Abstract
Starvation of diploid cells of the budding yeast Saccharomyces cerevisiae induces them to enter meiosis and differentiate into haploid spores. During meiosis, the precise timing of gene expression is controlled at the level of transcription, and also translation. If cells are returned to rich medium after they have committed to meiosis, the transcript levels of most meiotically upregulated genes decrease rapidly. However, for a subset of transcripts whose translation is delayed until the end of meiosis II, termed protected transcripts, the transcript levels remain stable even after nutrients are reintroduced. The Ime2-Rim4 regulatory circuit controls both the delayed translation and the stability of protected transcripts. These protected mRNAs localize in discrete foci, which are not seen for transcripts of genes with different translational timing and are regulated by Ime2. These results suggest that Ime2 and Rim4 broadly regulate translational delay but that additional factors, such as mRNA localization, modulate this delay to tune the timing of gene expression to developmental transitions during sporulation.
INTRODUCTION
Formation of haploid gametes from diploid cells through the specialized cell division of meiosis is central to the life cycle of sexually reproducing organisms. Gametogenesis involves exit from the mitotic cell cycle, progression through the meiotic divisions, and differentiation into specialized gametes that can later undergo fertilization to restore diploidy. In Saccharomyces cerevisiae gametogenesis, haploid genomes are packaged into gametes called spores, and the process is referred to as sporulation. Shared characteristics of sporulation and gametogenesis in metazoans include the dynamics of chromosome behavior in the meiotic prophase, postmeiotic hypercondensation of chromatin, and generation of specialized gametes (1–3).
Sporulation is triggered by nitrogen starvation in the presence of a poor carbon source (1). These starvation signals lead to the transcription of IME1, which encodes a transcription factor that controls entry into meiosis (4). Ime1 induces expression of a set of genes that are required for premeiotic DNA synthesis, as well as the initial steps of meiosis, particularly those involved in recombination during the meiotic prophase (5, 6). A key target of Ime1 is the gene encoding the Ime2 protein kinase (4, 7). The combined action of Ime1 and Ime2 leads to the induction of a second transcription factor, encoded by NDT80 (8). Ndt80 upregulates its own expression, as well as that of ∼300 additional genes termed the NDT80 regulon (8, 9). This regulon includes genes required for entry into the meiotic divisions, and thus, deletion of NDT80 results in the arrest of cells in the meiotic prophase (8, 10). NDT80 also governs the induction of genes whose products are required for late meiosis events, such as the packaging of daughter nuclei into spores, and postmeiotic events, such as spore wall development (9).
After induction of the NDT80 regulon, there are two other temporally regulated sets of transcriptionally induced genes, termed the mid-late genes and late genes (11). However, after meiotic prophase, the fine control of the timing of gene expression appears to be performed predominantly at the level of translational regulation rather than transcription (12). To obtain the high degree of synchrony necessary to distinguish differences in timing of translation during the meiotic divisions, an inducible NDT80 system (NDT80-IN) was used for ribosome-profiling studies (13–15). NDT80-IN results from the combination of NDT80 fused to the GAL1 promoter and the presence of a GAL4-estradiol receptor gene (ER) fusion gene (13). Transcription of NDT80 can therefore be controlled by addition of estradiol to the sporulation medium (SPM). Ribosome profiling has demonstrated that genes within the NDT80 regulon may be coordinately transcribed, but the translational efficiency of these messages is differentially regulated so that protein production is coordinated with development (12).
A regulatory pathway has been defined that controls a set of transcripts, including CLB3 and SPO20, that are delayed in translation until the onset of meiosis II (15). This pathway involves the Ime2 kinase and the RNA binding protein Rim4 (16). Binding of Rim4 to the 5′ untranslated region (UTR) of the CLB3 transcript represses CLB3 translation (15). Ime2 activity increases as cells progress through the meiotic divisions (13). Phosphorylation of Rim4 by Ime2 destabilizes Rim4, allowing the translation of Clb3 and other messages translated at the onset of meiosis II, when Ime2 kinase becomes active (15). Many additional Ndt80-regulated transcripts, with translational timing distinct from that of CLB3, bind Rim4 (15). How the translation of these other transcripts is controlled and whether the Ime2/Rim4 regulatory system contributes to their regulation have not been described.
At some point during meiosis, cells become committed to the process of sporulation. Commitment was discovered by the use of “return-to-growth” experiments that involve inducing cells to undergo meiosis and then transferring them at different times into rich medium. Committed cells are defined as those that complete the process of sporulation even when the inducing signal (starvation) is removed (17). While the precise moment of commitment has not been defined, it occurs after induction of NDT80, as cells up to that point return to growth when transferred to rich medium (18). Surprisingly, comparison of transcript levels between committed cells before and after transfer to rich medium shows that committed cells returned to rich medium in fact display an extensive response to the change in environment (19). In particular, transcript levels for ribosomal genes and other genes associated with cell cycle entry increase, while transcript levels for genes in the NDT80 regulon (as well as NDT80 itself) decrease significantly within 40 min. Despite these changes in transcript abundance, the cells complete sporulation.
Within the NDT80 regulon, a subset of genes was seen whose transcript levels remained high after transfer to rich medium (19). These genes were referred to as “insulated,” since they were refractory to the change in environmental cues (19). Because the term “insulation” has been used in other contexts to describe a different regulatory phenomenon in both yeast and higher cells (20, 21), we refer to these genes instead as “protected” genes. This work shows that protection of genes from nutrient signals correlates with a delay in their translation until the end of meiosis II. Thus, the return-to-growth assay reveals an aspect of the regulation of these transcripts that may account for their extended translational delay. Our results indicate that the Ime2/Rim4 pathway may provide a general mechanism to delay translation during meiosis and that sequestration of specific messages can further tune the timing of the start of translation.
MATERIALS AND METHODS
Yeast media, strains, and plasmids.
Unless otherwise noted, standard media and growth conditions were used (22). All the yeast strains used are listed in Table 1. Diploid strains carrying PSPS4-5′ UTRSPO20-SPO20, PSPS4-5′ UTRSPS4-SPO20, or PSPO20-5′ UTRSPS4-SPO20 at the SPO20 locus; SPS4-3×HA, SPS4-GFP, PSPO20-5′ UTRSPS4-SPS4, or PSPO20-5′ UTRSPO20-SPS4 at the SPS4 locus; or IME2ΔC241 were constructed by PCR-based integration in the haploid parents A14154 and A14155 and subsequent mating of the transformants (14, 23). To introduce the SPO20 or SPS4 upstream region, the plasmids pFA6a-HIS3MX6-PSPS4, pFA6a-HIS3MX6-PSPO20, and pFA6a-KanMX6-PSPO20 were constructed for use as PCR templates by replacing the GAL promoter in the pFA6a-HIS3MX6-PGAL1 and pFA6a-KanMX6-PGAL1 plasmids (24) with 1 kb of sequence upstream of the translational start site from SPO20 and SPS4, respectively. The positions of the transcriptional start sites used for SPO20 and SPS4 were based on an earlier study defining the 5′ ends of transcripts in sporulating cells (25). Strains carrying bacteriophage MS2 loop-tagged mRNAs were generated using CRISPR/Cas reagents provided by B. Futcher. Detailed descriptions of their construction will be presented elsewhere (G. Zhao and B. Futcher, unpublished data). We first generated plasmid pRS425-Cas9-SkHIS3-381. This plasmid expresses both the Streptococcus pyogenes cas9 gene under the control of the yeast TEF2 promoter and a noncoding guide RNA with a sequence that targets Cas9 for cleavage of the Saccharomyces kluyveri HIS3 sequence. MS2 loop sequences were amplified from the plasmid pLOXHIS5MS2L (26) with oligonucleotides that contained 5′ sequences homologous to the fusion junctions created by insertion of the green fluorescent protein (GFP) gene and S. kluyveri HIS3 in construction of the GFP-tagged strain collection (27). When pRS425-Cas9-SkHIS3-381 and the MS2 loop PCR product are cotransformed into strains from the GFP collection, Cas9 generates double-stranded breaks within the S. kluyveri HIS3 gene adjacent to the GFP sequence, and these breaks can be repaired by recombination integrating the MS2 PCR fragment. The resulting strains have lost the coding regions of both GFP and S. kluyveri HIS3, and in their place at the 3′ end of the open reading frame (ORF) are (in order) 30 nucleotides (nt) encoding the first 10 residues of GFP, a stop codon, the MS2 loops, 48 nucleotides from the original GFP tagging vector, and, finally, the genomic 3′ UTR. Details of both the Cas9 plasmid construction and this technique will be described elsewhere (Zhao et al., unpublished). The final integrations in all strains were verified by PCR and sequencing. These MS2 loop-tagged strains in the BY4741 background were then mated with the SK-1 background strain AN117-4B carrying plasmids expressing a GFP-tagged MS2 coat protein (MS2CP) (pMS2-CP-GFP [26]) and a red fluorescent protein (RFP) prospore membrane marker (pRS426-SPO20-mCherry [28]) and sporulated to observe mRNA localization. To make yLJ92, the ime2-as (analog-sensitive) allele was first PCR amplified from KBY516 (13), using an oligonucleotide 500 bp upstream of the translational start site and a downstream oligonucleotide that introduced a stop codon, followed by 30 nt of the wild-type IME2 3′ UTR immediately after codon 241. The PCR product was then cotransformed with the plasmid pRS425-Cas9-skHIS3-381 into the IME2ΔC241::HIS3MX6 strains. After screening for transformants that had lost the HIS3 marker, correct integrations in all the strains mentioned above were verified by PCR and sequencing. To make yLJ159, PCR-mediated integration was first used to replace codon 349 of RIM4 with a kanamycin resistance cassette in both yLJ97 and AN117-4B. The rim4 F349L allele was then amplified by PCR from strain A31421, and this product was cotransformed with the plasmid pRS425-Cas9-Kan-280 into the strains with the kanamycin insertions in RIM4. Correct integrations were identified by loss of G418 resistance and then verified by PCR and sequencing. Finally, the two resulting haploids were mated.
TABLE 1.
Strains used in this study
| Strain name | Genotype | Reference |
|---|---|---|
| AN120 | MATa/MATα ura3/ura3 his3ΔSK/his3ΔSK trp1::hisG/trp1::hisG arg4-NSP1/ARG1 lys2/lys2 hoΔ::LYS2/hoΔ::LYS2 rme1Δ::LEU2/RME1 leu2/leu2 | 36 |
| AN117-4B | MATα ura3 his3 trp1::hisG leu2 arg4-NSP1 lys2 hoΔ::LYS2 rme1Δ::LEU2 | 36 |
| A14201 | MATa/MATα hoΔ::LYS2/hoΔ::LYS2 lys2/lys2 ura3/ura3 leu2::hisG/leu2::hisG his3::hisG/his3::hisG trp1::hisG/trp1::hisG GAL-NDT80::TRP1/GAL-NDT80::TRP1 ura3::pGPD1-GAL4(848).ER::URA3/ura3::pGPD1-GAL4(848).ER::URA3 | 14 |
| A31421 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2::hisG/leu2::hisG his3::hisG/his3::hisG trp1::hisG/trp1::hisG GAL-NDT80::TRP1/GAL-NDT80::TRP1 ura3::pGPD1-GAL4(848).ER::URA3/ura3::pGPD1-GAL4(848).ER::URA3 CLB3-3HA::Kan/CLB3-3HA::Kan rim4F349L-3V5/rim4F349L-3V5 | 15 |
| yLJ28 | As A14201 plus HIS3MX6-PSPS4-5′ UTRSPS4-SPO20/HIS3MX6-PSPS4-5′ UTRSPS4-SPO20 | This study |
| yLJ29 | As A14201 plus HIS3MX6-PSPS4-5′ UTRSPO20-SPO20/HIS3MX6-PSPS4-5′ UTRSPO20-SPO20 | This study |
| yLJ40 | As A14201 plus HIS3MX6-PSPO20-5′ UTRSPS4-SPS4/HIS3MX6-PSPO20-5′ UTRSPS4-SPS4 | This study |
| yLJ41 | As A14201 plus HIS3MX6-PSPO20-5′ UTRSPO20-SPS4/HIS3MX6-PSPO20-5′ UTRSPO20-SPS4 | This study |
| yLJ44 | As A14201 plus KanMX6-PSPO20-5′ UTRSPS4-SPO20/KanMX6-PSPO20-5′ UTRSPS4-SPO20 | This study |
| yLJ50 | As A14201 plus IME2ΔC241::HIS3MX6/IME2ΔC241::HIS3MX6 | This study |
| yLJ80 | As A14201 plus SPS4-3HA-HIS3MX6/SPS4-3HA-HIS3MX6 | This study |
| yLJ89 | As A14201 plus IME2ΔC241::HIS3MX6/IM2ΔC241::HIS3MX6 SPS4-3HA-KanMX/SPS4-3HA-KanMX | This study |
| yLJ92 | As A14201 plus IME2ΔC241(M146G)-as/IME2ΔC241(M146G)-as | This study |
| yJL97 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 SPS4-12×MS2L | This study |
| yLJ99 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPS4-12×MS2L/SPS4 | This study |
| yLJ111 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPR6-12×MS2L/SPR6 | This study |
| yLJ112 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 LDS2-12×MS2L/LDS2 | This study |
| yLJ113 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPS1-12×MS2L/SPS1 | This study |
| yLJ119 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SGA1-12×MS2L/SGA1 | This study |
| yLJ120 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 CTS2-12×MS2L/CTS2 | This study |
| yLJ121 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPO20-12×MS2L/SPO20 | This study |
| yLJ122 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPR28-12×MS2L/SPR28 | This study |
| yLJ123 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPS4-12×MS2L/SPS4 IME2ΔC241::KanMX6/IME2ΔC241::KanMX6 | This study |
| yLJ137 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPO77-12×MS2L/SPO77 | This study |
| yLJ139 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SSP2-12×MS2L/SSP2 | This study |
| yLJ141 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 CDA1-12×MS2L/CDA1 | This study |
| yLJ159 | MATa/MATα his3Δ1/his3 leu2Δ0/leu2 met15Δ0/MET15 ura3Δ0/ura3 trp1::hisG/TRP1 arg4-NSP1/ARG4 lys2/LYS2 ho::LYS2/ho rme1::LEU2/RME1 SPS4-12×MS2L/SPS4 rim4-F349L/rim4-F349L | This study |
| yLJ167 | As A31421 plus SPS4-GFP-HIS3MX6/SPS4-GFP-HIS3MX6 | This study |
Return-to-growth conditions.
For return-to-growth experiments with GAL-NDT80 strains, cells were grown in yeast extract-peptone-dextrose (YPD) overnight at 30°C and then transferred to yeast extract-peptone-acetate (YPA) at an optical density at 660 nm (OD660) of 0.3. After incubation at 30°C for 16 to 18 h, the cells were washed and resuspended in SPM at a final OD660 of 1.6. The cells were incubated at 30°C for 6 h to allow the population to accumulate in meiotic prophase, and then 1 mM β-estradiol was added to the cell culture to induce meiotic entry. At each time point, the cells were washed once with distilled water (dH2O), resuspended in a 2× volume of YPD, and incubated at 30°C. For strains carrying ime2-as1, the inhibitor 3-methylbenzyl-PP1 (EMD Millipore) was add to the YPD at a final concentration of 50 μM at the time of transfer from SPM. The experiments examining commitment were performed three times (for a representative example, see Fig. 6).
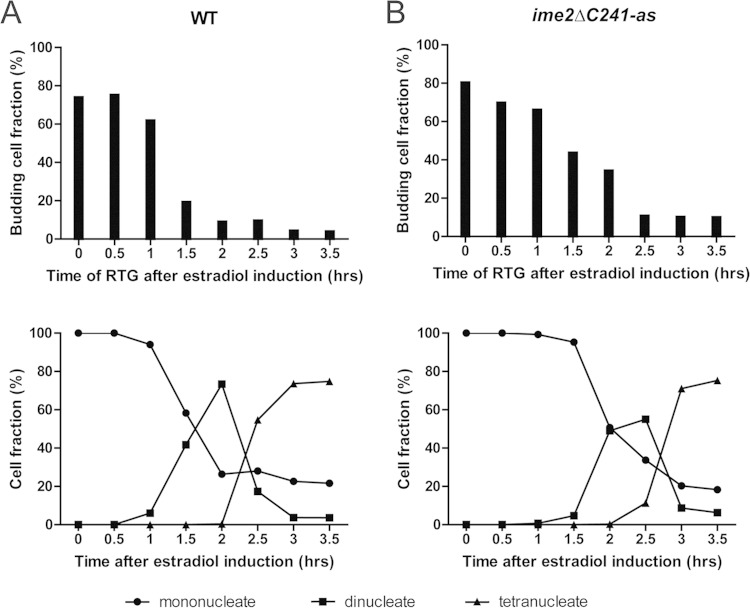
FIG 6.
Commitment to meiosis in wild-type and ime2ΔC241-as cells. (A) (Top) At the indicated times after estradiol addition, commitment was analyzed by transfer of cells to rich medium and determination of the budding index using light microscopy. For NDT80-IN cells (A14201), the percentage of budding cells was determined after 3 h of incubation in rich medium. (Bottom) Progression of the population through the meiotic divisions in the same time course. The percentages of cells containing one nucleus (before MI), two nuclei (MI), or four nuclei (MII or later) were determined by DAPI staining. (B) Commitment monitored in NDT80-IN ime2ΔC241-as cells (yLJ92). The cells were treated as for panel A, except that 50 μM 3-MB-PP1 was added to the rich medium upon return to growth in order to inactivate Ime2-as.
mRNA localization.
To observe the localization of MS2 loop-tagged mRNAs, diploids heterozygous for the tagged mRNAs and carrying the MS2CP-GFP and Spo2051–91-mCherry plasmids were grown in YPD overnight at 30°C and then diluted in YPA to an OD660 of 0.2. After incubation at 30°C overnight, the cells were washed and resuspended in SPM at an OD660 of 1.2 and incubated at 30°C. After 6 h of incubation, samples were placed on microscope slides, and images were collected on a Zeiss Axioplan2 microscope with a Zeiss mRM digital camera. The images were processed using Axiovision 4.0 software.
Western blot assays.
For Western blots, the NDT80-IN strains were sporulated as described above. At intervals, 5 ml of sporulating culture was collected, pelleted, resuspended in 5 ml of 5% trichloroacetic acid, and incubated at 4°C for 10 min. The cells were then pelleted and washed with 1 ml acetone, and the pellet was allowed to air dry for 2.5 h. The pellets were then resuspended in 100 μl freshly made lysis buffer (50 mM Tris, pH 7.5, 1 mM EDTA, 27.5 mM dithiothreitol, 11 mM phenylmethylsulfonyl fluoride, 2-fold-concentrated EDTA-free cOmplete protease inhibitor cocktail tablets [Roche]). The cells were broken by addition of 50 μl of zirconia beads, followed by two pulses at 6 m/s for 40 s in a FastPrep-24 high-speed benchtop homogenizer (MP Biomedicals). Then, 50 μl 3× SDS sample buffer was added, and each lysate was boiled for 5 min before loading on an SDS polyacrylamide gel. Sps4-GFP was detected using monoclonal anti-GFP antibodies (ClonTech) at 1:1,000 dilution. As a loading control, porin was detected by antiporin antibodies (Molecular Probes) at 1:1,000 dilution or Arp7 was detected using polyclonal anti-Arp7 antibodies (Santa Cruz Biotechnology) at 1:5,000 dilution. Quantitation was performed using an ImageQuant 4000 (GE Healthcare).
Microarrays.
Total RNA from cells pelleted at each time point was extracted and purified using a RiboPure yeast kit (Ambion). Cy3- and Cy5-labeled cRNAs were produced using an Agilent QuickAmp labeling kit (Agilent) and purified using an RNeasy minikit (Qiagen). Probes were hybridized to an Agilent yeast gene expression 8,000 by 15,000 microarray (Agilent) using an Agilent gene expression hybridization kit and hybridization oven. After hybridization, the arrays were scanned using an Agilent DNA microarray scanner, and the fluorescence was analyzed and normalized using Agilent feature extraction software. Basic analysis was performed using the LIMMA package in R. Clustering was performed using Cluster 3.0, and clustering data were visualized in Java Treeview.
qPCR.
Five milliliters of cells was collected at each time point. Total RNA was extracted and purified with a RiboPure yeast kit (Ambion), and then cDNAs were synthesized using the Transcriptor first-strand cDNA synthesis kit (Roche) and treated with RNase A (0.5 μg/μl) for 30 min at 37°C. After synthesis, the cDNA was purified using a PCR purification kit (Qiagen), and the cDNA concentration was then measured and adjusted to 1 ng/μl. The mRNA level of each gene was determined by quantitative PCR (qPCR) using a Mastercycler EP Realplex (Eppendorf) and LightCycler 480 DNA Sybr green I PCR master mix (Roche). All time points were assayed in triplicate in each experiment, and all experiments were performed at least twice.
RESULTS
Protected transcripts exhibit delayed translation.
Friedlander et al. (19) defined a set of 24 protected transcripts within the NDT80 regulon whose levels were not reduced when cells committed to sporulation were transferred into rich medium. Ribosome profiling throughout a sporulation time course demonstrated that, while the NDT80 regulon is transcriptionally induced at the same time, the timing of peak translation varies between messages (12). There is a sizable overlap between the protected gene set defined by return-to-growth experiments and a cluster of NDT80-induced genes that are translated very late in sporulation (with peak translation around 3 h after NDT80 induction) (12, 19). Comparisons between these two studies may not provide a complete picture, however, because of differences in synchrony between the strains. The return-to-growth study identifying protected transcripts used a wild-type yeast strain and lacked the degree of synchrony achieved by the ribosome-profiling experiments using the NDT80-IN system.
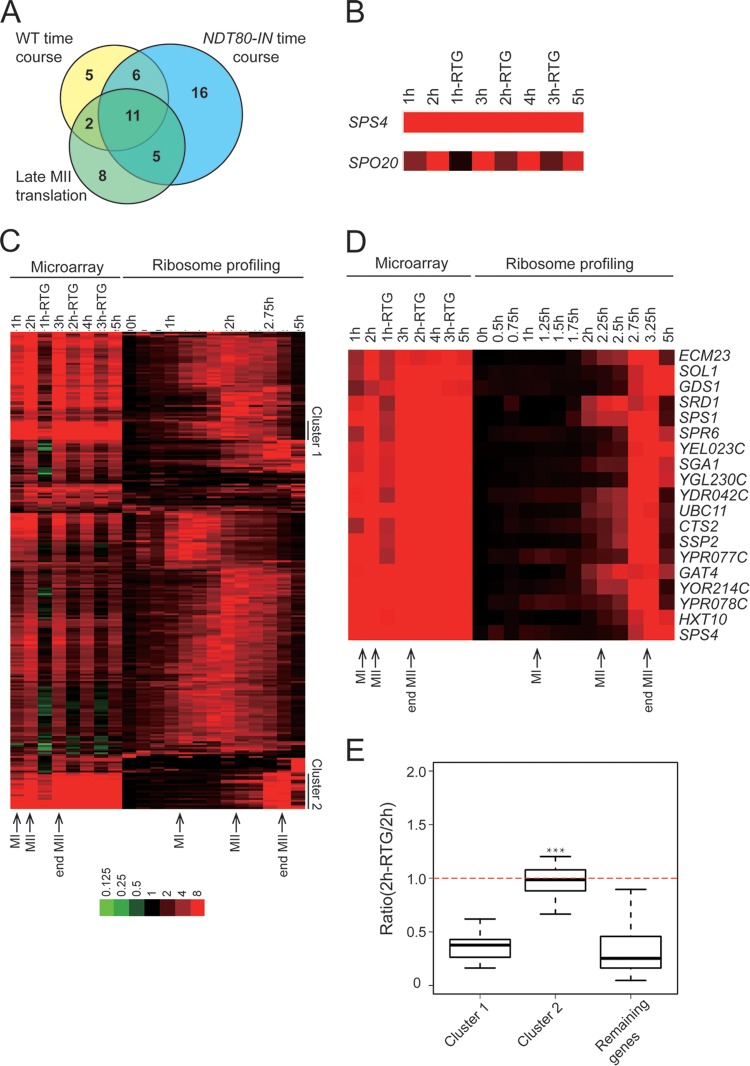
To allow direct comparison between the ribosome-profiling data and protected gene expression, a return-to-growth experiment was performed in the NDT80-IN background and transcript levels were analyzed using microarrays. Cells were arrested in meiotic prophase by incubation in sporulation medium for 6 h, and then NDT80 expression was induced by addition of estradiol. mRNA was isolated from cells taken immediately prior to estradiol addition, labeled with Cy3, and used as the normalizing control. At 1-h intervals after addition of estradiol, cells were taken directly from the sporulation medium or transferred to rich medium for 1 h. mRNA was then isolated from these cells, labeled with Cy5, mixed with the Cy3-labeled control mRNAs, and hybridized to microarrays. This experiment revealed a larger number of genes than in the previous study whose transcripts appeared protected upon return to growth (Fig. 1A) (19). Nonetheless, when the set of protected transcripts seen in this experiment is compared with the protected transcripts from Friedlander et al. and with the very late-translated genes from the ribosome-profiling data of Brar et al. (12), there is extensive overlap (Fig. 1A).
FIG 1.
A set of protected transcripts is translated at the end of meiosis II (MII). (A) Venn diagram showing the overlap between the set of protected transcripts identified in return-to-growth experiments using either NDT80-IN (A14210) or NDT80 (WT [wild-type] time course) (19) and the cluster of genes showing peak translation in late MII (Late MII translation) (12). The numbers indicate the number of genes in each subset. (B) Behaviors of a protected gene, SPS4, and a nonprotected gene, SPO20, in the microarray experiment. At the 1, 2, and 3-hour time points, aliquots were transferred to rich medium for 1 h prior to mRNA extraction. For example, “2 h-RTG” indicates cells that were transferred to YPD 2 h after addition of β-estradiol. RTG, return to growth. (C) Coclustering of the NDT80-IN return-to-growth expression data with the ribosome-profiling data for the NDT80 regulon from reference 12. Most of the protected transcripts fall into one of two translational clusters, indicated on the right. The color intensity scale (log2) is shown at the bottom. The times of meiosis I, meiosis II, and the end of meiosis II in the two time courses are indicated. The data used to generate the heat map are provided in Table S1 in the supplemental material. (D) Heat map of cluster 2 genes that are both protected and delayed in translation until the end of meiosis II. (E) Box plot of expression ratios (expression at 2 h RTG/expression at 2 h) for genes in cluster 1 and cluster 2 and the remaining genes in the microarray. The thick horizontal lines denote median values, the boxes represent the middle 50%, and the whiskers mark the 95% limits. The asterisks indicate that the distribution in cluster 2 is significantly different than those for both cluster 1 and the remaining genes (Student's t test; P < 0.001).
In our microarray data, the mRNA levels for most of the messages, such as SPO20, dropped 1 h after return to growth, but for protected transcripts, such as SPS4, message levels remained high (Fig. 1B). Clustering of the microarray data from the NDT80-IN return-to-growth experiment with the ribosome-profiling data from Brar et al. (12) revealed that the majority of protected transcripts fall primarily into one of two clusters (Fig. 1C). Transcripts in cluster 1 show a broad peak of ribosomal association centered 1.5 to 2 h after the addition of estradiol, corresponding to meiosis I (12). The second, larger cluster contains 19 genes (Fig. 1D) whose translation is delayed until more than 2.5 h after the induction of NDT80, a time point that corresponds to the end of the meiosis II division. Importantly, when the extent of protection of the transcripts in each cluster was assessed by measuring the ratio of the transcript levels before and after return to growth, only the transcripts in cluster 2 showed significant protection (Fig. 1E). Further, qPCR analysis of three genes in cluster 1 (CDA2, SMK1, and SMA2) demonstrated that their transcript levels dropped after return to growth (L. Jin, unpublished observation). Thus, cluster 1 transcripts, though they appear red in the heat map, are reduced after the introduction of nutrients, and cluster 2 represents the strongly protected transcripts. Cluster 2 is enriched (8/19) for genes involved in spore wall assembly, consistent with the idea that these protected transcripts encode proteins that function in postmeiotic stages of spore development. The strong correlation between protection and prolonged translational delay suggests they may be related phenomena.
IME2 and RIM4 regulate the translational delay of SPS4.
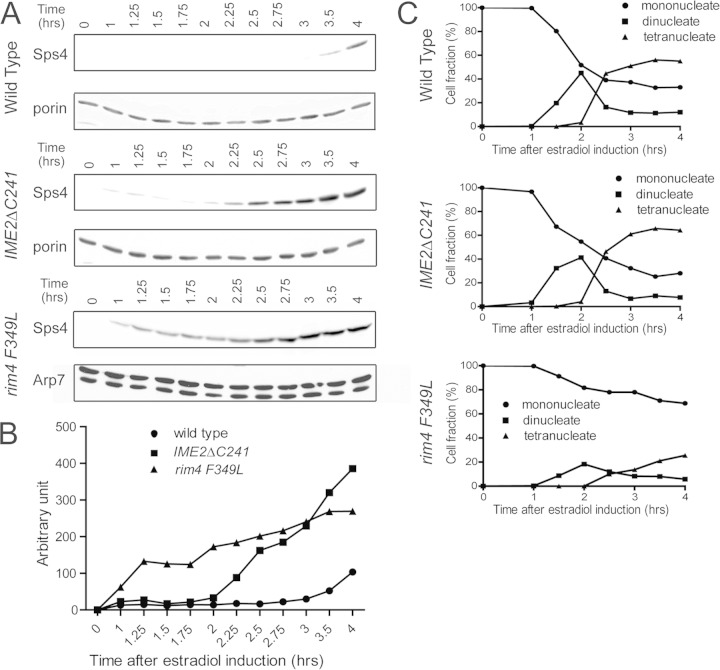
Transcripts exhibiting translational delays can be divided broadly between those that are delayed until early meiosis II and those that are delayed until the end of meiosis II. Only transcripts in the latter class show protection. A question, then, is whether the mechanisms of translational delay are the same in the two groups. Previous work has demonstrated that several nonprotected NDT80-regulated genes, including SPO20 and the cyclin gene CLB3, are delayed in translation until the onset of meiosis II and that this delay requires the RNA binding protein Rim4, the 5′ UTR of the transcript, and the absence of Ime2 kinase activity (15). A carboxy-terminal truncation of Ime2 (IME2ΔC241) creates a stable, hyperactive form of the kinase that leads to early translation of CLB3, SPO20, and several other transcripts with the same delay (15, 29). Ime2 promotes translation by antagonizing the RNA binding protein Rim4 (15). A point mutant in one of the Rim4 RNA binding domains (rim4-F349L) reduces RNA binding and leads to early translation of the CLB3 message, similar to premature activation of Ime2 (15, 25). rim4-F349L alters one of two RNA binding domains within Rim4, and in contrast to the rim4 deletion, which blocks gametogenesis prior to meiosis, a fraction of rim4-F349L cells in the NDT80-IN background are able to escape this early block and progress through meiosis (16). To test whether the same pathway also regulates the translational delay of protected transcripts, the timing of SPS4 translation was compared in wild-type, IME2ΔC241, and rim4-F349L cells. An in-frame fusion of sequences encoding three tandem copies of the hemagglutinin (HA) epitope was created at the 3′ end of SPS4 in both the wild-type and IME2ΔC241 strains in the NDT80-IN background. To monitor Sps4 protein levels in the rim4-F349L mutant strain, an SPS4::GFP fusion was used. The three cultures were transferred to sporulation medium for 6 h, and then samples were removed at 1 h and then every 15 min after the addition of estradiol. In the wild-type strain, Sps4-HA did not appear until 3 h after estradiol addition, consistent with the ribosome-profiling data (12) (Fig. 2A). In contrast, constitutive activation of Ime2 or mutation of RIM4 results in detectable Sps4 protein 1 h after NDT80 induction and accumulation of the protein more than an hour earlier than in the wild type (Fig. 2A and B). This is due to premature translation and not to an effect on meiotic progression or the transcriptional induction of SPS4, as these are unaffected by the IME2ΔC241 allele (Fig. 2C and data not shown). For the rim4-F349L strain, only about 20% of the cells progress into meiosis, probably due to defects in the premeiotic role of RIM4 (16). Nonetheless, in the fraction of cells that progress, meiosis I and meiosis II occur with kinetics similar to those of the wild type (Fig. 2C). These results indicate that at least one mechanism for translational control is shared by both nonprotected and protected transcripts.
FIG 2.
Timing of Sps4 translation in wild-type and IME2ΔC241 cells. (A) The levels of Sps4-3HA (strains yLJ80 and yLJ89) or Sps4-GFP (strain yLJ167) were monitored by Western blotting. Cells were incubated in SPM for 6 h prior to the addition of β-estradiol (time zero), and aliquots were removed at the indicated times after induction. As a loading control, the same samples were probed with antibodies to the mitochondrial Por1 protein. (B) Quantitation of the levels of Sps4 in panel A. The values are normalized to the amount of Por1 protein in each lane for yLJ80 and yLJ89 and the amount of Arp7 protein for yLJ167. (C) DAPI (4′,6-diamidino-2-phenylindole) staining was performed to monitor the progression of cells through the meiotic divisions in the same time courses. The percentages of cells containing one nucleus (before MI), two nuclei (MI), or four nuclei (MII or later) are indicated.
IME2 and RIM4 regulate protection.
If translational delay is linked to protection, then premature activation of Ime2 should cause not only early translation but also loss of protection. This idea was tested by comparing mRNA protection in wild-type and IME2ΔC241 diploids by qPCR using primers specific for the open reading frames of various genes. Two hours after NDT80 induction, cells were transferred to rich medium for 1 h. Transcript levels were then compared before and after return to growth (Fig. 3). In wild-type cells, for the protected transcripts SPS4 and GAT4, the transcript levels did not drop after return to growth, consistent with the microarray data (Fig. 3). In contrast, the GAT4 and SPS4 transcripts were no longer protected in the IME2ΔC241 mutant, as transcript levels decreased after transfer to rich medium, similar to unprotected SPO20 (Fig. 3). Further support for the idea that translational delay is a requirement for protection comes from the observation that protection of the GAT4 and SPS4 transcripts is lost in rim4-F349L diploids (Fig. 3). These results demonstrate that the Ime2/Rim4-mediated mechanism for translational delay is necessary for protection.
FIG 3.
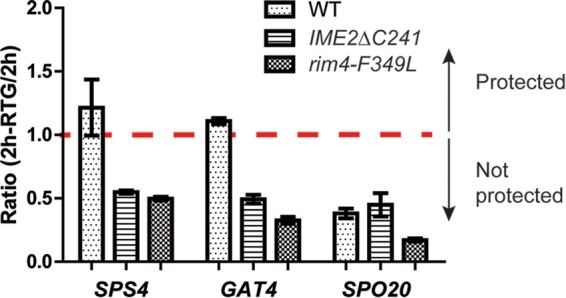
The effect of hyperactive Ime2 or mutation of RIM4 on protection. The levels of two protected transcripts, SPS4 and GAT4, and one nonprotected transcript, SPO20, were examined in a wild-type NDT80-IN strain (A14201) or in IME2ΔC241 (yLJ50) or rim4-F379L (A31421) derivatives. Two hours after addition of estradiol, the cultures were split; half was harvested, and half was transferred to rich medium for 1 h. The expression levels at all time points were measured by qPCR and normalized to the expression at time zero for each gene. The graph displays the ratios of the expression levels before and after transfer. Ratios greater than or equal to 1 indicate protection. The error bars represent the ranges of values from two independent experiments.
Protection of SPS4 requires the 5′ UTR.
For the CLB3 gene, control of translational timing by the Ime2/Rim4 pathway is exerted through binding of Rim4 to the 5′ UTR of the CLB3 mRNA. Moreover, fusion of 1,000 bp upstream of the translational start site of SPS4 to the NDT80 open reading frame is sufficient to protect NDT80 transcripts, suggesting that protection is provided either by the promoter or by the 5′ UTR of the SPS4 transcript (19). To map the cis elements involved in protection more precisely, a series of chimeras between the protected gene SPS4 and the nonprotected gene SPO20 were constructed in the NDT80-IN background and assayed for mRNA protection using qPCR.
For SPO20, mRNA levels decrease when cells are transferred to rich medium 2 h after NDT80 induction compared to cells in SPM, whereas for SPS4, the levels do not drop after return to growth (Fig. 1B and 3). One set of chimeras replaced 1,000 bp upstream of the AUG translation start codon from SPS4 with the corresponding sequence from SPO20 and vice versa. In both cases, transcript behavior was correlated with the upstream region and not the ORF. That is, the SPO20 transcripts generated using the SPS4 upstream region are protected, whereas the SPS4 transcripts fused to SPO20 upstream are not (Fig. 4C, D, and H). This 1,000-bp region contains both the promoter and the 5′ UTR. Chimeras were therefore constructed which retained the 5′ UTR for each ORF (at bp −133 from the translational start site for SPO20; bp −64 for SPS4) (25). In this case, protection was correlated with the presence of the 5′ UTR. SPO20 expressed from the SPS4 promoter region but carrying its own 5′ UTR was not protected, while transcripts from an SPS4 gene expressed from the SPO20 promoter but retaining the SPS4 5′ UTR were protected (Fig. 4E, F, and H).
FIG 4.
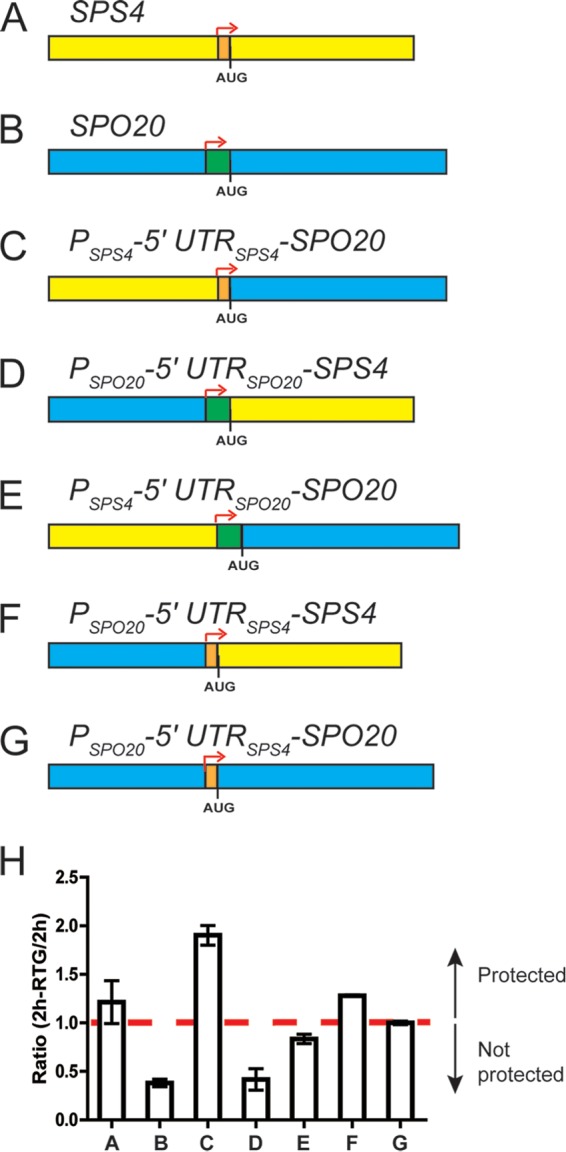
Mapping of the cis-acting determinants of protection. A series of SPS4 (yellow, with the 5′ UTR indicated in orange)-SPO20 (blue, with the 5′ UTR indicated in green) chimeric genes were constructed in the NDT80-IN background (A14201). Sequence swaps were made at the translational start site (AUG) or the reported transcriptional start sites (indicated by the red arrows). (A) SPS4. (B) SPO20. (C) SPS4 upstream region fused to the SPO20 coding region. (D) SPO20 upstream region fused to the SPS4 coding region. (E) SPS4 promoter fused to the SPO20 5′ UTR and coding region. (F) SPO20 promoter fused to the SPS4 5′ UTR and coding region. (G) SPO20 promoter and coding region with the SPS4 5′ UTR. (H) Transcript levels before and after return to growth were assayed for each construct. The values for bars A and B are from Fig. 3. The dashed line indicates a ratio of 1. The error bars represent the range of values from the two experiments.
These results suggest that the regulatory element that confers protection is harbored within the SPS4 5′ UTR. To determine if the 5′ UTR is sufficient for protection, the 5′ UTR of SPO20 was replaced with the 5′ UTR of SPS4. Indeed, transcripts derived from PSPO20-5′ UTRSPS4-SPO20 were protected, similar to those from the endogenous SPS4 gene (Fig. 4G and H). Thus, the 64 nucleotides of the SPS4 5′ UTR are necessary and sufficient for protection.
The SPS4 transcript is localized in IME2-regulated foci.
Though Ime2 and Rim4 regulate both translational delay and protection, many genes, such as SPO20, exhibit translational delay without protection, suggesting that an additional mechanism is required to establish protection. mRNAs whose translation is regulated temporally or spatially often exhibit specific localizations within the cell (30, 31). Therefore, one possible mechanism for protection from nutrient-induced turnover is sequestration of mRNAs from the degradation machinery. To investigate mRNA localization, transcripts were tagged by insertion of multiple copies of a hairpin sequence from the bacteriophage MS2 into their 3′ UTRs. These hairpin loops are specific binding sites for the MS2CP. Localization of transcripts can then be indirectly determined using the localization of an MS2CP-3×GFP fusion expressed in the same cells (26). Localization of six protected transcripts (SPS4, SSP2, SPR6, CTS2, SGA1, and SPS1) and five nonprotected transcripts (LDS2, SPO20, CDA1, SPR28, and SPO77) was monitored in strains containing a red fluorescent marker for the prospore membrane (Spo2051–91-mCherry) (28). Prospore membranes form and grow during the second meiotic division, so the mCherry reporter allows the identification of cells in meiosis II (1). In mid-meiosis II, all the protected transcripts are present in discrete foci clustered near the open ends of the prospore membranes (Fig. 5A and data not shown). In contrast, all the nonprotected transcripts show dispersed MS2CP-3×GFP fluorescence throughout the cytosol, similar to control cells with no tagged message (Fig. 5A and data not shown). Thus, there is perfect correlation between transcript protection and localization to foci within the cell.
FIG 5.
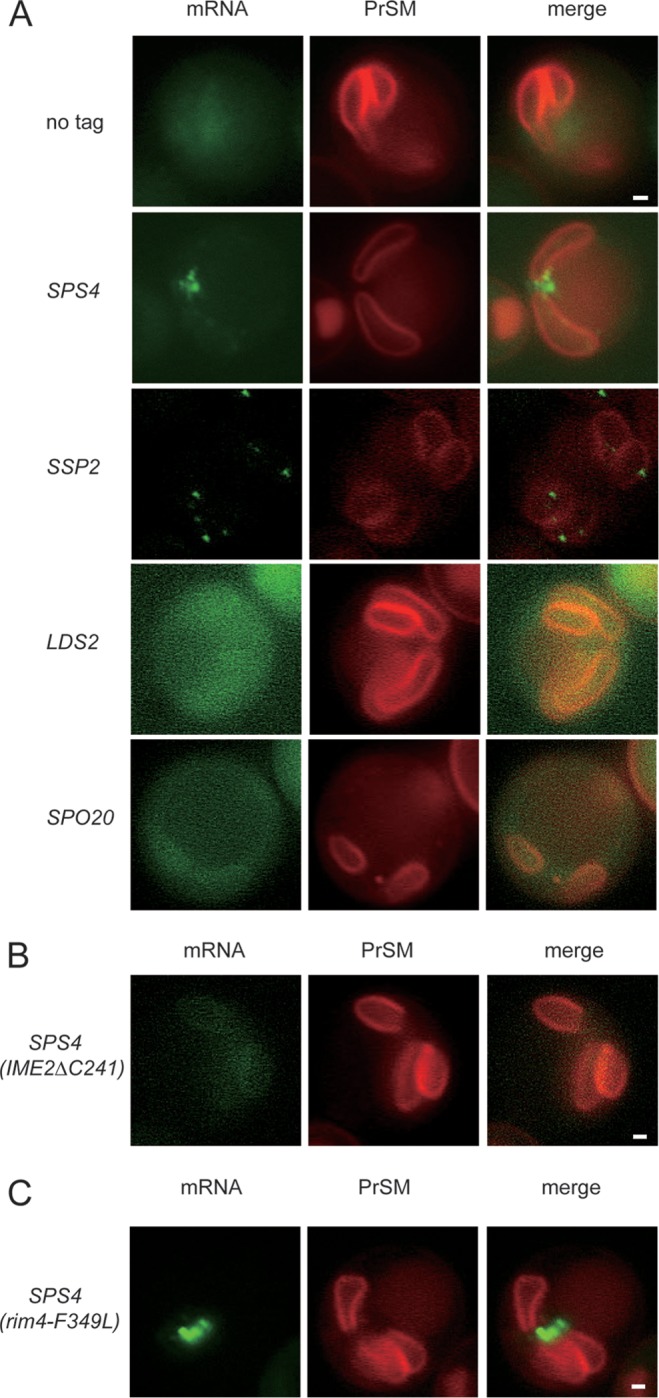
Localization of mRNAs during meiosis. (A) Untagged (AN120), SPS4 tag (yLJ99), SSP2 tag (yJL139), LDS2 tag (yLJ112), or SPO20 tag (yLJ121) diploids containing MS2CP-GFP and the prospore membrane marker SPO2051–91-mCherry were sporulated at 30°C. Cells in mid- to late meiosis II were identified by the prospore membrane morphology and the specific mRNA localization revealed by GFP fluorescence. SPS4 and SSP2 are protected transcripts. LDS2 and SPO20 are nonprotected members of the NDT80 regulon. (B) Effect of IME2ΔC241 on SPS4 mRNA foci. Strain yJL123, carrying the SPS4-MS2 loop transcriptional fusion and homozygous for IME2ΔC241, was sporulated and examined for localization of the SPS4-MS2 mRNA. (C) Effect of rim4-F349L on SPS4 mRNA foci. Strain yJL159, carrying the SPS4-MS2 loop transcriptional fusion and homozygous for rim4-F349L, was sporulated and examined for localization of the SPS4-MS2 mRNA. For all strains, the image shown for each tagged mRNA is representative of the pattern seen in >95% of the meiosis II cells, with >20 cells scored in each strain. Scale bar, 1 μm.
Given that translation delay is required for protection, the question arises whether it is also necessary for transcript localization to foci. In fact, activation of Ime2 using IME2ΔC241 abolished SPS4 transcript foci, with the mRNA instead exhibiting some concentration near the prospore membrane with diffuse cytoplasmic localization similar to that of nonprotected transcripts (Fig. 5B). This result indicates that loss of translational delay and protection correlates with loss of focus formation. In contrast, when SPS4 foci were examined in a rim4-F349L background, no changes in the transcript localization were seen (Fig. 5C), despite the fact that this mutation removes both protection and translational delay (Fig. 2 and 3). This result is considered further in Discussion below.
Loss of protection does not cause loss of meiotic commitment.
The identification of mutants that lose protection allowed us to test whether the maintenance of certain transcripts is important for commitment to differentiation in the NDT80-IN background. At half-hour intervals after the addition of estradiol, cells were transferred to rich medium, and after 3 h in rich medium, the budding index of each culture was determined by light microscopy. As cells enter meiosis from the G1 phase of the cell cycle, sporulating cells are unbudded, and committed cells, which finish the process of sporulation before reentering the cell cycle, do not produce buds within 3 h. However, uncommitted cells return more quickly to the mitotic cycle and can produce buds in this time window. In the wild-type strain, cells transferred to rich medium before the addition of estradiol, or within 1 h of estradiol addition, showed ∼70% budding (Fig. 6A). Between 60 and 90 min after estradiol addition, this response dropped to below 20%. indicating that the bulk of the population had undergone commitment. This timing correlates with the appearance of binucleate cells in the culture. Thus, in the wild type, commitment occurs at the time the population passes through the first meiotic division. This timing of commitment is consistent with that found in a recent study examining commitment in single cells rather than populations (32).
Return-to-growth experiments cannot be performed using IME2ΔC241 cells, because active Ime2 blocks bud emergence, and therefore, no budding is seen in cultures shifted back to rich medium even from premeiotic (and therefore precommitment) time points (33; L. Jin, unpublished observation). To assay commitment in the presence of constitutively active Ime2, an analog-sensitive (as) version of IME2CΔ241 was used. This mutation creates a conditional form of Ime2 that can be inhibited by addition of the purine analog, 3-methylbenzyl-PP1 (13). Thus, the constitutive activity of Ime2ΔC241-as can be used to prevent protection, but its activity can be inhibited when cells are returned to growth by addition of 3-methylbenzyl-PP1 to the rich medium. Cells carrying ime2ΔC241-as were analyzed for commitment in the budding assay described above, except that 3-methylbenzyl-PP1 was added at the time of transfer to YPD to inactivate Ime2. Addition of the inhibitor at the same time as return to growth did not restore protection in these cells; thus, commitment can be monitored in the absence of protection (data not shown). Under this protocol, the ime2ΔC241-as cells became committed to meiosis between 1 h and 2.5 h after addition of estradiol (Fig. 6B). This delay in commitment relative to the wild type mirrors a delay in the appearance of binucleate cells in the ime2ΔC241-as cells. Thus, commitment occurs in these cells and does so at the same time with respect to meiotic progression as in the wild type. These results indicate that protection and late translation of transcripts are not required for cells to commit to differentiation.
DISCUSSION
As yeast cells progress from the meiotic prophase into the meiotic divisions, control of translation becomes the critical process controlling the timing of gene expression (12). The results presented here demonstrate that the Ime2/Rim4 regulatory circuit controls the expression timing not only of mRNAs whose translation is delayed until the onset of meiosis II but also of mRNAs such as SPS4, whose translation is delayed until the end of the meiotic divisions.
Unlike most genes in the NDT80 regulon, the levels of these very late-translated mRNAs are stable when meiotic cells are returned to rich medium. The earlier observation that creating a protected form of NDT80 (by fusion to the SPS4 upstream region) does not alter the disappearance of other mRNAs in the NDT80 regulon when cells are transferred to rich medium (19) suggests that the mechanism of protection likely represents, not continued transcription, but rather resistance of specific messages to degradation. Thus, protected messages are sequestered away from the degradation machinery.
Earlier studies that provide the absolute level of mRNAs in sporulating cells suggest that the genes encoding protected transcripts, as a set, tend to be more highly expressed than those encoding nonprotected transcripts; however, there is significant overlap in expression levels between the two sets of genes (12, 25). In particular, SPO20 is expressed at a higher level than GAT4. Therefore, the differing responses of these mRNAs to introduction of nutrients is not simply a matter of the transcript levels.
The Ime2/Rim4 circuit is required for protection. As protected mRNAs are enriched in Rim4 precipitates (15), these results suggest that binding of Rim4 and establishing a translational delay are prerequisites for protection. Binding of Rim4 is not sufficient for protection, however, as translationally delayed Rim4-bound mRNAs, such as SPO20, are not protected. A strong correlation was observed between protection and localization of transcripts into discrete intracellular foci, suggesting that localization into these foci may be important for protection. The only exception to this correlation is that the rim4-F349L allele loses protection and translation delay but does not alter the localization of the SPS4 transcript into foci. The rim4-F349L allele is a partial loss-of-function allele (16). It may be that protection and translation are more sensitive assays of Rim4 activity than focus formation and that this accounts for the separation of these phenotypes in this strain. Alternatively, it may be that Rim4 regulates the translation and stability of the transcripts only after they are released from foci, similar to RIM4-mediated translational delay of SPO20.
These results suggest a model in which the Ime2/Rim4 circuit is the primary regulator of translational delay during meiosis, but additional layers of regulation must be necessary to “tune” the timing of translational onset. In the case of the protected transcripts, we propose that sequestration of the transcripts into foci extends the delay in translation until the end of meiosis II. Rim4 might act in conjunction with additional factors to organize the protected transcripts into foci, and upon Ime2-stimulated destruction of Rim4, the retention of the transcripts in foci would maintain their translational repression. The model predicts that loss of these additional factors would lead to loss of protection and of foci but that these transcripts might still exhibit a translational delay similar to that of SPO20. There are a number of genes in the NDT80 regulon that contain RNA binding motifs. These gene products are strong candidates to play a role in protection and modulation of translational timing.
Protection and differentiation.
Loss of protection does not block the commitment of cells to differentiation. Thus, maintaining the levels of protected transcripts is not required to stop cells from returning to mitotic growth. Similarly, though expression of Ime2 can interfere with mitotic growth (33), our data suggest that the activity of the Ime2 kinase is not required to stop cells from reentering mitosis because commitment occurs even in the presence of inhibited Ime2-as. As many of the proteins encoded by protected transcripts are known to be important for spore wall assembly, it is possible that protection, though unnecessary for commitment, is important for successful completion of sporulation under return-to-growth conditions. In this case, loss of protection would lead to cells that complete meiosis but fail to form proper spores. Unfortunately, though meiotic progression is efficient, completion of spore formation is highly variable in the NDT80-IN background, making it difficult to establish whether loss of protection affects spore formation under return-to-growth conditions.
Translational regulation during gametogenesis.
In midsporulation, the yeast cell switches from fine-grained control of gene expression based primarily on differential transcription to differential translation (12). One possible reason for this change is that the nuclear chromatin undergoes compaction, mediated by both modification of core histones and increased levels of histone H1, as cells progress through meiosis (3, 34). It may be that transcriptional regulation becomes more problematic in this more compacted chromatin so that translational control is preferred. By sequestering certain transcripts from the translational machinery, the cell may also ensure sufficient expression of those gene products to complete sporulation even if nutritional conditions change. Hypercompaction is also seen during spermatogenesis in many metazoans, where the chromatin is marked by histone modifications similar to those seen in sporulation in yeast, and at late stages, many of the histones are replaced by protamines (3, 35). It is unlikely that active transcription is occurring in these hypercompact nuclei. It will be interesting to learn if regulated translation is used to control proper timing of gene expression in the later stages of spermatogenesis, as well.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ed Winter (Thomas Jefferson University) for strains and Jeff Gerst (Weizmann Institute) for plasmids. We are grateful to Angelika Amon, Luke Berchowitz, and Nancy Hollingsworth for comments on the manuscript and to Bruce Futcher, Ed Luk, and Wali Karzai for helpful discussions.
This work was supported by P01 GM088297 to R.S. and A.M.N.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00189-15.
REFERENCES
- 1.Neiman AM. 2011. Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 189:737–765. doi: 10.1534/genetics.111.127126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerr GW, Sarkar S, Arumugam P. 2012. How to halve ploidy: lessons from budding yeast meiosis. Cell Mol Life Sci 69:3037–3051. doi: 10.1007/s00018-012-0974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnamoorthy T, Chen X, Govin J, Cheung WL, Dorsey J, Schindler K, Winter E, Allis CD, Guacci V, Khochbin S, Fuller MT, Berger SL. 2006. Phosphorylation of histone H4 Ser1 regulates sporulation in yeast and is conserved in fly and mouse spermatogenesis. Genes Dev 20:2580–2592. doi: 10.1101/gad.1457006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith HE, Mitchell AP. 1989. A transcriptional cascade governs entry into meiosis in Saccharomyces cerevisiae. Mol Cell Biol 9:2142–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell AP, Driscoll SE, Smith HE. 1990. Positive control of sporulation-specific genes by the IME1 and IME2 products in Saccharomyces cerevisiae. Mol Cell Biol 10:2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Primig M, Williams RM, Winzeler EA, Tevzadze GG, Conway AR, Hwang SY, Davis RW, Esposito RE. 2000. The core meiotic transcriptome in budding yeasts. Nat Genet 26:415–423. doi: 10.1038/82539. [DOI] [PubMed] [Google Scholar]
- 7.Shin ME, Skokotas A, Winter E. 2010. The Cdk1 and Ime2 protein kinases trigger exit from meiotic prophase in Saccharomyces cerevisiae by inhibiting the Sum1 transcriptional repressor. Mol Cell Biol 30:2996–3003. doi: 10.1128/MCB.01682-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu S, Herskowitz I. 1998. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol Cell 1:685–696. doi: 10.1016/S1097-2765(00)80068-4. [DOI] [PubMed] [Google Scholar]
- 9.Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Ajimura M, Padmore R, Klein C, Kleckner N. 1995. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol Cell Biol 15:6572–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell AP. 1994. Control of meiotic gene expression in Saccharomyces cerevisiae. Microbiol Rev 58:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brar GA, Yassour M, Friedman N, Regev A, Ingolia NT, Weissman JS. 2012. High-resolution view of the yeast meiotic program revealed by ribosome profiling. Science 335:552–557. doi: 10.1126/science.1215110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin KR, Zhang C, Shokat KM, Herskowitz I. 2003. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev 17:1524–1539. doi: 10.1101/gad.1101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlile TM, Amon A. 2008. Meiosis I is established through division-specific translational control of a cyclin. Cell 133:280–291. doi: 10.1016/j.cell.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berchowitz LE, Gajadhar AS, van Werven FJ, De Rosa AA, Samoylova ML, Brar GA, Xu Y, Xiao C, Futcher B, Weissman JS, White FM, Amon A. 2013. A developmentally regulated translational control pathway establishes the meiotic chromosome segregation pattern. Genes Dev 27:2147–2163. doi: 10.1101/gad.224253.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soushko M, Mitchell AP. 2000. An RNA-binding protein homologue that promotes sporulation-specific gene expression in Saccharomyces cerevisiae. Yeast 16:631–639. doi:. [DOI] [PubMed] [Google Scholar]
- 17.Simchen G, Pinon R, Salts Y. 1972. Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res 75:207–218. doi: 10.1016/0014-4827(72)90538-1. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya D, Lacefield S. 2013. Cdk1 modulation ensures the coordination of cell-cycle events during the switch from meiotic prophase to mitosis. Curr Biol 23:1505–1513. doi: 10.1016/j.cub.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Friedlander G, Joseph-Strauss D, Carmi M, Zenvirth D, Simchen G, Barkai N. 2006. Modulation of the transcription regulatory program in yeast cells committed to sporulation. Genome Biol 7:R20. doi: 10.1186/gb-2006-7-3-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips-Cremins JE, Corces VG. 2013. Chromatin insulators: linking genome organization to cellular function. Mol Cell 50:461–474. doi: 10.1016/j.molcel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simms TA, Dugas SL, Gremillion JC, Ibos ME, Dandurand MN, Toliver TT, Edwards DJ, Donze D. 2008. TFIIIC binding sites function as both heterochromatin barriers and chromatin insulators in Saccharomyces cerevisiae. Eukaryot Cell 7:2078–2086. doi: 10.1128/EC.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose MD, Fink GR. 1990. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 23.Lorenz MC, Muir RS, Lim E, McElver J, Weber SC, Heitman J. 1995. Gene disruption with PCR products in Saccharomyces cerevisiae. Gene 158:113–117. doi: 10.1016/0378-1119(95)00144-U. [DOI] [PubMed] [Google Scholar]
- 24.Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961. doi:. [DOI] [PubMed] [Google Scholar]
- 25.Miura F, Kawaguchi N, Sese J, Toyoda A, Hattori M, Morishita S, Ito T. 2006. A large-scale full-length cDNA analysis to explore the budding yeast transcriptome. Proc Natl Acad Sci U S A 103:17846–17851. doi: 10.1073/pnas.0605645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haim-Vilmovsky L, Gerst JE. 2009. m-TAG: a PCR-based genomic integration method to visualize the localization of specific endogenous mRNAs in vivo in yeast. Nat Protoc 4:1274–1284. doi: 10.1038/nprot.2009.115. [DOI] [PubMed] [Google Scholar]
- 27.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. 2003. Global analysis of protein localization in budding yeast. Nature 425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 28.Suda Y, Nakanishi H, Mathieson EM, Neiman AM. 2007. Alternative modes of organellar segregation during sporulation in Saccharomyces cerevisiae. Eukaryot Cell 6:2009–2017. doi: 10.1128/EC.00238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sari F, Heinrich M, Meyer W, Braus GH, Irniger S. 2008. The C-terminal region of the meiosis-specific protein kinase Ime2 mediates protein instability and is required for normal spore formation in budding yeast. J Mol Biol 378:31–43. doi: 10.1016/j.jmb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Cody NA, Iampietro C, Lecuyer E. 2013. The many functions of mRNA localization during normal development and disease: from pillar to post. Wiley Interdiscip Rev Dev Biol 2:781–796. doi: 10.1002/wdev.113. [DOI] [PubMed] [Google Scholar]
- 31.Singer-Kruger B, Jansen RP. 2014. Here, there, everywhere: mRNA localization in budding yeast. RNA Biol 11:1031–1039. doi: 10.4161/rna.29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuchiya D, Yang Y, Lacefield S. 2014. Positive feedback of NDT80 expression ensures irreversible meiotic commitment in budding yeast. PLoS Genet 10:e1004398. doi: 10.1371/journal.pgen.1004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolte M, Steigemann P, Braus GH, Irniger S. 2002. Inhibition of APC-mediated proteolysis by the meiosis-specific protein kinase Ime2. Proc Natl Acad Sci U S A 99:4385–4390. doi: 10.1073/pnas.072385099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryant JM, Govin J, Zhang L, Donahue G, Pugh BF, Berger SL. 2012. The linker histone plays a dual role during gametogenesis in Saccharomyces cerevisiae. Mol Cell Biol 32:2771–2783. doi: 10.1128/MCB.00282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathke C, Baarends WM, Awe S, Renkawitz-Pohl R. 2014. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta 1839:155–168. doi: 10.1016/j.bbagrm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Neiman AM, Katz L, Brennwald PJ. 2000. Identification of domains required for developmentally regulated SNARE function in Saccharomyces cerevisiae. Genetics 155:1643–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.