Abstract
The aim of this study was to examine the impact of different neural systems on monetary decision-making in frequent poker gamblers, who vary in their degree of problem gambling. Fifteen frequent poker players, ranging from non-problem to high-problem gambling, and fifteen non-gambler controls were scanned using fMRI while performing the Iowa Gambling Task (IGT). During IGT deck selection, between-groups fMRI analyses showed that frequent poker gamblers exhibited higher ventral–striatal but lower dorsolateral prefrontal and orbitofrontal activations, as compared to controls. Moreover, by using functional connectivity analyses, we observed higher ventral striatal connectivity in poker players, and in regions involved in attentional/motor control (posterior cingulate), visual (occipital gyrus) and auditory (temporal gyrus) processing. In poker gamblers, scores of problem gambling severity were positively associated with ventral-striatal activations and with the connectivity between the ventral-striatum seed and the occipital fusiform gyrus and the middle temporal gyrus. Present results are consistent with findings from recent brain-imaging studies showing that gambling disorder is associated with heightened motivational-reward processes during monetary decision-making, which may hamper one’s ability to moderate his level of monetary risk-taking.
Keywords: Decision-making, fMRI, problem gambling severity, ventral striatum
INTRODUCTION
Poker players may be one of the most intriguing populations to study among gamblers. Indeed, by contrast to non-strategic gambling (e.g., slots, roulette), deciding to raise or call in poker gambling may not only trigger reward anticipation and emotion regulation processes (as in other forms of non-strategic gambling), but should also be highly dependent on high-order cognitive processes (e.g., the use of working memory and planning in order to keep track of cards played to determine odds of receiving a certain card; Grant et al., 2012). Hence, because poker gambling might have conceivably different executive-control demands that non-strategic gambling, one could infer that poker players might be best able at exerting willpower during gambling, that is, to decide based on both short-terms and long-term consequences of an action (Bechara, 2005; Noël et al., 2010). Nevertheless, a significant proportion of poker gamblers become unable to shape and maintain their behaviors to avoid severe negative consequences and continue to gamble despite the accumulation of financial losses (APA, 2013; Dufour et al., 2013; Recher & Griffiths, 2012).
The present study aimed at examining neural systems that drive monetary decision-making during gambling in poker players, and how this dynamic varies according to the degree of problem gambling severity. We used a well-known gambling-related experimental paradigm: the Iowa Gambling Task (IGT; Bechara et al., 1994). The IGT is reminiscent of poker playing in terms of dealing with uncertainty - within a strategic gambling-like context of monetary rewards and losses - with some risky choices being advantageous in the short-term (high reward), but disadvantageous in the long run (higher punishment); other choices are less attractive in the short-term (low reward), but advantageous in the long run (lower punishment).
The phase of decision-making during the IGT (i.e., to choose among four decks of cards), and especially choosing from risky decks (versus less risky decks), has been shown to be underlined by a complex neurocognitive circuitry encompassing multiple neural systems (Bechara et al., 1999; Bolla et al., 2003; Christakou et al., 2009; Lawrence et al., 2009; Li et al., 2010; Power et al., 2012; Tanabe et al., 2007), and with a certain degree of dynamic balance among them (Bechara, 2005; Verdejo-Garcia & Bechara, 2009). More specifically, according to the somatic-marker hypothesis (Bechara & Damasio, 2005; Damasio, 1994), the elaboration of a decision triggers immediate and future prospects of an option, which are underlined by numerous affective/emotional (somatic) signals that conflict with each other. Based on this theoretical framework, there are at least two underlying types of dysfunctions where this overall somatic signal turns in favor of immediate outcomes (Bechara, 2005): (1) a hyperactivity in the impulsive system, which exaggerates the rewarding impact of available immediate incentives (referred as primary inducers; Bechara & Damasio, 2005; Damasio, 1995; Verdejo-Garcia & Bechara, 2009) through activation within the amygdala (a region involved in the reactivity to primary inducers; Bechara et al., 2003) and the ventral-striatum (VS; involved in reward anticipation and feedback processing; Haruno & Kawato, 2006), and (2) hypoactivity in the prefrontal cortex or reflective system, which forecasts the long term consequences of a given action through reactivation of emotions triggered by previous choices outcomes (referred as secondary inducers; Bechara & Damasio, 2005; Damasio, 1995; Verdejo-Garcia & Bechara, 2009). The action of the reflective system involves the reactivation of emotions triggered by previous choices outcomes (orbitofrontal cortex, OFC; ventromedial prefrontal cortex, vmPFC), conflict monitoring (the anterior cingulate cortex, ACC), working memory and motor response inhibition (dorsolateral prefrontal cortex, DLPFC) (Bechara, 2005). More recent evidence suggests that there is a third neural system mediated through the insular cortex (Naqvi & Bechara, 2009, 2014; Noël et al., 2013a, 2013b; Verdejo-Garcia et al., 2012). The insula is thought to play a key role in translating body states into what one subjectively may experience as a feeling of urge (i.e. embodied cognitions) (Craig, 2002, 2009). This state of urge might increase the drive toward immediate rewards by sensitizing the activity automatic emotional (amygdala) and incentive (ventral striatum) processes within the impulsive system, and by disabling prefrontal cortex regions necessary for reflective control processes (Naqvi & Bechara, 2009, 2014; Verdejo-Garcia et al., 2012). These latter findings on the role of the insula in addiction are consistent with, and complimentary to the Verdejo-Garcia and Bechara (2009) somatic marker proposal except that they provide more details on the mechanisms by which the insula (a component of the somatic marker neural circuit) influences decision-making.
With regard to the population of gamblers, behavioral studies on the IGT have found that problem gambling is usually associated with a preference for risky decks (for review, see Brevers et al., 2013). Moreover, a brain imaging study highlighted a diminished vmPFC activation was during IGT deck selection in pathological gamblers with comorbid substance disorder (Tanabe et al., 2007). However, since this study did not focus on “pure” gamblers, we cannot disentangle the specific effect of gambling addiction on observed diminished vmPFC activity. In another fMRI study, it has been showed that, during high-risk choice in the IGT, pathological gamblers exhibited increased activation in regions encompassing the extended reward pathway, including the mesial and most posterior aspects of the OFC, the amygdala, and the VS (Power et al., 2012). However, a main limitation of this study is that components of decision-making during the IGT have not been broken down into different steps of decision-making (Brevers et al., 2013; Dymond et al., 2013). More specifically, it is unclear whether enhanced brain activation is related to outcome anticipation (i.e., when the subject is pondering potential options before making a decision) or outcome processing (i.e., the subject has made a decision and is waiting for the outcome). This aspect seems crucial since recent brain imaging studies on gambling disorder showed that, while gambling, pathological gamblers exhibit a increased fronto-striatal activation during toward the anticipation of high-uncertain monetary rewards (Brevers et al., 2015; Miedl et al., 2010; van Hoslt et al., 2012), and a reduction of cerebral activity in the brain reward pathway during the processing of monetary gambling rewards and losses (de Ruiter et al., 2012; Reuter et al., 2005).
In summary, we aimed to examine the dynamics among multiple brain systems implicated in decision-making during IGT deck selection in a sample of frequent poker players, ranging from non-problem to high-problem gambling. We hypothesized that, due to the “gambling-like” aspect of the IGT, poker players would show higher brain activity than controls in regions commonly activated during IGT deck selection. Specifically, based on influential theoretical accounts on the somatic marker theory of addiction (Bechara et al., 2005; Verdejo-Garcia & Bechara, 2009), we expected that poker players would show a relative increase in neural activity within systems associated with reward processing, reactivity to emotional cues, and interoception and the experience of an urge, namely the VS and amygdala, and the insula, respectively. However, this increase would be accompanied by a relative decrease in neural activity within neural regions implicated in decision-making, conflict monitoring, and executive control, namely the DLPFC, vmPFC, OFC, and ACC. As further support for the hypothesis that poker players exhibit increased activity in key components of the so-called “impulsive” system, and given the implication of the VS in motivational goal-directed behaviors (e.g., Haber & Knutson, 2010) and in monetary decision-making in problem gamblers (Chase & Clark, 2012; Reuter et al., 2005; van Holst et al., 2012, 2014), we would expect a positive correlation between problem gambling severity score and ventral-striatal and brain regions associated activation during monetary decision-making.
MATERIALS and METHODS
Participants
Fifteen regular poker players (9 females and 6 males, on average 24.67±5.32 years of age) and 15 non-gambler controls participated in this study (9 females and 6 males, on average 22.07±1.67 years of age). All participants gave informed consent to the experimental procedure, which was approved by the University of Southern California Institutional Review Board. Participants received $40 dollars for their participation.
Poker gamblers were recruited on the Internet through advertisements displayed on online forums for poker players based in Los Angeles. The ads asked for participants who “played poker frequently” to participate in a one-day study to explore factors associated with decision-making in poker gambling. An email-screening interview was conducted by means of a locally developed screening tool (see also Brevers et al., 2012a, 2012b), which included an examination of frequency of gambling behavior and comorbid psychiatric disorders. We excluded any subject from the gambling group who a) reported gambling in casino settings less than twice a week or less than four times a month during the past 18 months. In addition, subjects were judged to be physically healthy on the basis of their medical history. Substance use was examined on the basis of items taken from the Addiction Severity Index Short Form. Medical history was examined via completion of an MRI screening form.
Control participants were recruited by word of mouth from the community. They were free of neurological or psychiatric history, and gave informed consent to the experimental procedure. All subjects were right handed and had normal or corrected-to-normal vision. Problem gambling severity was assessed the day of study with the South Oaks Gambling Screen (SOGS; Lesieur and Blume, 1987). All controls scored zero on the SOGS. Poker players’ SOGS scores and information on their frequency of poker playing (per week) and minimum amount of money spent on poker (per week) is depicted in Table 1.
Table 1.
SOGS score, poker playing frequency, weekly poker budget in the poker player group (n = 15).
| SOGS score (average) | M = 3.60, SD = 3.48 |
| Non-problem gambling (SOGS score ranging from 0 to 1) | n = 4 |
| Low problem gambling (SOGS score ranging from 2 to 4) | n = 6 |
| High problem gambling (SOGS score > 5) | n = 5 |
| Poker playing frequency (day per week) | M = 3.53, SD = 1.34 |
| Minimum amount of money spent on poker (in dollars per week) | M = 128.67, SD = 46.73 |
SOGS, South Oaks Gambling Screen; M, mean; SD, Standard Deviation; n, number of subject.
The Iowa Gambling Task (IGT)
On each trial, the participant selects a card from any of the four decks cards, labeled A, B, C, and D, on the screen. Each card selection yields a gain, but it can also yield a loss. The amounts won and lost are then displayed, and the display also includes the overall cumulative payoff, which is updated with each trial. Decks A and B yield gains of $100 (in “play money”) with every selection. At the same time, Deck A incurs a 0.5 probability of losing $250, and Deck B incurs a 0.1 probability of losing $1250. Therefore choosing from these two decks – referred to as the “disadvantageous” decks – leads to a net loss. Decks C and D yield smaller gains of $50 with every selection. They also incur smaller losses: Deck C incurs a 0.5 probability of losing $50, and Deck D incurs a 0.1 probability of losing $250. Therefore choosing from these two decks – referred to as the “advantageous” decks – leads to a net gain. Participants were given written instructions in which they were told that some decks were worse than others, and that they should avoid these decks in order to succeed in the task.
Procedure
Participants lay supine on the fMRI scanner bed, and viewed the task back-projected onto a screen through a mirror attached onto the head coil. Foam pads were used to minimize head motion. Stimulus presentation and timing of all stimuli and response events were achieved using Matlab 7.14 and Psychtoolbox 3.0 on an IBM-compatible PC. Participants’ responses were collected online using an MRI-compatible button box. An event-related design of IGT was used, with standard IGT instructions. Each trial was divided into a decision stage and a feedback stage. At the decision stage, a message (“Pick a Card”) was displayed at the center of screen, and participants were asked to choose a card from Decks A, B, C or D by pressing the corresponding button. Response had to be made within 3–7 (mean=4) s (this interval varied randomly between trials). At the feedback stage, participants were informed how much money they won or lost by their selected card. The feedback stage lasts for 3 s. For win-only trials (no loss), the win feedback (“you win $X”) was displayed for 1.5 s, followed by a 1.5-second blank screen. For win-but-loss trials, the win feedback (“you win $X”) was displayed for 1.5 s, followed by a 1.5 s display of the loss feedback (“but you also lose $X”). The inter-trial interval, i.e., the time between the 3 s feedback stage and the start of the next trial (“pick a card”) varied randomly between 1.1, 2.3, and 3.2 s. The sequence was optimized for design efficiency using an in-house program. In total, participants completed 100 trials and the task lasted for 15 min.
fMRI data acquisition
fMRI imaging was conducted in a 3T Siemens MAGNETOM Tim/Trio scanner in the Dana and David Dornsife Cognitive Neuroscience Imaging Center at the University of Southern California. Functional scanning used a z-shim gradient echo EPI sequence with PACE (Prospective Acquisition Correction). This specific sequence is dedicated to reduce signal loss in the prefrontal and orbitofrontal areas. The PACE option can help reduce the impact of head motion during data acquisition. The parameters are: TR/TE=2000/25 ms; flip angle = 90°; 64×64 matrix size with resolution 2×2×2 mm3. Thirty-one 3.5-mm axial slices were used to cover the whole cerebral cortex and most of the cerebellum with no gap. The slices were tilted about 30° clockwise along the AC–PC plane to obtain better signals in the orbitofrontal cortex. The anatomical T1-weighted structural scan was done using an MPRAGE sequence (TR/TE/TI=2530/3.1/800 ms; flip angle 10°; 208 sagittal slices; 256×256 matrix size with spatial resolution as 1×1×1 mm3).
GLM analyses
Image preprocessing and statistical analysis were carried out using FEAT (fMRI Expert Analysis Tool), a part of the FSL package (FMRIB software library, www.fmrib.ox.ac.uk/fsl). fMRI images were realigned to compensate for small residual head movements that were not captured by the PACE sequence. Translational movement parameters never exceeded 1 voxel in any direction for any participant. Data were spatially smoothed using a 5-mm full-width-half-maximum (FWHM) Gaussian kernel. The data were filtered in the temporal domain using a nonlinear high pass filter with a 100-second cut-off. A two-step registration procedure was used whereby EPI images were first registered to the MPRAGE structural image, and then into standard MNI space, using affine transformations (Jenkinson & Smith, 2001). Registration from MPRAGE structural image to standard space was further refined using FNIRT nonlinear registration. The data were modeled at the first level using a general linear model within FSL’s FILM module. In the first-level analysis, the following two trial types were modeled: advantageous decks (deck A + deck B selections) and disadvantageous decks (deck C + deck D selections). In this paper, we were particularly interested in the BOLD responses related to deck selection (i.e., the decision-making phase of the IGT). Consequently, each type of trial was only modeled at the deck selection level. The event onsets were convolved with a canonical hemodynamic response function (HRF, double-gamma) to generate the regressors used in the GLM. Temporal derivatives were included as covariates of no interest to improve statistical sensitivity (Frinston et al., 1998). Null events were not explicitly modeled, and therefore constituted an implicit baseline. The OLS (Ordinary Least Squares) simple mixed effect with automatic outlier detection (Woolrich, 2008) was used as second-level random effects analysis. To examine task-related brain activations, we contrasted: 1) decision-making (selections of decks A, B, C and D) versus baseline; 2) advantageous decision-making (selections of deck C and deck D) versus disadvantageous decision-making (selections of deck A and deck B), and vice-versa. Within-group images were thresholded using cluster detection statistics, with a height threshold of z > 2.3 and a cluster probability of p < .05, corrected for whole-brain multiple comparisons based on Gaussian Random Field Theory (GRFT). Between-groups images were thresholded using cluster detection statistics, with a height threshold of z > 2.3 and a cluster probability of p < .05, using a-priori region of interest (ROIs) constructed as spheres ranging from 5mm to 10 mm and centered based on peak coordinates from previous brain imaging studies on the IGT (Bolla et al., 2003; Christakou et al., 2009; Li et al., 2010; Power et al., 2012; Tanabe et al., 2007). ROIs comprised the DLPFC (10mm spheres, coordinates: ±40,28,30), the vmPFC (7mm spheres, coordinates: ±16,35,−8), the OFC (7mm spheres, coordinates: ±32,30,−13), the insular cortex (7mm spheres, coordinates: ±34,8,−8), the VS (5mm spheres, coordinates: ±10,16,−2), and the amygdala (5mm spheres, coordinates; ±30,2,−12).
Functional Connectivity Analyses
Psycho-physiological interaction analyses (PPI) were performed for the decision minus baseline contrast, with seed regions comprising a similar bilateral ventral striatal ROI as was used for the between-groups analyses. For each subject, a first level PPI model was set up using FSL including the following user-specified regressors: 1) the time course of the seed region, 2) a regressor coding for the decision condition (selections of decks A, B, C and D), 3) the interaction term, i.e. the multiplication of regressors 1 and 2. Single-subject contrast images for each of these three regressors were created. Each subject’s PPI contrast image for the interaction regressor were then entered into a second-level random-effects analysis to test for group effects. Connectivity analyses were thresholded at p < .05 whole-brain cluster-level corrected.
Behavioral Statistical Analyses
The IGT score was calculated as the number of choices from safe (C+D) minus risky (A+B) decks by 5 blocks of 20 choices. Due to the small sample size and non-normal distributions, non-parametric tests were used to examine IGT scores, and for correlation analyses between problem gambling severity score and VS activation and brain regions coupled with VS activation (extraction of parameter estimates). Friedman tests were performed separately in each group in order to examine the IGT score difference across each blocks. Mann–Whitney U tests were then performed to examine between-groups differences on the IGT scores on each block. Spearmann Rho was used to examine the association between SOGS scores and parameter estimates of brain activations. These analyses were carried out using SPSS software (SPSS, Inc., Chicago, Illinois).
RESULTS
In scanner behavior
In controls, Friedman tests revealed that the proportion of advantageous choices was higher: in the third block of 20 trials (Mean Rank = 1.73) than in the first block (Mean Rank = 1.27; χ2 (1,15) = 4.46, p = .035); in the fifth block (Mean Rank = 1.80) than in the third block (Mean Rank = 1.20; χ2 (1,15) = 5.40, p = .020), and in the fifth block (Mean Rank = 1.77) than in the first block of 20 trials (Mean Rank = 1.23; χ2 (1,15) = 4.57, p = .033). In the poker player group, we observed that the proportion of advantageous choices was higher: in the fifth block (Mean Rank = 1.77) than in the third block (Mean Rank = 1.23, χ2 (1,15) = 4.57, p = .033), and in the fifth block (Mean Rank = 1.77) than in the second block of 20 trials (Mean Rank = 1.23; χ2 (1,15) = 4.57, p = .033). No other significant result was observed. These findings indicate that both controls and poker players had learned to perform better during the task. Mann–Whitney U tests revealed no significant difference between poker players and controls for each blocks (all p > .05). In addition, there were no differences between the control and the poker player groups in their deck selection reaction time (in milliseconds) for each block (all p > .01).
fMRI BOLD responses
Within-group whole-brain analysis
Deck-selection minus baseline
The controls showed significant activation in brain areas encompassing the superior, middle and inferior frontal gyrus and the cingulate gyrus (see Figure 1 and Table 2). Poker players showed significant activation in the superior, middle, medial and inferior frontal gyrus, frontal orbital cortex, the insular cortex, the caudate, the putamen, the nucleus accumbens and the cingulate gyrus (see Figure 1 and Table 3).
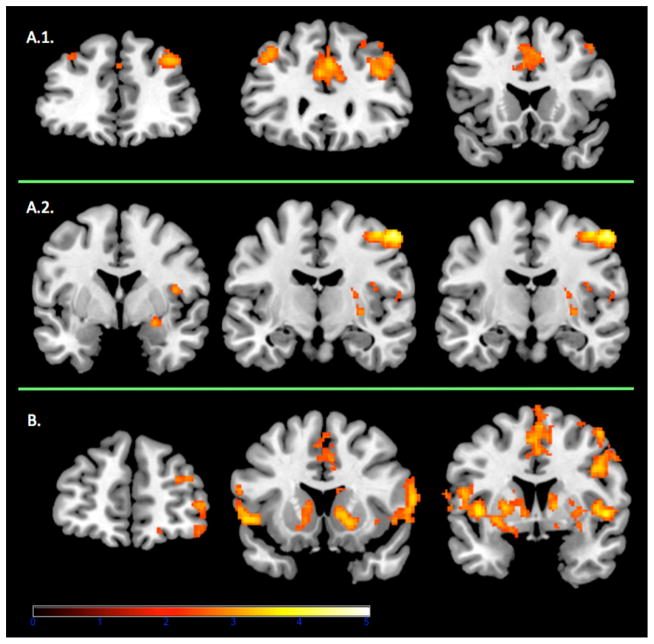
Figure 1.
Panel A.1 Coronal view (at Y = 40, 30, 20) of the frontal brain activations observed in controls for the whole brain contrast of deck selection minus baseline. Panel A.2. Coronal view (at Y = 0, −10, −15) of the amygdalar, striatal and insular brain activations observed in controls for the whole brain contrast of disadvantageous deck selection minus advantageous deck selection. Panel B. Coronal view (at y = 50, 15, 5) of the frontal, striatal and insular brain activations observed in poker players for the whole brain contrast of deck selection minus baseline. All contrast maps are thresholded at p < .05, corrected for whole-brain multiple comparisons. Red/Amber color demonstrates areas where subjects show relatively greater activation. The right side of the brain is on the right.
Table 2.
Significant brain activation within the control group.
| MNI Coordinates
|
||||||||
|---|---|---|---|---|---|---|---|---|
| IGT Contrast | Structure | Left/right | BA | k | x | y | z | Z value |
| Deck Selection vs. Baseline | Cerebellum/Occipital Gyrus | L | - | 24968 | −34 | −52 | −30 | 4.77 |
| Dorsolateral Prefrontal cortex/Middle Frontal Gyrus | R | 9 | 883 | 36 | 38 | 38 | 3.51 | |
| Anterior Cingulate | R | 32 | 788 | 4 | 28 | 34 | 3.51 | |
| Middle Frontal Gyrus | L | 8 | 457 | −30 | 62 | 28 | 3.14 | |
| Precentral Gyrus/Inferior Frontal Gyrus | L | 6/9 | 318 | −50 | 2 | 34 | 3.10 | |
| Bad Deck minus Good Deck | Cerebellum | L | - | 1430 | −18 | −56 | −20 | 4.77 |
| Postcentral Gyrus | R | 3 | 891 | 54 | −10 | 54 | 4.71 | |
| Insula/Central Opercular Cortex/Postcentral Gyrus | R | 13 | 569 | 48 | −20 | 20 | 3.82 | |
| Thalamus/Putamen/Amygdala | R | - | 380 | 16 | −20 | 0 | 3.28 | |
IGT, Iowa Gambling task; BA, Brodmann area; k, voxel cluster size (each voxel = 2mm3); L, left; R, right; MNI, Montreal Neurological Institute.
Table 3.
Significant brain activation within the poker player group.
| MNI Coordinates
|
||||||||
|---|---|---|---|---|---|---|---|---|
| IGT Contrast | Structure | Left/right | BA | k | x | y | z | Z value |
| Deck Selection vs. Baseline | Middle Occipital Gyrus/Postcentral Gyrus | L | - | 18315 | −46 | −74 | 4 | 4.52 |
| Postcentral Gyrus/Angular Gyrus/Parietal Operculum Cortex | R | - | 3947 | 52 | −14 | 52 | 3.77 | |
| Putamen/Inferior Frontal Gyrus/Insula | L | - | 1566 | −28 | 0 | −4 | 3.90 | |
| Central Opercular Cortex/Insula/Putamen/Caudate/Frontal Orbital Cortex/Accumbens | R | 13 | 1373 | 50 | 4 | 0 | 3.91 | |
| Cingulate Gyrus/Medial Frontal Gyrus | R | 24 | 971 | 0 | 0 | 52 | 3.95 | |
| Superior Frontal Gyrus/Middle Frontal Gyrus | R | 10 | 819 | 32 | 64 | −4 | 3.52 | |
IGT, Iowa Gambling task; BA, Brodmann area; k, voxel cluster size (each voxel = 2mm3); L, left; R, right; MNI, Montreal Neurological Institute.
Advantageous decks vs. disadvantageous decks
The controls showed significant activation in the insular cortex, the putamen, and the amygdala (see Table 2 and Figure 1). The poker player group showed no activation approaching significance for the contrast of disadvantageous decks minus disadvantageous decks selection. For both groups separately, we did not find significant activation for the advantageous decks minus disadvantageous decks contrast.
Between-groups ROI analysis
Deck selection minus baseline
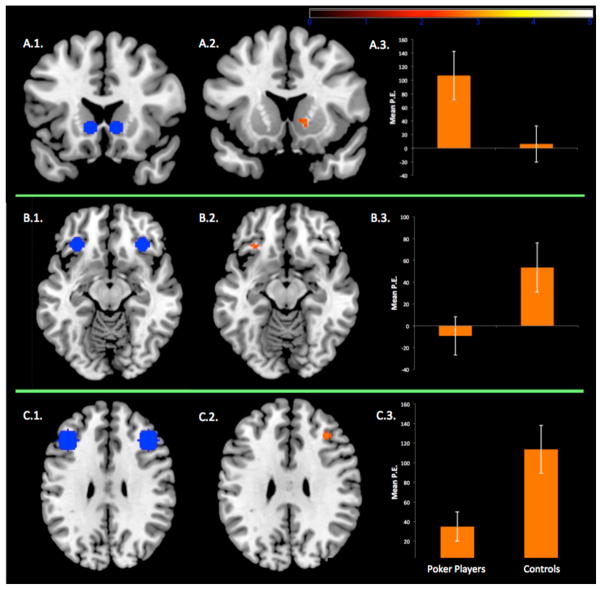
Comparison of activation between poker players and controls in our ROIs showed significant increased activation in controls for the right orbitofrontal cortex (BA 47), the right dorsolateral prefrontal cortex (BA 9) and significant increased activation in poker players in the right ventral striatum (see Figure 2). We found no significant between-groups activation within ROIs in the vmPFC, insular cortex, and amygdala. Importantly, in the poker player group, we observed a significant correlation between ventral striatal brain activation (extraction of parameter estimates) and scores of problem gambling severity (Spearmann Rho = .745, p < .001; see Figure 3).
Figure 2.
Panel A.1 Coronal view of ventral striatal regions of interest (ROIs). Panel A.2. Compared to controls, poker players showed stronger brain activation in the right ventral striatum (X = 12, Y = 12, Z = 0, k = 10, z = 2.69, at p < .05, corrected) for the contrast of deck selection minus baseline. Panel A.3. Mean parameter estimates (P.E.) extracted from the right ventral striatum for each group separately. Panel B.1. Horizontal view of orbitofrontal ROIs. Panel B.2. Compared to poker players, controls showed stronger brain activation in the left orbitofrontal cortex (X = −32, Y = 28, Z = −12, k = 11, z = 2.72, at p < .05, corrected) for the contrast of deck selection minus baseline. Panel B.3. Mean P.E. extracted from the left orbitofrontal cortex for each group separately. Panel C.1. Horizontal view of dorsolateral prefrontal ROIs. Panel C.2. Compared to poker players, controls showed stronger brain activation in the right dorsolateral prefrontal cortex (X = 36, Y = 30, Z = 28, k = 53, z = 2.96, at p < .05, corrected) for the contrast of deck selection minus baseline. Panel C.3. Mean P.E. extracted from the right dorsolateral prefrontal cortex for each group separately. Error bars are the standard errors of the mean.
Figure 3.
Fig. 5. In the poker player group (n = 15), gambling severity was positively correlated with P.E. extracted from the right ventral striatum ROI (Spearmann Rho = .745, p < .001).
Advantageous deck vs. disadvantageous deck
Comparison of activation between poker players and controls in ROIs did not reach significant activation for the advantageous deck vs. disadvantageous deck contrasts.
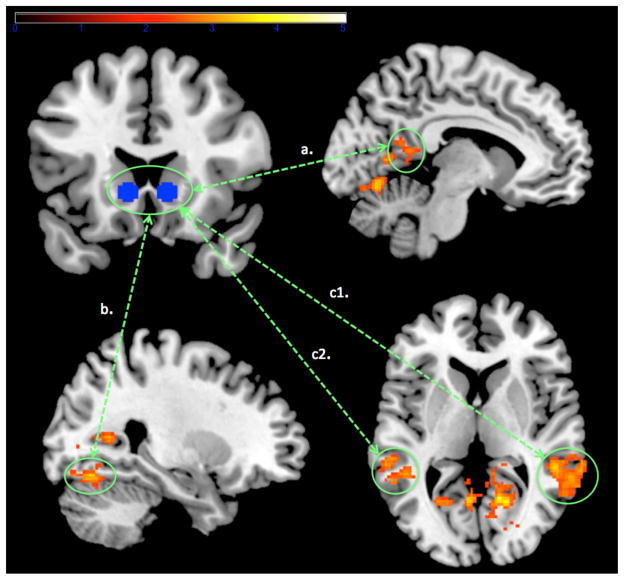
Group differences in ventral striatal functional connectivity during deck selection
We analyzed bilateral ventral striatal connectivity for the deck selection minus baseline contrast. Regions including the occipital fusiform gyrus, posterior cingulate, superior temporal gyrus and middle temporal gyrus showed significant higher increases in ventral striatal coupling in poker players, as compared with controls (see Table 4 and Figure 4). In addition, we observed significant correlation between scores of problem gambling severity and activations found in the occcipital Fusiform Gyrus (Spearmann Rho = .55, p < .05) and in the middle temporal gyrus (Spearmann Rho = .59, p < .05). No regions showed greater ventral striatal functional connectivity in controls minus poker players (at both p < .05 corrected and p < .001, uncorrected).
Table 4.
Group Differences for the ventral strialal PPI analyses: poker players minus controls.
| MNI Coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| Structure | Left/right | BA | k | x | y | z | Z value |
| Occipital Fusiform Gyrus/Lingual Gyrus | R/L | 18 | 818 | 22 | −70 | −14 | 3.97 |
| Posterior Cingulate/Precuneus Cortex/Lingual Gyrus | R | 30 | 687 | 14 | −60 | 4 | 4.01 |
| Superior Temporal Gyrus/Middle Temporal Gyrus/Supramarginal Gyrus | R | 22 | 484 | 56 | −38 | 8 | 3.54 |
| Middle Temporal Gyrus/Superior Temporal Gyrus/Supramarginal Gyrus/Angular Gyrus | L | 21 | 356 | −56 | −48 | 10 | 3.82 |
IGT, Iowa Gambling task; BA, Brodmann area; k, voxel cluster size (each voxel = 2mm3); L, left; R, right; MNI, Montreal Neurological Institute.
Figure 4.
As compared with controls, higher ventral striatal connectivity (p < .05, corrected) in poker players in the left posterior cingulate cortex (pathway a.), the occipital fusiform gyrus (pathway b.). and the middle/superior temporal gyrus (pathway c.).
DISCUSSION
In this study, we examined the levels that different neural systems play in monetary decision-making in frequent poker gamblers, who vary in their degree of problem gambling using the Iowa Gambling Task (IGT). We studied a sample of frequent poker players - ranging from non-problem to high-problem gambling – and a sample of non-gambler controls.
Consistent with our hypothesis, as compared to controls, poker players demonstrated increased activity within key component of the “impulsive” system, namely the VS, coupled with diminished activation in key components of the “reflective” system, namely the DLPFC and the OFC. These findings suggest that, as compared to controls, monetary decision-making in poker gamblers triggered higher motivational-reward processes but lower emotional (reactivation of emotions from by previous choices outcomes through the OFC) and cognitive/executive (through DLPFC activation) self-regulatory processes. This assumption is further supported by findings from our functional connectivity analyses, which showed higher ventral striatal connectivity in poker players (as compared to controls), in regions involved with bottom-up sensory processes including attentional/motor control (posterior cingulate; e.g., Leech & Sharp, 2013), visual (occipital gyrus; e.g., Bordier et al., 2013) and auditory (temporal gyrus; e.g., Bordier et al., 2013) saliency. Importantly, our findings also supported our second hypothesis that VS activity was positively associated with problem gambling severity. Scores of problem gambling severity were also positively associated with connectivity with the VS seed and the occipital Fusiform Gyrus and the middle temporal gyrus. Hence, the more severe an individual’s gambling problem is, the more IGT deck selection is underlined by bottom-up reward-salient processes.
Within-group fMRI analyses also revealed results that were consistent with this general finding. These analyses showed that, in non-gambler control participants, IGT deck selection was associated with activations in prefrontal (DLPFC, middle and inferior frontal gyrus) and anterior cingulate areas. This suggests that, in our sample of controls, deck selection during the IGT essentially recruited areas involved in self-controlled decision-making, that is, voluntary choices based on previous actions and outcomes (e.g., McClure et al., 2007; Hare et al., 2009). Moreover, by contrast to advantageous deck selection, choosing from disadvantageous decks was associated with heightened insula, putamen and amygdala activity. Hence, in controls, taking choices featuring high rewards and losses heightened neural activation within an emotion-arousal brain circuitry (Verdejo-Garcia & Bechara, 2009), as compared with choices featuring low reward and losses. This suggests that our control subjects were sensitive to the level of monetary risk-taking associated with their forthcoming choice. For instance, the heightened activation within the insular cortex prior to disadvantageous choice is consistent with several neuroimaging studies on decision-making under uncertainty, which observed an activation within the insula when anticipating both monetary loss (e.g., Paulus, 2003) and gain (e.g., Izuma et al, 2008). Other studies have shown that the insula is sensitive to risk level (Xue et al., 2010) and triggered by excessive product price, when deciding on whether or not to purchase an item (Knutson et al., 2007).
The maps of activations observed in frequent poker players during deck selection, were starkly different to those found within the control group. More specifically, while choosing a deck, poker players exhibited activation in inferior prefrontal cortex, orbitofrontal cortex, insula and striatum (putamen, caudate, accumbens). This pattern of activations might reflect motivational-arousal saliency for gambling-like choices in poker gamblers. Importantly, we highlighted that the type of deck selection (i.e., advantageous versus disadvantageous) did not trigger differential brain activation in poker gamblers. This suggests that choosing from advantageous or disadvantageous decks did not trigger differential brain processing in poker players (for a compatible finding in gambling disordered subjects, see Brevers et al., 2015).
Altogether, these findings support the notion that poker players exhibited a cue-induced signal increase toward the monetary decision-making during the IGT. To a broader extent, the present results are in line with the somatic marker hypothesis (Damasio, 1994) and with recent neurocognitive models of addiction (Naqvi & Bechara, 2009; Noël et al., 2013a, 2013b; Verdejo-Garcia and Bechara, 2009), which all advanced that hyperactivity in motivational-arousal brain areas toward immediate salient outcome (i.e., primary inducers) might disable the operation of the reflective high-order control system, necessary to forecasts the long term consequences of a given action through reactivation of emotions triggered by previous choices outcomes (i.e., secondary inducers). Noteworthy, between-groups findings highlighted in the present study seem partly inconsistent with previous fMRI reports on gambling disorder. More specifically, using gambling-like experimental paradigms (card games; Brevers et al., 2015; Miedl et al., 2010; van Holst et al., 2012), several studies showed higher activation in both the ventral-striatum and the OFC during decision-making in problem gamblers. This discrepancy might be explained by the structure of the experimental tasks used to examine the neural correlates of monetary decision-making in gamblers. More specifically, during the IGT, participants had to retrieve memories from previous choices outcomes. This suggests that, during the IGT, OFC activity might essentially underlie self-regulatory affective processes (Bechara et al., 2000). By contrast, during the card games used by previous fMRI studies (Brevers et al., 2015; Miedl et al., 2010; van Holst et al., 2012), participants computed their choices based on explicit information (probabilities and values of the potential rewards/losses displayed on the screen), and (ultimately) independently of previous choices outcomes. During these more simple card-games, the OFC might be primarily involved in cue reactivity (see also Goudriaan et al., 2010). In other words, decreased OFC activity observed in gamblers during the IGT might reflect a weakened strength of cognitive control, and increased OFC activity during simple card-games might reflect enhanced salience for gambling-related cues.
One strength of this study is that present sample of gamblers ranged at both extremities of problem gambling scores. This allowed us to highlight significant association between problem gambling severity and VS activations. Nevertheless, no significant association was found between problem gambling severity and behavioral performance during the IGT. One possible explanation is that, despite heightened VS activation, and despite observing a concomitant decrease in prefrontal activity, problem gambler participants still maintained reasonably normal IGT performance, mainly because the changes are perhaps so subtle, and the IGT is not behaviorally sensitive to detect these subtle changes in a manner similar to, for example, patients with prefrontal lesions. Another potential moderator of poker gamblers’ IGT behavior is that participants were not remunerated according to their performances. This could have lowered poker gamblers’ motivation to gamble and might have decreased the saliency associated with the action of gambling during the IGT. For instance, between-groups analyses revealed no increased insular activation in the poker player group, as compared to controls. Put differently, the experience of a strong urge to gamble could increase the saliency directed at options featuring high rewards (disadvantageous decks). This hypothesis is held by a triadic approach to addiction (Naqvi & Bechara, 2009, 2014; Noël et al., 2013a, 2013b), which argue that the experience of a state of craving, through heightened activation within the insular cortex, plays a key role in heightening the strengths of motivational-reward brain circuit toward the enactment of addiction-related behaviors. Thus, future brain imaging studies should manipulate and monitor the intensity of gambling urge during the IGT and examine the impact of this experienced state of craving on the association between gambling dependence severity and decision-making, with a special focus on the insular cortex.
Acknowledgments
Funding for this study was provided by the National Institute of Drug Abuse (NIDA; Grant R01-DA16708). NIDA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The authors thank Alex Hollihan for her help in recruiting participants.
Footnotes
AUTHORS CONTRIBUTION
Damien Brevers, Xavier Noël, James Melrose, Qinghua He and Antoine Bechara designed the study and wrote the protocol. Brevers Damien conducted literature searches and provided summaries of previous research studies. Damien Brevers recruited the participants and collected the data. Damien Brevers, Xavier Noël, James Melrose, Qinghua He and Antoine Bechara conducted the statistical analysis. Damien Brevers wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
All authors report no biomedical financial interests or potential conflicts of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bechara A. Decision Making, Impulse Control and Loss of Willpower to Resist Drugs: A Neurocognitive Perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Game Econ Behav. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision-making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi CS, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performaing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C, Puja F, Macaluso E. Sensory processing during viewing of cinematographic material: computational modeling and functional neuroimaging. Neuroimage. 2013;67:213–226. doi: 10.1016/j.neuroimage.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevers D, Cleeremans A, Goudriaan AE, Bechara A, Kornreich C, Verbanck P, Noël X. Decision making under ambiguity but not under risk is related to problem gambling severity. Psychiatry Res. 2012a;200:558–574. doi: 10.1016/j.psychres.2012.03.053. [DOI] [PubMed] [Google Scholar]
- Brevers D, Cleeremans A, Verbruggen F, Bechara A, Kornreich C, Verbanck P, Noël X. Impulsive action but impulsive choice determines problem gambling severity. Plos One. 2012b;7:e50647. doi: 10.1371/journal.pone.0050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A, Noël X. Iowa Gambling Task (IGT): twenty years after – gambling disorder and IGT. Front Psychol. 2013;4:665. doi: 10.3389/fpsyg.2013.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Hermoye L, Divano L, Kornreich C, Verbanck P, Noël X. Comfort for Uncertainty in pathological gamblers: A fMRI study. Behav Brain Res. 2015;278:262–270. doi: 10.1016/j.bbr.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Clark L. Gambling severity predicts midbrain response to near-miss outcomes. J Neurosci. 2010;30:6180–6187. doi: 10.1523/JNEUROSCI.5758-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Giampietro V, Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J Neurosci. 2009;29:11020–11028. doi: 10.1523/JNEUROSCI.1279-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: emotions, reason, and the human brain. New York: Avon Books; 1994. [Google Scholar]
- Dufour M, Brunelle N, Roy E. Are Poker Players All the Same? Latent Class Analysis. J Gambl Stud. 2013 doi: 10.1007/s10899-013-9429-y. [DOI] [PubMed] [Google Scholar]
- Dymond S, Lawrence NS, Yuen KSL. Neurocognitive mechanisms of impaired decision making in pathological gambling. Front Psychiatry. 2013;4:12. doi: 10.3389/fpsyt.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, De Ruiter MB, Van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Chamberlain SR, Schreiber LRN. Neurocognitive dysfunction in strategic and non-strategic gamblers. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:336–340. doi: 10.1016/j.pnpbp.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95:948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cereb Cortex. 2009;19:1134–114310. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2013;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D’Argembeau A, Nig M, Bechara A. The Iowa Gambling Task in FMRI images. Hum Brain Mapp. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedl SF, Fehr T, Meyer G, Herrmann M. Neurobiological correlates of problem gambling in a quasi-realistic blackjack scenario as revealed by fMRI. Psychiatry Res Neurimaging. 2010;181:165–173. doi: 10.1016/j.pscychresns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X, Bechara A, Brevers D, Verbanck P, Campanella S. Alcoholism and the Loss of Willpower: A Neurocognitive Perspective. J Psychophysiol. 2010;24:240–248. doi: 10.1027/0269-8803/a000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013a;23:632–638. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X, Brevers D, Bechara A. A Triadic Neurocognitive Approach to Addiction for Clinical Interventions. Front Psychiatry. 2013b;4:179. doi: 10.3389/fpsyt.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Power Y, Goodyear B, Crockford D. Neural correlates of pathological gamblers preference for immediate rewards during the iowa gambling task: an FMRI study. J Gambl Stud. 2012;28:623–636. doi: 10.1007/s10899-011-9278-5. [DOI] [PubMed] [Google Scholar]
- Recher J, Griffiths MD. An exploratory qualitative study of online poker professional players. Social Psychol Rev. 2012;14:13–25. [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum Brain Mapp. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, Veltman DJ, Büchel C, Van den Brink W, Goudriaan AE. Distorted expectancy coding in problem gambling: is the addictive in the anticipation? Biol Psychiatry. 2012;71:741–748. doi: 10.1016/j.biopsych.2011.12.030. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, Chase HW, Clark L. Striatal connectivity changes following gambling wins and near-misses: Associations with gambling severity. Neuroimage Clin. 2014;5:232–239. doi: 10.1016/j.nicl.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]