Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer and is characterized by remarkable desmoplasia. The desmoplasia is composed of extracellular matrix (ECM) proteins, myofibroblastic pancreatic stellate cells, and immune cells associated with a multitude of cytokines, growth factors, and ECM metabolizing enzymes. The mechanisms of participation of this complex matrix process in carcinogenesis are only starting to be appreciated. Recent studies showed key roles for stellate cells in the production of ECM proteins as well as cytokines and growth factors that promote the growth of the cancer cells all present in the desmoplastic parts of PDAC. In addition, interactions of ECM proteins and desmoplastic secreted growth factors with the cancer cells of PDAC activate intracellular signals including reactive oxygen species that act to make the cancer cells resistant to dying. These findings suggest that the desmoplasia of PDAC is a key factor in regulating carcinogenesis of PDAC as well as responses to therapies. A better understanding of the biology of desmoplasia in the mechanism of PDAC will likely provide significant opportunities for better treatments for this devastating cancer.
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer and the fourth leading cause of cancer-related death in the United States, with a 5-year survival of less than 5%.1 The poor prognosis of this cancer is due largely to its propensity for early local invasion and metastasis. Only aggressive therapy with surgical approaches, sometimes combined with chemotherapy, results in any 5-year survival for patients with this cancer.2
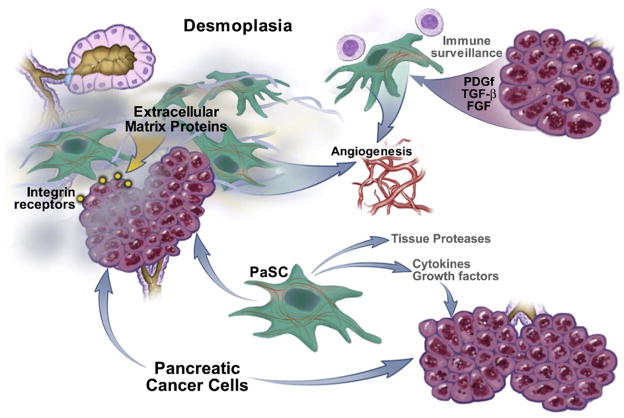
This cancer is also unique among solid tumors because of the extremely dense desmoplastic reaction that surrounds the cancer cell glands of this tumor. One report showed that more extensive fibroblastic cell proliferation in PDAC correlates with poorer disease outcome.3 Desmoplasia containing extracellular matrix (ECM) proteins, myofibroblastic pancreatic stellate cells (PaSCs), and immune cells could modulate the growth of PDAC by providing a scaffold for the cancer cells to grow as well as growth factors and immune modulators (Figure 1). For these reasons, there has been increasing interest in the role of desmoplasia in the biologic characteristics of pancreatic cancer, as discussed in this review. Although there are potentially many factors that make this cancer biologically aggressive and resistant to treatment, the prominence of the desmoplastic reaction has attracted attention of investigators in recent years as a potential source of factors responsible for the severe and malignant biologic behavior of this cancer. Importantly in this regard, there is increasing recognition that the local environment plays an active role in cancer initiation, progression, and metastasis,4,5 and that in many tumors desmoplasia plays an important role in these processes as well as in chemoresistance.5–7 However, despite this increased interest there is lack of critical understanding of the specific roles and effects of desmoplasia in pancreatic cancer. In this article, we summarize what is known about PDAC desmoplasia along with a discussion of issues that should be addressed to advance our knowledge to provide opportunities for therapeutic solutions for this cancer.
Figure 1.
Components of desmoplasia in PDAC. Pancreatic cancer cells and pancreatic duct cells promote each others’ growth and proliferation and together regulate processes of ECM deposition, angiogenesis, and disordered immune surveillance.
Composition of Pancreatic Cancer Desmoplasia
In PDAC there is a remarkable increase in ECM proteins including types I and V collagen and fibronectin.8 Lymph node and liver metastases have a similar increase in ECM proteins. The amount and type of collagens in PDAC desmoplasia are similar to those found in alcoholic chronic pancreatitis and tumor-associated chronic pancreatitis tissue.9 Of note, a comparative analysis of gene expression in human pancreatic adenocarcinoma, chronic pancreatitis, normal pancreas, and pancreatic cancer cell lines demonstrated that gene expression profile of PDAC desmoplasia and chronic pancreatitis is similar.10,11
A major participant in the desmoplasia of PDAC is the PaSC. Although the cancer cells of PDAC are able to produce ECM proteins, recent evidence demonstrates that the PaSC is the main producer of ECM proteins as well as cytokines, chemokines, and growth factors during the development and progression of PDAC. The PaSC and its role in PDAC are discussed in more detail in the next section.
The role of inflammatory/immune cells contained in the desmoplasia of pancreatic cancer is poorly understood. A morphologic analysis of the PDAC specimens12 found that there is a sparse mononuclear cell infiltrate present around cancer glands, with a tendency to cluster at the peripheral invasive edge of the tumor. These cells consist mainly of mast cells, macrophages, and some lymphocytes and plasma cells. Importantly, there was a tendency for poorer survival in patients with tumors with greater numbers of mast cells. The biologic roles that these inflammatory/immune cells play in PDAC are not established but likely include regulation of angiogenesis (mast cells contain angiogenic factors such as vascular endothelial growth factor [VEGF])12 and immune surveillance. Of importance is the finding that a large number of patients with pancreatic cancer have tumor antigen–specific and functionally competent T cells in both peripheral blood and bone marrow that have cytotoxic effects on cultured pancreatic cancer cells.13 These findings suggest that the complex cancer microenvironment prevents the potential beneficial effects of these cytotoxic T cells and thus protects the cancer cells from tumor surveillance probably by elaborating cytokines such as VEGF, interleukin-10, and transforming growth factor–β among other factors.14 A more thorough understanding of the genesis and regulation of factors that undermine immune surveillance is necessary.
Pancreatic Stellate Cell and Cancer Desmoplasia
A topic that has been receiving increased attention in recent years is the presence of PaSCs in the desmoplasia of PDAC and the role of these cells in the biology of this cancer.7,15 PaSCs are one of several cell types normally present in the exocrine pancreas. They are normally located in the periacinar space in a quiescent state, having long cytoplasmic processes that encircle the base of the pancreatic acinus. They can also be found in perivascular and periductal regions of the pancreas.16 In the quiescent state the PaSC has a low rate of proliferation and a low rate of production of ECM proteins, growth factors, and cytokines. PaSCs participate in disease pathogenesis after transforming from their normal quiescent state into an activated state (also known as a myofibroblastic state).17 Activated PaSCs proliferate, migrate, and produce ECM proteins, cytokines, chemokines, and growth factors.18 During disease pathogenesis, the PaSCs maintain the activated state through exposure to cytokines and growth factors likely produced by acinar cells, inflammatory cells, platelets, endothelial cells, cancer cells, and PaSCs themselves.18 Thus, the activated state is initiated and maintained by both paracrine and autocrine mechanisms.18 Of note, isolation and characterization of stromal cells from human pancreatic adenocarcinoma and alcohol-induced chronic pancreatitis samples demonstrated that cells from both sources had the same characteristic morphology, cytofilament expression, and capacity to synthesize ECM proteins.15 Further evidence that the stromal cells in the desmoplasia of PDAC are activated PaSCs comes from findings that cells expressing α-smooth muscle actin, a specific marker of the activated state of the PaSC, co-localize with mRNA encoding procollagen α1I19 in the stromal component of PDAC. These findings have contributed to the general consensus that PaSCs in their activated state are the major contributors to the production of ECM proteins that constitute the desmoplasia.10,15,20–23
Evidence is developing that there is a symbiotic relationship between pancreatic adenocarcinoma cells and PaSCs of PDAC that results in an overall increase in the rate of growth of the tumor and possibly metastasis. For example, culture supernatants from human pancreatic tumor cell lines stimulate PaSC proliferation and production of ECM proteins via the ability of the cancer cells to produce and secrete platelet-derived growth factor, which promotes the activated state and proliferation of PaSCs; and transforming growth factor-β1 and fibroblast growth factor-2, which promote ECM protein production in PaSCs.15,19 In addition, the growth rate of tumor cells injected subcutaneously into nude mice (mice that lack T cells and are severely immunocompromised) is markedly increased when PaSCs are included in the inoculum.15 In contrast to the tumors that form when only cancer cells are injected, the tumors that form when both cancer cells and PaSCs are used have a desmoplasia similar to that observed in human PDAC adenocarcinoma.15
In a “converse” approach to determine the effects of interactions between stromal and cancer cells of PDAC, conditioned media taken from PaSC cultures promoted pancreatic cancer cell proliferation, migration, invasion, and anchorage independent growth.21 Furthermore, the PaSC conditioned media decreased the effectiveness of radiation and chemotherapy (gemcitabine). These findings indicate that PaSCs produce and secrete soluble factors that promote the cancer phenotype of pancreatic cancer cells. These early findings point out important roles for the desmoplasia of PDAC in the mechanism of carcinogenesis.
Of note, in addition to soluble cytokines and growth factors secreted by PaSCs and cancer cells that promote each others’ growth, another possible mechanism by which tumor desmoplasia might promote pancreatic adenocarcinoma cell growth is that PaSCs and tumor cells produce matrix metalloproteinases and tissue serine proteases, such as members of the plasminogen activator system. These mediators can degrade ECM proteins and might promote tumor cell invasion and metastases, as has been postulated for other tumors.24,25
Reactive Oxygen Species Mediate the Effects of Desmoplasia in Cancer Cells
The complete network of mechanisms by which PaSCs and the components of desmoplasia enhance the growth of tumor cells in the PDAC is complex and only partly understood. A potential mechanism by which desmoplasia promotes PDAC is through direct action of ECM proteins and growth factors coming from the stroma on the cancer cells. For example, we have found that both ECM proteins (such as laminin and fibronectin) and growth factors (such as insulin-like growth factor-1) promote survival and prevent death of pancreatic cancer cells.26–28 The effects of laminin and fibronectin come from their interaction with cancer cell integrin receptors. When bound to ECM proteins, the integrin receptors transactivate a key growth factor receptor (for insulin-like growth factor receptor-1) that mediates the intracellular events that promote cancer cell survival and growth.29
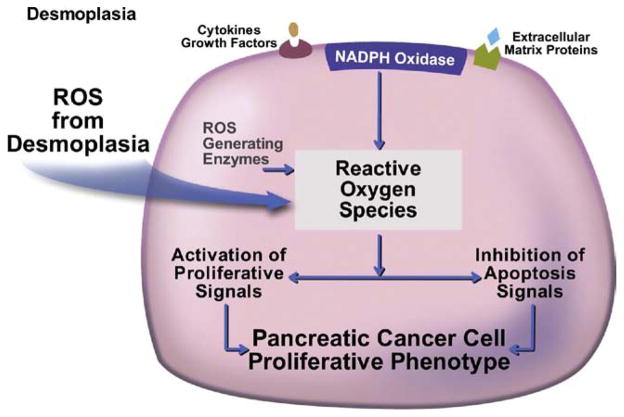
Importantly, our studies revealed that both ECM proteins and growth factors promote survival of the cancer cells through activation of intracellular reactive oxygen species (ROS) generating systems in the pancreatic cancer cells.26–28,30 Key generators of ROS are the non-phagocytic nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes that are regulated by the ECM proteins and growth factors. An important and novel mechanism of ROS action is their effect on an oxidant sensitive group of phosphatases, the protein tyrosine phosphates (PTPs).30 By inhibiting PTP activity, ROS promote and sustain phosphorylation and activity of key cancer-associated kinases such as JAK2 and Akt.30 These activated kinases then mediate the effects of ROS on cancer cell survival and proliferation. These findings show how components of the desmoplasia of PDAC can engage prosurvival and proliferative signals in pancreatic cancer cells by using the generation of ROS to regulate cancer signaling pathways known to have procarcinogenic properties in this and other cancers (Figure 2). Activation of NADPH oxidase by growth factors occurs in both cancer and stellate cells.31 Of note, it has recently been demonstrated that activation of NADPH oxidase in stellate cells mediates liver fibrosis.32 Because stellate cells from both liver and pancreas are likely identical, such results point to a key role for ROS in the actions of PaSCs in the desmoplasia of PDAC.17
Figure 2.
ROS promote the pancreatic cancer cell phenotype. ROS from both desmoplasia and through desmoplasia stimulation of cancer cells through cytokines, growth factors, and ECM proteins mediate the pancreatic cancer cell phenotype.
Conclusions and Further Directions
This brief review presents observations suggesting key roles for desmoplasia in the pathogenesis and severity of this highly malignant cancer. Desmoplasia appears to provide survival benefits for the cancer cells, resistance of the cancer to treatment, and probably immune surveillance dysfunction. Our understandings of the mechanisms that mediate these effects of desmoplasia are only partially understood. However, because it is likely that desmoplasia plays a key role in promoting the resistance of this cancer to treatment, progress in this disease will require much better knowledge about how desmoplasia mediates its effects. If sufficiently understood, it is conceivable that adjunctive therapies targeted to arresting the functions of desmoplasia will improve outcomes.
Acknowledgments
Funding
Supported by the Department of Veterans Affairs, Los Angeles; Hirshberg Foundation for Pancreatic Cancer Research; UCLA Center for Excellence in Pancreatic Diseases (P01AT003960); Southern California Research Center for ALPD and Cirrhosis (P60 AA11999); NIH Grants R01CA119025, R03 AA016008, R21AA016840, R03 AA016008.
Abbreviations used in this paper
- ECM
extracellular matrix
- NADPH
nicotinamide adenine dinucleotide phosphate
- PaSC
pancreatic stellate cell
- PDAC
pancreatic ductal adenocarcinoma
- PTP
protein tyrosine phosphate
- ROS
reactive oxygen species
- VEGF
vascular endothelial growth factor
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ahlgren JD. Epidemiology and risk factors in pancreatic cancer. Semin Oncol. 1996;23:241–250. [PubMed] [Google Scholar]
- 3.Watanabe I, Hasebe T, Sasaki S, et al. Advanced pancreatic ductal cancer: fibrotic focus and beta-catenin expression correlate with outcome. Pancreas. 2003;26:326–333. doi: 10.1097/00006676-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- 5.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 6.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 7.Vonlaufen A, Phillips PA, Xu Z, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 8.Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Imamura T, Iguchi H, Manabe T, et al. Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas. 1995;11:357–364. doi: 10.1097/00006676-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Binkley CE, Zhang L, Greenson JK, et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 12.Esposito I, Menicagli M, Funel N, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz-Winnenthal FH, Volk C, Z’graggen K, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 14.Kleeff J, Beckhove P, Esposito I, et al. Pancreatic cancer micro-environment. Int J Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 15.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Watari N, Hotta Y, Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat Jpn. 1982;58:837–858. doi: 10.2535/ofaj1936.58.4-6_837. [DOI] [PubMed] [Google Scholar]
- 17.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 19.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 21.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koninger J, Giese T, di Mola FF, et al. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun. 2004;322:943–949. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida S, Yokota T, Ujiki M, et al. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem Biophys Res Commun. 2004;323:1241–1245. doi: 10.1016/j.bbrc.2004.08.229. [DOI] [PubMed] [Google Scholar]
- 24.Schneiderhan W, Diaz F, Fundel M, et al. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120:512–519. doi: 10.1242/jcs.03347. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto H, Itoh F, Adachi Y, et al. Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology. 1997;112:1290–1296. doi: 10.1016/s0016-5085(97)70143-4. [DOI] [PubMed] [Google Scholar]
- 26.Edderkaoui M, Hong P, Vaquero EC, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137–G1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 27.Vaquero EC, Edderkaoui M, Nam KJ, et al. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188–1202. doi: 10.1016/s0016-5085(03)01203-4. [DOI] [PubMed] [Google Scholar]
- 28.Vaquero EC, Edderkaoui M, Pandol SJ, et al. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 29.Edderkaoui M, Hong P, Lee JK, et al. Insulin-like growth factor-I receptor mediates the prosurvival effect of fibronectin. J Biol Chem. 2007;282:26646–26655. doi: 10.1074/jbc.M702836200. [DOI] [PubMed] [Google Scholar]
- 30.Lee JK, Edderkaoui M, Truong P, et al. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Hu R, Wang YL, Edderkaoui M, et al. Ethanol augments PDGF-induced NADPH oxidase activity and proliferation in rat pancreatic stellate cells. Pancreatology. 2007;7:332–340. doi: 10.1159/000105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Minicis S, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology. 2006;131:272–275. doi: 10.1053/j.gastro.2006.05.048. [DOI] [PubMed] [Google Scholar]