of a presynaptic nerve terminal, a postsynaptic muscle and perisynaptic glial cells. Emerging evidence has also demonstrated an existence of perisynaptic fibroblast-like cells at the NMJ. In this review, we discuss the importance of Schwann cells, the glial component of the NMJ, in the formation and function of the NMJ. During development, Schwann cells are closely associated with presynaptic nerve terminals and are required for the maintenance of the developing NMJ. After the establishment of the NMJ, Schwann cells actively modulate synaptic activity. Schwann cells also play critical roles in regeneration of the NMJ after nerve injury. Thus, Schwann cells are indispensable for formation and function of the NMJ. Further examination of the interplay among Schwann cells, the nerve and the muscle will provide insights into a better understanding of mechanisms underlying neuromuscular synapse formation and function.
Keywords: electron microscopy, mutant mice, neuregulin–erbB signalling, synaptic transmission, synaptogenesis, vertebrate neuromuscular junction
Introduction
Chemical synapses are specialized cell–cell junctions where a neuron conveys information to its target cell by releasing neuro-transmitters. Most synapses are composed of a presynaptic neuron, a postsynaptic target and nearby glial cells. In this review, we focus on the glial component, Schwann cells, and their roles in the formation and function of the NMJ (neuromuscular junction). The NMJ is a synapse between a motoneuron and a muscle cell and is one of the best-studied model synapses. Studies over the last several decades have identified a number of signalling molecules crucial for co-ordinated interactions among synaptic components during neuromuscular synaptogenesis [1–8]. Some of the signalling molecules identified at the NMJ are also involved in synaptogenesis in central synapses [9]. At the NMJ, Schwann cells are involved in the maintenance of the NMJ during development and the re-establishment of the NMJ after nerve regeneration. A large body of evidence has also demonstrated that Schwann cells modulate synaptic activity at the NMJ. Thus Schwann cells actively participate in both formation and function of the NMJ.
Terminal Schwann Cells: The Glial Component of the NMJ
Initially the presence of additional cells besides the nerve and muscle cells at the NMJ had been controversial [10]. Only in the past two decades have glial cells been recognized as an integral component of the NMJ [11]. Synapse-associated non-myelinating Schwann cells are variously called teloglia, perisynaptic Schwann cells or terminal Schwann cells. In this section, we provide a detailed description of ultrastructure of the developing and mature NMJs to illustrate the cellular organization of the NMJ, including the presynaptic nerve terminal, the postsynaptic muscle cell, the Schwann cells accompanying the nerve terminal and the perisynaptic fibroblast-like cells [12–14].
Origin and development of the Schwann cells
During embryonic development, Schwann cells are originated from the neural crest cells. In mice, the generation of Schwann cell precursors takes place at E12–E13 (embryonic day 12– 13), followed by immature Schwann cells at E13–E15, which persist till birth [15]. During postnatal development, immature Schwann cells further differentiate to either myelinating axonal Schwann cells or non-myelinating terminal Schwann. The fate of immature Schwann cells is determined by the axons associated with them: for example, immature Schwann cells associated with single large-diameter axons become myelinated. In contrast, the differentiation of non-myelinating terminal Schwann cells is less understood. The transition from immature Schwann cells to these two mature types is thought to be reversible. On nerve injury, both mature types de-differentiate into immature-like reactive Schwann cells and play an important role in reinnervation [16]. Interestingly, terminal Schwann cells express myelin proteins, such as protein 0 and myelin-associated glycoprotein, indicating their Schwann cell origin [17].
The presence of Schwann cells at nerve–muscle contacts during development
Shortly before the formation of primary myotubes, motor axons arrive at the developing muscle along with Schwann cells. The nerve extends primary intramuscular nerve through the central region of the muscle vertical to the long axis of the myotubes and then further extends secondary and tertiary nerves from the primary nerve. The distribution pattern of the developing Schwann cells can be monitored by using anti-S100 antibodies (Figure 1). During early postnatal development, terminal Schwann cells proliferate and increase the number of their soma per NMJ significantly [18,19]. The Schwann cell soma exists only in half of NMJs at birth, whereas typical adult NMJs have three to four terminal Schwann cell somata in frogs and rodents [18–20]. The number of terminal Schwann cells per NMJ is tightly regulated and related to the end-plate size that they cover [21,22]. Recently NT-3 (neurotrophin 3) produced by muscle has been proposed to determine the number of terminal Schwann cells [23].
Figure 1. Distribution of Schwann cells along the nerve during neuromuscular synaptogenesis.
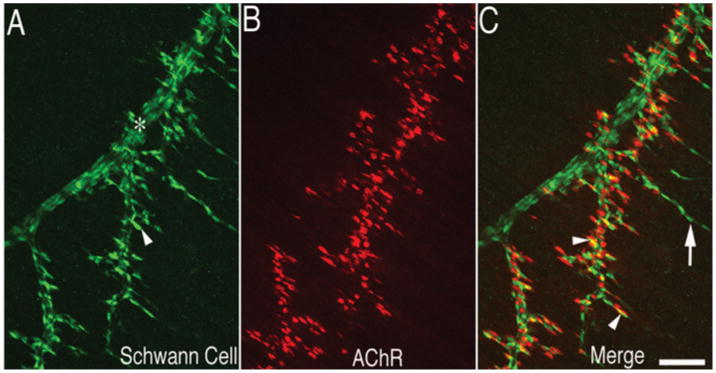
Mouse diaphragm muscle at E18.5 was immunolabelled with anti-S100 antibody (in green) for Schwann cells (A) and Texas Red-conjugated α-bungarotoxin (in red) for postsynaptic AChR (B). Schwann cells (arrowhead in A) delineanate both the nerve trunk (*) and the nerve branches and are present at the NMJ (arrowheads in C) as well as the tip of the nerve branches extended beyond the NMJ (arrow in C). Scale bar, 100 μm.
Since Schwann cells migrate along the nerve, they are present at the earliest nerve–muscle contact. Figure 2(A) illustrates an early stage of synapse formation (E15.5 of mouse diaphragm). A cluster of unmyelinated axons wrapped loosely by immature Schwann cells makes close apposition to the surface of myotubes. Synaptic contacts appear very simple, and there is little sign of presynaptic and postsynaptic specialization, although the basal lamina is clearly seen at the synaptic cleft. Nerve terminals are small and contain only a few synaptic vesicles. Neither junctional folds nor subsynaptic sarcoplasmic area are present; hence, a myofibril can be seen just beneath the nerve–muscle contact. Figure 2(B) shows more advanced embryonic stage (E18.5 of mouse diaphragm). Primitive junctional folds are found in some NMJs as shown here (arrowheads in Figure 2B). Subsynaptic sarcoplasma becomes apparent accumulating many organelles, such as mitochondria and endoplasmic reticulum. Multiple nerve terminals make synaptic contact and even a cluster of synaptic vesicles can be seen at the presynaptic membrane (arrow in Figure 2B). The numbers of synaptic vesicles in the terminals are variable. Basal lamina is clearly present in the synaptic cleft. The processes of immature Schwann cytoplasm tightly wrap the cluster of nerve terminals.
Figure 2. Ultrastructure of developing NMJs in embryonic muscles in mice.
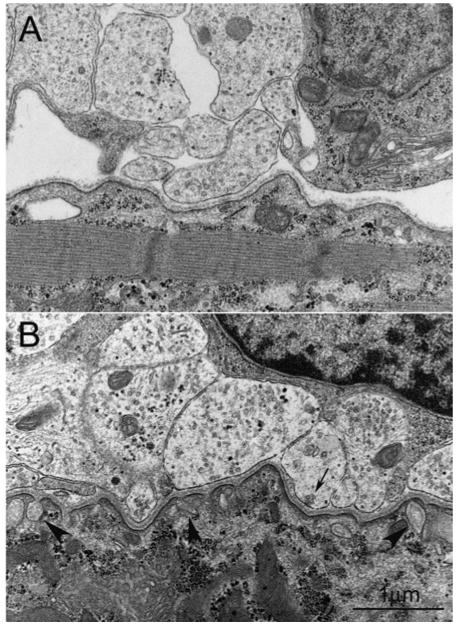
(A) A nerve–muscle contact depicting an early stage of synaptogenesis (E15.5 diaphragm). A cluster of axons loosely wrapped by immature Schwann cell attaches on to the muscle membrane. Although basal lamina is clearly seen at the synaptic cleft, presynaptic and postsynaptic specialization are barely recognizable. Only a few synaptic vesicles are present in the nerve terminal. (B) An immature NMJ (E18.5 diaphragm) illustrating primitive junctional folds (arrowheads) and organelles in the subsynaptic salcoplasm. Nerve terminals contain more synaptic vesicles compared with earlier stage shown in (A). Some synaptic vesicles (arrow) are clustered at the presynaptic membrane, presumably the active zone. Scale bar, 1 μm.
Cellular organization of the NMJ
As NMJs matured, the tripartite arrangement of the NMJ is established. This is illustrated in Figure 3(A), a low-magnification electron micrograph of an adult mouse NMJ. The spatial arrangement has also been revealed by scanning electron microscopy [24]. Multiple nerve terminal profiles, which are half embedding in a gutter of the muscle membrane, appose to the postsynaptic specialization, in which the receptors for neuro-transmitter acetylcholine are localized. Junctional sarcoplasma contains subsynaptic muscle nucleus and other organelles. In this instance, Schwann cell soma is present above the nerve terminals. Outside of the basal lamina above the Schwann cell, thin processes can be seen over the NMJ area (Figure 3A, arrows). These processes are likely the newly identified NMJ-capping cells [12]. Figure 3(B) is a higher magnification of one of the nerve terminals in Figure 3(A). The nerve terminal contains abundant synaptic vesicles, mitochondria and occasionally vesicular structures. Elaborated junctional folds are evident in the postsynaptic specialization. The top portion of the nerve terminal is covered by Schwann cells process directly without basal lamina (arrows in Figure 3B).
Figure 3. Ultrastructure of a mature NMJ in adult muscle in mice.
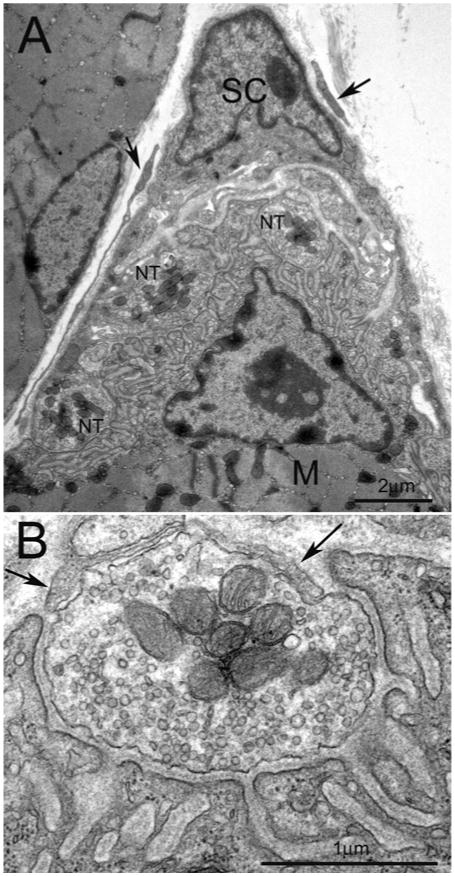
(A) Low magnification of electron micrograph illustrating typical arrangement of the NMJ. The Schwann cell (SC) with its electro-dense nucleus cap the nerve terminals (NT) apposing the postsynaptic specialization of the muscle (M). The processes (arrows) around Schwann cell may be the NMJ-capping cells recently characterized by Court et al. [12]. (B) Higher magnification of one of the nerve terminals shown in (A). The nerve terminal, half buried in the surface of muscle fibre, contains plentiful synaptic vesicles and mitochondria. Postsynaptic muscle membrane displays fully elaborated junctional folds. Basal lamina appears in the synaptic cleft. Opposing the muscle, thin processes of Schwann cell (arrows) cap the nerve terminal. Scale bar, 2 μm in (A) and 1 μm in (B).
A fibroblast-like cell, recently referred to as kranocyte [12], caps the terminal Schwann cells and extends its cytoplasmic processes over the entire end-plate area (arrows in Figure 3A). Every adult NMJ is covered by one or two kranocytes in rodents. Kranocytes are evenly distributed in newborn mouse muscle, but become restricted to NMJ area during postnatal development. Whether kranocytes play any role in synapse formation is not known. However, after denervation, kranocytes quickly proliferate and spread over the NMJ area before Schwann cell sprouting, suggesting they may play a role in NMJ repair after nerve injury. Similar cell accumulation at the NMJ has been observed after denervation in rat and frog, and these cells have been suggested to be of fibroblast origin [13,14]. Notably kranocytes are positively immunostained by anti-NRG (neuregulin) antibody [12]. This raises the possibility that kranocytes may interact with terminal Schwann cells through NRG signalling.
Role of Schwann Cells During the Formation of the NMJ
As discussed in the previous section, Schwann cells are present at the beginning of nerve–muscle contact, suggesting their involvement in neuromuscular synapse formation. Genetic studies of NRG1 and its receptor (erbB2 and erbB3) in mice provide valuable information for the role of Schwann cells in the formation of the NMJ. NRG1–erbB signalling is essential for the survival of the entire Schwann cell lineage [15]; disruption of NRG1–erbB signalling causes severe deficiency of Schwann cells. Both NRG1 and erbB mutant mice lack Schwann cells in the periphery [25–29]. In the absence of Schwann cells, motor axons still project and reach the target muscles, but are markedly defasciculated. This suggests that Schwann cells are dispensable for axon path finding but are essential for nerve fasciculation.
In erbB2, erbB3 and cysteine-rich-domain-containing NRG1 mutant mice, the NMJs are initially established but fail to be maintained [25–29]. Thus, Schwann cells are dispensable for the initial nerve–muscle contacts but are essential for subsequent growth and maintenance of the developing synapses. The maintenance role of terminal Schwann cells has been further supported by observation of the developing NMJ in tadpoles, in which the extension of Schwann cell processes always precedes AChR (acetylcholine receptor) deposition and synaptic growth, thus appearing to guide nerve terminals [20]. When terminal Schwann cells were selectively killed in the muscle of living tadpoles using complement-mediated cell lyses, synaptic growth was markedly reduced and terminal retractions were widespread [30]. Thus Schwann cells promote the growth and maintenance of the developing NMJ [31].
Role of Schwann Cells in the Re-Establishment of the NMJ after Nerve Injury
Emerging evidence indicates that the restoration of the NMJ after motor nerve injury depends greatly on terminal Schwann cells. Terminal Schwann cells profusely extend their processes on muscle denervation [32] and induce and guide the growth of nerve sprouts [33,34]. Both terminal and axonal Schwann cells respond similarly on denervation, as they de-differentiate into immature-like reactive Schwann cells. An important feature of terminal Schwann cell processes is ‘bridge’ formation between denervated and innervated NMJs after partial denervation. Terminal Schwann cells associated with denervated NMJs extend their processes to adjacent innervated NMJs forming the Schwann cell bridge, which induces axonal sprouting from the innervated NMJs along the bridge to the denervated NMJs [35]. In vivo repeated observations have further confirmed the guidance role of terminal Schwann cells for axonal sprouts [36,37].
The importance of terminal Schwann cells in restoration of the NMJ is highlighted in neonatal rodent muscles, which suffer unsuccessful reinnervation after muscle denervation unlike in adult. Thompson and co-workers have shown that the loss of nerve induces apoptosis of terminal Schwann cells and that the absence of Schwann cells results in the lack of axonal sprouts and severely impaired reinnervation and muscle function [38,39]. This is due to the fact that terminal Schwann cells are still dependent on a nerve-derived factor, NRG, for their survival in the early postnatal period. These experiments, exploited somehow unique situation, present strong evidence for indispensable role of terminal Schwann cells for NMJ restoration in neonatal animals. Bridge formation is affected by activity, such as blockade of transmitter release, muscle stimulation or exercise [35,40–42], although the mechanisms of these effects are not well understood.
The mechanism that triggers terminal Schwann cell sprouting is not clear. NRG1–erbB signalling may play a role in inducing terminal Schwann cell sprouting since NRG1 expression in Schwann cells is up-regulated after denervation [43]. Exogenous application of NRG1 to neonatal muscles or induction of con-stitutively active erbB2interminal Schwann cells induces profuse sprouting of terminal Schwann cells resembling that seen after denervation [44,45]. Induction of constitutively active erbB2 in muscle during embryonic development is lethal showing severely impaired synapse formation [46]. These experiments display the ability of NRG1 to alter Schwann cell behaviour. However, recently Schwann cell specific ablation of erbB2 in adult mice has shown no detectable effects on the maintenance of myelin-ated nerves or on the proliferation and survival of Schwann cells after axotomy [47]. Terminal Schwann cells were not examined in that study. In addition, muscle-specific conditional knockout experiments suggest that NRG1 signalling in the muscle is dispensable for NMJ formation [48,49]. It will be of interest to test the reaction of terminal Schwann cells after nerve injury in these animals [50]. Beside NRG1, application of CNTF (ciliary neur-otrophic factor) induces nerve terminal sprouting [51]; however, CNTF-null mice show both terminal Schwann cells and axonal sprouting after nerve injury or muscle inactivity [52]. Thus, the involvement of CNTF is unlikely. Additionally, several molecules are up-regulated in Schwann cells after denervation, including GAP-43 (growth-associated protein-43) [53], GFAP (glial fibril-lary acidic protein) [54], low-affinity NGF (nerve growth factor) receptor p75 [55], nestin [56], cell adhesion molecule CD44 [57] and transcription factor zinc-finger proliferation 1 [58]. Some of them have been used as reactive Schwann cell markers, but whether they are involved in Schwann cell sprouting remains to be examined.
NO (nitric oxide) might be involved in reinnervation of the NMJ, because, in the mice treated with NO synthase inhibitor, Schwann cell failed to extend their processes and nerve terminal sprouting was barely observed after nerve injury [59]. This may be the reason why Schwann cell bridge formation is impaired in mdx mice, a model for Duchenne muscular dystrophy, in which NO synthase is deficient owing to the defects of dystrophin–glycoprotein complex [60]. Notably, NO are involved in Schwann cell modulation of neurotransmitter release [61].
Interestingly, chemorepellent Semaphorin 3A is selectively expressed in terminal Schwann cells at fast-fatigable muscle fibres after nerve injury or muscle inactivity [62]. It is proposed that this muscle-fibre-type-specific expression may contribute to suppressing nerve terminal plasticity and vulnerability of the fast fibres in pathological conditions, such as ALS (amyotrophic lateral sclerosis).
Schwann cells may contribute to postsynaptic differentiation by expressing NRG2, a homologue of NRG1, which appears to promote AChR transcription [63]. In addition, Schwann cells express active agrin after nerve injury in frog [64].
Role of Schwann Cells In Modulating Neuromuscular Synaptic Transmission
Because glial cells are traditionally regarded as non-excitable, their involvement in synaptic activity was difficult to examine before advanced imaging techniques became available. In this section, we summarize the evidence for the involvement of terminal Schwann cells in neuromuscular transmission.
How do Schwann cells detect neuromuscular transmission?
Similarly to astrocytes in the central nervous system [65], Schwann cells at the NMJ respond with a transient elevation in the intracellular Ca2+ level on high-frequency nerve stimulation in frogs [66,67] and in mice [68]. Since this phenomenon is observed without extracellular Ca2+, the major source of Ca2+ transient is thought to be the internal Ca2+ store [66,68], although Schwann cells possess voltage-sensitive Ca2+ channels [69]. Neurotransmitter ACh and other molecules co-released from nerve terminals during transmitter release are responsible for eliciting a Ca2+ response in terminal Schwann cells. Local applications of ACh, ATP, adenosine and substance P, all induce a Ca2+ response in terminal Schwann cells, and various muscarinic and purinergic receptors have been identified using pharmacological tools [70]. In addition, a recent study has shown that NTs, brain-derived neurotrophic factor and NT-3, but not NGF, also induce Ca2+ transient in terminal Schwann cells [71]. These studies demonstrate that terminal Schwann cells sense and respond to synaptic activity and neurotrophic factors.
How do Schwann cells modulate neuromuscular transmission?
What is the outcome of the Ca2+ transient in terminal Schwann cells? Castonguay and Robitaille [72] have carried out series of experiments in this field. The Ca2+ transient in terminal Schwann cells was induced by treatments of thapsigargin, an inhibitor of the Ca2+-ATPase pump or inositol 1,4,5-triphosphate, while the neuromuscular transmission was continuously monitored at frog NMJs [72]. The increased Ca2+ level in terminal Schwann cells enhanced nerve-evoked transmitter release, which was prevented by microinjections of the Ca2+ che-lator BAPTA [1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid] into Schwann cells before thapsigargin application [72]. These experiments show that the Ca2+ transient in terminal Schwann cells is sufficient to modulate neuromuscular transmission.
Besides this Ca2+ transient, what are the signalling pathways responsible for Schwann cell modulation of transmitter release? Because many of the receptors of Schwann cells that convey Ca2+ transient are G-protein-coupled, manipulation of G-protein signalling may affect neuromuscular transmission. Indeed, microinjection of GTP-γS (a non-hydrolysable analogue of GTP) into terminal Schwann cells decreased nerve-evoked transmitter release, whereas microinjection of GDP-βS (a non-hydrolysable analogue of GDP) reduced synaptic depression during high-frequency stimulation at frog NMJs [73]. Thus, stimulating or inhibiting G-protein activity in terminal Schwann cells influences synaptic transmission.
How does Ca2+ transient in Schwann cells affect neurotransmitter release? The evidence shows complicated mechanisms involving the muscle for synaptic depression: a Ca2+ transient in terminal Schwann cells induces glutamate release from the Schwann cells, which activates metabotrophic receptors on the postsynaptic muscle membrane [74]. The activation of the receptors stimulates synthesis of NO in the muscle. The NO diffuses to the nerve terminal and causes the depression of transmitter release [61]. For potentiation of transmitter release, it is proposed that prostaglandins produced by the Schwann cells directly act on the nerve terminal [75].
Although the studies discussed above have shown that terminal Schwann cells are capable of increasing or decreasing transmitter release dependent on which pathway is activated, it is not clear as to what determines which pathway would be activated. Using a novel approach, Reddy et al. [30] have shown that terminal Schwann cell ablation by complement-mediated cell lyses does not affect acute neuromuscular synaptic transmission, but causes chronic nerve retraction and a reduction in neuromuscular synaptic transmission. Because this experiment focused on the outcome in whole muscle level in the absence of terminal Schwann cells, the lack of acute effect may reflect the net effect, since Schwann cells affect transmitter release both negative and positive ways. The complexity of Schwann cell modulation is underlined in the study examining the glial response to altered long-term activity. Chronic blockade of postsynaptic AChR using α-bungarotoxin or chronic nerve stimulation, surprisingly, do not affect terminal Schwann cell Ca2+ responses [76]. However, further analysis has revealed complex changes in sensitivities to muscarinic and purinergic components in the terminal Schwann cells [76]. Therefore the altered long-term activities do cause the changes in how Schwann cells respond to synaptic transmission, but these changes are not revealed by the overall Ca2+ responses in the Schwann cells.
In addition, Schwann cell intrinsic factor appears to affect Schwann cell responses [77]. Schwann cells in fast-twitch muscles always showed stronger Ca2+ response compared with those in slow-twitch muscle regardless of stimulation frequency or level of transmitter release [77]. Thus the characteristic of synaptic activity is not the only determinant but the muscle type also affects Schwann cell modulation. Recently, fast muscle selective expression of Semaphorin 3A in terminal Schwann cells has been reported [62]. How muscle properties affect Schwann cell behaviours remains unknown.
Summary and Perspectives
In the present review, we have summarized the role of Schwann cells in the formation and function of the NMJ. During development, Schwann cells are accompanied by motor axons during their migration to the target muscles, and are present at nascent nerve–muscle contacts. Schwann cells are required for the maintenance of the NMJ. In the absence of the Schwann cells, neuromuscular synapses degenerate shortly after the establishment of initial synaptic contacts. Following the formation of the synapses, Schwann cells modulate synaptic activity at the NMJ. Schwann cells also play critical roles in reinnervation of the muscle after nerve injury; they extend their processes to induce and guide axonal sprouts for re-establishing neuromuscular synapses. Thus Schwann cells play important roles at various stages during the formation and function of the NMJ. However, molecular signalling pathways responsible for the interactions among Schwann cells, the presynaptic nerve terminal and the postsynaptic muscle cell remain to be further elucidated. Uncovering the underlying molecular mechanisms will provide vital insights into a better understanding of the biology of the NMJ as well as synapses in the central nervous system.
Acknowledgments
We thank Professor George Augustine (Laboratory of Synaptic Circuitry, Program in Neuroscience and Behavioral Disorders, Duke-NUS Graduate Medical School, Singapore) and Dr Yun Liu (Department of Neuroscience, University of Texas Southwestern Medical Center, Dallas, TX, U.S.A.) for valuable suggestions and critical comments on the manuscript before submission.
Abbreviations used
- AChR
acetylcholine receptor
- CNTF
ciliary neurotrophic factor
- NGF
nerve growth factor
- E12 etc
embryonic day 12 etc
- NMJ
neuromuscular junction
- NRG
neuregulin
- NT-3
neurotrophin 3
References
- 1.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 2.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 3.Burden SJ. Building the vertebrate neuromuscular synapse. J Neurobiol. 2002;53:501–511. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- 4.Witzemann V. Development of the neuromuscular junction. Cell Tissue Res. 2006;326:263–271. doi: 10.1007/s00441-006-0237-x. [DOI] [PubMed] [Google Scholar]
- 5.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hughes BW, Kusner LL, Kaminski HJ. Molecular architecture of the neuromuscular junction. Muscle Nerve. 2006;33:445–461. doi: 10.1002/mus.20440. [DOI] [PubMed] [Google Scholar]
- 7.Ribchester RR. Mammalian neuromuscular junctions: modern tools to monitor synaptic form and function. Curr Opin Pharmacol. 2009;9:297–305. doi: 10.1016/j.coph.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai KO, Ip NY. Central synapse and neuromuscular junction: same players, different roles. Trends Genet. 2003;19:395–402. doi: 10.1016/S0168-9525(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 10.Ko CP, Sugiura Y, Feng Z. The biology of perisynaptic (terminal) Schwann cells. In: Armati P, editor. The Biology of Schwann Cells. Cambridge University Press; New York: 2007. pp. 72–99. [Google Scholar]
- 11.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 12.Court FA, Gillingwater TH, Melrose S, Sherman DL, Greenshields KN, Morton AJ, Harris JB, Willison HJ, Ribchester RR. Identity, developmental restriction and reactivity of extralaminar cells capping mammalian neuromuscular junctions. J Cell Sci. 2008;121:3901–3911. doi: 10.1242/jcs.031047. [DOI] [PubMed] [Google Scholar]
- 13.Connor E. Developmental regulation of interstitial cell density in bullfrog skeletal muscle. J Neurocytol. 1997;26:23–32. doi: 10.1023/a:1018507324217. [DOI] [PubMed] [Google Scholar]
- 14.Connor EA, McMahan UJ. Cell accumulation in the junctional region of denervated muscle. J Cell Biol. 1987;104:109–120. doi: 10.1083/jcb.104.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 16.Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56:1518–1531. doi: 10.1002/glia.20778. [DOI] [PubMed] [Google Scholar]
- 17.Georgiou J, Charlton MP. Non-myelin-forming perisynaptic schwann cells express protein zero and myelin-associated glycoprotein. Glia. 1999;27:101–109. doi: 10.1002/(sici)1098-1136(199908)27:2<101::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Hirata K, Zhou C, Nakamura K, Kawabuchi M. Postnatal development of Schwann cells at neuromuscular junctions, with special reference to synapse elimination. J Neurocytol. 1997;26:799–809. doi: 10.1023/a:1018570500052. [DOI] [PubMed] [Google Scholar]
- 19.Love FM, Thompson WJ. Schwann cells proliferate at rat neuromuscular junctions during development and regeneration. J Neurosci. 1998;18:9376–9385. doi: 10.1523/JNEUROSCI.18-22-09376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera AA, Qiang H, Ko CP. The role of perisynaptic Schwann cells in development of neuromuscular junctions in the frog (Xenopus laevis) J Neurobiol. 2000;45:237–254. doi: 10.1002/1097-4695(200012)45:4<237::aid-neu5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Lubischer JL, Bebinger DM. Regulation of terminal Schwann cell number at the adult neuromuscular junction. J Neurosci. 1999;19:RC46. doi: 10.1523/JNEUROSCI.19-24-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan CL, Williams TJ. Testosterone regulates terminal Schwann cell number and junctional size during developmental synapse elimination. Dev Neurosci. 2001;23:441–451. doi: 10.1159/000048731. [DOI] [PubMed] [Google Scholar]
- 23.Hess DM, Scott MO, Potluri S, Pitts EV, Cisterni C, Balice-Gordon RJ. Localization of TrkC to Schwann cells and effects of neurotrophin-3 signaling at neuromuscular synapses. J Comp Neurol. 2007;501:465–482. doi: 10.1002/cne.21163. [DOI] [PubMed] [Google Scholar]
- 24.Desaki J, Uehara Y. The overall morphology of neuromuscular junctions as revealed by scanning electron microscopy. J Neurocytol. 1981;10:101–110. doi: 10.1007/BF01181747. [DOI] [PubMed] [Google Scholar]
- 25.Lin W, Sanchez HB, Deerinck T, Morris JK, Ellisman M, Lee KF. Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc Natl Acad Sci U S A. 2000;97:1299–1304. doi: 10.1073/pnas.97.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 27.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 28.Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW. Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- 30.Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 2003;40:563–580. doi: 10.1016/s0896-6273(03)00682-2. [DOI] [PubMed] [Google Scholar]
- 31.Feng Z, Ko CP. The role of glial cells in the formation and maintenance of the neuromuscular junction. Ann N Y Acad Sci. 2008;1132:19–28. doi: 10.1196/annals.1405.016. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds ML, Woolf CJ. Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. J Neurocytol. 1992;21:50–66. doi: 10.1007/BF01206897. [DOI] [PubMed] [Google Scholar]
- 33.Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 34.Son YJ, Trachtenberg JT, Thompson WJ. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci. 1996;19:280–285. doi: 10.1016/S0166-2236(96)10032-1. [DOI] [PubMed] [Google Scholar]
- 35.Love FM, Thompson WJ. Glial cells promote muscle reinnervation by responding to activity-dependent postsynaptic signals. J Neurosci. 1999;19:10390–10396. doi: 10.1523/JNEUROSCI.19-23-10390.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Malley JP, Waran MT, Balice-Gordon RJ. In vivo observations of terminal Schwann cells at normal, denervated, and reinnervated mouse neuromuscular junctions. J Neurobiol. 1999;38:270–286. [PubMed] [Google Scholar]
- 37.Kang H, Tian L, Thompson W. Terminal Schwann cells guide the reinnervation of muscle after nerve injury. J Neurocytol. 2003;32:975–985. doi: 10.1023/B:NEUR.0000020636.27222.2d. [DOI] [PubMed] [Google Scholar]
- 38.Trachtenberg JT, Thompson WJ. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379:174–177. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- 39.Lubischer JL, Thompson WJ. Neonatal partial denervation results in nodal but not terminal sprouting and a decrease in efficacy of remaining neuromuscular junctions in rat soleus muscle. J Neurosci. 1999;19:8931–8944. doi: 10.1523/JNEUROSCI.19-20-08931.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Love FM, Son YJ, Thompson WJ. Activity alters muscle reinnervation and terminal sprouting by reducing the number of Schwann cell pathways that grow to link synaptic sites. J Neurobiol. 2003;54:566–576. doi: 10.1002/neu.10191. [DOI] [PubMed] [Google Scholar]
- 41.Tam SL, Gordon T. Neuromuscular activity impairs axonal sprouting in partially denervated muscles by inhibiting bridge formation of perisynaptic Schwann cells. J Neurobiol. 2003;57:221–234. doi: 10.1002/neu.10276. [DOI] [PubMed] [Google Scholar]
- 42.Tam SL, Archibald V, Jassar B, Tyreman N, Gordon T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J Neurosci. 2001;21:654–667. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 1997;17:1642–1659. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trachtenberg JT, Thompson WJ. Nerve terminal withdrawal from rat neuromuscular junctions induced by neuregulin and Schwann cells. J Neurosci. 1997;17:6243–6255. doi: 10.1523/JNEUROSCI.17-16-06243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayworth CR, Moody SE, Chodosh LA, Krieg P, Rimer M, Thompson WJ. Induction of neuregulin signaling in mouse schwann cells in vivo mimics responses to denervation. J Neurosci. 2006;26:6873–6884. doi: 10.1523/JNEUROSCI.1086-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponomareva ON, Ma H, Vock VM, Ellerton EL, Moody SE, Dakour R, Chodosh LA, Rimer M. Defective neuromuscular synaptogenesis in mice expressing constitutively active ErbB2 in skeletal muscle fibers. Mol Cell Neurosci. 2006;31:334–345. doi: 10.1016/j.mcn.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, Suter U. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci. 2006;26:2124–2131. doi: 10.1523/JNEUROSCI.4594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escher P, Lacazette E, Courtet M, Blindenbacher A, Landmann L, Bezakova G, Lloyd KC, Mueller U, Brenner HR. Synapses form in skeletal muscles lacking neuregulin receptors. Science. 2005;308:1920–1923. doi: 10.1126/science.1108258. [DOI] [PubMed] [Google Scholar]
- 49.Jaworski A, Burden SJ. Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1. J Neurosci. 2006;26:655–661. doi: 10.1523/JNEUROSCI.4506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rimer M. Neuregulins at the neuromuscular synapse: past, present, and future. J Neurosci Res. 2007;85:1827–1833. doi: 10.1002/jnr.21237. [DOI] [PubMed] [Google Scholar]
- 51.English AW. Cytokines, growth factors and sprouting at the neuromuscular junction. J Neurocytol. 2003;32:943–960. doi: 10.1023/B:NEUR.0000020634.59639.cf. [DOI] [PubMed] [Google Scholar]
- 52.Wright MC, Son YJ. Ciliary neurotrophic factor is not required for terminal sprouting and compensatory reinnervation of neuromuscular synapses: re-evaluation of CNTF null mice. Exp Neurol. 2007;205:437–448. doi: 10.1016/j.expneurol.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolf CJ, Reynolds ML, Chong MS, Emson P, Irwin N, Benowitz LI. Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the growth-associated protein GAP-43. J Neurosci. 1992;12:3999–4010. doi: 10.1523/JNEUROSCI.12-10-03999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgiou J, Robitaille R, Trimble WS, Charlton MP. Synaptic regulation of glial protein expression in vivo. Neuron. 1994;12:443–455. doi: 10.1016/0896-6273(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 55.Hassan SM, Jennekens FG, Veldman H, Oestreicher BA. GAP-43 and p75NGFR immunoreactivity in presynaptic cells following neuromuscular blockade by botulinum toxin in rat. J Neurocytol. 1994;23:354–363. doi: 10.1007/BF01666525. [DOI] [PubMed] [Google Scholar]
- 56.Kang H, Tian L, Son YJ, Zuo Y, Procaccino D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, et al. Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. J Neurosci. 2007;27:5948–5957. doi: 10.1523/JNEUROSCI.0621-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorlewicz A, Wlodarczyk J, Wilczek E, Gawlak M, Cabaj A, Majczynski H, Nestorowicz K, Herbik MA, Grieb P, Slawinska U, et al. CD44 is expressed in non-myelinating Schwann cells of the adult rat, and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol Dis. 2009;34:245–258. doi: 10.1016/j.nbd.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Ellerton EL, Thompson WJ, Rimer M. Induction of zinc-finger proliferation 1 expression in non-myelinating Schwann cells after denervation. Neuroscience. 2008;153:975–985. doi: 10.1016/j.neuroscience.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 59.Marques MJ, Pereira EC, Minatel E, Neto HS. Nerve-terminal and Schwann-cell response after nerve injury in the absence of nitric oxide. Muscle Nerve. 2006;34:225–231. doi: 10.1002/mus.20576. [DOI] [PubMed] [Google Scholar]
- 60.Personius KE, Sawyer RP. Terminal Schwann cell structure is altered in diaphragm of mdx mice. Muscle Nerve. 2005;32:656–663. doi: 10.1002/mus.20405. [DOI] [PubMed] [Google Scholar]
- 61.Thomas S, Robitaille R. Differential frequency-dependent regulation of transmitter release by endogenous nitric oxide at the amphibian neuromuscular synapse. J Neurosci. 2001;21:1087–1095. doi: 10.1523/JNEUROSCI.21-04-01087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Winter F, Vo T, Stam FJ, Wisman LA, Bar PR, Niclou SP, van Muiswinkel FL, Verhaagen J. The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol Cell Neurosci. 2006;32:102–117. doi: 10.1016/j.mcn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Rimer M, Prieto AL, Weber JL, Colasante C, Ponomareva O, Fromm L, Schwab MH, Lai C, Burden SJ. Neuregulin-2 is synthesized by motor neurons and terminal Schwann cells and activates acetylcholine receptor transcription in muscle cells expressing ErbB4. Mol Cell Neurosci. 2004;26:271–281. doi: 10.1016/j.mcn.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Yang JF, Cao G, Koirala S, Reddy LV, Ko CP. Schwann cells express active agrin and enhance aggregation of acetylcholine receptors on muscle fibers. J Neurosci. 2001;21:9572–9584. doi: 10.1523/JNEUROSCI.21-24-09572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fields RD, Stevens-Graham B. New insights into neuron–glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jahromi BS, Robitaille R, Charlton MP. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron. 1992;8:1069–1077. doi: 10.1016/0896-6273(92)90128-z. [DOI] [PubMed] [Google Scholar]
- 67.Reist NE, Smith SJ. Neurally evoked calcium transients in terminal Schwann cells at the neuromuscular junction. Proc Natl Acad Sci U S A. 1992;89:7625–7629. doi: 10.1073/pnas.89.16.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rochon D, Rousse I, Robitaille R. Synapse-glia interactions at the mammalian neuromuscular junction. J Neurosci. 2001;21:3819–3829. doi: 10.1523/JNEUROSCI.21-11-03819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robitaille R, Bourque MJ, Vandaele S. Localization of L-type Ca2+ channels at perisynaptic glial cells of the frog neuromuscular junction. J Neurosci. 1996;16:148–158. doi: 10.1523/JNEUROSCI.16-01-00148.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robitaille R. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J Neurosci. 1995;15:7121–7131. doi: 10.1523/JNEUROSCI.15-11-07121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Todd KJ, Auld DS, Robitaille R. Neurotrophins modulate neuron–glia interactions at a vertebrate synapse. Eur J Neurosci. 2007;25:1287–1296. doi: 10.1111/j.1460-9568.2007.05385.x. [DOI] [PubMed] [Google Scholar]
- 72.Castonguay A, Robitaille R. Differential regulation of transmitter release by presynaptic and glial Ca2+ internal stores at the neuromuscular synapse. J Neurosci. 2001;21:1911–1922. doi: 10.1523/JNEUROSCI.21-06-01911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robitaille R. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron. 1998;21:847–855. doi: 10.1016/s0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]
- 74.Pinard A, Levesque S, Vallee J, Robitaille R. Glutamatergic modulation of synaptic plasticity at a PNS vertebrate cholinergic synapse. Eur J Neurosci. 2003;18:3241–3250. doi: 10.1111/j.1460-9568.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- 75.Rousse I, Robitaille R. Calcium signaling in Schwann cells at synaptic and extra-synaptic sites: active glial modulation of neuronal activity. Glia. 2006;54:691–699. doi: 10.1002/glia.20388. [DOI] [PubMed] [Google Scholar]
- 76.Belair EL, Vallee J, Robitaille R. In vivo long-term synaptic plasticity of glial cells. J Physiol. 2010;588:1039–1056. doi: 10.1113/jphysiol.2009.178988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rousse I, St-Amour A, Darabid H, Robitaille R. Synapse–glia interactions are governed by synaptic and intrinsic glial properties. Neuroscience. 2010;167:621–632. doi: 10.1016/j.neuroscience.2010.02.036. [DOI] [PubMed] [Google Scholar]


