Abstract
Chemotaxis affords motile cells the ability to rapidly respond to environmental challenges by navigating cells to niches favoring growth. Such a property results from the activities of dedicated signal transduction systems on the motility apparatus, such as flagella, type IV pili, and gliding machineries. Once cells have reached a niche with favorable conditions, they often stop moving and aggregate into complex communities termed biofilms. An intermediate and reversible stage that precedes commitment to permanent adhesion often includes transient cell-cell contacts between motile cells. Chemotaxis signaling has been implicated in modulating the transient aggregation of motile cells. Evidence further indicates that chemotaxis-dependent transient cell aggregation events are behavioral responses to changes in metabolic cues that temporarily prohibit permanent attachment by maintaining motility and chemotaxis. This minireview discusses a few examples illustrating the role of chemotaxis signaling in the initiation of cell-cell contacts in bacteria moving via flagella, pili, or gliding.
INTRODUCTION
Motile bacterial cells have developed various strategies to navigate away from environments in which nutrients or other conditions limit growth or, alternatively, to implement cellular responses that allow them to persist under these conditions. Examples of such adaptive responses include transition from vegetative states to surface-attached communities in biofilms, flocculation in liquid cultures, and the formation of dormant spores or stress-resistant cysts (1–3). These responses correspond to long-term adaptation to persistent growth-limiting conditions and are regulated by complex regulatory networks. A great deal of attention has been paid to the mechanisms controlling the transition of cells from growth to long-term-survival mode, and in particular, to the “swim-or-stick” transitions of motile cells into nonmotile communities that adhere to surfaces (biofilm) or other cells (flocs) (4). Flocculated and biofilm-bound cells are functionally similar (5), and both have enhanced resistance to a variety of environmental stressors, with implications ranging from medicine (5) to agriculture (6). Extracellular structures, such as exopolysaccharides (EPS) and surface adhesins, directly trigger the permanent attachment of cells. Cell-cell and cell-surface contacts can also be mediated indirectly by eliciting changes in cellular behaviors, such as motility. An increasing number of reports document motility contributing to the ability of bacteria to form biofilms or to flocculate. Irreversible attachment is accompanied by a loss of motility, and given the competitive advantage that motility provides bacteria, permanent attachment of motile cells to surfaces or other cells is tightly controlled. Beyond motility, bacterial chemotaxis, which is the ability to direct motility in gradients of effectors, has also been implicated in modulating attachment (7–11). Before committing to a sessile biofilm or to flocculate, many motile bacteria first initiate transient cell-cell and cell-surface contacts to produce dynamic aggregates of still-motile cells. By controlling the activity of the motility apparatus, chemotaxis can actively promote the initiation of cell-cell contacts during aggregation and, as a result, regulate transient cell aggregation prior to irreversible adhesion. Here, I review selected examples that illustrate how chemotaxis signal transduction promotes transient aggregation in bacteria motile by flagella, pili, or gliding.
CHEMOTAXIS SIGNALING AND MOTILITY APPARATUS
Chemotaxis allows motile bacteria to rapidly escape conditions that limit growth by orienting their movement toward a more favorable niche. Chemotaxis thus promotes the transient accumulation of cells within a particular region, increasing the probability of cell-cell interactions, including transient attachment. The coordinated chemotaxis response of a population of motile cells may result in the formation of clusters around transient nutrient sources (12–14) and of traveling bands of cells that rapidly metabolize ephemeral sources of nutrients (13, 15). As a result, chemotaxis may significantly impact nutrient cycles in soils and oceans (16, 17). Chemotaxis signal transduction pathways are conserved, and the genes encoding them are found in the genomes of bacteria mobile by flagella (swimming or swarming), pili (twitching), or other mechanisms that occur in the absence of identified appendages, referred to as gliding (18, 19). Regardless of the motility apparatus under control, chemotaxis signal transduction functions to link environmental sensing to changes in the motility pattern and to bias movement toward attractants or away from repellants.
Chemotaxis signaling pathways control flagellar motility by regulating the frequency at which the flagellar motor changes its direction of rotation or the speed at which the flagellar motor rotates. This mode of control is conserved across flagellated bacteria, regardless of flagellar arrangement or number (19). Chemotaxis signaling also controls twitching, the movement of cells on moist surfaces mediated by type IV pili (TFP), but the mechanisms involved are distinct from those controlling flagellum-dependent chemotaxis (20–22). First, twitching motility is primarily a social form of movement. Twitching involves cell-cell interactions and movement along the long axis of the cells, with little to no movement being observed in isolated cells. Second, twitching results from a sequence of extension, tethering, and retraction of TFP (23–27). Several distinct chemotaxis signaling pathways have been implicated in the control of the direction of twitching. In Myxococcus xanthus, TFP are assembled in an alternating manner at either pole of the cells to establish the direction of movement (21, 28, 29). The Frz chemotaxis pathway coordinates the frequency at which pilus assembly occurs at the leading pole of the cell (21, 28, 30). However, this is not a universal mechanism. In the spherical unicellular cyanobacterium Synechocystis sp. strain PCC6803 (here Synechocystis), twitching depends on TFP that are arranged around the cell surface, and reversals in the twitching direction are not observed (31, 32). Chemotaxis signaling via a system named Tax was proposed to function to polarize TFP activity or modulate TFP localization around the cells to bias movement in the direction of incident light (33). As expected from their different signaling outputs, Tax and Frz are not homologous pathways, despite both of them regulating twitching activity in gradients. Twitching bacteria can also secrete slimes that are left as tracks on the surface traveled by a moving cell. The slimes comprise EPS and were shown to affect the activity of TFP in some bacteria. In the twitching bacterium M. xanthus, an individual pilus binds EPS, and EPS binding triggers pilus retraction (34). This mechanism may be more widespread, given that most twitching bacteria secrete slimes. The chemotaxis systems that control twitching also typically coordinate the production of EPS to ensure TFP function (23, 28).
While the role of EPS during twitching may be to enhance the function of TFP, other forms of motility, controlled by chemotaxis pathways, rely exclusively on EPS production. Bacterial motility that does not depend on flagella or pili is referred to as gliding. In filamentous cyanobacteria, such as Nostoc punctiforme, Phormidium spp., Anabaena spp., or Oscillatoria spp., gliding motile multicellular filaments move together over surfaces in a direction parallel to their long axis and orient with respect to light intensity, quality, and/or direction by phototaxis (35). Secretion of slime was suggested to power the motility via a mechanism likened to “jet propulsion,” in which the extruded slime expands as it hydrates, providing enough force to propel the filament forward (36). In N. punctiforme, rings of electron-dense pores located between cells were identified as structures that potentially secrete the slime (36). A putative EPS secretion machinery (called Hsp) that localized as ring-like structures at the junctions between cells in the hormogonia (motile forms) of the filamentous cyanobacterium N. punctiforme was recently characterized (37). A chemotaxis system named Hmp controls the secretion of EPS by regulating the transcription of hsp genes (38), linking chemotaxis control of gliding to EPS secretion in these organisms. Gliding motility occurs by an entirely distinct mechanism in M. xanthus. In this organism, cells moving as individuals display a form of gliding called adventurous motility (or A-motility). Gliding depends on distributed motors localized within the cell envelope that move in helical tracks and push on the surface upon contact to generate thrust to move the cell forward. The gliding motors use proton motive force, but the nature of the helical tracks on which these motors move is not known; however, evidence suggests that they may include cytoskeleton proteins, such as MreB (39–41). To switch the direction of movement of the cell, the gliding motors must move in the opposite direction. A switch in cell polarity brings about changes in the direction of movement so that the lagging cell pole becomes the leading pole for movement (28). The Frz chemotaxis pathway controls the switch in cell polarity and thus coordinates both twitching and gliding motility in M. xanthus (21, 28, 42).
CHEMOTAXIS CONTROL OF SURFACE APPROACH AND TRANSIENT CELL AGGREGATION
The role of motility in mediating the attachment of bacteria to surfaces has been best described for flagellated bacteria and is discussed below. Attachment of motile bacteria to surfaces to form biofilms or to other cells to form flocs can be described as a two-step process. The first step is reversible; i.e., cells remain motile and may leave the surface after a variable period of time. The second step involves more specific interactions and is characterized by a permanent loss of motility and the production of additional extracellular structures that anchor the cells to the surfaces. The first reversible step depends on physicochemical interactions between the bacteria and the surface and is strongly influenced by both hydrodynamic effects and Brownian motion (43). Motile bacteria can move toward surfaces and, when sufficiently close, may experience the hydrodynamic effects of the surface and become entrapped, spending longer times swimming close to the surface (44). Prolonged residence near a surface enhances the probability of weak adhesion by means of van der Waals, electrostatic, and hydrophobic interactions (5, 43). Hydrodynamic trapping of swimming bacteria may thus promote transient cell-surface contacts. It is likely that many of the hydrodynamic effects and Brownian diffusion that can take place between motile cells and abiotic surfaces will be relevant to the interactions between two cells that closely approach one another. A recent model predicts that for bacteria swimming in a very confined space, the activity of bacterial flagella alone can alter the hydrodynamic conditions to such an extent that it might trigger aggregation under these conditions (45). Chemotaxis signaling can thus modulate reversible cell-cell contacts by promoting the initiation of these interactions (8, 46–48), which can be maintained through hydrodynamics and the Brownian effect. Chemotaxis can also facilitate the disruption of cell-cell contacts, since active motility, especially changes in the direction of the rotation of flagella, can license the cells to leave the surface. The initial weak adhesions might lead to irreversible attachment via mechanisms that likely involve sensing of surfaces (4), such as hindrance of flagellar rotation acting as a mechanosensing signal for the cell to deploy EPS and adhesins for permanent attachment. Increased cell density in cell aggregates might also trigger attachment via quorum sensing. Chemotaxis toward the AI-2 quorum-sensing molecule that is mediated by a chemotaxis receptor in Escherichia coli might be relevant (49).
Transient cell aggregations prior to permanent attachment also occur in cells moving across surfaces by TFP or gliding (23, 28, 50). Interestingly, the aggregates described on surfaces also form in liquid media, with a similar requirement for motility (50–53). This observation indicates that properties associated with the motility machinery play a direct role in the establishment of cell-cell contacts. Chemotaxis directing twitching and gliding might thus direct motility to concentrate groups of bacteria and orient them via regulating TFP activity and EPS production. The growing cell density might increase the number of contacts that TFP (twitching), EPS (twitching and gliding), and adhesion complexes (gliding) can make with other cells and with surfaces. During twitching, it is the coordinated retraction of multiple TFP that allows movement (23). Increased cell density might thus alter the frequency at which TFP bind and retract, progressively causing a reduction in cell motility and thus trapping groups of cells within aggregates. In addition, twitching bacteria and gliding cells often secrete slimes that can modify the surfaces on which cells move and thus further alter motility. Slime secretion might further alter the patterns and frequency of TFP binding-retraction and cause a progressive loss of motility.
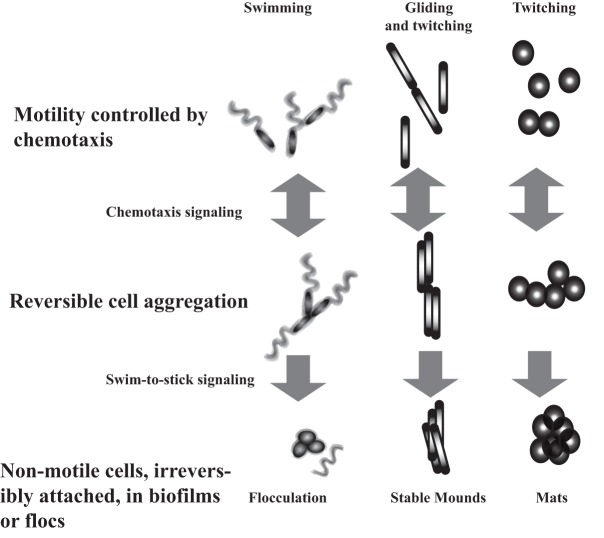
The hallmark of chemotaxis is the reversible control of motility patterns. Given the interplay between motility and fluid hydrodynamics near surfaces and the role of chemotaxis in altering motility patterns, chemotaxis might ultimately modulate how cells approach surfaces or other cells, making cell-cell or cell-surface contacts more or less likely. A role for chemotaxis in controlling these transient behaviors might also be advantageous, as reversibly attached cells remain motile and free to move away in response to specific environmental cues (Fig. 1). The alternative would be transition to permanent attachment accompanied by a loss of motility.
FIG 1.
Chemotaxis-dependent cell aggregation. Chemotaxis signaling, by modulating motility patterns, can promote transient cell-cell interactions between cells motile by flagella, by type IV pili, or by gliding. The examples shown are discussed in the text and illustrate transient cell aggregation in A. brasilense (left), M. xanthus (middle), and Synechocystis (right). The double arrows indicate reversible events modulated by chemotaxis signal transduction. The unidirectional arrows represent a committed transition to irreversible attached states.
CHEMOTAXIS CONTROL OF FLAGELLAR MOTILITY AND CLUMPING IN AZOSPIRILLUM BRASILENSE
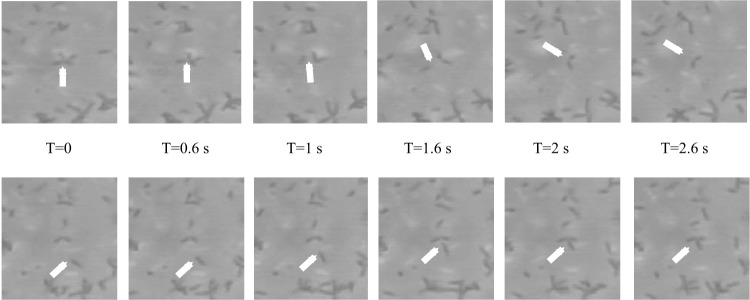
A. brasilense is a motile soil bacterium able to colonize the roots of cereals. This bacterium swims using a single polar flagellum, the activity of which is controlled by chemotaxis (54). A. brasilense is microaerophilic, and elevated oxygen concentrations are detrimental to its metabolism (8, 55). Under conditions of elevated aeration, motile A. brasilense cells clump in transient cell-cell contacts between motile cells (56). The production of clumps is thought to temporarily protect the cells from elevated oxygen by reducing the surface-to-volume ratio of the cells to limit diffusion (8). Clumping in A. brasilense is controlled by chemotaxis signaling (56). Mutations in genes that encode proteins of the chemotaxis signaling pathway, named Che1, caused a defect in chemotaxis and in clumping and flocculation. During chemotaxis, A. brasilense transiently increases swimming speed, and Che1 signaling controls this behavior (56). Experimental evidence and modeling indicate that the role of Che1 in controlling transient increases in swimming speed alone recapitulates the observed effects on clumping (56, 57). Clumping initially involves two or three motile cells, which remain in contact for about 1 s before swimming apart (Fig. 2). If conditions of aeration stress persist, the clumps of motile cells become more cohesive (up to 8 to 10 cells remaining within the clumps for >2 s) and progressively include more nonmotile cells (Fig. 2). Clumping between motile cells is also required for flocculation in A. brasilense (56). Clumping represents an intermediate step that precedes an irreversible attachment of cells and loss of motility in flocculation, which occurs if elevated aeration persists (8). While the production of EPS is likely involved in stabilizing the clumps (56, 58), the exact mechanism by which the cells adhere to one another at their nonflagellated pole is not known. One possibility is that adhesins, which are present in the polar region of the cells or secreted at this location, stabilize the cell-cell contacts in clumps, akin to a similar adhesion described in closely related alphaproteobacteria (59–61). Flocculation is widespread in many motile bacteria capable of chemotaxis, and chemotaxis defects have been linked to increased cell aggregation in many bacterial species (62–64). It is thus likely that chemotaxis-dependent clumping occurs in other bacterial species.
FIG 2.
Clumping in A. brasilense. The image sequences represent frames taken at 0.6-s intervals (T, time) from a video recording of free-swimming A. brasilense cells. The cultures were prepared by growing A. brasilense from a single colony, under elevated aeration, as described previously (8). The images were obtained by dark-field microscopy at ×40 magnification. Cells in transient stable clumps are visible. The arrows point to instances when a motile cell leaves a clump (top row) or joins a clump (bottom row). Cells in the suspension and the clumps were motile.
CHEMOTAXIS CONTROL OF TWITCHING, GLIDING, AND AGGREGATION IN M. XANTHUS
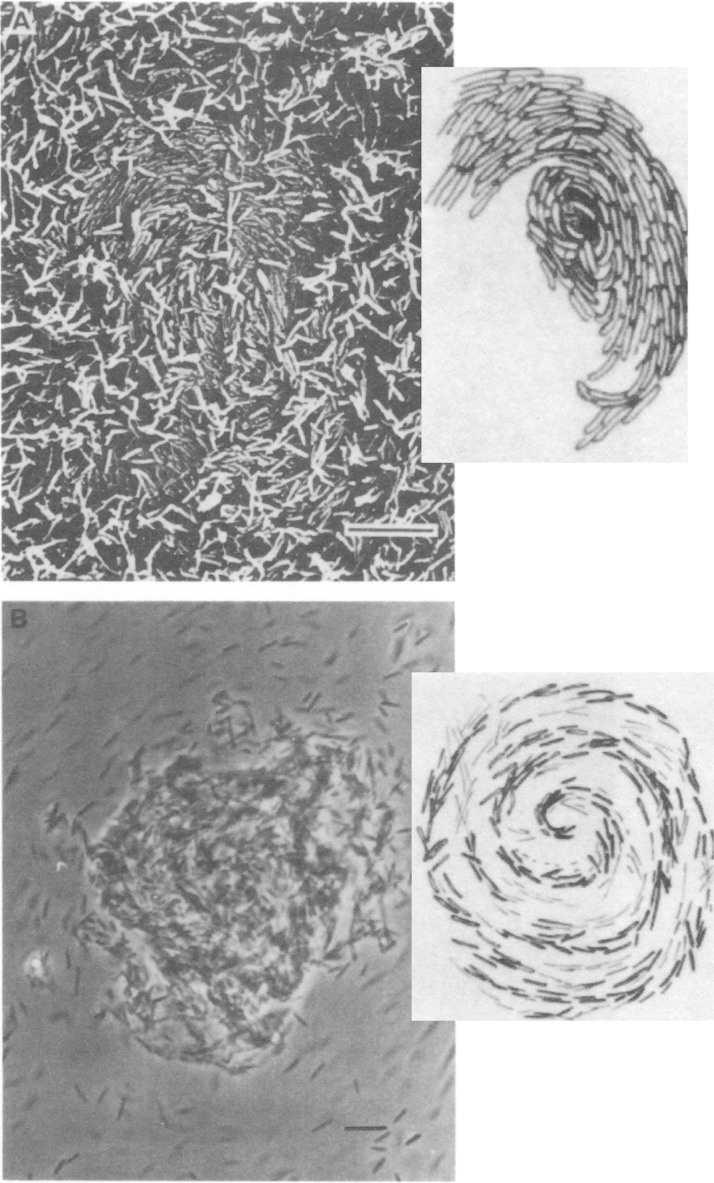
M. xanthus is a predatory bacterium in soil that is able to undergo various forms of development (28). When food sources become scarce, for example, following the consumption of prey, M. xanthus cells converge into large aggregates that will eventually produce fruiting bodies. Motile cells initially aggregate into large and unstable mounds. These mounds assemble and disassemble several times before some of the cells at the center of large aggregates become trapped and progressively lose motility before eventually forming fruiting bodies, probably owing to additional exchanges of signals (28). The mounds are thus initially transient cell aggregates between motile cells (Fig. 3). Both twitching and gliding motility are required for M. xanthus to bring cells together in large aggregates during development into fruiting bodies (28). The Frz pathway, which coordinates twitching and gliding, is required for the initiation of aggregation between motile cells (28). Mutations that impair Frz function cause defects in both twitching and gliding motility and affect development, because cells fail to aggregate into mounds, instead forming “frizzy” filaments that do not develop further. Experimental evidence and mathematical modeling suggest that reduced cell velocity and parallel alignment of cells are required for the formation of aggregates (7). Reduction in velocity might result from a change in the activity of the motility engines via Frz signaling, but evidence also suggests that Frz alters the alignment of cells relative to one another during initial aggregation. First, the FrzCD receptor, thought to provide sensory input to the Frz system, forms protein clusters that align when moving cells are brought in contact, and this event activates Frz signaling (65). Second, FrzCD methylation levels correlate with the extent of aggregation (66). Last, the alignment of adjacent cells is abolished by a mutation within the central regulator of the Frz signaling pathway. Therefore, Frz signaling is required for transient aggregation in M. xanthus by controlling changes in motility to bring cells together and by orienting them in aggregates.
FIG 3.
Twitching and gliding and cell aggregation in M. xanthus. Scanning electron (top) and phase-contrast (bottom) micrographs and artist sketches from the prints illustrating the initiation of aggregation during M. xanthus development. The movement of motile cells in spiral patterns depends on transient cell contacts, and motile cells may leave or remain within the aggregates. Scale bar, 10 μm. Adapted from reference 78 with permission.
CHEMOTAXIS CONTROL OF TWITCHING AND AGGREGATION IN CYANOBACTERIA
Many photosynthetic cyanobacteria form mats in various environments, and the maintenance of these multicellular communities depends on motility and the ability to move in response to light direction or quality (intensity and wavelength) by phototaxis. Phototaxis is the oriented movement of motile cells in gradients of light. Studies of diverse organisms have shown that light signals are processed by dedicated receptors and are integrated with chemical signal inputs by chemotaxis signal transduction pathways to ultimately alter motility patterns (22, 67, 68). Mats are heterogeneous environments and comprise both motile cells and permanently attached nonmotile cells in biofilms. Within the mats, motile cells are able to move vertically or laterally to adjust their position with respect to light to ensure maximum photosynthesis (53). Not only is motility essential for the formation of colonial aggregates, but it also contributes to the structure of the mats (50). In both unicellular and filamentous cyanobacteria, the motility of the aggregates depends on chemotaxis signaling pathways to bring the cells together and, ultimately, to enable phototaxis (Fig. 4). Despite their dissimilar motility apparatuses, unicellular and filamentous cyanobacteria employ functionally similar strategies of cell aggregation to respond to light gradients.
FIG 4.
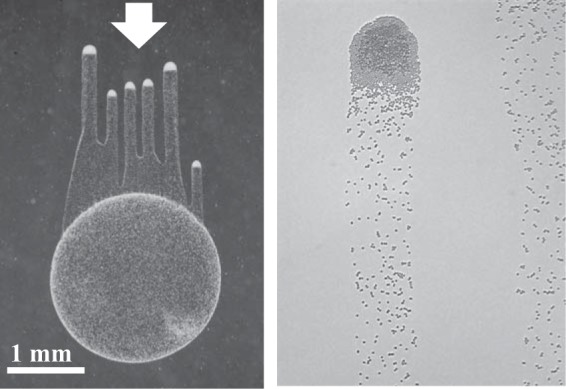
Phototaxis and aggregation in twitching Synechocystis cells. Left, communities of Synechocystis cells exhibit phototaxis, i.e., they move directionally toward white light (incident from the top). This image was taken 2 days after a drop of cells was placed on a low-concentration (0.4%) agarose plate. The original drop can be seen, as well as the finger-like projections of cells moving toward the light. Right, image of single cells within these finger-like projections moving toward white light (incident from the top), taken at ×20 magnification under the microscope. Time-lapse video microscopy of the cell populations can be used to track the motility behavior of cells (33, 70). Courtesy of Rosanna Chau and Devaki Bhaya, reproduced with permission.
Phototaxis in Synechocystis depends on Tax signaling (22, 32) and relies on TFP and the production of EPS. Twitching Synechocystis cells leave a track of slime as they move, and the presence of the slime alone can stimulate motility (69, 70). In Synechocystis, aggregates of motile cells are observed as finger-like projections on the surfaces of agar plates (Fig. 4). Microscopically, these aggregates correspond to groups of motile cells that move at a greater speed than that of smaller aggregates or single cells (31, 33). Aggregate sizes are initially variable, suggesting that initial cell-cell contacts are transient and dynamic, with some aggregates becoming sufficiently large to form finger-like projections (31, 70). The role of chemotaxis in regulating aggregation is illustrated by the observation that mutations in tax genes cause a null phototaxis phenotype due to a lack of aggregation and failure to form finger-like projections (22). The dependence of motility speed on the number of cells in aggregates provides additional support linking TFP function and slime/EPS production, because an increase in local slime concentration produced by a group of cells may be required to ensure movement. It has been proposed that such slime production, possibly EPS, might be a prerequisite to movement to overcome frictional drag (69, 70).
Like unicellular cyanobacteria, filamentous cyanobacteria, such as N. punctiforme, Phormidium spp., Anabaena spp., or Oscillatoria spp., move as aggregates during phototaxis, and motility is required for initial aggregation (35). The aggregates comprise motile cells that move in gradients of light as units but move back and forth in a random manner under diffuse light. These aggregates can thus assemble or disassemble depending on the presence of a light gradient, highlighting their transient nature and linking this behavior to chemotaxis signaling. A dispersed suspension of motile gliding cells (trichomes) of the filamentous Oscillatoria terebriformis forms clumps within a few seconds by cell-cell interactions, and the rate of clumping is directly proportional to the rate of gliding motility (71). Cell aggregation in this species is thus intimately connected to motility, suggesting that the gliding motility apparatus itself or its function promotes cell adhesion. Hormogonium filaments of N. punctiforme also clump together immediately upon differentiation into motile forms. Hormogonia glide as individuals or groups of cells but are capable of phototaxis only as masses of cells that form ripple patterns on agar surfaces (38). Mutations in the Hmp chemotaxis system controlling EPS secretion for jet propulsion motility abolish gliding, and thus phototaxis, in hormogonia (38). In N. punctiforme, EPS secretion might thus function both to power cell motility and to ensure cell-cell interactions and cohesion during phototaxis (38).
RECEPTORS AND CUES FOR CHEMOTAXIS-DEPENDENT CELL AGGREGATION
A straightforward consequence of the control of transient aggregation by a chemotaxis signaling system is that dedicated chemotaxis receptors are expected to provide sensory input that ultimately regulates these behaviors. Consistent with this hypothesis, chemotaxis receptors modulating aggregation have been identified in Synechocystis (72), N. punctiforme (73), M. xanthus (74), and A. brasilense (75, 76).
The cues detected by these receptors correspond to physicochemical conditions related to energy metabolism, such as redox status (receptors from A. brasilense) and light (receptors from Synechocystis and N. punctiforme). This is consistent with the aggregation of motile cells occurring when cells experience a change in energy metabolism. For example, starvation induces aggregation in M. xanthus (28). Red light stimulates photosynthesis, phototaxis, and aggregation in Synechocystis (32). Elevated oxygen concentrations reduce metabolism and trigger clumping in A. brasilense (8). A recent study using a fluorescent marker for respiration and flow cytometry to track short-time-scale bacterial adhesion events showed that E. coli experiences a decrease in respiration immediately upon single cell-cell or cell-surface contacts (77). Changes in metabolism may thus accompany cell adhesion to any surface, with chemotaxis signal transduction, via dedicated receptors, providing an early behavioral indication of deteriorating environmental conditions.
CONCLUSIONS
The formation of cell aggregates by motile cells as a result of chemotaxis signaling is a transient behavior in which the cells are not yet committed to permanent adhesion. This behavior is likely advantageous to the cells since, depending on environmental conditions, the cell-cell contacts between motile cells may transition into permanent adhesion, or cells may return to moving in gradients. Transient cell-cell contacts in aggregates might facilitate short-distance signaling and function as behavioral checkpoints between reversible and irreversible attachment. Close cell-cell contacts in aggregates might also allow the signal exchange of otherwise poorly diffusible molecules or molecules embedded within the cell envelope. Aggregation between motile cells often occurs when cells experience a change in metabolism. Such conditions might represent a major cue processed by chemotaxis signal transduction into changes in motility patterns that lead to aggregation. This is also consistent with the link between flocculation and biofilm formation and nutrient limitations. These behaviors thus provide an insightful and tractable model to decipher how cells integrate metabolism with behavioral changes.
ACKNOWLEDGMENTS
I thank Devaki Bhaya and Larry Shimkets for sharing unpublished material and information. I also thank Bob Belas, Mark Gomelsky, and Emilia Maurellio for insightful discussions, constructive suggestions, and continued encouragement. I thank three anonymous reviewers for comments on previous versions of the manuscript.
Research in my laboratory is supported by National Science Foundation grant NSF-MCB 1330344.
Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation.
REFERENCES
- 1.Kjelleberg S, Albertson N, Flärdh K, Holmquist L, Jouper-Jaan Å, Marouga R, Ostling J, Svenblad B, Weichart D. 1993. How do non-differentiating bacteria adapt to starvation? Antonie Van Leeuwenhoek 63:333–341. doi: 10.1007/BF00871228. [DOI] [PubMed] [Google Scholar]
- 2.He K, Bauer CE. 2014. Chemosensory signaling systems that control bacterial survival. Trends Microbiol 22:389–398. doi: 10.1016/j.tim.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson CN, Mandel MJ, Silhavy TJ. 2005. Escherichia coli starvation diets: essential nutrients weigh in distinctly. J Bacteriol 187:7549–7553. doi: 10.1128/JB.187.22.7549-7553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belas R. 2014. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol 22:517–527. doi: 10.1016/j.tim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8:623–633. [DOI] [PubMed] [Google Scholar]
- 6.Sadasivan L, Neyra CA. 1985. Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J Bacteriol 163:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sliusarenko O, Zusman DR, Oster G. 2007. Aggregation during fruiting body formation in Myxococcus xanthus is driven by reducing cell movement. J Bacteriol 189:611–619. doi: 10.1128/JB.01206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bible AN, Stephens BB, Ortega DR, Xie Z, Alexandre G. 2008. Function of a chemotaxis-like signal transduction pathway in modulating motility, cell clumping, and cell length in the alphaproteobacterium Azospirillum brasilense. J Bacteriol 190:6365–6375. doi: 10.1128/JB.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrova OE, Cherny KE, Sauer K. 2014. The Pseudomonas aeruginosa diguanylate cyclase GcbA, a homolog of P. fluorescens GcbA, promotes initial attachment to surfaces, but not biofilm formation, via regulation of motility. J Bacteriol 196:2827–2841. doi: 10.1128/JB.01628-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merritt PM, Danhorn T, Fuqua C. 2007. Motility and chemotaxis in Agrobacterium tumefaciens surface attachment and biofilm formation. J Bacteriol 189:8005–8014. doi: 10.1128/JB.00566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratt LA, Kolter R. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn N, Fenchel T, Mitchell J. 1998. Microscale nutrient patches in planktonic habitats shown by chemotactic bacteria. Science 282:2254–2256. doi: 10.1126/science.282.5397.2254. [DOI] [PubMed] [Google Scholar]
- 13.Stocker R, Seymour JR, Samadani A, Hunt DE, Polz MF. 2008. Rapid chemotactic response enables marine bacteria to exploit ephemeral microscale nutrient patches. Proc Natl Acad Sci U S A 105:4209–4214. doi: 10.1073/pnas.0709765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker R, Seymour JR. 2012. Ecology and physics of bacterial chemotaxis in the ocean. Microbiol Mol Biol Rev 76:792–812. doi: 10.1128/MMBR.00029-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saragosti J, Calvez V, Bournaveas N, Perthame B, Buguin A, Silberzan P. 2011. Directional persistence of chemotactic bacteria in a traveling concentration wave. Proc Natl Acad Sci U S A 108:16235–16240. doi: 10.1073/pnas.1101996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JR, Stocker R. 2012. Trade-offs of chemotactic foraging in turbulent water. Science 338:675–679. doi: 10.1126/science.1219417. [DOI] [PubMed] [Google Scholar]
- 17.Buchan A, Crombie B, Alexandre GM. 2010. Temporal dynamics and genetic diversity of chemotactic-competent microbial populations in the rhizosphere. Environ Microbiol 12:3171–3184. doi: 10.1111/j.1462-2920.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 18.Wuichet K, Zhulin IB. 2010. Origins and diversification of a complex signal transduction system in prokaryotes. Sci Signal 3:ra50. doi: 10.1126/scisignal.2000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadhams GH, Armitage JP. 2004. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5:1024–1037. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- 20.Darzins A. 1994. Characterization of a Pseudomonas aeruginosa gene cluster involved in pilus biosynthesis and twitching motility: sequence similarity to the chemotaxis proteins of enterics and the gliding bacterium Myxococcus xanthus. Mol Microbiol 11:137–153. doi: 10.1111/j.1365-2958.1994.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Zusman DR, Shi W. 2000. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr Biol 10:1143–1146. doi: 10.1016/S0960-9822(00)00705-3. [DOI] [PubMed] [Google Scholar]
- 22.Bhaya D, Takahashi A, Grossman AR. 2001. Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC6803. Proc Natl Acad Sci U S A 98:7540–7545. doi: 10.1073/pnas.131201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol 66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 24.Skerker JM, Berg HC. 2001. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A 98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morand PC, Bille E, Morelle S, Eugene E, Beretti JL, Wolfgang M, Meyer TF, Koomey M, Nassif X. 2004. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J 23:2009–2017. doi: 10.1038/sj.emboj.7600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merz AJ, So M, Sheetz MP. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 27.Jin F, Conrad JC, Gibiansky ML, Wong GC. 2011. Bacteria use type-IV pili to slingshot on surfaces. Proc Natl Acad Sci U S A 108:12617–12622. doi: 10.1073/pnas.1105073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Ducret A, Shaevitz J, Mignot T. 2012. From individual cell motility to collective behaviors: insights from a prokaryote, Myxococcus xanthus. FEMS Microbiol Rev 36:149–164. doi: 10.1111/j.1574-6976.2011.00307.x. [DOI] [PubMed] [Google Scholar]
- 29.Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. 2002. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A 99:16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackhart BD, Zusman DR. 1985. “Frizzy” genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc Natl Acad Sci U S A 82:8767–8770. doi: 10.1073/pnas.82.24.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burriesci M, Bhaya D. 2008. Tracking phototactic responses and modeling motility of Synechocystis sp. strain PCC6803. J Photochem Photobiol B 91:77–86. doi: 10.1016/j.jphotobiol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Bhaya D, Bianco NR, Bryant D, Grossman A. 2000. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol Microbiol 37:941–951. doi: 10.1046/j.1365-2958.2000.02068.x. [DOI] [PubMed] [Google Scholar]
- 33.Chau RMW, Ursell T, Wang S, Huang KC, Bhaya D. 2015. Maintenance of motility bias during cyanobacterial phototaxis. Biophys J 108:1623–1632. doi: 10.1016/j.bpj.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Sun H, Ma X, Lu A, Lux R, Zusman D, Shi W. 2003. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc Natl Acad Sci U S A 100:5443–5448. doi: 10.1073/pnas.0836639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoiczyk E. 2000. Gliding motility in cyanobacterial: observations and possible explanations. Arch Microbiol 174:11–17. doi: 10.1007/s002030000187. [DOI] [PubMed] [Google Scholar]
- 36.Hoiczyk E, Baumeister W. 1998. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr Biol 8:1161–1168. doi: 10.1016/S0960-9822(07)00487-3. [DOI] [PubMed] [Google Scholar]
- 37.Risser DD, Meeks JC. 2013. Comparative transcriptomics with a motility-deficient mutant leads to identification of a novel polysaccharide secretion system in Nostoc punctiforme. Mol Microbiol 87:884–893. doi: 10.1111/mmi.12138. [DOI] [PubMed] [Google Scholar]
- 38.Risser DD, Chew WG, Meeks JC. 2014. Genetic characterization of the hmp locus, a chemotaxis-like gene cluster that regulates hormogonium development and motility in Nostoc punctiforme. Mol Microbiol 92:222–233. doi: 10.1111/mmi.12552. [DOI] [PubMed] [Google Scholar]
- 39.Nan B, McBride MJ, Chen J, Zusman DR, Oster G. 2014. Bacteria that glide with helical tracks. Curr Biol 24:R169–R173. doi: 10.1016/j.cub.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauriello EM, Mouhamar F, Nan B, Ducret A, Dai D, Zusman DR, Mignot T. 2010. Bacterial motility complexes require the actin-like protein, MreB and the Ras homologue, MglA. EMBO J 29:315–326. doi: 10.1038/emboj.2009.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nan B, Chen J, Neu JC, Berry RM, Oster G, Zusman DR. 2011. Myxobacteria gliding motility requires cytoskeleton rotation powered by proton motive force. Proc Natl Acad Sci U S A 108:2498–2503. doi: 10.1073/pnas.1018556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mignot T, Merlie JP Jr, Zusman DR. 2005. Regulated pole-to-pole oscillations of a bacterial gliding motility protein. Science 310:855–857. doi: 10.1126/science.1119052. [DOI] [PubMed] [Google Scholar]
- 43.Conrad JC. 2012. Physics of bacterial near-surface motility using flagella and type IV pili: implications for biofilm formation. Res Microbiol 163:619–629. doi: 10.1016/j.resmic.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Vigeant MA, Ford RM, Wagner M, Tamm LK. 2002. Reversible and irreversible adhesion of motile Escherichia coli cells analyzed by total internal reflection aqueous fluorescence microscopy. Appl Environ Microbiol 68:2794–2801. doi: 10.1128/AEM.68.6.2794-2801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsang AC, Kanso E. 2014. Flagella-induced transitions in the collective behavior of confined microswimmers. Phys Rev E Stat Nonlin Soft Matter Phys 90:021001. doi: 10.1103/PhysRevE.90.021001. [DOI] [PubMed] [Google Scholar]
- 46.McClaine JW, Ford RM. 2002. Reversal of flagellar rotation is important in initial attachment of Escherichia coli to glass in a dynamic system with high- and low-ionic-strength buffers. Appl Environ Microbiol 68:1280–1289. doi: 10.1128/AEM.68.3.1280-1289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moorthy S, Watnick PI. 2004. Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol Microbiol 52:573–587. doi: 10.1111/j.1365-2958.2004.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnenschein EC, Syit DA, Grossart HP, Ullrich MS. 2012. Chemotaxis of Marinobacter adhaerens and its impact on attachment to the diatom Thalassiosira weissflogii. Appl Environ Microbiol 78:6900–6907. doi: 10.1128/AEM.01790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hegde M, Englert DL, Schrock S, Cohn WB, Vogt C, Wood TK, Manson MD, Jayaraman A. 2011. Chemotaxis to the quorum-sensing signal AI-2 requires the Tsr chemoreceptor and the periplasmic LsrB AI-2-binding protein. J Bacteriol 193:768–773. doi: 10.1128/JB.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castenholz RW. 1982. Motility and taxis, p 413–439. In Carr NG, Whitton BA (ed), The biology of cyanobacteria. Blackwell Scientific Publications, Oxford, United Kingdom. [Google Scholar]
- 51.Kim SH, Ramaswamy S, Downard J. 1999. Regulated exopolysaccharide production in Myxococcus xanthus. J Bacteriol 181:1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnold JW, Shimkets LJ. 1988. Cell surface properties correlated with cohesion in Myxococcus xanthus. J Bacteriol 170:5771–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson LL, Castenholz RW. 1987. Diel vertical movements of the cyanobacterium Oscillatoria terebriformis in a sulfide-rich hot spring microbial mat. Appl Environ Microbiol 53:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexandre G, Greer SE, Zhulin IB. 2000. Energy taxis is the dominant behavior in Azospirillum brasilense. J Bacteriol 182:6042–6048. doi: 10.1128/JB.182.21.6042-6048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okon Y, Houchins JP, Albrecht SL, Burris RH. 1977. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol 98:87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- 56.Bible A, Russell MH, Alexandre G. 2012. The Azospirillum brasilense Che1 chemotaxis pathway controls swimming velocity, which affects transient cell-to-cell clumping. J Bacteriol 194:3343–3355. doi: 10.1128/JB.00310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi X, Nellas RB, Byrn MW, Russell MH, Bible AN, Alexandre G, Shen T. 2013. Swimming motility plays a key role in the stochastic dynamics of cell clumping. Phys Biol 10:026005. doi: 10.1088/1478-3975/10/2/026005. [DOI] [PubMed] [Google Scholar]
- 58.Edwards AN, Siuti P, Bible AN, Alexandre G, Retterer ST, Doktycz MJ, Morrell-Falvey JL. 2011. Characterization of cell surface and extracellular matrix remodeling of Azospirillum brasilense chemotaxis-like 1 signal transduction pathway mutants by atomic force microscopy. FEMS Microbiol Lett 314:131–139. doi: 10.1111/j.1574-6968.2010.02156.x. [DOI] [PubMed] [Google Scholar]
- 59.Langille SE, Weiner RM. 1998. Spatial and temporal deposition of Hyphomonas strain VP-6 capsules involved in biofilm formation. Appl Environ Microbiol 64:2906–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G, Brown PJ, Tang JX, Xu J, Quardokus EM, Fuqua C, Brun YV. 2012. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol Microbiol 83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Kim J, Koestler BJ, Choi J-H, Waters CM, Fuqua C. 2013. Genetic analysis of Agrobacterium tumefaciens unipolar polysaccharide production reveals complex integrated control of the motile-to-sessile switch. Mol Microbiol 89:929–948. doi: 10.1111/mmi.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahman H, King RM, Shewell LK, Semchenko EA, Hartley-Tassell LE, Wilson JC, Day CJ, Korolik V. 2014. Characterisation of a multi-ligand binding chemoreceptor CcmL (Tlp3) of Campylobacter jejuni. PLoS Pathog 10:e1003822. doi: 10.1371/journal.ppat.1003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi Y, Kim S, Hwang H, Kim KP, Kang DH, Ryu S. 2015. Plasmid-encoded MCP is involved in virulence, motility, and biofilm formation of Cronobacter sakazakii ATCC 29544. Infect Immun 83:197–204. doi: 10.1128/IAI.02633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao J, Allen C. 2007. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J Bacteriol 189:6415–6424. doi: 10.1128/JB.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mauriello EM, Astling DP, Sliusarenko O, Zusman DR. 2009. Localization of a bacterial cytoplasmic receptor is dynamic and changes with cell-cell contacts. Proc Natl Acad Sci U S A 106:4852–4857. doi: 10.1073/pnas.0810583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Astling DP, Lee JY, Zusman DR. 2006. Differential effects of chemoreceptor methylation-domain mutations on swarming and development in the social bacterium Myxococcus xanthus. Mol Microbiol 59:45–55. doi: 10.1111/j.1365-2958.2005.04926.x. [DOI] [PubMed] [Google Scholar]
- 67.Rudolph J, Oesterhelt D. 1995. Chemotaxis and phototaxis require a CheA histidine kinase in the archaeon Halobacterium salinarium. EMBO J 14:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang ZY, Gest H, Bauer CE. 1997. Chemosensory and photosensory perception in purple photosynthetic bacteria utilize common signal transduction components. J Bacteriol 179:5720–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galante A, Wisen S, Bhaya D, Levy D. 2012. Modeling local interactions during the motion of cyanobacteria. J Theor Biol 309:147–158. doi: 10.1016/j.jtbi.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ursell T, Chau RMW, Wisen S, Bhaya D, Huang KC. 2013. Motility enhancement through surface modification is sufficient for cyanobacterial community organization during phototaxis. PLoS Comput Biol 9:e1003205. doi: 10.1371/journal.pcbi.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castenholz RW. 1968. The behavior of Oscillatoria terebriformis in hot springs. J Phycol 4:132–139. doi: 10.1111/j.1529-8817.1968.tb04687.x. [DOI] [PubMed] [Google Scholar]
- 72.Ng WO, Grossman AR, Bhaya D. 2003. Multiple light inputs control phototaxis in Synechocystis sp. strain PCC6803. J Bacteriol 185:1599–1607. doi: 10.1128/JB.185.5.1599-1607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campbell EL, Hagen KD, Chen R, Risser DD, Ferreira DP, Meeks JC. 2015. Genetic analysis reveals the identity of the photoreceptor for phototaxis in hormogonium filaments of Nostoc punctiforme. J Bacteriol 197:782–791. doi: 10.1128/JB.02374-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bustamante VH, Martinez-Flores I, Vlamakis HC, Zusman DR. 2004. Analysis of the Frz signal transduction system of Myxococcus xanthus shows the importance of the conserved C-terminal region of the cytoplasmic chemoreceptor FrzCD in sensing signals. Mol Microbiol 53:1501–1513. doi: 10.1111/j.1365-2958.2004.04221.x. [DOI] [PubMed] [Google Scholar]
- 75.Russell MH, Bible AN, Fang X, Gooding JR, Campagna SR, Gomelsky M, Alexandre G. 2013. Integration of the second messenger c-di-GMP into the chemotactic signaling pathway. mBio 4(2):e00001-13. doi: 10.1128/mBio.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie Z, Ulrich LE, Zhulin IB, Alexandre G. 2010. PAS domain containing chemoreceptor couples dynamic changes in metabolism with chemotaxis. Proc Natl Acad Sci U S A 107:2235–2240. doi: 10.1073/pnas.0910055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geng J, Beloin C, Ghigo JM, Henry N. 2014. Bacteria hold their breath upon surface contact as shown in a strain of Escherichia coli, using dispersed surfaces and flow cytometry analysis. PLoS One 9:e102049. doi: 10.1371/journal.pone.0102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Connor KA, Zusman DR. 1989. Patterns of cellular interactions during fruiting-body formation in Myxococcus xanthus. J Bacteriol 171:6013–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]