Abstract
We previously showed that minimal residual disease (MRD) detection pre- hematopoietic cell transplant (HCT) and acute graft versus host disease (aGVHD) independently predicted risk of relapse in pediatric ALL. In this study we further define risk by assessing timing of relapse and the effects of leukemia risk category and post-HCT MRD. By multivariate analysis, pre-HCT MRD < 0.1% and aGVHD by day +55 were associated with decreased relapse and improved EFS. Intermediate leukemia risk status predicted decreased relapse, and improved EFS and OS. Patients with pre-HCT MRD ≥ 0.1% who did not develop aGVHD compared to those with MRD <0.1% who did develop aGVHD had much worse survival (2yr EFS 18 vs. 71%; p=0.001, 2yr OS 46 vs. 74%; p=0.04). Patients with pre-HCT MRD <0.1% who did not experience aGVHD had higher rates of relapse if they did not develop aGVHD (40% vs 13%; p= 0.008). Post-HCT MRD led to a substantial increase in relapse risk (HR=4.5, p<0.01). Patients at high risk of relapse can be defined after transplant using leukemia risk category, presence of MRD pre- or post-HCT, and occurrence of aGVHD. An optimal window to initiate intervention to prevent relapse occurs between day +55 and +200 after HCT.
Introduction
Published classification schemes for childhood acute lymphoblastic leukemia (ALL) describing when allogeneic hematopoietic cell transplantation (HCT) is recommended are based upon response to therapy and genetic characteristics.1, 2 After a first or subsequent relapse, further delineation of risk of failure of therapy has been made based upon timing and site of relapse, immunophenotype, and persistence of minimal residual disease (MRD) after reinduction therapy.3–6 Although high rates of post-HCT relapse have been noted when persistent MRD is present prior to the HCT preparative regimen, the effect of MRD on established leukemia risk groups has not been explored. In addition, little is known about whether post-HCT events such as graft versus host disease (GVHD) alter predicted outcomes.
Although often debated, several studies7–15 have suggested a positive effect of GVHD in decreasing relapse after allogeneic HCT for ALL. We confirmed this finding in a prospective phase III of adding sirolimus for GVHD prophylaxis.16–20
Past attempts to de-escalate GVHD prophylaxis and encourage GVL have sometimes shown success,21 but can lead to significant morbidity associated with GVHD.22 Any child subjected to either immune withdrawal or a potentially dangerous targeted agent early after HCT should be at very high risk for relapse in order to minimize unnecessary toxicity. Because both aGVHD after transplant and MRD pre-HCT and post-HCT may significantly effect relapse risk,23, 24 careful study of the interplay between these factors is needed to define high-risk populations where interventions to reduce relapse could be performed. In this study we look at the effect of established leukemia disease risk classification groups, pre- and post-HCT MRD detection, and the occurrence of GVHD on relapse and survival. We then identify target populations and timeframes post-HCT where intervention to prevent relapse would have the greatest impact.
Patients and Methods
Protocol COG ASCT0431 (PBMTC ONC051) was conducted from 2007–2011 and was approved by the NCI Central IRB and local IRBs as applicable. The trial was open to ages 1–21 with ALL in CR1 and CR2, utilizing related and unrelated bone marrow or cord blood. Three leukemia risk categories of patients were allowed: High Risk CR1 (Ph+ ALL, extreme hypodiploidy [<44 chromosomes], or induction failure [either M3 BM at day +29 or M2 or minimal residual disease (MRD)>1% at day +43]); High Risk CR2 (B-cell BM relapse <36m from diagnosis, T-cell or Ph+ BM relapse at any time, T-cell isolated extramedullary [IEM] relapse <18m from diagnosis); and Intermediate Risk CR2 (B-cell BM relapse ≥36m from diagnosis, B-cell IEM <18m from diagnosis). Details regarding transplant approach, GVHD grading, and 6-color multi-channel flow cytometry MRD were published previously.23, 25–27 MRD was measured on BM pre-HCT and at 1,3 and 8–12m post-HCT, and was performed at a single COG central flow laboratory (Johns Hopkins). Centers were blinded to pre- and post-HCT MRD results, and modification of the protocol based upon local MRD results was not allowed.
Statistical Approach
Patients who did not experience an event were censored at the time of last contact. Event free survival (EFS) and overall survival (OS) were estimated by the Kaplan-Meier method.28 The log-rank statistic was used to compare EFS and OS between standard and experimental treatment groups. The HRs, CIs, and tests of significance associated with patient characteristics were assessed using proportional hazards regression modeling.29 Time-dependent coding of GVHD occurrence and post-HCT MRD status was used to appropriately account for timing of these factors and number of post-HCT MRD assessments was included in all regression models evaluating post-HCT MRD status. Cause-specific outcome probabilities of relapse or TRM over time were estimated using non-parametric methods of Aalen and Johannsen.30
To provide graphical comparisons of outcome probabilities over time by aGVHD status, the landmark analysis curve29 approach was used in which outcome probabilities were computed from day +55 forward with aGVHD status determined at day +55. Day +55 was chosen because by then 93% of those who developed aGVHD had experienced peak grade and determination of risk classification at this time would allow sufficient time for implementing further treatment strategies. Landmark analyses curves starting anywhere between days +50–80 were essentially equivalent. Eight patients had died of TRM by day +55 (5.5%), with 2 of the 8 having developed aGVHD before death. Two relapses had occurred, meaning 10 out of the 143 patients (7%) did not contribute to the landmark analysis. The use of landmark curves avoids bias that would occur comparing Kaplan-Meier estimates starting at HCT based on aGVHD status, a condition that occurs after HCT.29 P-values given in landmark figures are from the Cox regression analysis with aGVHD as a time-dependent covariate and uses all the available data. HRs for post-HCT MRD and relapse were computed using Cox regression with MRD status and the number of MRD assessments included as time-dependent covariates.
Table 1 shows the patient population involved in the analysis. This phase III trial compared tacrolimus/methotrexate with sirolimus/tacrolimus/methotrexate for GHVD prophylaxis. The populations in both arms were balanced for key factors associated with outcome.23 Although there was less grade I and II aGVHD noted on the sirolimus arm, rates of relapse, survival, and TRM were similar between the two arms. In addition, the effect of aGVHD in decreasing relapse was similar between the treatment arms (p=0.65).23 Rates of MRD and the effect of MRD on outcomes were also noted to be similar in both arms (p=0.21). With this in mind, we combined both arms for this analysis of the effect of GVHD and MRD. The main data analysis of risk groups was performed on patients with pre-HCT MRD data present. Twenty seven percent of patients did not provide pre-HCT BM MRD samples, most often because they had already undergone pre-HCT BM sampling prior to consent and refused a second procedure. Table 1 shows no differences between the pre-HCT MRD present population and the full cohort.
Table 1.
Patient characteristics (percent) in all patients and in patients with pre-HCT MRD assessment.
| All patients | Pre-HCT MRD known | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Percent | N | Percent | |||
|
|
||||||
| Total | 143 | 100% | 105 | 100% | ||
| Age | ||||||
| 1–9 | 76 | 53% | 55 | 52% | ||
| 10+ | 67 | 47% | 50 | 48% | ||
| Sex | ||||||
| Female | 59 | 41% | 43 | 41% | ||
| Male | 84 | 59% | 62 | 59% | ||
| Immunophenotypes | ||||||
| B-Precursor | 113 | 79% | 89 | 85% | ||
| T-Cell | 27 | 19% | 15 | 14% | ||
| Mixed Lineage (MLL) | 3 | 2% | 1 | 1% | ||
| Extramedullary disease at diagnosis | ||||||
| No | 119 | 83% | 91 | 87% | ||
| Yes | 24 | 17% | 14 | 13% | ||
| Leukemia risk group/donor type | ||||||
| High risk CR1 | 49 | 34% | 39 | 37% | ||
| High risk CR2 | 69 | 48% | 46 | 44% | ||
| Intermediate risk CR2 | 25 | 17% | 20 | 19% | ||
| Stem cell source/HLA matching | ||||||
| Matched sibling | 78 | 55% | 57 | 54% | ||
| Other related donor BM (1 PBSC) | 4 | 3% | 2 | 2% | ||
| Unrelated donor – BM (5 PBSC) | 34 | 24% | 27 | 26% | ||
| Unrelated donor - cord blood | 27 | 19% | 19 | 18% | ||
| Leukemia risk group/stem cell source | ||||||
| Risk group | Stem cell source | |||||
| High risk CR1 | sib | 29 | 20% | 22 | 21% | |
| High risk CR1 | non-cord non-sib | 17 | 12% | 14 | 13% | |
| High risk CR1 | cord | 3 | 2% | 3 | 3% | |
| High risk CR2 | sib | 24 | 17% | 15 | 14% | |
| High risk CR2 | non-cord non-sib | 21 | 15% | 15 | 14% | |
| High risk CR2 | cord | 24 | 17% | 16 | 15% | |
| Intermediate risk CR2 | sib | 25 | 17% | 20 | 19% | |
| MRD status pre-transplant | ||||||
| Negative | 75 | 52% | 75 | 71% | ||
| Positive, <0.1% | 13 | 9% | 13 | 12% | ||
| Positive, 0.1%+ | 17 | 12% | 17 | 16% | ||
| Not donea | 38 | 27% | 0 | 0% | ||
| Grade of acute GVHD | ||||||
| None | 88 | 62% | 63 | 60% | ||
| 1 | 20 | 14% | 14 | 13% | ||
| 2 | 19 | 13% | 16 | 15% | ||
| 3 | 9 | 6% | 7 | 7% | ||
| 4 | 7 | 5% | 5 | 5% | ||
| Chronic GVHD | ||||||
| No | 107 | 75% | 76 | 72% | ||
| Yes | 36 | 25% | 29 | 28% | ||
| aGVHD and cGVHD | ||||||
| aGVHD | cGVHD | |||||
| None | No | 73 | 51% | 51 | 49% | |
| None | Yes | 15 | 10% | 12 | 11% | |
| Grade I–III | No | 29 | 20% | 21 | 20% | |
| Grade I–III | Yes | 19 | 13% | 16 | 15% | |
| Grade IV | No | 5 | 3% | 4 | 4% | |
| Grade IV | Yes | 2 | 1% | 1 | 1% | |
Results
Individual and Combined Effects of aGVHD and pre-HCT MRD on Relapse and Survival
As part of the primary manuscript reporting outcomes of COG ASCT0431/PBMTC ONC051, a multivariate analysis defined independent benefits for relapse and survival from 1) low pre-HCT MRD (absent or <0.1% by flow cytometry) and 2) the occurrence of grades I–III aGVHD (grade IV aGVHD decreased relapse but decreased survival due to TRM).23 In this analysis we sought to determine in more detail how these clinical factors combine to identify risk of poor outcomes. We chose to perform a landmark analysis of patients alive with or without aGVHD at day +55 as outlined in the methods section. Figure 1a shows a dramatic difference in risk of relapse between patients with pre-HCT MRD ≥ 0.1% who did not develop aGVHD compared to those with MRD <0.1% who did develop aGVHD (CI of relapse 73% vs 13%; p=<0.0001). It is also notable that there is a significant difference in relapse between those previously considered to be “good risk” patients (pre-HCT MRD <0.1%) who did and did not experience aGVHD (13% vs.40%; p= 0.008).
Figure 1.
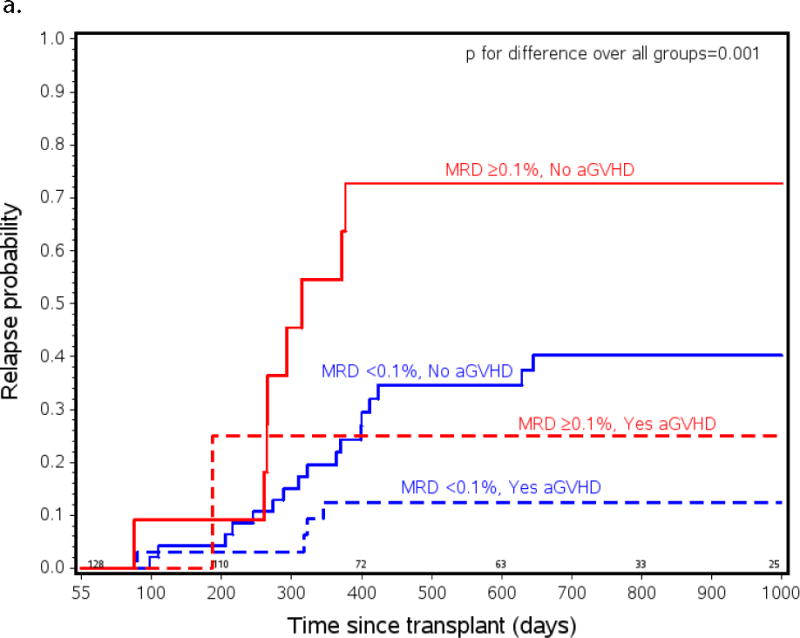
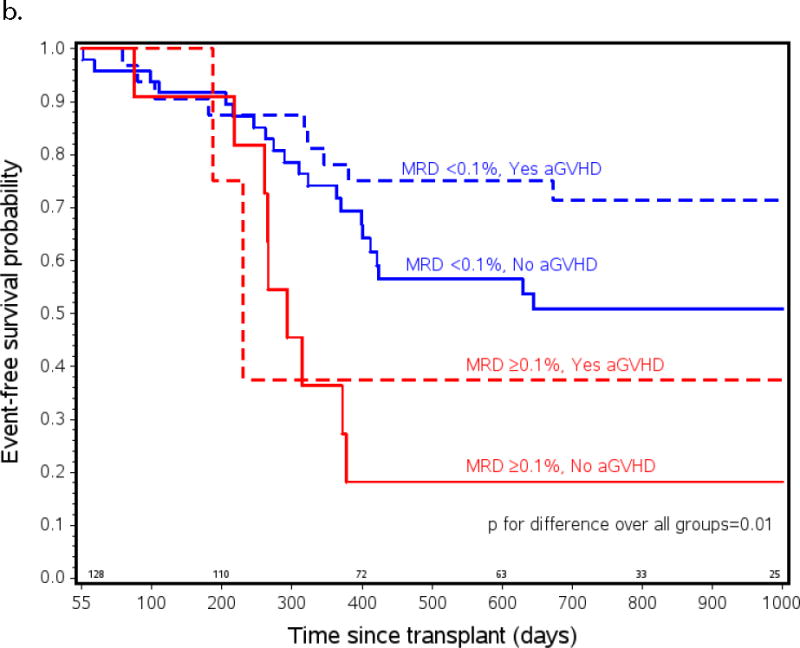
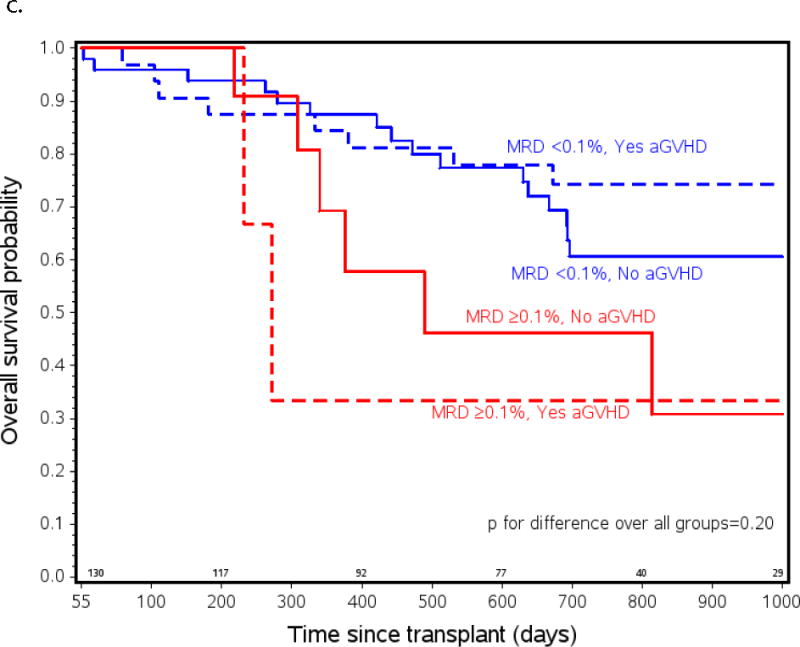
Probability of a) relapse b) event free (EFS) and c) overall survival (OS) in ALL patients from all risk categories who had pre-HCT flow MRD measured and lived to day +55 after transplant (n=105). Patients listed as MRD+ had ≥ 0.1% measured by flow cytometry in BM within 2 weeks initiating their preparative regimen. Patients listed as experiencing aGVHD had onset of any grade of aGVHD before day +55. MRD <0.1% and no aGVHD n=51, MRD <0.1% and yes aGVHD n=37, MRD ≥0.1% and no aGVHD n=12, MRD ≥0.1% and yes aGVHD n=5.
The changes in relapse risk based on these two clinical factors translated into similar changes in EFS. Figure 1b shows a marked difference in survival between patients who had pre-HCT MRD ≥ 0.1% who did not develop aGVHD compared to those with MRD <0.1% who did develop aGVHD (2yr EFS 18 vs. 71%; p=0.001, 2yr OS 46 vs. 74%; p=0.04), and a trend toward increased EFS in the patients who were MRD <0.1% who did or did not develop aGVHD (71% vs 51%; p=0.07). There were only 5 patients who had pre-HCT MRD ≥ 0.1% who did develop aGVHD so possibly due to low numbers, no statistical differences in relapse risk and EFS between this group and any of the others were found.
The Combined Effect of Leukemia Risk Group, aGVHD and pre-HCT MRD on Relapse and Survival
With the noted effects of aGVHD and pre-HCT MRD on outcomes, we asked whether current risk classification groups remain relevant, or if risk could be better predicted using these factors alone. COG ALL risk classifications (high risk [HR] CR1, HR CR2, and intermediate risk [IR] CR2) are based upon time to relapse, immune phenotype, cytogenetics, and site of relapse. Definitions of these leukemia risk groups are detailed in the methods section and are very similar to European classification approaches.4 Hazard ratios estimated in multivariate analyses looking at leukemia risk group, aGVHD, and pre-HCT MRD with aGVHD as a time-dependent covariate, are shown in Table 2. Of note, all three of these factors: leukemia risk group, the presence of high levels of MRD pre-HCT, and the occurrence of grade I–III aGVHD after HCT, independently influenced outcome. Within the risk groups, HR CR1 and HR CR2 ALL were very similar in outcome, but patients with IR CR2 experienced less relapse and better EFS and OS independent of aGVHD and MRD effects. With this in mind, the HR CR1/CR2 groups were combined for further analyses.
Table 2.
Hazard ratios (95% confidence intervals) for leukemia risk group, pre-HCT MRD, and aGVHD in the multivariate model.
| Relapse | TRM | EFS | OS | |
|---|---|---|---|---|
|
|
||||
| IR CR2 vs HR CR2 or HR CR1 | 0.33 0.12–0.94 | 0.21 0.03–1.64 | 0.30 0.12–0.75 | 0.31 0.11–0.86 |
| Pre-HCT MRD <0.1% vs ≥0.1% | 2.72 1.23–5.99 | 1.41 0.39–5.05 | 2.16 1.11–4.20 | 1.80 0.85–3.82 |
| Grade I–III aGVHD vs No aGVHD | 0.40 0.19–0.83 | 0.85 0.29–2.46 | 0.48 0.26–0.89 | 0.56 0.29–1.08 |
| Grade IV aGVHD vs No aGVHD | 0.0 – | 8.33 2.86–24.27 | 2.35 0.97–5.70 | 2.87 1.17–7.09 |
The first 3 lines of Table 3 show the 2-year risk of relapse based on the key pre-HCT factors, leukemia risk group and pre-HCT MRD. In HR CR1 and CR2 patients, the EFS difference in children with low MRD (<0.1%) compared to high MRD (≥0.1%) was more than 30% (47% vs 15%, p<0.0001). Intermediate leukemia risk patients with pre-HCT MRD < 0.1% had an excellent prognosis (>80% EFS). The lower portion of Table 3 shows the modifying effect of aGVHD occurring by day +55 on patient risk of experiencing an event. Patients with grade I–III aGVHD had an approximately 20% higher 2-year EFS probability than patients with no aGVHD in each leukemia risk category and MRD status (Table 2, p=0.02).
Table 3.
Two year EFS probability, relapse risk, TRM risk, and overall survival probability among patients alive and relapse-free at day 55+ by leukemia risk group, pre-HCT MRD.* The top part of the table does not take aGVHD into consideration. The lower part assesses outcome with and without aGVHD prior to day 55 and gives the outcome probabilities from day 55 to 2 years for patients alive and relapse free at day 55.
| Leukemia Risk Group | Pre-HCT MRD | N | aGVHD by day 55 | EFS probability | Relapse risk | TRM risk | Survival probability | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| High risk CR1 or CR2 | <0.1% | 64 | 47.0% | (34.4–58.7) | 30.0% | (19.0–41.7) | 23.0% | (13.7–33.6) | 55.1% | (41.9–66.6) | |
| High risk CR1 or CR2 | ≥0.1% | 16 | 14.6% | (3.2–34.0) | 64.5% | (34.0–83.7) | 20.9% | (6.1–41.4) | 36.3% | (13.3–60.1) | |
| Intermediate risk CR2 | <0.1% | 19 | 83.3% | (57.9–94.1) | 16.7% | (4.8–34.8) | 0.0% | – | 87.5% | (60.0–96.6) | |
|
| |||||||||||
| High risk CR1 or CR2 | <0.1% | 43 | None | 47.0% | (30.2–62.2) | 41.8% | (25.4–57.3) | 11.2% | (3.7–23.5) | 59.9% | (42.1–73.8) |
| High risk CR1 or CR2 | <0.1% | 21 | Grade I–III | 65.8% | (39.5–82.8) | 17.0% | (4.3–37.0) | 17.2% | (4.5–36.8) | 65.6% | (39.1–82.7) |
| High risk CR1 or CR2 | ≥0.1% | 11 | None | 11.7% | (1.8–31.7) | 73.2% | (36.9–90.7) | 15.1% | (3.2–35.4) | 40.8% | (13.5–66.9) |
| High risk CR1 or CR2 | ≥0.1% | 5 | Grade I–III | 36.5% | (1.0–80.4) | 46.7% | (1.1–87.9) | 16.8% | (3.7–38.1) | 30.1% | (1.0–73.3) |
| Intermediate risk CR2 | <0.1% | 8 | None | 72.8% | (30.6–91.8) | 27.2% | (5.8–55.2) | – | 71.4% | (29.9–91.1) | |
| Intermediate risk CR2 | <0.1% | 11 | Grade I–III | 90.6% | (50.8–98.6) | 9.4% | (0.7–32.8) | – | 100.0% | (.–100.0) | |
Grade IV aGVHD outcomes: 1 IR CR2, MRD <0.1% (1 died); 4 H CR2, MRD <0.1% (4 died), 2 HR CR2, MRD not done (1 died)
The population at highest risk for poor outcome was HR CR1/CR2 patients who were MRD+ pre-HCT who then did not experience grade I–III aGVHD (EFS 12%). Patients with the best outcome were those in the intermediate risk category who were MRD <0.1% who experienced grades I–III aGVHD, with EFS above 90%. Of note, HR CR1/CR2 patients who had low MRD, a group thought to have good outcome, had a relatively poor EFS of 47% if they did not get grade I–III aGVHD.
Effect of Post-Transplant Detection of MRD on Relapse
There were a total of 262 post-HCT MRD assessments done in 112 patients (95, 88, and 68 assessments at 1, 3, and 8–12m, respectively), among whom 21 had at least one positive MRD test. Of these 21 patients, 20 had one positive MRD test while one had three positive MRD tests. Detection of any level of post-transplant MRD led to a substantial increase in relapse risk (HR=4.5, p<0.01). Multivariate analysis including pre-HCT MRD and the occurrence of aGVHD showed that the presence of detectable MRD after transplant led to an increase in HR of 3.5 (p=0.003) independent of other factors.
A positive or negative post-HCT MRD test was predictive of relapse or non-relapse, but over time the predictive power of the MRD measurement was lost. Comparing patients whose last MRD test was in the past 60 days, those who tested MRD+ had much higher relapse rates than patients who tested negative (HR 4.5, p=0.01). However, comparing patients whose last MRD test was over 60 days in the past, relapse rates were similar in those who tested positive or negative (HR 1.1, p=0.87). Among the 88 patients who were MRD negative at 1-month post-HCT, 30 eventually relapsed, but none did so within 60 days of the MRD assessment (minimum time was 69 days). In this cohort, there were only two patients who relapsed within 60 days of a negative MRD test.
Flow MRD detected in BM at 8–12 months after HCT was more strongly predictive of relapse than earlier MRD assessments. Risk of relapse by 2 years for patients with MRD detected at 8–12 months post-HCT was 73 vs. 33% (p=0.01), while relapse rates between those with very early post-HCT MRD positivity and those who were MRD negative were not significantly different. Finally, the level of MRD measured post-HCT may be important. Of the 19 MRD+ post-HCT patients with MRD quantitation available, 10 had MRD <0.1% and 9 had MRD ≥0.1% with 2 (20%) and 6 (67%) relapsing in each group (p=0.07).
Defining Target Populations and Optimal Timing for Post-Transplant Interventions
Figure 2a shows a non-parametric patient status analysis of probabilities of 1) relapse-free survival (RFS), 2) relapse, and 3) TRM in patients the first 2 years after transplant, and the effect of the presence or absence of aGVHD on these events. The graph shows 100% of the patient population and follows them over the first few years post-HCT. All patients started alive, without aGVHD (blue lines = no aGVHD), and without relapse. Over time, a portion of patients developed aGVHD (thus moving to the red lines; red lines = experienced aGVHD). Patients with or without aGVHD either stayed alive (solid lines), relapsed (dot-dash), or experienced TRM (dashed lines). The most striking observation was that the probability of relapse after getting aGVHD was low, resulting in remarkable stability of the “alive, yes aGVHD” probability state over time. In contrast, when patients did not get aGVHD their relapse risk was high (as shown by the upward slope of the “relapse, no aGVHD” curve), especially after day +100, and remained high until just over 400 days after transplant. Of note, 59% of all relapses occurred between days +100 and +400 in patients who did not experience aGVHD, implying that a successful intervention to prevent relapse after transplant in this population could have a significant impact on survival.
Figure 2.
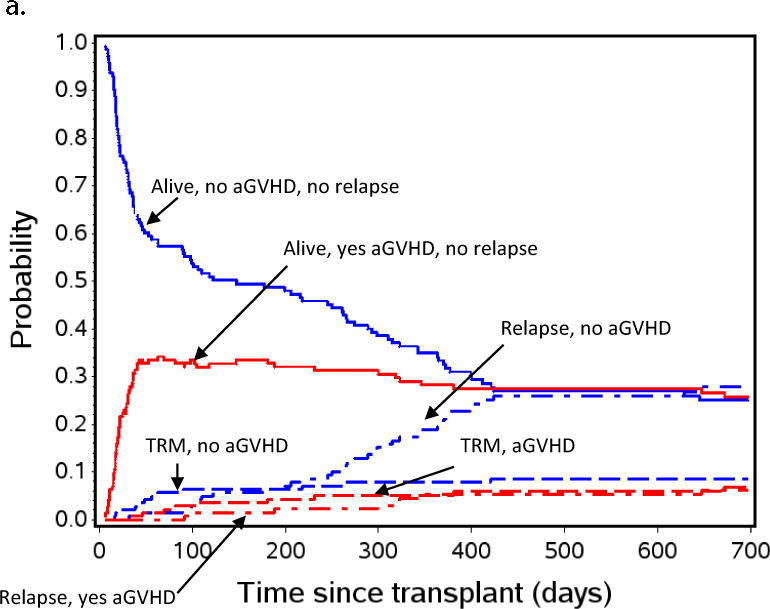
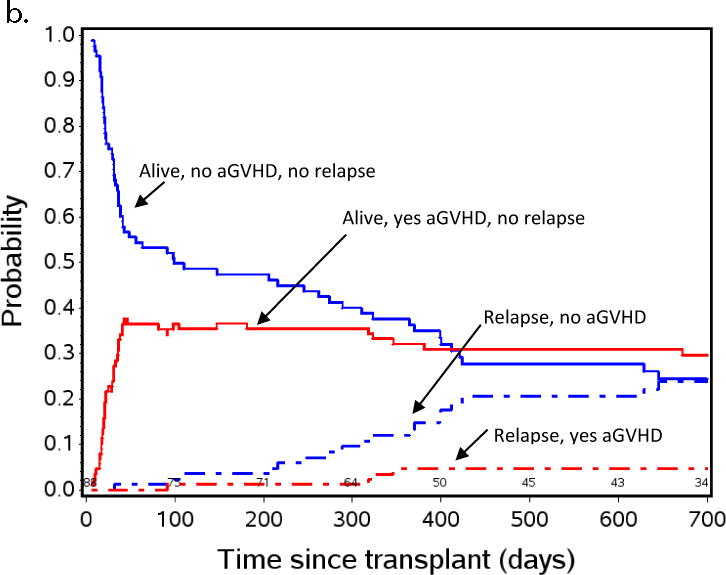
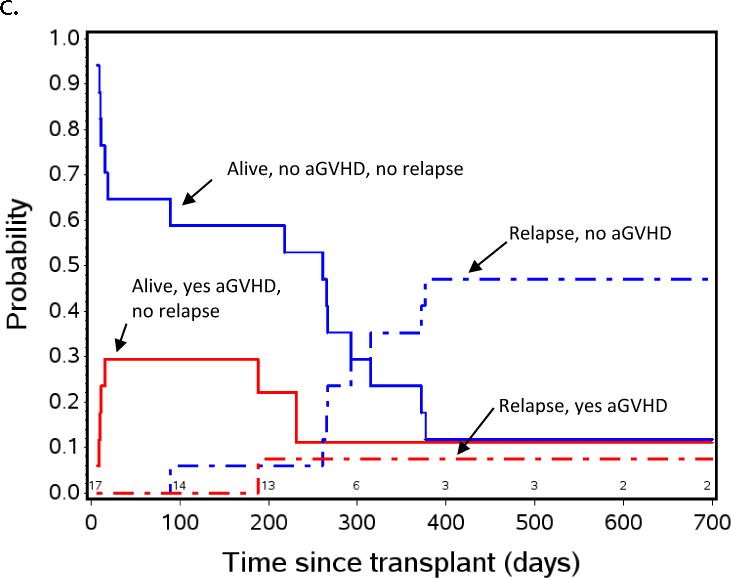
Aalen-Johansen non-parametric patient state probabilities: a) all patients (N=143) are represented through day +700 after HCT. b) patients with low or absent MRD pre-HCT, and c) patients with pre-HCT MRD≥ 0.1% measured by flow cytometry in BM within 2 weeks initiating their preparative regimen. Red and blue lines indicate patients who did (red) or did not (blue) experience aGVHD by day +55. Solid red and blue lines show patients with relapse-free survival, dashed lines represent TRM, dot-dash lines represent relapse. Because TRM was noted to be low in the overall population represented in Figure 2a, patients, this curve was not included in Figures 2b and 2c to emphasize the effect of aGVHD on relapse.
Figures 2b and 2c are similar patient state probability curves for those with pre-HCT MRD < 0.1% or MRD ≥ 0.1%, respectively. These figures show the clear pattern of increased risk of relapse in patients not developing aGVHD and low risk in those who do develop aGVHD, regardless of the pre-HCT MRD. This method of analysis demonstrates a window of opportunity for intervention to prevent relapse between day +55 and day +200, when those at high risk for relapse have been defined, and most patients who will relapse have yet to do so.
Discussion
With promising immunological and cellular therapeutic approaches coming into more general use, it is important to define timing and target populations for intervention to prevent relapse after HCT. This analysis clarifies that both pre-HCT and post-HCT factors (disease risk, pre- and post-HCT MRD, and aGHVD) can be used to define populations with poor outcome. Weaknesses of this study come from the fact that subdividing our cohort created some small clinical subgroups (e.g. pre-HCT MRD+ with aGVHD), follow up may not be sufficient to detect differences in some of our subgroups, and one must always approach unplanned comparisons with caution. That said the clinical factors we describe (pre-HCT MRD, post-HCT aGVHD, IR vs. HRCR1/CR2) define populations with marked and highly significant differences in outcome, allowing for consideration of interventional trials for these patients.
This is the first multicenter study correlating post-HCT flow MRD with relapse adding data to other studies correlating PCR-based post-HCT MRD with outcomes.31 Measurement of MRD by flow cytometry is the standard technique across pediatric ALL trials in the US, as it is well suited for rapid turnover. Negative BM flow MRD after HCT provides reassurance regarding relapse for at least a few months, however, high level (≥ 0.1%) or late (>8months after HCT) detection of flow-MRD is associated with poor outcome. Although the 6-color flow cytometry used in this study detected disease uniformly to a level of 0.01% or lower,32 we do not know if these results are directly comparable to PCR methods. We also do not know whether higher channel flow cytometry or next generation sequencing MRD approaches33 would modify our conclusions regarding risk, but we anticipate that more sensitive approaches will only strengthen the predictive ability of pre- and post- HCT MRD.
Because aGVHD alters a given patient’s risk profile, use of the absence of aGVHD as a criterion to choose a population for post-HCT intervention is an attractive approach. In this study, the patients most likely to benefit from intervention were those in HR CR1/CR2 with MRD ≥0.1% pre-HCT who did not develop aGVHD (overall survival <20%). Of note, the majority of relapses (58%) occurred in patients who had low (<0.1%) or negative MRD pre-HCT. Because patients with low/negative MRD pre-HCT and no aGVHD have a relapse rates >40%, including this population in relapse-reduction studies may be appropriate. If a strategy of treating post-HCT MRD positivity is adopted for patient selection, our data suggest that monitoring would need to be performed every 2 months until at least day +450. Further study using more frequent post-HCT MRD monitoring of later timepoints (from day +100-+450) is needed to better define the predictive power of this assay after HCT.
Two approaches could be taken for post-HCT intervention. The first would be a “maintenance” strategy, employing agents that do not require intact adaptive immunity (e.g. targeted immunotoxins, moxetumomab34 or inotuzumab35). A second approach includes two steps: 1) speeding up the rate of immune taper, and if successfully off immune suppression without significant GVHD, 2) introduction of an agent that uses adaptive immunity to stimulate a GVL effect or targets cancer (e.g. blinatumomab36 or CAR-modified T cells37–39). Either approach should occur early post-HCT in order to avoid the high risk of relapse between day +100 and +450 (Figure 2). Whether such post-transplant approaches will improve survival requires further study. While some studies have suggested improved survival with early immunological interventions for worsening mixed chimerism,40, 41 others using similar interventions for pre-HCT MRD have shown delay in relapse, but no change in survival.42
In conclusion, we have identified strong interactions between leukemia risk groups, the occurrence of aGVHD, and MRD pre- and post-HCT in determining relapse risk and survival in children with ALL after HCT. By day +55, specific populations at very high risk for relapse can be defined. There is a window between day +55 and day +100–200 when the large majority of at-risk patients have not relapsed. Study of interventions with maintenance courses of immunotoxins or weaning immune suppression followed by agents designed to enhance anti-tumor effect may prevent relapse in appropriately defined high-risk populations.
Supplementary Material
Figure 3.
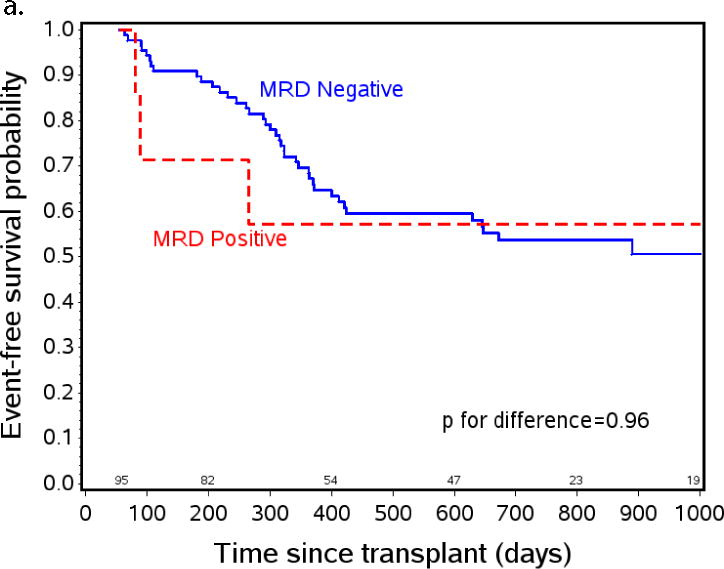
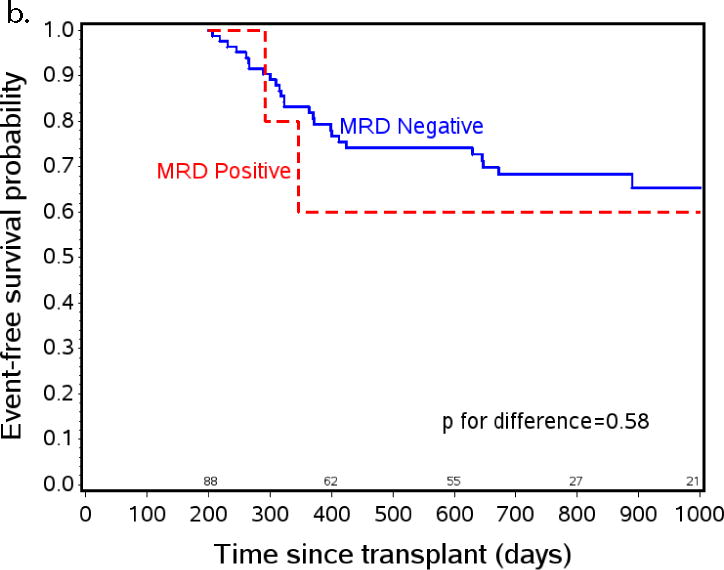
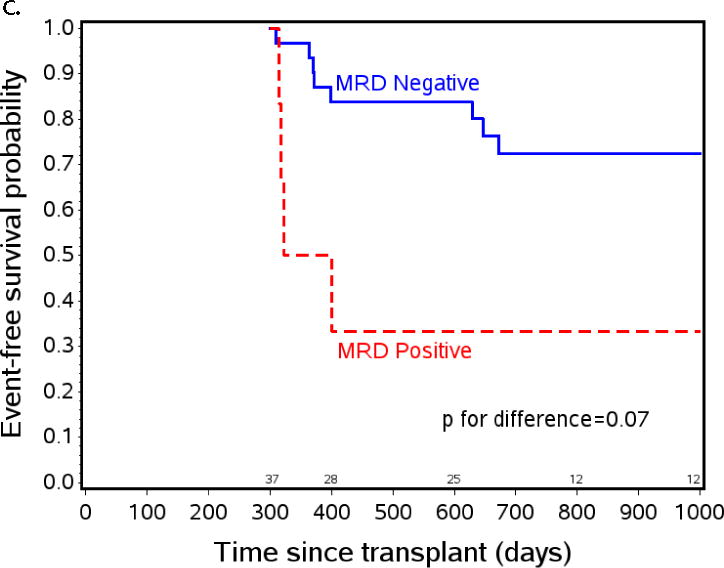
The effect of post-HCT MRD positivity on event free survival (EFS) when tested at specific time points after transplant. a) EFS from day +55 by post-engraftment MRD status. At day +55 patients on study n=131, patients with MRD assessment prior to day +55 (planned day +30, range day 18–54) n=95, MRD negative n=88, MRD positive n=7. b) EFS from day +200 by three month MRD status. At day +200 patients on study n=131, patients with MRD assessment between day +55 and day +200 (planned day +100, range day 76–181) n=95, MRD negative n=88, MRD positive n=7. c) EFS from day +300 by eight month MRD status. At day +300 patients on study n=93, patients with MRD assessment between day +200 and day +300 (optional assessment day +240, range day 226–290) n=37, MRD negative n=31, MRD positive n=6.
Acknowledgments
This study was supported in part by N01 HC-45220/HHSN268200425220C, U10 CA098543 and R01CA1116660. PBMTC activities were supported by 2U01HL069254 and a consortium grant from the St. Baldrick’s Foundation.
Research Support: This study was supported in part by NCI grants N01 HC-45220/HHSN268200425220C, U10 CA098543 and R01CA1116660. PBMTC activities were supported by NCI/NHLBI 2U01HL069254 and the St. Baldrick’s Foundation.
The study is registered with ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT00382109).
Footnotes
Presented in part at the American Society of Hematology Meeting, December 2012 and the Second International Workshop: Biology Prevention and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation, Nov. 5–6, 2012, Bethesda MD, Plenary Oral Presentation, Best Abstracts Session.
The Authors have no conflicts of interest to report.
Supplementary information is available at BMT’s website
References
- 1.Pulsipher MA, Peters C, Pui CH. High-risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? Biol Blood Marrow Transplant. 2011;17(1 Suppl):S137–48. doi: 10.1016/j.bbmt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120(14):2807–16. doi: 10.1182/blood-2012-02-265884. [DOI] [PubMed] [Google Scholar]
- 3.Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101(10):3835–9. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 4.Schrauder A, von Stackelberg A, Schrappe M, Cornish J, Peters C. Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. Bone Marrow Transplant. 2008;41(Suppl 2):S71–4. doi: 10.1038/bmt.2008.58. [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009–17. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, et al. Reinduction platform for children with first marrow relapse in acute lymphoblastic lymphoma. J Clin Oncol. 2008;26(24):3971–8. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62. [PubMed] [Google Scholar]
- 8.Cornelissen JJ, Carston M, Kollman C, King R, Dekker AW, Lowenberg B, et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood. 2001;97(6):1572–7. doi: 10.1182/blood.v97.6.1572. [DOI] [PubMed] [Google Scholar]
- 9.Passweg JR, Tiberghien P, Cahn JY, Vowels MR, Camitta BM, Gale RP, et al. Graft-versus-leukemia effects in T lineage and B lineage acute lymphoblastic leukemia. Bone Marrow Transplant. 1998;21(2):153–8. doi: 10.1038/sj.bmt.1701064. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Cho BS, Kim SY, Choi SM, Lee DG, Eom KS, et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(9):1083–94. doi: 10.1016/j.bbmt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Nordlander A, Mattsson J, Ringden O, Leblanc K, Gustafsson B, Ljungman P, et al. Graft-versus-host disease is associated with a lower relapse incidence after hematopoietic stem cell transplantation in patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2004;10(3):195–203. doi: 10.1016/j.bbmt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Zikos P, Van Lint MT, Lamparelli T, Gualandi F, Occhini D, Bregante S, et al. Allogeneic hemopoietic stem cell transplantation for patients with high risk acute lymphoblastic leukemia: favorable impact of chronic graft-versus-host disease on survival and relapse. Haematologica. 1998;83(10):896–903. [PubMed] [Google Scholar]
- 13.Gustafsson Jernberg A, Remberger M, Ringden O, Winiarski J. Graft-versus-leukaemia effect in children: chronic GVHD has a significant impact on relapse and survival. Bone Marrow Transplant. 2003;31(3):175–81. doi: 10.1038/sj.bmt.1703808. [DOI] [PubMed] [Google Scholar]
- 14.Locatelli F, Zecca M, Messina C, Rondelli R, Lanino E, Sacchi N, et al. Improvement over time in outcome for children with acute lymphoblastic leukemia in second remission given hematopoietic stem cell transplantation from unrelated donors. Leukemia. 2002;16(11):2228–37. doi: 10.1038/sj.leu.2402690. [DOI] [PubMed] [Google Scholar]
- 15.Dini G, Zecca M, Balduzzi A, Messina C, Masetti R, Fagioli F, et al. No difference in outcome between children and adolescents transplanted for acute lymphoblastic leukemia in second remission. Blood. 2011;118(25):6683–90. doi: 10.1182/blood-2011-05-354233. [DOI] [PubMed] [Google Scholar]
- 16.Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J, et al. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112(5):2020–3. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, et al. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc Natl Acad Sci U S A. 2003;100(25):15113–8. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, et al. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107(3):1149–55. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulsipher MA, Wall DA, Grimley M, Goyal RK, Boucher KM, Hankins P, et al. A phase I/II study of the safety and efficacy of the addition of sirolimus to tacrolimus/methotrexate graft versus host disease prophylaxis after allogeneic haematopoietic cell transplantation in paediatric acute lymphoblastic leukaemia (ALL) Br J Haematol. 2009;147(5):691–9. doi: 10.1111/j.1365-2141.2009.07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014;123(13):2017–25. doi: 10.1182/blood-2013-10-534297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locatelli F, Zecca M, Rondelli R, Bonetti F, Dini G, Prete A, et al. Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood. 2000;95(5):1572–9. [PubMed] [Google Scholar]
- 22.Pulsipher MA, Peters C, Pui CH. High-risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? Biol Blood Marrow Transplant. 2011;17(1 Suppl):S137–48. doi: 10.1016/j.bbmt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase III COG/PBMTC trial. Blood. 2014 doi: 10.1182/blood-2013-10-534297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–84. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 25.Borowitz MJ, Wood BL, Devidas M, Loh M, Raetz E, Nachman JB, et al. Improved Post-Induction Chemotherapy Does Not Abrogate Prognostic Significance of Minimal Residual Disease (MRD) for Children and Young Adults with High Risk Acute ymphoblastic Leukemia (ALL). A Report From Children’s Oncology Group (COG) Study AALL0232. Blood. 2011;18(21) Abstract 1440. [Google Scholar]
- 26.Dworzak MN, Froschl G, Printz D, Mann G, Potschger U, Muhlegger N, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99(6):1952–8. doi: 10.1182/blood.v99.6.1952. [DOI] [PubMed] [Google Scholar]
- 27.Borowitz MJ, Pullen DJ, Shuster JJ, Viswanatha D, Montgomery K, Willman CL, et al. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors. A Children’s Oncology Group study. Leukemia. 2003;17(8):1566–72. doi: 10.1038/sj.leu.2403001. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 30.Aalen O, Johansen S. An empirical transition matrix for nonhomogeneous Markov chains based on censored observations. Scandinavian Journal of Statistics. 1978;5:141–150. [Google Scholar]
- 31.Bader P, Kreyenberg H, von Stackelberg A, Eckert C, Salzmann-Manrique E, Meisel R, et al. Monitoring of Minimal Residual Disease After Allogeneic Stem-Cell Transplantation in Relapsed Childhood Acute Lymphoblastic Leukemia Allows for the Identification of Impending Relapse: Results of the ALL-BFM-SCT 2003 Trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.4631. [DOI] [PubMed] [Google Scholar]
- 32.Bruggemann M, Schrauder A, Raff T, Pfeifer H, Dworzak M, Ottmann OG, et al. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia. 2010;24(3):521–35. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- 33.Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–80. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30(15):1822–8. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–5. doi: 10.1038/sj.leu.2404866. [DOI] [PubMed] [Google Scholar]
- 36.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 37.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 2013 doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grupp SA, June CH. Adoptive cellular therapy. Curr Top Microbiol Immunol. 2011;344:149–72. doi: 10.1007/82_2010_94. [DOI] [PubMed] [Google Scholar]
- 40.Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22(9):1696–705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 41.Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood. 2011;118(20):5681–8. doi: 10.1182/blood-2011-04-348805. [DOI] [PubMed] [Google Scholar]
- 42.Lankester AC, Bierings MB, van Wering ER, Wijkhuijs AJ, de Weger RA, Wijnen JT, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. 2010;24(8):1462–9. doi: 10.1038/leu.2010.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


