Abstract
Cerebellar injury is increasingly recognized as an important complication of very preterm birth. However, the neurodevelopmental consequences of early life cerebellar injury in prematurely born infants have not been well elucidated. We performed a literature search of studies published between 1997 and 2014 describing neurodevelopmental outcomes of preterm infants following direct cerebellar injury or indirect cerebellar injury/underdevelopment. Available data suggests that both direct and indirect mechanisms of cerebellar injury appear to stunt cerebellar growth and adversely affect neurodevelopment. This review also provides important insights into the highly integrated cerebral-cerebellar structural and functional correlates. Finally, this review highlights that early life impairment of cerebellar growth extends far beyond motor impairments and plays a critical, previously underrecognized role in the long-term cognitive, behavioral, and social deficits associated with brain injury among premature infants. These data point to a developmental form of the cerebellar cognitive affective syndrome previously described in adults. Longitudinal prospective studies using serial advanced magnetic resonance imaging techniques are needed to better delineate the full extent of the role of prematurity-related cerebellar injury and topography in the genesis of cognitive, social-behavioral dysfunction.
Keywords: Cerebellar injury, Cerebellar development, Cerebellum, Prematurity, Neurodevelopment, Outcome
Background
Cerebellar development follows a precisely programmed series of critical developmental processes of cellular migration, proliferation, and arborization [1]. The third trimester of pregnancy is characterized by a highly dynamic period for cerebellar development, during which time the cerebellum undergoes its most rapid growth unparalleled by any other cerebral structure [2-5]. This vulnerable developmental period renders the cerebellum to a host of potential insults that can disrupt its highly regulated programmed course. Extremely preterm infants are particularly susceptible to impaired cerebellar development given that these critical phases of cerebellar development occur within the hazards of early exposure to the extrauterine environment [6].
Over the past decade, the increased adoption of a mastoid view on neonatal cranial ultrasound imaging [7] and greater availability of magnetic resonance imaging (MRI) have enhanced our ability to reliably detect cerebellar injury in surviving preterm infants. In fact, up to 19 % of premature infants born before <32 weeks of gestation have been shown by MRI to have cerebellar injury [8], with higher rates among extremely low birth weight (<750 g) infants [6, 9].
Disturbances in cerebellar development of premature infants are thought to occur by three mechanisms: (1) direct cerebellar injury (CBI), (2) indirect cerebellar injury or cerebellar underdevelopment associated with cerebral injury, (3) cerebellar underdevelopment in the absence of direct CBI or cerebral injury. Direct cerebellar injury is often hemorrhagic and will invariably result in tissue loss (cerebellar atrophy) and subsequent cerebellar growth failure (cerebellar disruption) [6, 10, 11] (Fig. 1). Secondly, indirect cerebellar injury can occur in the absence of direct CBI but in the presence of a cerebral parenchymal injury, likely resulting in crossed cerebellar diaschisis [12]. Crossed cerebellar diaschisis is associated with reduced blood flow and metabolism in the cerebellar hemisphere contralateral to a cerebral injury resulting in cerebellar hypoplasia and decreased growth [13-15], and has been described in ex-premature infants [5, 16]. Thirdly, cerebellar underdevelopment has also been described in surviving preterm infants in the absence of direct CBI or cerebral injury, suggesting that prematurity itself is associated with impaired cerebellar development [17, 18]. Possible mechanisms/mediators underlying this form of cerebellar underdevelopment include injury below the current resolution of clinical MRI, maternal-placental growth factors, genetic or chromosomal anomalies, and factors associated with a compromised immature cerebral/systemic circulation [6, 19-21].
Fig. 1.
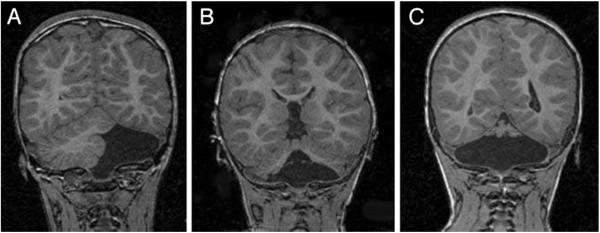
Follow-up brain MRIs (coronal spoiled gradient recalled T1-weighted) of infants with isolated cerebellar hemorrhagic injury on neonatal cranial ultrasound. a Complete absence of the left cerebellar hemisphere with preservation of the right cerebellar hemisphere and vermis. b Absence of the inferior cerebellar vermis and inferior portions of both cerebellar hemispheres. c Near-total cerebellar destruction with only a small amount of superior cerebellar vermis present (Reprint from [22] Copyright 2001 by American Academy of Pediatrics. Reprint with permission.)
Despite the increased recognition of cerebellar injury in survivors of preterm birth, the neurodevelopmental consequences of cerebellar injury have been largely unexplored. The primary objective of this paper is to review our current understanding of the functional consequences of cerebellar injury (direct and indirect) in survivors or preterm birth.
Methods
To delineate cerebellar structure-function relationships in expreterm infants, a structured review of the literature was performed. Figure 2 outlines the Quorum flowchart used. First, Pubmed (Medline), CINAHL, and PsycINFO searches were performed using the combination of the following medical subject headings: “premature” infant and “cerebellum.” These searches were limited to articles published in English between 1970 and June 2014, and to studies conducted in human subjects under the age of 18 years. From these searches, a total of 231 publications were identified, and their titles and abstracts were screened for the following inclusion criteria: (i) prematurely born subjects (<37 weeks gestational age (GA)), (ii) evaluation of the cerebellar development or CBI by neuroimaging, (iii) developmental outcome assessments. Sixty original research reports met the inclusion criteria and underwent a subsequent full review to verify that the initial screening criteria were satisfied. This full review eliminated an additional 38 studies (see Fig. 2). The reference lists of these retrieved articles were screened for additional publications that met inclusion criteria; this resulted in one additional study. Therefore, a total of 23 studies were selected and further analyzed. Data extracted included information such as the study design, sample characteristics, neuroimaging modalities, neurodevelopmental assessments, variables under study, statistical tests, and main findings. A summary of these studies are presented in Tables 1, 2, and 3. A quality assessment of the studies was performed following the McMaster University framework for critical review of quantitative studies [4]. Given that different methodologies and outcome measures were used across studies, statistical analysis of the results was not performed.
Fig. 2.
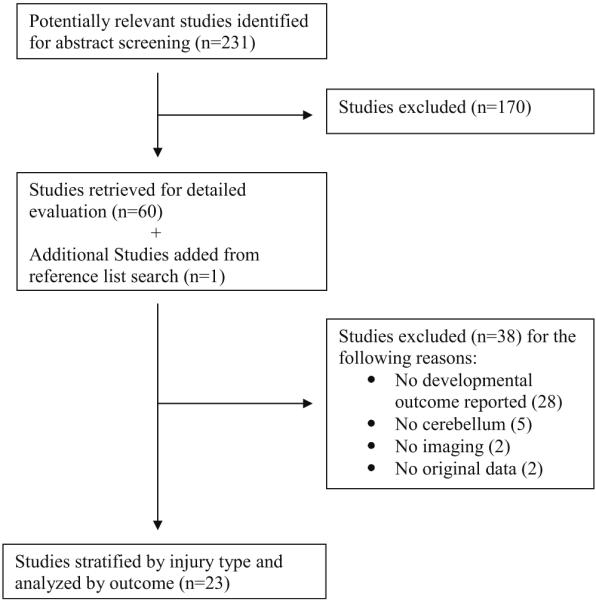
Quorum flowchart
Table 1.
Summary of studies reporting direct cerebellar injury (n=12)
| Studies | Design | Populations (complete data) | Lesions | Imaging | Cerebellar outcomes |
Developmental outcomes | Conclusions |
|---|---|---|---|---|---|---|---|
| Bednarek [24] | Retrospective case series |
6 PT 24–28 w GA with CBI | CBI, cerebral injury |
HUS | Presence of CBI | Heterogeneous evaluation and variable time |
6/6 (100 %) PT children with CBI presented with adverse outcome: 3 died and 3 had impaired neurological development. |
| Dyet [28] | Prospective cohort |
119 PT <30 w GA | CBI, cerebral injury |
ptMRI and tMRI | Presence of CBI | 18–36 months: Griffiths Mental Developmental Scales, CP |
6/8 (75 %) PT children with CBI presented with adverse outcome: 3 died and 3 had severe developmental delay including 1 with CP. |
| Johnsen [32] | Retrospective case series |
67 PT <28 w GA, ≤1,000 g with CP |
CBI, cerebral injury |
Follow-up MRI | Presence of CBI | 1.5–20 years Neurological status at clinical follow-up |
PT born subjects with CBI were more unlikely to walk (59 %) or talk (77 %) than those with a normal CBL (24 % and 19 %). |
| Limperopoulos [22] |
Retrospective case–control |
35 PT ≤32 w GA with isolated CBI, 35 controls PT ≤32 w with no CBI or cerebral injury, 16 PT with CBI + cerebral injury |
CBI, cerebral injury |
HUS, follow-up MRI for PT with an abnor- mal HUS |
Presence of isolated CBI or combined CBI with cerebral injury |
2–5 years: Neuro exam MSEL PDMS VABS CBCL M-CHAT SCQ |
Isolated CBI was significantly associated with severe developmental delay in motor skills (40–54 %), language (37–40 %), and cognition (40–46 %); deficits in daily living activities performance (40 %), internalizing behavioral problems (34 %) and autistic features (37 %). Cerebral injury and more extended cerebellar lesions (bilateral vs unilateral) were associated with greater deficits. Vermal injury was associated with socialization difficulties (VABS) and positive autism screening (M-CHAT+SCQ). |
| Limperopoulos [26] |
Prospective cohort |
40 PT ≤32 w GA with isolated CBI |
CBI | HUS, follow-up MRI |
Regional cerebral volume contralateral to CBI |
18–63 months: Neuro exam MSEL PDMS CBCL M-CHAT |
Greater dorsolateral prefrontal volume was associated with better M-CHAT and CBCL internalizing scores. Greater sensorimotor volume was associated with higher PDMS gross motor score. Greater premotor volume was associated with higher Mullen cognitive scores. Greater midtemporal volume was associated with higher Mullen Expressive language score. |
| Mercuri [11] | Retrospective case series |
10 PT ≤33 w GA with CBI | CBI, cerebral injury |
HUS, follow-up MRI |
Presence of CBI | 7 month–8 years: Neuro exam, motor development |
All PT children with CBI had mild (40 %) to severe (60 %) motor delay. |
| Messerchmidt [23] |
Retrospective case–control |
31 PT ≤31 w GA with severe volume reduction in CBL on HUS; 31 PT GA and gender matched with cerebral injury but normal CBL on HUS |
CBL volume reduction, cerebral injury |
HUS | Presence of CBI | 24–36 months: Neuro exam BSID-II: Motor + cognitive composites |
PT children with CBI had significantly worse neurodevelopmental outcomes than children without CBI. All children with CBI (100 %) had CP and had neurodevelopmental delay (motor and cognitive). |
| Steggerda [9] | Prospective cohort |
108 PT <32 w GA | CBI, cerebral injury |
HUS, tMRI | Presence of small CBI |
2 years: Neuro exam GMFCS BSID-III: Motor + cognitive composites CBCL |
In PT infants with small CBI, behavioral problems were found in 15–23 %, motor delay in 15 %and mild or severe neurodevelopmental outcome in 61.5 %. None of the children with small CBI had cognitive delay. Small CBI was not significantly associated with poorer neurodevelopmental outcomes or problematic behavior when compared to preterm without CBI. |
| Tam [29] | Prospective cohort |
94 PT ≤34 w GA | CBI, cerebral injury |
HUS, ptMRI & tMRI |
Presence of CBI | 3–6 years: Neuro exam WPPSI-III (IQ) |
Of the 8 PT infants with CBI detectable on MRI only, 4 (50 %) presented with abnormal neurological examination findings at 3–6 years of age. CBI was significantly associated with neurological anomalies, but not with IQ evaluated at follow-up. |
| Van Kooji [30] | Prospective cohort |
112 PT <31 w GA | CBI, cerebral injury |
tMRI, | Presence of CBI, CBL volume and H1-MRS |
2 years: BSID-III: Motor + cognitive composites |
No significant differences in BSID-III scores between PT children with and without CBI. Cognitive scores were associated with CBL volume and CBL NAA/Cho ratio but not CBI. No significant association was found between CBL variables and the motor composite. |
| Zafeiriou [31] | Retrospective case series |
12 PT <28 w GA with pontocerebellar hypoplasia |
Pontocerebellar hypoplasia, cerebral injury |
Follow-up MRI | Presence of pontocerebellar hypoplasia |
21–90 months: Neuro exam, motor development |
All PT children with pontocerebellar hypoplasia (100 %) had CP and presented with movement disorders. |
| Zayek [25] | Retrospective cohort |
1120 PT <28 w GA | CBI, cerebral injury |
HUS | Presence of CBI | 12–18 months: BSID-II or BSID-III: Motor + cognitive composites |
PT infants with CBI had significantly more neurodevelopmental impairments. Of the 32 children with CBI, 15 (50 %) had cognitive impairment, 18 (56 %) motor impairment including CP in 10 (32 %). Lesions isolated to the cerebellar hemispheres were associated with lower cognitive scores, but not with motor skills. Lesions involving the vermis were associated with motor and cognitive impairments. |
BSID Bayley Scale for Infant Development, CBCL Child Behavior Checklist, CBI cerebellar injury, CBL cerebellum, CP cerebral palsy, GA gestational age, H1 -MRS proton magnetic resonance spectroscopy, HUS head ultra sound, M-CHAT Modified Checklist for Autism in Toddlers, MSEL Mullen Scales for Early Learning, PDMS Peabody Developmental Motor Scales, PT preterm, ptMRI preterm MRI, SCQ Social Communication Questionnaire, tMRI term or term-equivalent MRI, VABS Vineland Adaptative Behavior Scale, w week, WPPSI Wechsler Preschool and Primary Scale of Intelligence
Table 2.
Summary of studies reporting cerebellar underdevelopment/hypoplasia secondary to cerebral injury (n=8)
| Studies | Design | Populations (complete data) | Lesions | Imaging | Cerebellar outcomes | Develop. outcomes | Conclusions |
|---|---|---|---|---|---|---|---|
| Gadin [33] | Prospective cohort |
20 PT ≤30 w GA, 20 NICU admitted ≥37 w GA |
Cerebral injury | tMRI | CBL volume | 6 months: BSID-III: Motor composite |
No significant association was found between CBL volume and the motor composite score. |
| Lind [34] | Prospective cohort |
97 PT <37 w GA ≤1,500 g, 161 term-born controls |
Cerebral injury on HUS only |
HUS, tMRI | CBL volume | 5 years: NEPSY-II FTF |
PT children had significantly lower scores on each subscale than term children. In PT children, greater CBL volume was significantly associated with better motor skills and executive functioning of the FTF. No significant association was found between CBL volume and any of the NEPSY-II sub- scales. |
| Lind [35] | Prospective cohort |
164 PT <37 w GA ≤1,500 g |
Cerebral injury on HUS only |
HUS, tMRI | CBL volume | 2 years: BSID-II: cognitive composite Hammersmith Infant Neurological Examination |
Smaller CBL volume was significantly associated with lower scores on the Hammersmith Infant Neurological Examination. When controlling for cerebral injury, smaller CBL volume continue to be associated with more neurological abnormalities. CBL volume was not significantly associated with the cognitive composite. |
| Northman [36] | Prospective cohort |
27 PT ≤33 w GA with CI on HUS, 22 PT≤ 33 w GA without CI, 26 term-born controls |
CBI, cerebral injury | HUS, follow-up MRI |
CBL volume | 16 years: WASI (IQ) |
PT born adolescents with cerebral injury on neonatal HUS had significantly lower IQ than the two other groups. In PT born adolescents with cerebral injury on neonatal HUS, larger CBL volume was significantly associated with higher IQ. |
| Nosarti [39] | Prospective cohort |
207 PT≤33 w GA 104 term-born controls |
Cerebral injury on HUS only |
Follow-up MRI | VBM: CBL GM and WM concentration |
14–15 years: Various evaluation of: language, executive functions, memory, visual motor integration. Neuromotor assessment (in 116 PT and 35 controls) |
PT born adolescents had significantly lower scores on the language and executive function evaluations. No regional volumetric difference in the CBL was found to be significantly associated with the neuromotor or cognitive outcome. |
| Shah [37] | Prospective cohort |
83 PT ≤32 w GA 13 term-born controls |
CBI, cerebral injury | tMRI | CBL volume | 2 years: BSID-II: Motor + cognitive composites |
In PT infants, greater CBL volume was significantly associated with better motor scores. However, the associations did not remain significant after adjusting for WMI and intracranial volume. No significant association was found between CBL volume and the cognitive composite. |
| Spittle [40] | Prospective cohort |
83 PT ≤30 w GA | Cerebral injury | tMRI | Cerebellar transverse diameter |
1 and 3 months: General Movement assessments |
After adjusting for WMI, GA and gender, smaller CBL transverse diameter was significantly associated with abnormal General Movement score at 3 months. |
| Taylor [38] | Prospective cohort |
37 PT ≤750 g, 35 PT 750–1,499 g, 36 term-born controls |
Cerebral injury on HUS |
Follow-up MRI | CBL volume | 16 years: Various evaluations of: IQ, language, memory, perceptual- motor organization and executive func- tions |
In PT born adolescents, greater CBLWM volume was significantly associated with better total IQ, memory, executive functions and perceptual motor skills but not with language ability. In PT born adolescents, greater CBL GM volume was significantly associated with better executive functions. |
CBCL Child Behavior Checklist, CBI cerebellar injury, CBL cerebellum, FTF Five to Fifteen questionnaire, GA gestational age, GM gray matter, GMFCS Gross Motor Function Classification System, HUS head ultra sound, NEPSY-II Developmental NEuroPSYchological Assessment, PT preterm, ptMRI preterm MRI, SDQ Strengths and Difficulties Questionnaire, tMRI term or term-equivalent MRI, VBM Voxel Base Morphometry, w week, WASI Wechsler Abbreviated Scale of Intelligence, WM white matter
Table 3.
Summary of studies reporting cerebellar underdevelopment/hypoplasia without evidence of cerebellar or cerebral injury (n=3)
| Studies | Design | Populations (complete data) | Lesions | Imaging | Cerebellar outcomes | Develop. outcomes | Conclusions |
|---|---|---|---|---|---|---|---|
| Allin [43] | Prospective cohort | 67 PT ≤33 w GA, 50 age-matched term- born controls |
NR | Follow-up MRI | CBL volume | 1 year: neuro exam 8 years: WISC-R (IQ) K-ABC 14–15 years: neuro exam verbal fluency reading spelling digit span |
In 8-year-old PT, greater CBL volume was sig- nificantly associated with higher score on the WISC-R full scale IQ and with the following subscales: similarities (verbal), block design, objective assembly (non-verbal); and with all the mental processing subcomponents of K- ABC. In 14–15 years old PT, CBL volume was significantly associated with better reading ability and greater digit span. No significant association between CBL volume and neuromotor outcome was found. |
| Martinussen [41] | Prospective cohort | 50 PT≤1,500 g, 49 term-born small for GA, 57 Term- born controls |
NR | Follow-up MRI | CBL volume | 15 years: WISC-III (IQ) VMI |
|
| In PT born | adolescents, greater CBL WM volume was significantly associated with higher total IQ, VMI and with VMI visual perception scores. |
||||||
| Parker [42] | Prospective cohort | 65 PT≤33 w GA, 34 term-born controls |
NR | 2 follow-up MRI | CBL volume and growth |
15 years and 18–19 years: WASI (IQ) Verbal fluency 18–19 years: General Health Questionnaire |
In PT born adolescents, greater CBL volume was significantly associated with higher full scale IQ at 15 and at 18 years of age. Associations were no more significant once adjusted for cerebral WM volume. In PT born adolescents, CBL volume reduction was significantly associated with worse behavioral and psychological outcome on the General Health Questionnaire. In PT born adolescents, CBL volume was not associated with verbal fluency. |
CBL cerebellum, GA gestational age, GM gray matter, K-ABC Kaufman Assessment Battery for Children, NR not reported, PT preterm, VMI Developmental Test of Visual-Motor Integration, WASI Wechsler Abbreviated Scale of Intelligence, WISC-III Wechsler Intelligence Scale for Children-Third edition, WM white matter, WISC-R Wechsler Intelligence Scale for Children-Revised
Results
To facilitate synthesis of the information extracted, the studies were categorized into three groups based on the different types of impaired cerebellar development described in survivors of prematurity. These categories were as follows: (1) direct CBI (i.e., primary cerebellar hemorrhage or infraction); (2) cerebellar underdevelopment/hypoplasia secondary to a cerebral injury; (3) cerebellar underdevelopment/hypoplasia without evidence of CBI or cerebral injury.
Direct Cerebellar Injury
Study Design
Twelve studies (two case–control studies, four retrospective case series, and six cohort studies (one retrospective and five prospective)) have evaluated the developmental outcomes of premature infants with direct CBI. The study/cohort characteristics extracted from these 12 studies are summarized in Table 1. All preterm cohorts were born at or before 34 weeks GA. The control group in the two case–control studies was a cohort of ex-preterm infants with a normal structural cerebrum and cerebellum based on MRI [22, 23].
Neuroimaging Modality and Findings
Cerebellar injury was detected by neonatal head ultrasound (HUS) [22, 24-26] or on T1/T2-weighted images obtained from a preterm (<36 weeks GA) or a term-equivalent MRI study [9, 22, 26-29]. The severity of CBI varied from small punctate cerebellar hemorrhages (<4 mm) [9, 29, 30] to more severe forms of cerebellar injury including cerebellar infarction and resulting atrophy on conventional MRI [11, 23, 31, 32].
Neurodevelopmental Assessments
Age at outcome testing varied considerably across studies, with testing performed anywhere from 1 to 20 years of age. Neuromotor development was a common study outcome which was determined by medical record review [11, 24, 31, 32], by formal neurologic examinations [9, 11, 22, 23, 26, 29, 31], or standardized developmental assessments [9, 22, 23, 25, 29, 30]. Other measured outcomes included cognition [9, 22, 23, 25, 26, 28-30], behavioral difficulties [9, 22], and evaluation of functional status [22].
Motor Outcomes
Overall, children with a severe CBI had significantly worse neuromotor outcomes when compared to preterm infants without CBI [22, 23, 25, 32]. Of the three studies [9, 29, 30] comparing children with and without punctate cerebellar hemorrhagic injury, only Tam et al. [29] found statistically significant worse motor outcomes in the injured group. Formal neurologic examinations revealed that 40–100 % of children with CBI had adverse neurologic outcomes such as difficulty walking and movement disorders [11, 23, 24, 28, 29]. Additionally, cerebral palsy was clinically diagnosed in 32–100 % of the children with CBI [23, 25, 31]. Using norm-referenced standardized developmental scales, motor delays were wide-ranging (15–100 %) in children with CBI [9, 22, 23, 25]. Using proton magnetic resonance spectroscopy (1H-MRS) in the cerebellum, one study revealed that N-acetylaspartate/choline ratio (NAA/Cho) was not associated with motor outcomes [30]. Interestingly, smaller regional volume in the sensorimotor cortex contralateral to a unilateral isolated CBI was associated with worse motor skills [26].
Cognitive Outcomes
Cognitive abilities were significantly lower in ex-preterm children with a direct CBI when compared to ex-preterm children without CBI [23, 25, 32]. Cognitive impairment was present in 40–100 % of the children with CBI [22–25, 28]. Unlike ex-preterm children with larger CBI, ex-preterm children with small punctate lesions did not exhibit significant cognitive impairment [9, 29, 30]. Zayek et al. [25] reported that CBI limited to one or both cerebellar hemispheres was associated exclusively with cognitive impairment as compared to lesions involving the vermis that were also associated with motor deficits. In general, the more severe the cerebellar lesion is (i.e., bilateral as opposed to unilateral), the more significant the cognitive impairment [22]. Interestingly, cognitive scores correlated strongly with cerebellar NAA/Cho ratio and cerebellar volume [30]. Lastly, in ex-preterm children with isolated CBI, smaller premotor cortical volume contralateral to the CBI was found to be associated with a lower cognitive score [26].
Language Outcomes
Only one study compared language abilities in ex-preterm children with and without CBI, and reported significant language impairments in children with CBI [22]. In this study, up to 40 % of the ex-preterm children with CBI demonstrated receptive and expressive language impairments. In a second study by Johnsen et al. [32], 77 % of the prematurely born subjects with CBI and cerebral palsy failed to develop language abilities. Lastly, in ex-preterm children with isolated CBI, regional reductions in the midtemporal cerebral regions contralateral to the CBI were found to be associated with greater difficulties in expressive language [26].
Socio-behavior Outcomes
Internalizing and externalizing behavioral problems have been described in up to one third of ex-preterm children with CBI [9, 22]. Additionally, 37 % of the children with isolated CBI showed early signs of autistic features [22]. When examining the topography of injury, vermian injury was strongly associated with socialization difficulties and early autism symptoms [22]. Limperopoulos et al. [22] also reported a significantly higher rate of behavioral problems (internalizing > externalizing) in preterm infants with severe CBI. Conversely, punctate CBI was not associated with significantly higher behavioral problems compared to children without CBI [9]. Lastly, in ex-preterm children with an isolated CBI, autistic symptoms and internalizing behavioral problems were associated with a lower regional dorsolateral prefrontal cortical volume contralateral to the CBI [26].
Summary of Findings
With the exception of punctate cerebellar lesions, collectively these data suggest that direct CBI is associated with long-term motor, cognitive, and language impairments, as well as socialization and behavioral difficulties. Moreover, secondary underdevelopment of cerebellar projection pathways to regional cerebral cortical areas of the contralateral cerebral hemisphere is significantly predictive of domain-specific long-term functional impairments.
Cerebellar Underdevelopment/Hypoplasia Secondary to Cerebral Injury
Study Design
We identified a total of eight prospective cohort studies that examined the relationship between cerebellar development and outcomes in survivors of preterm birth (<37 weeks GA) with cerebral injury. Six of these studies also included a comparison group of term-born (≥37 weeks GA) infants.
Neuroimaging Modality and Findings
Six out of the eight studies quantified cerebellar volume using a combination of automated and manual three-dimensional (3-D) segmentation methods [33–38]. Voxel-based morphometry (VBM) was used in one study to evaluate the differences in cerebellar white and gray matter volumes [39] while another study performed two-dimensional (2-D) measurements to evaluate cerebellar transverse diameter [40]. All quantitative 3-D volumetric and 2-D analyses were performed on MRI images obtained around term-equivalent age with the exception of Northam et al. [36] and Nosarti et al. [39] who performed their MRI analyses in ex-preterm adolescents.
Neurodevelopmental Assessments
Outcome evaluations varied across studies and follow-up ages ranged from 1 month to 16 years. Motor outcome evaluations were predominantly used when studying children under 2 years of age, while cognitive outcomes were primarily used in studies of children from 5 years through adolescence. Neuromotor evaluations included the following: assessment of early life spontaneous movements [40], standardized neurological evaluations [34, 39], and the Bayley Scales of Infant Development [33–35, 37]. Cognitive abilities were evaluated using a variety of age appropriate and skill-specific standardized evaluations (see details in Table 2) [34, 36, 38, 39]. Noteworthy, language skills [38] and behavior problems/functional status [34] were only evaluated in one study.
Motor Outcomes
Smaller cerebellar transverse diameter was found to be the most important predictor of abnormal generalized movement in ex-premature infants at 3 months corrected age [40]. Conversely, no significant association between cerebellar volume and motor score was found when evaluated at 6 months of age [33]. In studies performed in ex-preterm infants between 2 and 5 years of age, smaller cerebellar volume was significantly associated with lower neuromotor skills [34, 35]. Although Shah et al. [37] also reported significant associations between motor scores and cerebellar volume at 2 years of age, these associations were no longer significant once adjusted for cerebral white matter injury and intracranial volume. The authors speculated that white matter injury was a major determinant of developmental outcome in their study population.
Cognitive Outcomes
In ex-preterm children between 2 and 5 years of age, no significant associations were reported between cerebellar volume and cognitive outcomes [34, 35, 37]. Interestingly, a larger cerebellar volume was significantly associated with higher executive functioning scores in older children [34]. Similarly, there was a positive relationship between cerebellar volume and cognition in prematurely born adolescents [36, 38], while Nosarti et al. [39] reported no significant association between cognition and cerebellar gray and white matter concentrations as evaluated by VBM.
Language/Behavioral Outcomes
No significant association between cerebellar volume and language or behavior was reported in prematurely born children and adolescents with cerebral injury [34, 38].
Summary of Findings
To summarize, findings were inconsistent across studies in this group. Likely, this is due to the use of different outcome evaluations and patient demographics. Although cerebral injury was documented in these studies, only a small number of studies adequately controlled for it in their analyses, which could further explain the inconsistencies reported between the different studies. Nevertheless, preliminary findings suggest that cerebellar volume reduction secondary to cerebral injury appears to be associated with impaired neuromotor and cognitive performance in ex-preterm adolescents.
Cerebellar Underdevelopment/hypoplasia Without Evidence of CBI or Cerebral Injury
Study Design
Only three prospective cohort studies fell into this category. Subjects included prematurely born adolescents evaluated between 14 and 19 years of age. The main findings of these studies are summarized in Table 3. All studies included a comparison group of age-matched healthy term-born adolescents. In addition, Martinussen et al. [41] included a second comparison group of adolescents who were born small for gestational age.
Neuroimaging Modality and Findings
None of these studies reported neonatal imaging findings (HUS or MRI) and no structural parenchymal injury (cerebellar or cerebral) were described. Only Martinussen et al. [41] reported that a small subset (n = 4) of prematurely born adolescents in their sample (n = 50) had intraventricular hemorrhage, but they did not quantify the severity or control for it in their analyses. All studies quantified cerebellar volume using a combination of automated and manual 3-D segmentation analyses of MRI images obtained during adolescence. Parker et al. [42] performed serial MRI scans, one at 15 years and a second during early adulthood, which enabled them to examine cerebellar growth between these two time points.
Neurodevelopmental Assessments
The evaluations performed in this group targeted mainly higher order functions such as memory, language, reading ability, and verbal and non-verbal IQ assessments (see details in Table 3). In addition to neuromotor and cognitive assessments performed during adolescence, Allin et al. [43] incorporated motor items from a neurological evaluation performed at 1 year as well as the results from cognitive evaluations administered at 8 years of age. One study [42] evaluated behavioral problems and psychological distress using a self-administered questionnaire (i.e., the General Health Index).
Motor Outcomes
Only one study evaluated neuromotor outcomes, in which, no significant association between motor or neurological examination and cerebellar volume was found in prematurely born adolescents at 14–15 years of age [43].
Cognitive Outcomes
A common finding among the three studies was that cerebellar volume was positively associated with total IQ [41–43]. Cerebellar volume was also associated with performance on a number of cognitive subtests including similarities, block design, mental processing, reading ability, visual motor integration, and visual perception [41–43]. In the study by Parker et al. [42], these associations were no longer significant once they controlled for cerebral white matter volume. Conversely, in the two other studies, associations remained significant after controlling for total brain volume [43] or intracranial volume [41].
Language Outcomes
No significant association was found between verbal fluency and cerebellar volumetric growth [41–43].
Socio-behavioral Outcomes
Cerebellar volume reduction was significantly associated with behavioral and psychological problems in prematurely born young adults but not in their term-born peers [42].
Summary of Findings
Taken together, these studies demonstrate that smaller cerebellar volumes were consistently associated with lower cognitive abilities in prematurely born adolescents who had no documented history of direct CBI or cerebral injury. Additionally, available evidence suggests that cerebellar volume reduction is associated with greater behavioral problems and psychological distress in ex-preterm young adults.
Discussion
In this review, we analyzed 23 studies published between 1997 and 2014 that reported neurodevelopmental outcomes of prematurity-related cerebellar injury. We rigorously searched three major databases. We selected studies only published in English; therefore, it is possible that relevant studies printed in other languages may have been missed. Due to the heterogeneity in research methodology used among the studies, meta-analyses could not be performed. For example, gestational age at birth varied widely between studies (from 22 to 36 weeks). It is well known that cerebellar injury and perinatal complications are inversely related to gestational age at birth. Therefore, the wide-ranging neurodevelopmental impairments reported by the different studies could be due in part to the variable gestation age of the study cohorts, where impairments may be more prevalent in samples with a lower gestational age at birth compared to those with a higher gestational age. Hence, the reader should interpret the findings reported in this review of literature with caution. Nevertheless, to provide greater synthesis of the results, we stratified the studies into three categories of prematurity-related cerebellar injury. It is important to note that the study group assignment was entirely based on the information provided in the published reports, which could have introduced bias when information was not reported. Nonetheless, to our knowledge, this is the first review to extensively summarize the neurodevelopmental and functional consequences of early life cerebellar injury in ex-preterm survivors using a structured approach.
About half of the studies reviewed included information from neonatal cranial ultrasound. MRI was the most common brain imaging modality and was used by all studies except three that reported only HUS findings [23–25]. Most studies used a prospective longitudinal design and serial MRI was performed in only three studies [28, 29, 42]. Longitudinal serial imaging studies spanning the neonatal to adolescent period, coupled with standardized outcomes evaluations during childhood and/or adolescence, are currently lacking. Such studies are needed to determine to what extent neonatal imaging findings and subsequent neurodevelopmental impairments described herein are transient or persistent in nature. Understanding the long-term consequences of prematurity-related cerebellar underdevelopment is essential for determining the best clinical services for optimal development. Despite the heterogeneity among the analyzed studies, this review highlights a common, cross-cutting theme that prematurity-related cerebellar injury is associated w ith non-desirable motor and non-motor outcomes.
Motor Outcomes
Overall, neuromotor impairments were associated with severe direct CBI with a prevalence varying from 15 to 100 % [11, 22–25, 29, 31, 32]. Although less consistent, a similar trend was observed in studies evaluating cerebellar volume in the presence of cerebral injury; reduced cerebellar volume was associated with less favorable motor outcomes [34, 35, 40]. Despite the well-established role of the cerebellum in motor function, only 39 % (9/23) of studies evaluated motor performance using standardized developmental assessments and most did not differentiate between gross and fine motor deficits. The majority of studies relied on a formal neurologic examination which is more likely to detect major neurologic dysfunction, but may be less sensitive to the more subtle motor deficits.
Cognitive Outcomes
The results of this review demonstrate that prematurity-related CBI occurs on a continuum of severity and is associated with wide-ranging and far-reaching long-term neurocognitive impairments and disabilities. This review highlights a strong association between cerebellar volumetric growth impairment and cognitive disabilities as well as executive dysfunction secondary to direct and indirect CBI [34, 36, 38, 41–43]. These data corroborate previous reports from other pediatric populations including older children following cerebellar tumor resection who develop higher order cognitive deficits such as difficulties with task organization and problem solving [44, 45]. Moreover, a broad range of cognitive dysfunction including impairments in visuospatial processing, executive function, and memory has been reported in adults with cerebellar lesions [46].
Language Outcomes
Although a cerebellar role in language deficits has been increasingly reported in adults following cerebellar vascular lesions [47, 48], to date, very few studies in ex-preterm children following early life CBI have examined the association between the cerebellum and early language development. Available evidence [22, 32] suggests that direct CBI, and more specifically injury to the vermis [22], is associated with language deficits in ex-preterm children. Similarly, larger hemorrhagic lesions affecting the cerebellar vermis have been implicated in language deficits among term-born infants [49]. Interestingly, these findings differ from the lateralized right cerebellar hemispheric language pathway that has been reported in adults using functional MRI studies [50]. These intriguing topographic differences associated with injury to the immature versus the mature cerebellum deserve further investigation. Taken together, available evidence links direct CBI with expressive and receptive language deficits in preschool- and school-aged children; however, future studies are needed to better delineate the type of language deficits and their regional cerebellar topology.
Social-Behavioral Outcomes
Although behavioral problems are prevalent among survivors of preterm birth [14, 51, 52], behavioral outcomes in expreterm survivors of CBI were relatively unexplored in the studies included in this review. Only three studies examined the relationship between behavioral difficulties and direct/indirect CBI [22, 42] and two of them found strong associations between behavioral problems and cerebellar injury/underdevelopment [22, 42]. Moreover, socialization difficulties and early autistic features were also found to be highly prevalent and primarily related to vermian injury [22]. This atypical social-behavioral functioning in ex-preterm children following early life CBI is strongly suggestive of an autism spectrum disorders profile. Interestingly, similar associations between social/behavior dysfunction and the vermis have been described in young children with cerebellar malformations [53]. Converging evidence from other study populations has suggested that regional cerebellar vermis volume may be a substrate for autism spectrum disorders [46, 54].
The cerebellum is known to play a role in the pathogenesis of neurodevelopmental and neuropsychiatric conditions such as dyslexia, attention deficit hyperactivity disorder, schizophrenia, and autism [15, 46, 55, 56]. Taken together, converging lines of investigation point to an elevated increased risk among survivors of very premature infants for subsequent development of cognitive, learning, behavioral, and socio-affective disturbances. These data support an under-appreciated role for early life cerebellar injury in the high prevalence of long-term pervasive neurodevelopmental disabilities. Noteworthy, the degree to which these initial positive screen rates for autistic features are transient or reflective of true autism spectrum disorders remains to be determined. Ongoing studies are needed to examine the sensitivity, specificity, and predictive validity of early autism screeners in expreterm infants following a CBI.
Developmental Cerebellar Cognitive Affective Syndrome
Although previously thought to be exclusively involved in motor control, the cerebellum is now known to play a critical role in higher order cognitive and affective functions. Schmahmann and Sherman [57] were the first to describe the cerebellar cognitive affective syndrome characterized by a myriad of impairments in executive, visual spatial, linguistic, and affective function in adults [58, 59] and in older children [60–64] following cerebellar lesion. Relevant to the current review is an apparent ‘developmental’ form of cerebellar cognitive affective syndrome is survivors of prematurity-related CBI. The underpinnings of this developmental cerebellar cognitive affective profile suggest an overlap with the features of early autism described herein. Worthy of note is the fact that social-behavioral changes described in the cerebellar cognitive affective syndrome appear to be most prominent with injury to the cerebellar vermis and paravermian regions (described above). Higher order cognitive and social-behavioral deficits have also been described in children with cerebellar malformations [65]. Although most studies have reported non-specific global developmental delays, recent reports using comprehensive outcome evaluations suggest that visuospatial skills, language, and attention are also impaired in children with cerebellar malformations [53, 66, 67]. Moreover, as reported in children with prematurity-related CBI, smaller cerebellar volume was associated with lower motor and non-motor functioning in children with cerebellar malformations. In particular, smaller right cerebellar hemispheric volume was associated with lower expressive language and cognitive skills, while smaller vermian volume was associated with behavioral problems [68]. Collectively, these data suggest that a developmental cerebellar cognitive affective profile is present in children with both acquired and developmental cerebellar lesions.
Cerebellar Functional Topography
Although very few studies in this review described the topography of the cerebellar lesions, available data suggests that there appears to be a functional topography to cerebellar underdevelopment in ex-preterm survivors. Zayek et al. [25] reported that lesions confined to the lateral hemisphere, either unilaterally or bilaterally, were associated with cognitive impairment, while other studies found that injury that involved the vermis was associated with more extensive cognitive, language, and social-behavioral disturbances [22, 32]. Although the topography of neuromotor deficits was not directly explored in the studies reviewed herein, literature from adult populations suggests that motor abnormalities occur in the presence of midline and/or anterior lobe cerebellar lesions or malformations [47, 69]. The fastigial nuclei are important relays of the vestibulo-occular system. They are the most medially located cerebellar nuclei and, therefore, are more vulnerable in the event of vermian injury. Cerebellar strokes in adults are more frequent in the posterior inferior cerebellar artery (PICA) territory, which in turn has implicated the inferior part of the vermis and posterior sections of the cerebellar hemispheres [70]. There appears to be a similar predilection for infero-medial injury in prematurity-related CBI [6, 32], which may in part explain the predisposition for cognitive, language, and social impairments over motor problems in these infants.
Only one study in this review [26] performed regional cerebral parcellation and tissue segmentation in children with isolated direct CBI. This study showed regional decreases in cerebral cortical volume contralateral to unilateral CBI. These volume reductions were identified in apparently uninjured cerebral cortical regions and were highly significantly associated with language, motor, cognitive, and behavioral deficits. These results suggest that secondary impaired cerebral cortical volumetric growth following a direct contralateral CBI likely underlies the developmental impairments observed in these children. The authors postulated that transtentorial trophic withdrawal is associated with secondary growth impairment of cerebral cortical development after remote CBI. Remarkably, only about a third of the studies included in this review tentatively controlled for this crossed cerebellar diachisis effect by adjusting for cerebral volume or injury in their analyses [11, 22, 23, 25, 26, 29, 30, 36, 37]. Future studies combining advanced regional volume analyses of the cerebrum and cerebellum will help to better elucidate this phenomenon and its functional implications. Moreover, given the highly plastic properties of the cerebellum alongside its protracted developmental course, the potential role of early intervention to prevent secondary developmental disruption warrants further exploration.
Conclusion and Future Directions
This review highlights that cerebellar injury (direct or indirect) in very preterm born infants has far-reaching functional consequences among survivors. Collectively, the existing literature supports the notion of a developmental form of cerebellar cognitive affective syndrome in prematurity-related cerebellar injury. To date, cerebellar structure-function relationships have been explored almost exclusively using single measurement designs and primarily at a macrostructural level by qualitative evaluation of the cerebellar structure or 3-D volumetric MRI. Future prospective, longitudinal studies applying serial advanced quantitative MRI techniques such as diffusion tensor imaging (DTI), functional MRI, and 1H-MRS will provide critically important, currently unavailable insights at a microstructural, functional, and metabolic level. Likewise, ongoing research is needed in order to precisely delineate the relationship between prematurity-related cerebellar injury and the true prevalence of autism spectrum disorders. Collectively, this will allow clinicians to provide more informed prognostic counseling and anticipatory planning, as well as the development of more timely, tailored, and cost-effective models for early intervention that will lead to better allocation of resources.
Acknowledgments
Marie Brossard-Racine received post-doctoral fellowship support from the Canadian Institute of Health Research at the time of manuscript preparation. We also want to thank Dr. Maria Powell for her assistance with manuscript review/editing.
Footnotes
Conflict of Interest We, the authors, certify that we have no conflict of interest to disclose.
References
- 1.Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24(9):1085–104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tam EWY, Chau V, Ferriero DM, Barkovich AJ, Poskitt KJ, Studholme C, et al. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci Transl Med. 2011;3(105):105ra. doi: 10.1126/scitranslmed.3002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CH, Chang FM, Yu CH, Ko HC, Chen HY. Assessment of fetal cerebellar volume using three-dimensional ultrasound. Ultrasound Med Biol. 2000;26(6):981–8. doi: 10.1016/s0301-5629(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 4.Law M, MacDermid J. Evidence-based rehabilitation. 3rd Slack Inc; Thorofare: 2013. [Google Scholar]
- 5.Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116(4):844–50. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 6.Limperopoulos C, Benson CB, Bassan H, Disalvo DN, Kinnamon DD, Moore M, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005;116(3):717–24. doi: 10.1542/peds.2005-0556. [DOI] [PubMed] [Google Scholar]
- 7.Di Salvo DN. A new view of the neonatal brain: clinical utility of supplemental neurologic US imaging Windows. Radiographics. 2001;21(4):943–55. doi: 10.1148/radiographics.21.4.g01jl14943. [DOI] [PubMed] [Google Scholar]
- 8.Steggerda SJ, Leijser LM, Wiggers-de Bruïne FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. 2009;252(1):190–9. doi: 10.1148/radiol.2521081525. [DOI] [PubMed] [Google Scholar]
- 9.Steggerda SJ, De Bruine FT, van den Berg-Huysmans AA, Rijken M, Leijser LM, Walther FJ, et al. Small cerebellar hemorrhage in preterm infants: perinatal and postnatal factors and outcome. Cerebellum. 2013;12(6):794–801. doi: 10.1007/s12311-013-0487-6. [DOI] [PubMed] [Google Scholar]
- 10.Johnsen SD, Tarby TJ, Lewis KS, Bird R, Prenger E. Cerebellar infarction: an unrecognized complication of very low birthweight. J Child Neurol. 2002;17(5):320–4. doi: 10.1177/088307380201700502. [DOI] [PubMed] [Google Scholar]
- 11.Mercuri E, He J, Curati WL, Dubowitz LM, Cowan FM, Bydder GM. Cerebellar infarction and atrophy in infants and children with a history of premature birth. Pediatr Radiol. 1997;27(2):139–43. doi: 10.1007/s002470050085. [DOI] [PubMed] [Google Scholar]
- 12.Rollin NK, Wen TS, Domingues R. Crossed cerebellar atrophy in children: a neurologic sequela of extreme prematurity. Pediatr Radiol. 1995;25(suppl 1):S20–5. [PubMed] [Google Scholar]
- 13.Shamoto H, Chugani HT. Glucose metabolism in the human cerebellum: an analysis of crossed cerebellar diaschisis in children with unilateral cerebral injury. J Child Neurol. 1997;12:407–14. doi: 10.1177/088307389701200701. [DOI] [PubMed] [Google Scholar]
- 14.Cosentino-Rocha L, Klein VC, Linhares MB. Effects of preterm birth and gender on temperament and behavior in children. Infant Behav Dev. 2014;37(3):446–56. doi: 10.1016/j.infbeh.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Stoodley CJ, Stein JF. Cerebellar function in developmental dyslexia. Cerebellum. 2013;12(2):267–76. doi: 10.1007/s12311-012-0407-1. [DOI] [PubMed] [Google Scholar]
- 16.Tam EW. Potential mechanisms of cerebellar hypoplasia in prematurity. Neuroradiology. 2013;55(Suppl 2):41–6. doi: 10.1007/s00234-013-1230-1. [DOI] [PubMed] [Google Scholar]
- 17.de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012;54(4):313–23. doi: 10.1111/j.1469-8749.2011.04216.x. [DOI] [PubMed] [Google Scholar]
- 18.Reeber SL, Otis TS, Sillitoe RV. New roles for the cerebellum in health and disease. Front Syst Neurosci. 2013;7:83. doi: 10.3389/fnsys.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–95. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 20.Padilla N, Alexandrou G, Blennow M, Lagercrantz H, Aden U. Brain growth gains and losses in extremely preterm infants at term. Cereb Cortex (New York, NY: 1991) 2014 doi: 10.1093/cercor/bht431. doi:10.1093/cercor/bht431. [DOI] [PubMed] [Google Scholar]
- 21.Poretti A, Boltshauser E, Doherty D. Cerebellar hypoplasia: differential diagnosis and diagnostic approach. Am J Med Genet C: Semin Med Genet. 2014;166C(2):211–26. doi: 10.1002/ajmg.c.31398. [DOI] [PubMed] [Google Scholar]
- 22.Limperopoulos C, Bassan H, Gauvreau KK, Robertson RL, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–93. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 23.Messerschmidt A, Fuiko R, Prayer D, Brugger P, Boltshauser E, Zoder G, et al. Disrupted cerebellar development in preterm infants is associated with impaired neurodevelopmental outcome. Eur J Pediatr. 2008;167(10):1141–7. doi: 10.1007/s00431-007-0647-0. [DOI] [PubMed] [Google Scholar]
- 24.Bednarek N, Akhavi A, Pietrement C, Mesmin F, Loron G, Morville P. Outcome of cerebellar injury in very low birth-weight infants: 6 case reports. J Child Neurol. 2008;23(8):906–11. doi: 10.1177/0883073808318063. [DOI] [PubMed] [Google Scholar]
- 25.Zayek M, Benjamin JT, Maertens P, Trimm RF, Lal CV, Eyal FG. Cerebellar hemorrhage: a major morbidity in extremely preterm infants. J Perinatol. 2012;32(9):699–704. doi: 10.1038/jp.2011.185. [DOI] [PubMed] [Google Scholar]
- 26.Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex. 2012;24(3):728–36. doi: 10.1093/cercor/bhs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kooij BJM, de Vries LS, Ball G, van Haastert IC, Benders MJNL, Groenendaal F, et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. Am J Neuroradiol. 2012;33(1):188–94. doi: 10.3174/ajnr.A2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–48. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 29.Tam EW, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr. 2011;158(2):245–50. doi: 10.1016/j.jpeds.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kooij BJ, Benders MJ, Anbeek P, van Haastert IC, de Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54:260–6. doi: 10.1111/j.1469-8749.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 31.Zafeiriou DI, Ververi A, Anastasiou A, Soubasi V, Vargiami E. Pontocerebellar hypoplasia in extreme prematurity: clinical and neuroimaging findings. Pediatr Neurol. 2013;48(1):48–51. doi: 10.1016/j.pediatrneurol.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60–4. doi: 10.1177/08830738050200011001. [DOI] [PubMed] [Google Scholar]
- 33.Gadin E, Lobo M, Paul DA, Sem K, Steiner KV, Mackley A, et al. Volumetric MRI and MRS and early motor development of infants born preterm. Pediatr Phys Ther. 2012;24(1):38–44. doi: 10.1097/PEP.0b013e31823e069d. [DOI] [PubMed] [Google Scholar]
- 34.Lind A, Haataja L, Rautava L, Valiaho A, Lehtonen L, Lapinleimu H, et al. Relations between brain volumes, neuropsychological assessment and parental questionnaire in prematurely born children. Eur Child Adolesc Psychiatry. 2010;19(5):407–17. doi: 10.1007/s00787-009-0070-3. [DOI] [PubMed] [Google Scholar]
- 35.Lind A, Parkkola R, Lehtonen L, Munck P, Maunu J, Lapinleimu H, et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41:953–61. doi: 10.1007/s00247-011-2071-x. [DOI] [PubMed] [Google Scholar]
- 36.Northam GB, Liegeois F, Chong WK, Wyatt JS, Baldeweg T. Total brain white matter is a major determinant of IQ in adolescents born preterm. Ann Neurol. 2011;69(4):702–11. doi: 10.1002/ana.22263. [DOI] [PubMed] [Google Scholar]
- 37.Shah DK, Anderson PJ, Carlin JB, Pavlovic M, Howard K, Thompson DK, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60(1):97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- 38.Taylor HG, Filipek PA, Juranek J, Bangert B, Minich N, Hack M. Brain volumes in adolescents with very low birth weight: effects on brain structure and associations with neuropsychological outcomes. Dev Neuropsychol. 2011;36(1):96–117. doi: 10.1080/87565641.2011.540544. [DOI] [PubMed] [Google Scholar]
- 39.Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131(1):205–17. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 40.Spittle AJ, Doyle LW, Anderson PJ, Inder TE, Lee KJ, Boyd RN, et al. Reduced cerebellar diameter in very preterm infants with abnormal general movements. Early Hum Dev. 2010;86(1):1–5. doi: 10.1016/j.earlhumdev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Martinussen M, Flanders DW, Fischl B, Busa E, Lohaugen GC, Skranes J, et al. Segmental brain volumes and cognitive and perceptual correlates in 15-year-old adolescents with low birth weight. J Pediatr. 2009;155(6):848–53. doi: 10.1016/j.jpeds.2009.06.015. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker J, Mitchell A, Kalpakidou A, Walshe M, Jung H-Y, Nosarti C, et al. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain. 2008;131(5):1344–51. doi: 10.1093/brain/awn062. [DOI] [PubMed] [Google Scholar]
- 43.Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MHS, Stewart AL, et al. Cognitive and motor function and the size of the cerebellum in adolescent born very pre-term. Brain. 2001;124:60–6. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- 44.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(5):1041–50. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 45.Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123:1051–61. doi: 10.1093/brain/123.5.1051. Pt 5. [DOI] [PubMed] [Google Scholar]
- 46.O’Halloran CJ, Kinsella GJ, Storey E. The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol. 2012;34(1):35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- 47.Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11(2):336–51. doi: 10.1007/s12311-011-0247-4. [DOI] [PubMed] [Google Scholar]
- 48.Schmahmann JD, MacMore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162(3):852–61. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Limperopoulos C, Robertson RL, Sullivan NR, Bassan H, du Plessis AJ. Cerebellar injury in term infants: clinical characteristics, magnetic resonance imaging findings, and outcome. Pediatr Neurol. 2009;41(1):1–8. doi: 10.1016/j.pediatrneurol.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59(2):1560–70. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 52.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 53.Bolduc ME, Du Plessis AJ, Sullivan N, Khwaja OS, Zhang X, Barnes K, et al. Spectrum of neurodevelopmental disabilities in children with cerebellar malformations. Dev Med Child Neurol. 2011;53(5):409–16. doi: 10.1111/j.1469-8749.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 54.Courchesne E. Abnormal early brain development in autism. Mol Psychiatry. 2002;7(Suppl 2):S21–3. doi: 10.1038/sj.mp.4001169. [DOI] [PubMed] [Google Scholar]
- 55.Schmahmann J, Weilburg J, Sherman J. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6(3):254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 56.Becker EB, Stoodley CJ. Autism spectrum disorder and the cerebellum. Int Rev Neurobiol. 2013;113:1–34. doi: 10.1016/B978-0-12-418700-9.00001-0. [DOI] [PubMed] [Google Scholar]
- 57.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 58.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 59.Marien P, Verslegers L, Moens M, Dua G, Herregods P, Verhoeven J. Posterior fossa syndrome after cerebellar stroke. Cerebellum. 2013;12(5):686–91. doi: 10.1007/s12311-013-0478-7. [DOI] [PubMed] [Google Scholar]
- 60.Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(Suppl 9):33–5. doi: 10.1111/j.1528-1167.2007.01399.x. [DOI] [PubMed] [Google Scholar]
- 61.Grill J, Viguier D, Kieffer V, Bulteau C, Sainte-Rose C, Hartmann O, et al. Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg. 2004;101(2 Suppl):152–8. doi: 10.3171/ped.2004.101.2.0152. [DOI] [PubMed] [Google Scholar]
- 62.Turkel SB, Shu Chen L, Nelson MD, Hyder D, Gilles FH, Woodall L, et al. Case series: acute mood symptoms associated with posterior fossa lesions in children. J Neuropsychiatry Clin Neurosci. 2004;16(4):443–5. doi: 10.1176/jnp.16.4.443. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez VG, Stuebing K, Juranek J, Fletcher JM. Volumetric analysis of regional variability in the cerebellum of children with dyslexia. Cerebellum. 2013;12(6):906–15. doi: 10.1007/s12311-013-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex. 2010;46(7):933–46. doi: 10.1016/j.cortex.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Bolduc ME, Limperopoulos C. Neurodevelopmental outcomes in children with cerebellar malformations: a systematic review. Dev Med Child Neurol. 2009;51(4):256–67. doi: 10.1111/j.1469-8749.2008.03224.x. [DOI] [PubMed] [Google Scholar]
- 66.Poretti A, Dietrich Alber F, Brancati F, Dallapiccola B, Valente EM, Boltshauser E. Normal cognitive functions in Joubert Syndrome. Neuropediatrics. 2009;40:287–90. doi: 10.1055/s-0030-1249630. [DOI] [PubMed] [Google Scholar]
- 67.Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130(10):2646–60. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- 68.Bolduc M-E, Du Plessis AJ, Sullivan NR, Guizard N, Zhang X, Robertson RL, et al. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 2011;11(2):531–42. doi: 10.1007/s12311-011-0312-z. [DOI] [PubMed] [Google Scholar]
- 69.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barth A, Bogousslavsky J, Regli F. The clinical and topographic spectrum of cerebellar infarcts: a clinical—magnetic resonance imaging correlation study. Ann Neurol. 1993;33(5):451–6. doi: 10.1002/ana.410330507. [DOI] [PubMed] [Google Scholar]


