ABSTRACT
The Scc4 protein (CT663) of the pathogenic bacterium Chlamydia has been described as a type III secretion (T3S) chaperone as well as an inhibitor of RNA polymerase. To examine if these roles are connected, we first investigated physical interactions between Chlamydia trachomatis Scc4 and the T3S chaperone Scc1 and a T3S substrate, CopN. In a yeast 3-hybrid assay, Scc4, Scc1, and CopN were all required to detect an interaction, which suggests that these proteins form a trimolecular complex. We also detected interactions between any two of these three T3S proteins in a pulldown assay using only recombinant proteins. We next determined whether these interactions affected the function of Scc4 as an inhibitor of RNA transcription. Using Escherichia coli as a heterologous in vivo system, we demonstrated that expression of C. trachomatis Scc4 led to a drastic decrease in transcript levels for multiple genes. However, coexpression of Scc4 with Scc1, CopN, or both alleviated Scc4-mediated inhibition of transcription. Scc4 expression also severely impaired E. coli growth, but this growth defect was reversed by coexpression of Scc4 with Scc1, CopN, or both, suggesting that the inhibitory effect of Scc4 on transcription and growth can be antagonized by interactions between Scc4, Scc1, and CopN. These findings suggest that the dual functions of Scc4 may serve as a bridge to link T3S and the regulation of gene expression in Chlamydia.
IMPORTANCE This study investigates a novel mechanism for regulating gene expression in the pathogenic bacterium Chlamydia. The Chlamydia type III secretion (T3S) chaperone Scc4 has been shown to inhibit transcription by RNA polymerase. This study describes physical interactions between Scc4 and the T3S proteins Scc1 and CopN. Furthermore, Chlamydia Scc1 and CopN antagonized the inhibitory effects of Scc4 on transcription and growth in a heterologous Escherichia coli system. These results provide evidence that transcription in Chlamydia can be regulated by the T3S system through interactions between T3S proteins.
INTRODUCTION
Chlamydia trachomatis is the most prevalent cause of bacterial sexually transmitted infections in the United States (1, 2). In addition, it is the most common cause of preventable blindness in the world (3). Chlamydia is an unusual obligate intracellular bacterium that has two distinct forms, the infectious elementary body (EB) and the noninfectious reticulate body (RB) (4). Once the EB attaches and enters a susceptible host cell, it converts into an RB that replicates by binary fission, generating hundreds of progeny within the membrane-bound chlamydial inclusion.
Like other pathogenic Gram-negative bacteria, Chlamydia utilizes a type III secretion (T3S) system to deliver effector proteins into a eukaryotic cell (5). In Chlamydia, T3S is important for a number of steps in the intracellular infection (6). EB entry into the host cell is mediated in part by translocation of the T3S effector protein Tarp, which recruits actin at the site of EB attachment and likely aids in internalization (7). At early and middle stages of the developmental cycle, secretion of Inc proteins into the inclusion membrane is proposed to be important for establishing the inclusion and altering host membrane trafficking and signaling pathways (8). Late in the developmental cycle, upon conversion of RBs to EBs, the EBs are preloaded with T3S proteins in preparation for a new round of infection (9).
T3S chaperones are known to selectively regulate and bind to T3S translocator and effector proteins (10). These chaperones have multiple functions, including stabilization of T3S substrates, prevention of premature interactions between substrates through sequestration, and targeting substrates for secretion through the T3S apparatus (10). T3S chaperones are subdivided into several classes based on their substrate specificity. Class I chaperones bind to a single (class IA) or multiple (class IB) T3S effectors, class II chaperones bind translocator proteins, and class III chaperones interact with components of the T3S needle complex (11).
Scc1 and Scc4 are class IA chlamydial chaperones that form a heterodimer (12, 13). The Chlamydia pneumoniae Scc1 and Scc4 heterodimer interacts with the N terminus of CopN, which appears to have effector functions as well as serving as the putative “plug” for the T3S injectisome to prevent premature effector protein secretion (14–17). C. pneumoniae Scc1 and Scc4 have also been shown to facilitate CopN secretion in a heterologous Yersinia T3S system (12). In addition to having a chaperone function, C. trachomatis Scc4 binds RNA polymerase in region 4 of the σ subunit σ66 and the flap domain of the β subunit, which are involved in −35 promoter recognition during transcription initiation (18). In an in vitro transcription assay, Scc4 inhibited Escherichia coli RNA polymerase and a hybrid E. coli polymerase containing a portion of C. trachomatis σ66 (18). The significance of these relationships is not understood, but they suggest that T3S and gene expression in Chlamydia may be linked by T3S chaperones.
In this study, we examined whether the physical interactions between the C. trachomatis protein Scc4 and the T3S proteins Scc1 and CopN can affect its function as a transcriptional regulator. We report that Scc1 and CopN can antagonize the ability of Scc4 to block transcription and reverse Scc4-mediated growth inhibition in a heterologous in vivo assay. These findings provide molecular evidence of a mechanistic link between T3S and transcription in Chlamydia.
MATERIALS AND METHODS
E. coli strains and growth conditions.
E. coli strain XL1-Blue (Stratagene) was used for plasmid maintenance and propagation. E. coli strain BL21 Star(DE3) (Invitrogen) was used for expression and purification of recombinant proteins. E. coli strain T7 Express (New England Biolabs) was used for coexpression experiments analyzing gene expression and growth. All E. coli strains were grown in Luria-Bertani (LB) medium at 37°C with appropriate antibiotics.
C. trachomatis culture conditions.
C. trachomatis serovar D strain UW-3/Cx, obtained from the American Type Culture Collection (ATCC), was propagated in HeLa 229 cells (ATCC). HeLa 229 cells were grown in Eagle's minimal essential medium (EMEM; Life Technologies Corp.) supplemented with 5% fetal bovine serum (Atlanta Biologicals), 2 mM l-glutamine, and 50 μg/ml of gentamicin (Mediatech, Inc.). Chlamydia stocks were prepared by inoculating monolayers of HeLa 229 cells in 1-dram glass vials. After inoculation, monolayers were centrifuged at room temperature for 1 h at 800 × g, followed by the addition of EMEM containing cycloheximide (1 μg/ml). Cultures were incubated for 48 h at 37°C before being sonicated in SPG (200 mM sucrose, 20 mM sodium phosphate, 5 mM glutamate, pH 7.4) and centrifuged at 500 × g for 10 min. The supernatant was removed and stored at −80°C.
Yeast 3-hybrid assay (Y3H).
C. trachomatis copN (resulting in a full-length protein or one with an N- or C-terminal truncation) was cloned into the activation domain (AD) site of the pGADT7 “prey” vector, while C. trachomatis scc1, scc4, or scc3 or Yersinia sycE was cloned into the two binding domain (BD) sites of the pBridge Y3H “bait” vector (Table 1). The two vectors were cotransformed into the Saccharomyces cerevisiae Y2HGold strain according to the manufacturer's protocol (Clontech). Yeast cotransformants were plated on medium lacking histidine, leucine, methionine, and tryptophan and supplemented with 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (X-α-Gal) and aureobasidin A (AbA). A positive interaction was identified by growth of blue colonies.
TABLE 1.
C. trachomatis CopN, Scc1, and Scc4 demonstrate a trimolecular interaction in a yeast three-hybrid assay
| copN gene in pGADT7 (prey) AD | Gene in pBridge (bait) at: |
Interaction | |
|---|---|---|---|
| BD site 1 | BD site 2 | ||
| Full length | scc1 | scc4 | Positive |
| Full length | scc4 | scc1 | Positive |
| Full length | scc1 | Negative | |
| Full length | scc4 | Negative | |
| Full length | scc1 | sycEa | Negative |
| Full length | scc4 | sycEa | Negative |
| Coding for N terminus | scc4 | scc1 | Positive |
| Coding for N terminus | scc4 | Negative | |
| Coding for N terminus | scc1 | Negative | |
| Coding for N terminus | scc4 | scc1 | Negative |
| Coding for N terminus | scc3 | Positive | |
Yersinia ortholog of C. trachomatis scc1.
Protein purification.
The 6×His-tagged pET45b+ vector (Novagen-Merck KGaA) was used for expression of recombinant 6×His-tagged proteins. The expression vector pGEX4T-1 (GE Healthcare) was employed to produce glutathione S-transferase (GST) fusion proteins. N-terminally 6×His- or GST-tagged recombinant chlamydial T3S proteins were purified using an affinity technique. Briefly, E. coli BL21 was grown overnight in MagicMedia E. coli expression medium (Invitrogen) and collected by centrifugation at 5,000 × g for 10 min at 4°C. Pellets were washed and resuspended in TNGS buffer, pH 7.5 (20 mM Tris-HCl, 50 mM sodium chloride, 5% glycerol, 0.025% N-lauroyl sarcosine). Cells were disrupted by using a French press followed by centrifugation at 30,000 × g for 40 min at 4°C. Clarified extracts and pellets were checked by SDS-PAGE for the presence of recombinant proteins. Insoluble proteins found primarily in the pellet were purified using a urea-denaturing protocol, with subsequent renaturing by dialysis with TNGS buffer.
Pulldown assays with purified recombinant proteins.
GST-protein fusions were immobilized on glutathione-Sepharose beads (100 to 500 μl) by incubating the purified GST-protein fusions with glutathione-Sepharose beads equilibrated with TEN100 (20 mM Tris, pH 7.4, 0.1 mM EDTA, and 100 mM NaCl) at 4°C with rocking for 1 h. Charged beads were washed four times with 100 volumes of TEN100 to remove unbound material, resuspended in TEN100, and stored at 4°C. Approximately 500 μg of purified 6×His fusion proteins in TEN were individually mixed with the immobilized GST fusion proteins at 4°C with nutation for 1 h. The glutathione-Sepharose beads were then washed four times with 100 bed volumes of TEN buffer. Interacting proteins were eluted by boiling them in sample buffer and were subsequently separated by SDS-PAGE and visualized using Western blot analysis. The samples were separated by 10% SDS-PAGE and visualized by Western blotting using anti-His-Scc4, anti-His-Scc1, and/or anti-His-CopN or anti-GST-CopN mouse polyclonal antibodies.
Pulldown assay with chlamydial lysate.
Pulldown experiments were performed with purified Scc1 and Scc4 recombinant proteins, and extracts of HeLa 229 cells infected with C. trachomatis serovar D were prepared using a CryoMill (Retsch) and subsequently cleared by centrifugation at 30,000 × g for 1 h. The 6×His-tagged recombinant protein(s) alone or the proteins mixed together were bound on the HisPur cobalt resin (Thermo Scientific) previously blocked with 0.5% bovine serum albumin (BSA) in TNGS buffer. Nonspecific interactions were removed by washing the specimens with TNGS buffer. The Chlamydia cleared lysate (3 to 5 mg of total protein) in TNGS buffer was added to the recombinant-protein-charged beads and incubated for 1 h at 4°C with agitation. Unbound proteins were washed with TNGS buffer supplemented with 5 mM imidazole. The complexes bound to the HisPur cobalt resin were denatured with the addition of SDS sample buffer with 20 mM dithiothreitol (DTT) heated at 95°C for 10 min. The samples were separated by 10% SDS-PAGE and visualized by Western blotting using anti-His-Scc4, -Scc1, and/or -CopN mouse polyclonal antibodies.
E. coli growth inhibition and sample collection.
The E. coli T7 Express strain transformed with pST44, pMT1649, pMT1652, pMT1653, pMT1654, pMT1655, pMT1667, or pMT1668 (see Table S1 in the supplemental material) was grown in LB broth with 100 μg/ml of carbenicillin. Overnight cultures were diluted to an optical density at 600 nm (OD600) of 0.05 with or without 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) induction. The OD600 was monitored at 0.5, 1, 2, 3, and 4 h. At 4 h, aliquots of approximately 1 × 108 bacterial cells were collected from each culture. Bacterial cells were washed twice with ice cold phosphate-buffered saline (PBS), pelleted, and stored at −80°C. Aliquots were subsequently used to isolate DNA for genome copy quantification and RNA for reverse transcriptase quantitative PCR (qRT-PCR) and for Western blotting to demonstrate protein expression.
Isolation and processing of nucleic acids.
E. coli cells containing expression plasmids were grown without IPTG (uninduced) or with 0.1 mM IPTG (induced) for 4 h, and aliquots were collected. One aliquot for each experimental condition was processed using the DNeasy blood and tissue kit (Qiagen) according to the manufacturer's instructions to purify genomic DNA. DNA was eluted with 200 μl of diethyl pyrocarbonate (DEPC)-treated double-distilled water (ddH2O), giving 0.5% total DNA/μl, which was then diluted to 0.01% total DNA/μl and stored at −20°C. An additional aliquot was collected in parallel under each experimental condition for isolation of RNA using the RNeasy Plus minikit (Qiagen) according to the manufacturer's instructions, and RNA was eluted into 50 μl of DEPC-treated ddH2O. From total RNA, 2 μl (4%) was incubated with 10 units of RQ1 RNase-Free DNase (Promega) at 37°C for 60 min, after which an additional 10 units of DNase was added and incubated with RNA for another 60 min. Following DNase treatment, the RNA was repurified using the RNeasy Plus minikit and eluted into 200 μl of DEPC-treated ddH2O, giving a final concentration of 0.02% of total RNA per microliter.
qPCR.
For analysis of DNA from E. coli, quantitative PCR (qPCR) using the iQ SYBR green kit (Bio-Rad) was performed in triplicate with primers diluted to a final concentration of 250 nM and a total reaction volume of 20 μl. As a template, 2 μl (0.02%) of total genomic DNA was added to each reaction mixture. Reactions were carried out on a Bio-Rad iCycler with an initial denaturation step at 95°C for 5 min followed by 40 cycles of 30 s at 95°C and 30 s at 60°C. Fluorescent detection occurred during the annealing phase and subsequently during a dissociation curve analysis to confirm amplification of a single product. The threshold cycles (CT) were determined using the Bio-Rad iCycler software.
qRT-PCR.
For analysis of RNA transcript levels, quantitative reverse transcriptase PCR (qRT-PCR) using the iTaq Universal SYBR green one-step kit was performed in triplicate with primers diluted to a final concentration of 250 nM and a total reaction volume of 20 μl. cDNA was synthesized from 2 μl (0.04%) of DNase-treated RNA for 10 min at 50°C, followed by a 5-min denaturation cycle at 95°C, and 40 cycles of 30 s at 95°C and 30 s at 60°C. Fluorescent detection and dissociation curve analysis were performed as described for qPCR. Reaction mixtures lacking reverse transcriptase were performed in parallel for each RNA sample to confirm the absence of contaminating DNA.
qRT-PCR data analysis.
To normalize transcripts to genome copy number, standard curves were generated with each gene-specific primer pair by performing qPCR on E. coli genomic DNA ranging between 3 × 102 and 3 × 106 copies in each reaction mixture. The CT value obtained by adding 0.02% of total DNA for each sample was fit to the standard curve to determine the number of corresponding gene copies and then multiplied by a dilution factor of 5,000 (100%/0.02%) to yield the total number of genome copies in each E. coli aliquot. The CT value obtained by adding 0.04% of total RNA for each sample using qRT-PCR was also fit to the gene-specific standard curve to determine the number of transcript copies and then multiplied by a dilution factor of 2,500 to yield the total number of transcript copies in each E. coli aliquot. To calculate the relative number of transcripts per genome copy, the number of transcripts was divided by the number of genome copies for each paired set of aliquots. Fold change was then calculated as the relative number of transcripts per genome copy of induced samples divided by the relative number of transcripts per genome copy of uninduced samples ([transcripts/genome induced]/[transcripts/genome uninduced]). Statistical significance of fold change differences in qRT-PCR results was determined by comparing each condition to results for the Scc4-expressing E. coli strain using Student's t test.
RESULTS
We first investigated interactions between the C. trachomatis T3S proteins Scc4, Scc1, and CopN using a yeast 3-hybrid (Y3H) approach. A positive interaction with CopN required Scc4 and Scc1 to be present and was not detected with either Scc4 or Scc1 alone (Table 1; see also Fig. S1 in the supplemental material). There was no interaction when we replaced either Scc4 or Scc1 with SycE, an Scc1 ortholog from Yersinia, indicating that the interactions were Chlamydia specific. Using a truncated form of CopN, we mapped the interaction with Scc4 and Scc1 to the N-terminal half of CopN. As a control, we used the C terminus of CopN and found that it interacted with Scc3, as previously described (19), but not with Scc4 and Scc1.
To further understand the relationship between C. trachomatis Scc4, Scc1, and CopN, we examined two-way physical interactions between any two members of this trimolecular complex. These studies were performed with pulldown assays using recombinant purified proteins tagged at their N termini with either 6×His or GST (Table 2; see also Fig. S2 in the supplemental material). Interaction between Scc1 and CopN was detected in reciprocal experiments with either His-Scc1 and GST-CopN or His-CopN and GST-Scc1. His-Scc4 interacted with GST-Scc1 and interacted in a separate experiment with GST-CopN. However, there was no interaction detected when we used GST-Scc4 and either His-Scc1 or His-CopN, which may have been due to steric hindrance or conformational constraints caused by the GST tag. These results demonstrate that each of these three C. trachomatis T3S proteins can interact with one another under these in vitro conditions.
TABLE 2.
Interactions detected using a pulldown assay with recombinant chlamydial Scc4, Scc1, and CopN, each tagged at its N terminus with either 6×His or GST
| Protein | Interaction witha: |
||
|---|---|---|---|
| GST-Scc1 | GST-CopN | GST-Scc4 | |
| His-Scc1 | NT | Positive | Negative |
| His-CopN | Positive | NT | Negative |
| His-Scc4 | Positive | Positive | NT |
NT, not tested.
We then examined if we could detect interactions between Scc4, Scc1, and CopN in a pulldown assay using a lysate from Chlamydia-infected cells (Fig. 1). We supplemented the lysate with recombinant His-Scc4 and His-Scc1 (rScc4 and rScc1), bound the mixture to cobalt resin, and assayed for coisolation of CopN. Native CopN was detected when rScc4 and rScc1 were incubated with the chlamydial lysate, providing further evidence of a trimolecular complex. We were unable to detect CopN when we supplemented samples with either rScc4 or rScc1 alone, which suggests that the native proteins were at low abundance in the lysate.
FIG 1.
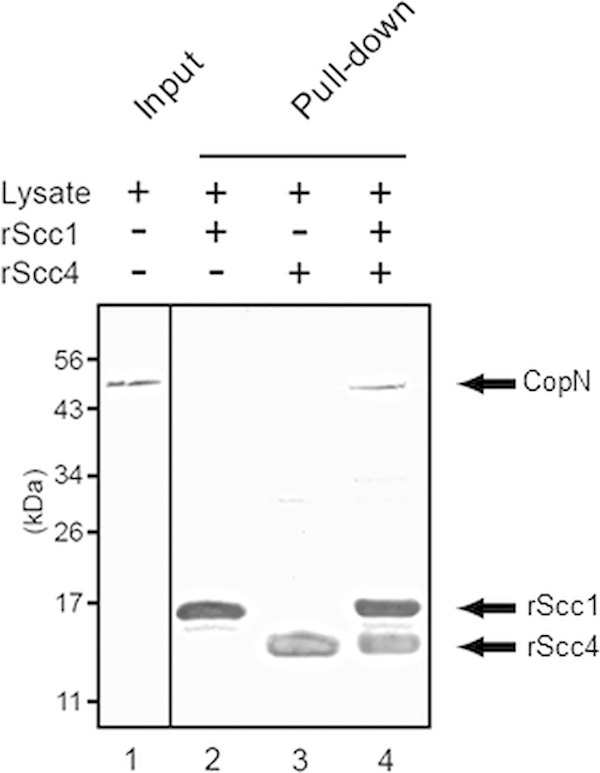
Pulldown assay showing protein-protein interactions for His-tagged Scc4 and Scc1 (rScc4 and rScc1) and native CopN. Lane 1 shows CopN present in the C. trachomatis lysate used in the pulldown assay (input). Lanes 2 to 4 show proteins recovered from the pulldown when the chlamydial lysate was incubated with cobalt resin and with rScc1 alone (lane 2), rScc4 alone (lane 3), or rScc1 and rScc4 (lane 4). Mouse polyclonal antibodies used to detect the proteins in the Western blot shown are as follows: lane 1, anti-His-CopN; and lanes 2 to 4, an antibody cocktail composed of anti-His-CopN, anti-His-Scc1, and anti-His-Scc4.
It has been reported that C. trachomatis Scc4 binds the β and σ subunits of RNA polymerase and inhibits transcription of the major form of chlamydial RNA polymerase, σ66 RNA polymerase, as well as E. coli σ70 RNA polymerase (18). Therefore, once we had established that these three T3S proteins physically interact, we examined whether Scc1 and CopN altered the ability of Scc4 to inhibit transcription. Using E. coli as a heterologous in vivo transcription assay, plasmids containing one, two, or all three of the chlamydial T3S genes, scc4, scc1, and copN, were expressed. We checked the IPTG-induced expression of the chlamydial proteins by Western blotting and verified that Scc4 protein levels were not affected by Scc1 or CopN coexpression (see Fig. S3 in the supplemental material). We then measured transcript levels of selected σ70-dependent E. coli genes, normalizing the results to E. coli genome copy number to control for any differences in growth rate. Finally, we examined if particular combinations of the chlamydial T3S proteins affected transcription by comparing transcript levels with and without IPTG induction of these proteins.
Scc4 by itself caused a 22-fold reduction in transcription of a constitutively expressed gene, recA (P < 0.05) (Fig. 2A). When Scc4 was coexpressed with Scc1, however, there was only a 4-fold decrease in recA transcript levels, consistent with partial rescue of Scc4-mediated transcriptional inhibition. In contrast, when Scc4 was coexpressed with CopN, or together with Scc1 and CopN, transcript levels were restored to baseline, demonstrating that inhibition of transcription by Scc4 had been completely reversed. In a control experiment, Scc1 and CopN in the absence of Scc4 did not alter recA transcription, indicating that these T3S proteins did not nonspecifically stimulate transcription.
FIG 2.
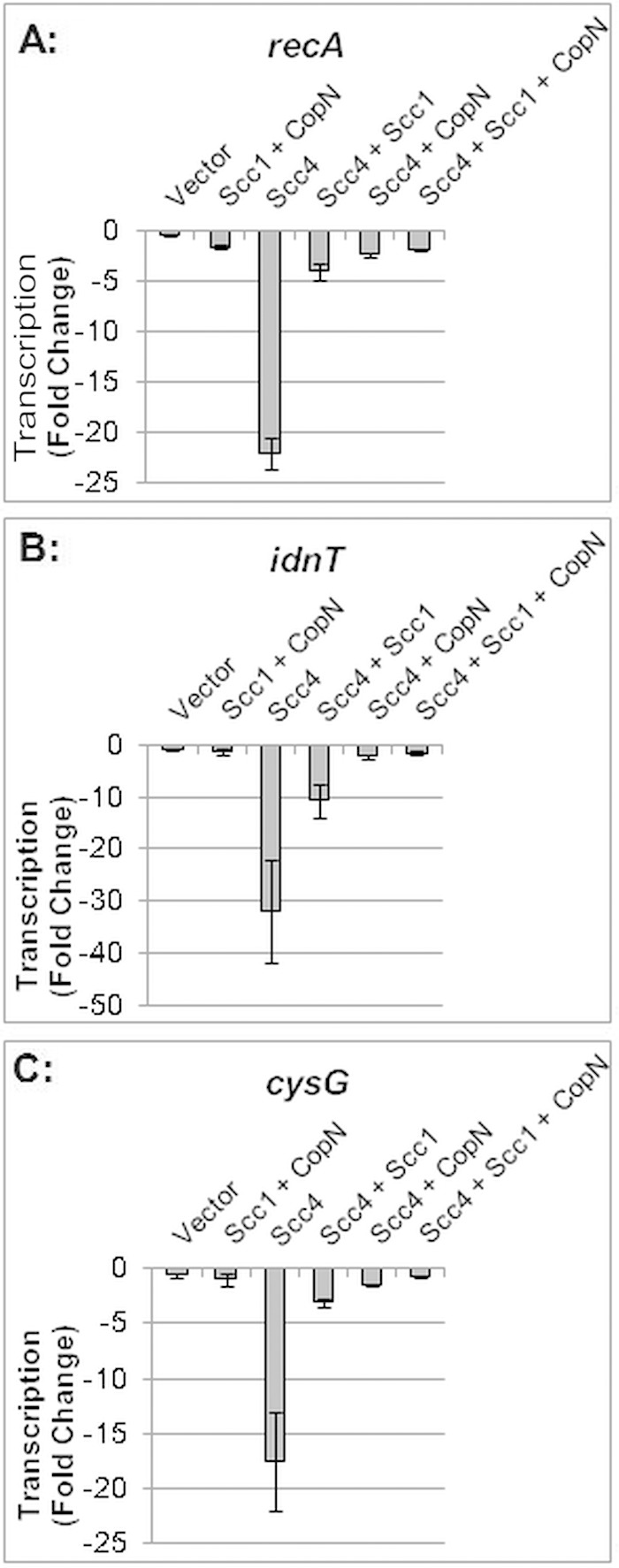
Effect of C. trachomatis Scc4, Scc1, and CopN in various combinations on the transcription of three E. coli genes. Transcripts were measured by qRT-PCR 4 h after expression of the chlamydial proteins was induced by IPTG. For each combination of chlamydial proteins, transcript levels were normalized to genome copy number and reported as a fold change from transcript levels in uninduced cells. Scc4 expression decreased transcription relative to that under all other experimental conditions (P < 0.05).
To examine if Scc1 and CopN had a general effect on the reversal of Scc4 transcriptional inhibition, we performed this analysis with two additional E. coli genes. We chose idnT and cysG because these genes are stably expressed during IPTG-induced recombinant protein expression (20). Expression of Scc4 alone decreased transcription of idnT by 32-fold and cysG by 18-fold (P < 0.05) (Fig. 2B and C). As with recA, Scc4-mediated inhibition was partially reversed by Scc1, and transcription was restored to baseline levels by CopN or Scc1 plus CopN. Together, these results demonstrate the ability of CopN and to a lesser extent Scc1 to antagonize Scc4-mediated transcriptional inhibition.
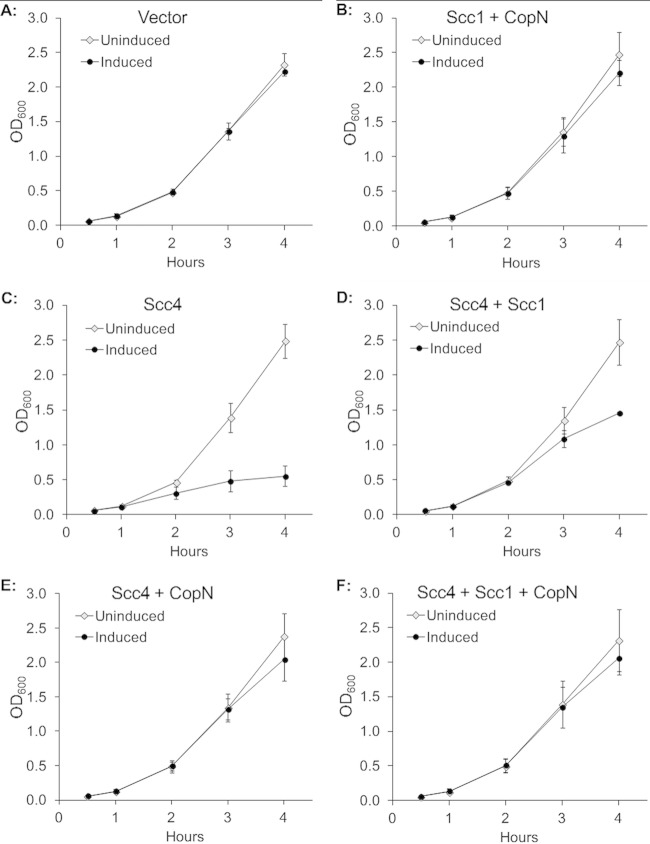
The pronounced transcriptional inhibition by Scc4 and the rescue of this inhibition by Scc1 and CopN led us to investigate the effect of these three chlamydial T3S proteins on the growth of E. coli. Scc4 expression inhibited E. coli growth, resulting in only a small increase in OD600 for up to 4 h postinduction compared to that of the uninduced control (Fig. 3C). Scc4-mediated growth inhibition was partially reversed by coexpression with Scc1 (Fig. 3D). In contrast, there was complete rescue of growth inhibition when Scc4 was coexpressed with CopN (Fig. 3E) or when Scc4 was coexpressed with Scc1 and CopN (Fig. 3F). In control experiments, Scc1 and CopN expression in the absence of Scc4 did not alter E. coli growth (Fig. 3B). In contrast, Scc4 was still able to inhibit E. coli growth when it was coexpressed with Yersinia pseudotuberculosis SycE, which is a T3S chaperone similar in size and function to chlamydial Scc1 (see Fig. S4 in the supplemental material). This result demonstrates that reversal of Scc4-mediated growth inhibition was due to Scc1 and CopN and was not a nonspecific effect from coexpression of an additional protein with Scc4. These findings indicate that the growth defect produced by Scc4 was likely due to its inhibitory effect on RNA polymerase and transcription. In addition, our data indicate that CopN and Scc1 are able to modulate these negative effects of Scc4 on transcription and growth, with CopN demonstrating the most pronounced effect.
FIG 3.
Effect of C. trachomatis Scc4, Scc1, and CopN in various combinations on E. coli growth as measured by OD600. Each graph shows the growth curve in the absence (uninduced) or presence (induced) of IPTG. The x axis shows the time after the addition of 0.1 mM IPTG to the induced sample. (A) Empty vector plasmid; (B) Scc1 and CopN; (C) Scc4 alone; (D) Scc4 and Scc1; (E) Scc4 and CopN; (F) Scc4, Scc1, and CopN.
DISCUSSION
A distinguishing characteristic of a Chlamydia infection is the temporal expression of chlamydial genes over the course of the intracellular developmental cycle. However, the signals that control gene regulation remain largely unknown. In this study, we focused on a regulator of chlamydial RNA polymerase that also functions as a T3S chaperone. We showed that C. trachomatis Scc4 interacts with the T3S proteins Scc1 and CopN in Y3H and pulldown assays, which is consistent with studies of their orthologs in C. pneumoniae (12). We then showed that these physical interactions have functional significance by demonstrating that Scc1 and CopN were able to antagonize the inhibitory effects of Scc4 on the transcription and growth of E. coli. These findings indicate that T3S and gene expression in Chlamydia may be linked by the dual functions of Scc4 as a T3S chaperone and a general inhibitor of transcription.
Our data support a model in which Scc1 and CopN prevent Scc4 from binding and inhibiting RNA polymerase. The experimental evidence indicates a functional role for the trimolecular Scc4-Scc1-CopN complex in sequestering Scc4 and modulating its activity. Our results also indicate that Scc4 can interact with CopN, and to a lesser extent with Scc1, in two-way interactions. However, these bimolecular interactions were detected using purified recombinant proteins in our pulldown assay and by overexpressing the chlamydial proteins in our in vivo transcription and growth studies. Thus, the physiologic relevance of the two-way interactions is unclear because the high concentrations of the chlamydial proteins in these studies may have exaggerated the physical interactions between these proteins. Our findings of two-way and three-way interactions between Scc4, Scc1, and CopN are mostly consistent with the results of published studies. For example, efficient secretion of C. pneumoniae CopN required Scc4 and Scc1, and all three proteins were required for interactions in a pulldown assay (12). Direct interactions between C. trachomatis Scc4 and Scc1 in a yeast two-hybrid assay have also been reported (13). However, we also discovered evidence of potential two-way interactions between CopN and either Scc1 or Scc4, which has not been previously reported.
We are aware that Scc4 may have caused an apparent decrease in transcription if there were sufficient numbers of dead bacteria to artifactually increase the genome copy number used to normalize our transcript levels. However, Scc4-expressing E. coli continued to divide, albeit slowly (Fig. 3), while showing very large decreases in transcription (>18-fold inhibition for all three genes) (Fig. 2). Furthermore, the ability of Scc1 and CopN to alleviate this transcriptional inhibition suggests that the inhibitory activity of Scc4 is due to a specific molecular mechanism.
T3S and gene expression are linked in a number of pathogenic Gram-negative bacteria (21, 22, 23), but the mechanism in Chlamydia appears to have some unique features. In a common scenario in other bacteria, the T3S chaperone interacts with a transcription factor, promoting selective activation of T3S genes. For example, in Shigella flexneri, secretion of the T3S effectors IpaB and IpaC releases the T3S chaperone IpgC, which then serves as a coactivator for the transcription factor MxiE, causing activation of T3S effectors (24). This coupling of T3S and gene expression in other bacteria is used to homeostatically regulate T3S protein levels. In contrast, Chlamydia Scc4 appears to be a global regulator of chlamydial transcription because it targets the core transcriptional machinery (18). Thus, Scc4 has the potential to inhibit all genes transcribed by σ66 RNA polymerase and not just T3S genes. Another difference is that the T3S chaperone typically plays a role in transcriptional activation in other bacteria, while Scc4 has a negative effect as an inhibitor of chlamydial transcription.
It is not known when Scc4 acts as a transcriptional inhibitor in the chlamydial developmental cycle, and so it is difficult to predict when its activity is modulated by Scc1 and CopN. Scc4 has been proposed to inhibit σ66 RNA polymerase at late times because Scc4 protein accumulates late in the developmental cycle (18). However, Scc4 is transcribed from a midcycle gene, leaving unexplained how σ66 RNA polymerase, which is the major form of chlamydial RNA polymerase, can transcribe midcycle and late genes if Scc4 is already present. Our results provide a possible mechanism by which Scc4 is bound and sequestered by Scc1 and CopN during midcycle, preventing it from inhibiting RNA polymerase at that time. Disruption of the Scc4-CopN-Scc1 complex after late genes have been transcribed may then be a very late event in which Scc4 is released to inhibit σ66 RNA polymerase at the end of the developmental cycle. This switch would be predicted to have a global effect in downregulating chlamydial transcription, although it may be selective because σ28 RNA polymerase, which transcribes a subset of late genes, is not inhibited by Scc4 (18). This switch would also affect the availability of CopN, which is the plug for the T3S apparatus and a secreted effector, but CopN localization at late times has not been determined.
In summary, we propose that the ability of Scc4 to inhibit transcription by the major chlamydial RNA polymerase can be regulated by its interactions with the T3S proteins CopN and Scc1. This mechanism is based on the dual functions of Scc4 as a T3S chaperone and a transcriptional regulator. This functional link between T3S and transcription provides new insight into how chlamydial gene expression is regulated and may be exploited in a novel antichlamydial strategy targeting the temporal control of transcription during the intracellular infection.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Eric Cheng, who initiated this work, and UyenPhoung Tran for her work with the Y3H system. We also thank Jennifer Lee, Chris Rosario, and Emilie Orillard for critical reading of the manuscript. Partho Ghosh generously provided anti-SycE antibodies.
This work was supported in part by NIH grant AI71104 (E.M.P.) and by NIH grant AI44198 (M.T.). B.R.H. was supported by NRSA postdoctoral fellowship F32-AI108097.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00379-15.
REFERENCES
- 1.Batteiger BE, Tan M. 2014. Chlamydia trachomatis (trachoma, genital infections, perinatal infections, and lymphogranuloma venereum), p 2154–2170. In Bennett JE, Dolin R, Mandell GL (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 8th ed Elsevier Inc, Philadelphia, PA. [Google Scholar]
- 2.Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onwen DH, Schley AW, Anderson WJ, Grigoryan A, Aranas AE, Wodajo MS, Abellera JP, Centers for Disease Control and Prevention (CDC). 2014. Summary of notifiable diseases—United States, 2012. MMWR Morb Mortal Wkly Rep 61:1–121. [PubMed] [Google Scholar]
- 3.Burton MJ, Mabey DC. 2009. The global burden of trachoma: a review. PLoS Negl Trop Dis 3:e460. doi: 10.1371/journal.pntd.0000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulder JW. 1991. Interaction of Chlamydiae and host cells in vitro. Microbiol Rev 55:143–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galán JE, Lara-Tejero M, Marlovits TC, Wagner S. 2014. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol 68:415–438. doi: 10.1146/annurev-micro-092412-155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller KE, Plano GV, Fields KA. 2014. New frontiers in type III secretion biology: the Chlamydia perspective. Infect Immun 82:2–9. doi: 10.1128/IAI.00917-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, Carabeo RA, Hackstadt T. 2004. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A 101:10166–10171. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valdivia RH. 2008. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr Opin Microbiol 11:53–59. doi: 10.1016/j.mib.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Saka HA, Thompson JW, Chen YS, Kumar Y, Dubois LG, Moseley MA, Valdivia RH. 2011. Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms. Mol Microbiol 82:1185–1203. doi: 10.1111/j.1365-2958.2011.07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fattori J, Prando A, Martins AM, Rodrigues FH, Tasic L. 2011. Bacterial secretion chaperones. Protein Pept Lett 18:158–166. doi: 10.2174/092986611794475048. [DOI] [PubMed] [Google Scholar]
- 11.Page AL, Parsot C. 2002. Chaperones of the type III secretion pathway: jacks of all trades. Mol Microbiol 46:1–11. doi: 10.1046/j.1365-2958.2002.03138.x. [DOI] [PubMed] [Google Scholar]
- 12.Silva-Herzog E, Joseph SS, Avery AK, Coba JA, Wolf K, Fields KA, Plano GV. 2011. Scc1 (CP0432) and Scc4 (CP0033) function as a type III secretion chaperone for CopN of Chlamydia pneumoniae. J Bacteriol 193:3490–3496. doi: 10.1128/JB.00203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spaeth KE, Chen YS, Valdivia RH. 2009. The Chlamydia type III secretion system C-ring engages a chaperone-effector protein complex. PLoS Pathog 5:e1000579. doi: 10.1371/journal.ppat.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archuleta TL, Du Y, English CA, Lory S, Lesser C, Ohi MD, Ohi R, Spiller BW. 2011. The Chlamydia effector chlamydial outer protein N (CopN) sequesters tubulin and prevents microtubule assembly. J Biol Chem 286:33992–33998. doi: 10.1074/jbc.M111.258426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewoody RS, Merritt PM, Marketon MM. 2013. Regulation of the Yersinia type III secretion system: traffic control. Front Cell Infect Microbiol 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida K, Matsuo J, Yamamoto Y, Yamaguchi H. 2014. Chlamydia pneumoniae effector chlamydial outer protein N sequesters fructose bisphosphate aldolase A, providing a benefit to bacterial growth. BMC Microbiol 14:330. doi: 10.1186/s12866-014-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nawrotek A, Guimaraes BG, Velours C, Subtil A, Knossow M, Gigant B. 2014. Biochemical and structural insights into microtubule perturbation by CopN from Chlamydia pneumoniae. J Biol Chem 289:25199–25210. doi: 10.1074/jbc.M114.568436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao X, Deighan P, Hua Z, Hu X, Wang J, Luo M, Wang J, Liang Y, Zhong G, Hochschild A, Shen L. 2009. A regulator from Chlamydia trachomatis modulates the activity of RNA polymerase through direct interaction with the beta subunit and the primary sigma subunit. Genes Dev 23:1818–1829. doi: 10.1101/gad.1784009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slepenkin A, de la Maza LM, Peterson EM. 2005. Interaction between components of the type III secretion system of Chlamydiaceae. J Bacteriol 187:473–479. doi: 10.1128/JB.187.2.473-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou K, Zhou L, Lim Q, Zou R, Stephanopoulos G, Too HP. 2011. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR. BMC Mol Biol 12:18. doi: 10.1186/1471-2199-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z, Chen G, Joshi S, Brutinel ED, Yahr TL, Chen L. 2007. Biochemical characterization of a regulatory cascade controlling transcription of the Pseudomonas aeruginosa type III secretion system. J Biol Chem 282:6136–6142. [DOI] [PubMed] [Google Scholar]
- 22.Tucker SC, Galán JE. 2000. Complex function for SicA, a Salmonella enterica serovar Typhimurium type III secretion-associated chaperone. J Bacteriol 182:2262–2268. doi: 10.1128/JB.182.8.2262-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson DM, Ramamurthi KS, Tam C, Schneewind O. 2002. YopD and LcrH regulate expression of Yersinia enterocolitica YopQ by a posttranscriptional mechanism and bind to yopQ RNA. J Bacteriol 184:1287–1295. doi: 10.1128/JB.184.5.1287-1295.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavris M, Page AL, Tournebize R, Demers B, Sansonetti P, Parsot C. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol Microbiol 43:1543–1553. doi: 10.1046/j.1365-2958.2002.02836.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.