Abstract
Percutaneous coronary intervention (PCI) based on fractional flow reserve (FFRcath) measurement during invasive coronary angiography (CAG) results in improved patient outcome and reduced healthcare costs. FFR can now be computed non-invasively from standard coronary CT angiography (cCTA) scans (FFRCT). The purpose of this study is to determine the potential impact of non-invasive FFRCT on costs and clinical outcomes of patients with suspected coronary artery disease in Japan. Clinical data from 254 patients in the HeartFlowNXT trial, costs of goods and services in Japan, and clinical outcome data from the literature were used to estimate the costs and outcomes of 4 clinical pathways: (1) CAG-visual guided PCI, (2) CAG-FFRcath guided PCI, (3) cCTA followed by CAG-visual guided PCI, (4) cCTA-FFRCT guided PCI. The CAG-visual strategy demonstrated the highest projected cost ($10,360) and highest projected 1-year death/myocardial infarction rate (2.4 %). An assumed price for FFRCT of US $2,000 produced equivalent clinical outcomes (death/MI rate: 1.9 %) and healthcare costs ($7,222) for the cCTA-FFRCT strategy and the CAG-FFRcath guided PCI strategy. Use of the cCTA-FFRCT strategy to select patients for PCI would result in 32 % lower costs and 19 % fewer cardiac events at 1 year compared to the most commonly used CAG-visual strategy. Use of cCTA-FFRCT to select patients for CAG and PCI may reduce costs and improve clinical outcome in patients with suspected coronary artery disease in Japan.
Keywords: Fractional flow reserve, Non-invasive diagnosis, Cost-effectiveness, Computational fluid dynamics, Coronary computed tomographic angiography
Introduction
Prior studies have shown clinical and economic benefits from assessing and utilizing invasive fractional flow reserve (FFRcath) measurements to guide percutaneous coronary intervention (PCI). In the randomized controlled fractional flow reserve versus angiography for multivessel evaluation (FAME) study including 1,005 patients, it was demonstrated that deferring PCI in vessels not associated with myocardial ischemia based on FFRcath resulted in improved clinical outcomes and lower costs [1, 2]. Furthermore, the FAME II study involving 888 patients demonstrated that PCI in vessels associated with myocardial ischemia based on FFRcath significantly reduced urgent revascularization when compared to medical therapy alone [3]. In all studies published to date, invasive FFR has been assessed during angiography (FFRcath). While FFRcath is widely recommended and offers clinical and economic benefits, it is not yet widely used due to inconvenience and costs [4].
A new technology based on standard coronary computed tomographic angiography (cCTA) allows FFR to be estimated non-invasively (FFRCT) before sending a patient to angiography. Three prospective, multicenter, validation studies have been performed (DISCOVER-FLOW—diagnosis of ischemia-causing stenosis obtained via non-invasive fractional flow reserve [5]; DeFACTO—determination of fractional flow reserve by anatomic computed tomographic angiography [6]; and HeartFlowNXT—HeartFlow analysis of coronary blood flow using CT angiography: NeXT steps [7] to determine the diagnostic accuracy of FFRCT using FFRcath as the reference standard. Good concordance between FFRCT and FFRcath was found with high diagnostic accuracy of FFRCT for the detection or exclusion of hemodynamically significant stenosis using FFR ≤0.80 as the reference standard.
An analysis of potential costs and consequences of utilizing FFRCT to guide clinical decision-making in the United States has suggested the possibility of meaningful cost savings and clinical benefits [8]. In the present paper, we report a similar analysis, using data from the most recently published trial, HeartFlowNXT [7], as well as Japanese procedure and device cost information. The primary objective of this analysis is to determine the potential magnitude of cost savings and clinical benefit which could be expected in Japan through utilization of FFRCT.
Methods
We used data from 254 patients enrolled in the HeartFlowNXT trial [7]. All patients had known or suspected stable coronary artery disease (CAD) and were scheduled for coronary angiography (CAG). Each patient in the trial was evaluated with cCTA, FFRCT, clinically-indicated CAG and FFRcath. Calculation of FFRCT from standard acquired cCTA images and evaluation of FFRcath were performed independently at core laboratories [7]. The study results demonstrated that FFRCT provided high per-patient diagnostic accuracy (81 %) for the detection of hemodynamically significant CAD with a sensitivity of 86 % (95 % CI 77–92 %) and specificity of 79 % (95 CI 72–84 %) using FFRcath as the reference standard. FFRCT also provided excellent discrimination of patients with and without lesion-specific ischemia with an area under the receiver-operating characteristics curve of 0.90 (95 % CI 0.87–0.94 %) [7]. Using patient-specific data from this study, we modeled four hypothetical diagnostic/treatment pathways for patients with known or suspected CAD who are scheduled for coronary angiography (Fig. 1):
Pathway 1: CAG-visual: all patients undergo coronary angiography as scheduled. Those with ≥50 % stenosis by visual assessment of angiographic images undergo PCI.
Pathway 2: CAG-FFRcath: all patients undergo angiography as scheduled. Those patients with ≥50 % stenosis undergo FFRcath and only those with FFRcath ≤0.80 undergo PCI.
Pathway 3: cCTA-CAG: all patients undergo cCTA. Only those with ≥50 % cCTA stenosis undergo CAG. Those with ≥50 % stenosis by visual assessment of the angiogram undergo PCI.
Pathway 4: cCTA-FFRCT-CAG: all patients undergo cCTA. Those with ≥50 % stenosis by cCTA undergo FFRCT. Only those with FFRCT ≤0.80 undergo CAG and PCI is performed after visual angiographic confirmation of the stenosis.
Fig. 1.
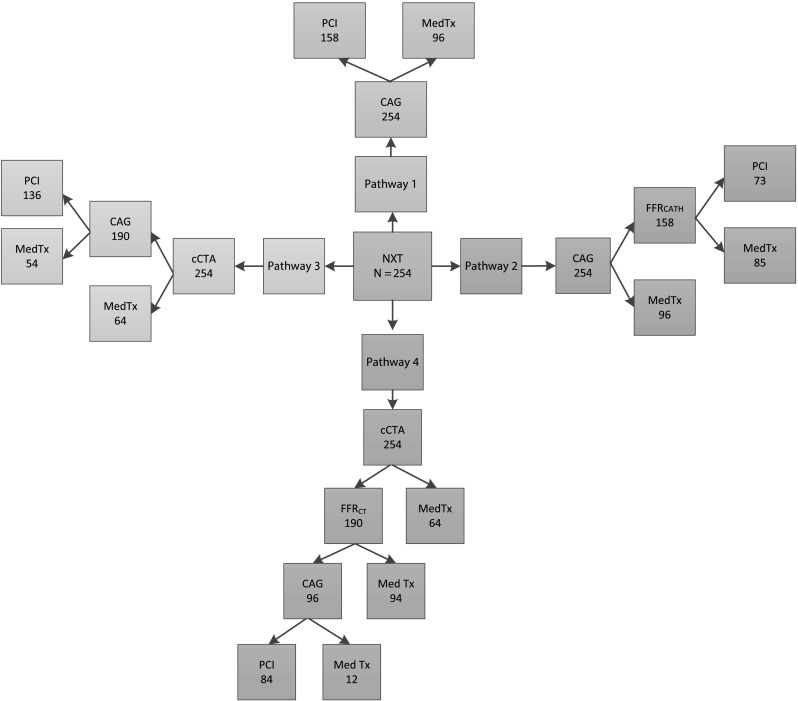
Outline of 4 hypothetical diagnostic/treatment strategies applied to the 254 patient population from the HeartFlowNXT clinical trial
For each pathway, we estimated the costs in Japan in accord with the Japanese Ministry of Health, Labour and Welfare (MHLW) medical insurance medical expense database [9]. We considered cost data from the detailed economic analysis in the 2 years follow-up report of the FAME study [1] and applied the conversion rate of 93.42 Yen/Dollar. The peri-procedural costs utilized in this study are shown in Table 1. While the actual cost of FFRCT analysis has not yet been determined, for the purposes of this analysis, we sought the cost of FFRCT which produced equivalence between the costs of Pathway 2 and Pathway 4. This was calculated to be $2,000, which is comparable to the FFRcath disposable costs + CAG procedure fee as reimbursed by the MHLW medical insurance. The analysis of each pathway involved totaling the costs for each test and procedure described for all patients in that pathway and dividing by the total number of patients (254) giving the average cost per patient.
Table 1.
Procedural costs used in the analysis
| Costs per procedure | Procedure fee | Device cost | Hospital stay | Total costs | ||
|---|---|---|---|---|---|---|
| Per night | Avg nights | Total | ||||
| Angio | $420 | $60 | $1,500 | 1.4 | $2,100 | $2,580 |
| PCI-1 vessel | $2,550 | $5,789 | $1,500 | 2.0 | $3,000 | $11,339 |
| PCI-2 vessel | $2,550 | $9,802 | $1,500 | 2.0 | $3,000 | $15,352 |
| PCI-3 vessel | $2,550 | $13,815 | $1,500 | 2.0 | $3,000 | $19,365 |
| cCTA | $400 | – | – | 0.0 | – | $400 |
| FFR | $42 | $1,800 | – | 0.0 | – | $1,842 |
| Price FFRct | – | $2,000 | – | 0.0 | – | $2,000 |
We estimated future event rates for appropriately and inappropriately treated patients using the FFR cutoff value of <0.80 drawing on data from the deferral versus performance of PTCA in patients without documented ischemia (DEFER) [10], FAME [11, 12] and providing regional observations to study predictors of events in the coronary tree (PROSPECT) [13] studies. We considered a coronary lesion to be significant by visual assessment of the CAG or cCTA if lumen stenosis was ≥50 % and defined functional significance of a lesion as either an FFRcath or FFRCT of ≤0.80. Accordingly, we used the following assumptions to estimate the combined 1 year death/MI rate for: (a) PCI in patients with FFRcath ≤0.80: 3 %; (b) PCI in patients with FFRcath >0.80: 3 %; (c) medical therapy in patients with FFRcath >0.80: 1 %; (d) medical therapy in patients with FFRcath ≤0.80: 5 %; (e) invasive measurement of FFRcath: 0.4 % [7, 14].
Results
A total of 254 patients from the international, multicenter HeartFlowNXT trial had complete information and were included in the analysis. Fifty-seven patients (22 %) in the study were from Japanese clinical sites. Patient characteristics are shown in Table 2. One-third of the patients (80/254) had ischemia-causing stenoses with FFR ≤0.80 and 21 % of the 484 vessels in which invasive FFR was measured had FFRcath ≤0.80.
Table 2.
Characteristics of study population (n = 254)
| Age ± SD (range) | 64 ± 10 years (32–84 years) |
| Men:women (%) | 162:92 (64:36 %) |
| Asian:Caucasian (%) | 86:163 (34:64 %) |
| Hyperlipidemia (%) | 200 (79 %) |
| Hypertension (%) | 174 (69 %) |
| Diabetes (%) | 58 (23 %) |
| Current smoking (%) | 46 (18 %) |
| Prior myocardial infarction (%) | 5 (2 %) |
| Angina within past month (%) | 197 (78 %) |
Utilization and costs
In this analysis, we modeled the expected cost to treat each patient. The overall cost for each patient is dependent on the test(s) utilized in a given pathway, the lesion measurements for each test, the order in which tests are performed and the treatment performed in accord with the test results. As discussed above, in this analysis we included four hypothetical clinical pathways. The average per-patient cost, for each clinical pathway is shown on Table 3.
Table 3.
Peri-procedural costs and 1 year clinical event rates
| CAG visual | CAG-FFRcath | cCTA-CAG | cCTA-FFRCT-CAG | |
|---|---|---|---|---|
| No. of patients undergoing CAG (per 100 pts) | 100 | 100 | 75 | 38 |
| No. of patients undergoing PCI (per 100 pts) | 62 | 29 | 54 | 33 |
| Vessels treated by PCI (per 100 pts) | 80 | 37 | 72 | 48 |
| Costs per patient | $10,360 | $7,222 | $9,128 | $7,222 |
| 1 year event rate | 2.4 % | 1.9 % | 2.2 % | 1.9 % |
In the most commonly used clinical strategy, CAG visual (Pathway 1), all patients would undergo CAG, 62 % would undergo PCI and 80 vessels per 100 patients would be treated based on visual assessment of the angiogram with an average cost of $10,360 per patient. In Pathway 2, FFRcath was used to select patients for PCI and only 29 % of patients would undergo PCI with 37 vessels per 100 patients requiring PCI, assuming strict adherence to the recommended threshold of FFRcath ≤0.80. This 54 % reduction in PCI would result in a potential average cost of $7,222 per patient corresponding to a 30 % savings per patient when compared to Pathway 1. In Pathway 3, cCCTA was used to select patients for CAG reducing the number of angiograms by 25 % and the number of patients undergoing PCI by 13 % compared to Pathway 1 (CAG visual). PCI was guided by visual CAG and the number of vessels treated was reduced by only 10 % with average cost savings of 12 % relative to Pathway 1. In Pathway 4, the strategy of initial cCCTA with FFRCT in patients with ≥50 % stenosis and CAG only in those with FFRCT ≤0.80 reduced the number of angiograms by 62 % and number of patients undergoing PCI by 47 %. Only 48 vessels per 100 patients needed PCI with an average cost per patient of $7,222, a 30 % cost savings per patient compared to Pathway 1.
Clinical events
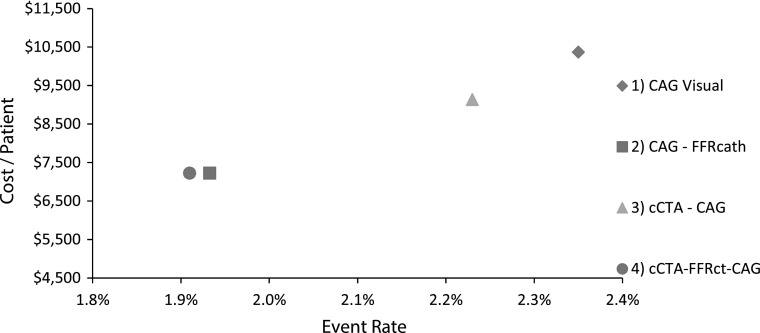
The estimated one-year rate of death or MI for the CAG visual-guided strategy (Pathway 1) was 2.4 % (Table 3). Use of cCTA to select patients for CAG (Pathway 3) reduced the death/MI rate to 2.2 % due to the reduction in number of CAG. The use of FFRcath or FFRCT to guide PCI treatment (Pathways 2 and 4) resulted in the greatest reduction (21 %) in death/MI rate to 1.9 % compared to visual angiography-guided treatment. The combined effects of cost and clinical event rate are shown in Fig. 2.
Fig. 2.
Combined per-patient cost and projected 1 year event rate (death/MI) for 4 clinical pathways modeled in this study. FFRcath and FFRCT guided clinical pathways demonstrated the lowest cost and clinical event rate compared to visual-guided treatment strategies
Discussion
Three prospective, multicenter clinical trials, comprising 609 patients and 1,050 vessels have evaluated the diagnostic accuracy of FFRCT using FFRcath as the reference standard [5–7]. Each study has shown FFRCT to have high diagnostic accuracy for the diagnosis of lesion-specific ischemia using FFRcath as the reference standard with significant improvement in the ability to discriminate patients with and without ischemia when compared to cCTA [5–7] and CAG [7]. The use of invasive FFRcath to identify lesion-specific ischemia and guide coronary intervention is now well-established and has been shown to not only improve patient outcome but also reduce costs [1]. It has been suggested that non-invasive FFRCT may be poised to assume the role of gatekeeper to the interventional catheterization laboratory, especially for intermediate stenosis [15], and a previously modeled analysis based on US data has suggested that the use of FFRCT may improve patient outcomes while reducing healthcare costs [8].
In this study we based our analysis on the most recently published experience with FFRCT which incorporates the latest refinements in software technology with automated image processing and improved physiologic modeling of coronary flow parameters [7]. The results show that the diagnostic performance of non-invasive FFRCT compares favorably to invasive FFRcath and can discriminate ischemia-causing stenoses from non-functional stenoses. According to European Society of Cardiology [16] and American Heart Association [17] practice guidelines, FFRcath is the gold standard for assessing the hemodynamic significance of coronary lesions and for interventional clinical decision-making; however, FFRcath is not practical in many cases for reasons of safety and time. FFRCT has potential value in selecting patients for CAG and interventional treatment with the potential of achieving significant reduction in costs and improving outcomes compared to visual angiography-guided treatment. As indicated by the model in this study, the utilization of FFRCT in Japan may result in fewer diagnostic catheterizations, fewer inappropriate PCI treatments, improved patient outcomes, and a 30 % reduction in average cost per patient relative to standard care (Pathway 1) if fully implemented.
In addition to advancing patient care, utilizing FFRCT technology in Japan may provide significant cost savings for the overall Japanese Healthcare System by safely deferring unnecessary CAG and identifying patients who would benefit from PCI. In 2011 it is estimated that 504,476 coronary angiographies and 181,991 non-emergent PCIs were performed in Japan [18]. If our analysis can be extrapolated to the larger population, the utilization of FFRCT might decrease coronary angiographies by as much as ~60 % and PCI procedures by ~40 %. Based on the results in this study, we estimate that widespread implementation of FFRCT in Japan has the potential to result in considerable cost savings to the Japanese Healthcare System while improving the clinical outcome for patients.
Study limitations
This study has several important limitations. First, this study is a simulation of possible costs and outcomes rather than documentation of costs incurred and outcomes experienced utilizing FFRCT in actual clinical practice. While direct assessment of cost-efficacy is not yet available for FFRCT or other non-invasive testing modalities in stable CAD, the source data for this study represents the largest clinical experience of any non-invasive testing modality using FFRcath as the reference standard. FFRCT has close direct correlation to measured FFR, which has well-documented outcome and cost data in more than 1,005 patients [11], but independent confirmation with actual outcome data utilizing FFRCT for clinical decision making is needed. The extent to which patients analyzed in this report may not precisely reflect the spectrum of patients undergoing CAG in Japan may limit the ability to extrapolate directly to expected outcomes in Japan. A prospective longitudinal study evaluating clinical outcomes, resource utilization and quality of life of FFRCT-guided evaluation and treatment of patients with suspected CAD is currently underway (PLATFORM trial, clinical trials.gov NCT01943903). Second, this study did not include patients with acute coronary ischemia, patients with prior PCI or CABG, and patients who are not suitable candidates for cCTA. Thus, the usefulness of FFRCT in this broader population of patients with CAD is unknown. Third, costs related to clinical adverse events during follow-up were not considered; however, the FFRCT guided pathway had the lowest event rate during follow-up. Fourth, FFRCT is not yet widely available and market pricing for this test has not yet been determined. This analysis uses an FFRCT price of $2,000, the price at which the costs of Pathways 2 and 4 were equivalent. The resulting average total cost of treating a patient in Pathway 4 (cCTA-FFRCT-CAG) is $7,222. If the price of FFRCT is modeled as $1,500, the average cost of treating a patient in this pathway decreases to $6,848, a 34 % savings compared to standard care (Pathway 1). If the price of FFRCT is modeled as $2,500, the average cost for a patient in this pathway is $7,596, a 27 % reduction compared to standard care (Pathway 1). Finally, this analysis does not consider the possibility of only partial or limited adoption of the FFRCT decision pathway, which inherently would limit the potential cost savings of this approach. For example, the decision to send a patient to CAG is made by comprehensive evaluation of the patient’s symptoms and physical findings, risk profile, and results of other non-invasive tests for myocardial ischemia and may cause the physician to override the results of the FFRCT. Similarly, physicians in Pathway 2 may choose to—not measure FFR in the cath lab or may override the results of FFRcath thus reducing the potential economic and outcome benefit. Thus, actual savings achieved may be limited by physicians’ adherence to the clinical decision making pathway. Further evaluations including prospective outcome studies are underway to better understand and quantify the potential clinical and economic improvements identified in this simulation.
Conclusion
Analysis of data from the HeartFlowNXT trial and using Japanese costs of goods and services suggest that utilization of non-invasive FFRCT for clinical decision making could improve clinical outcomes and decrease costs by more accurately identifying patients for CAG and PCI.
Acknowledgments
The NXT trial was funded by the HeartFlow, Inc.
Conflict of interest
Ben Forrest and Christopher K. Zarins are employees of HeartFlow, Inc.
References
- 1.Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, et al. Fractional flow reserve versus angiography for multivessel evaluation study I. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010;122:2545–2550. doi: 10.1161/CIRCULATIONAHA.109.925396. [DOI] [PubMed] [Google Scholar]
- 2.Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the fame (fractional flow reserve versus angiography for multivessel evaluation) study. J Am Coll Cardiol. 2010;56:177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Fearon WF, Shilane D, Pijls NH, Boothroyd DB, Tonino PA, Barbato E, et al. Fractional flow reserve versus angiography for multivessel evaluation I. Cost-effectiveness of percutaneous coronary intervention in patients with stable coronary artery disease and abnormal fractional flow reserve. Circulation. 2013;128:1335–1340. doi: 10.1161/CIRCULATIONAHA.113.003059. [DOI] [PubMed] [Google Scholar]
- 4.Kleiman NS. Bringing it all together: integration of physiology with anatomy during cardiac catheterization. J Am Coll Cardiol. 2011;58:1219–1221. doi: 10.1016/j.jacc.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter discover-flow (diagnosis of ischemia-causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol. 2011;58:1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic ct angiography. JAMA. 2012;308:1237–1245. doi: 10.1001/2012.jama.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S; NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014;63(12):1145–55. [DOI] [PubMed]
- 8.Hlatky MA, Saxena A, Koo BK, Erglis A, Zarins CK, Min JK. Projected costs and consequences of computed tomography-determined fractional flow reserve. Clin Cardiol. 2013;36:743–748. doi: 10.1002/clc.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health, Labour and Welfare. http://www.Mhlw.Go.Jp/bunya/iryouhoken/iryouhoken14/index.html.
- 10.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the defer study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 11.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 12.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the fame study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 14.Pijls NH, Sels JW. Functional measurement of coronary stenosis. J Am Coll Cardiol. 2012;59:1045–1057. doi: 10.1016/j.jacc.2011.09.077. [DOI] [PubMed] [Google Scholar]
- 15.Hecht HS. The game changer? J Am Coll Cardiol. 2014;63(12):1156–8. [DOI] [PubMed]
- 16.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31(20):2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 17.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 18.JCS National Survey on Management of Cardiovascular Diseases: Annual Report. http://www.J-circ.Or.Jp/jittai_chosa/jittai_chosa2011web.Pdf (2011).