Abstract
Although generally associated with cardiovascular regulation, angiotensin II receptor type 1 (AT1aR) blockade in mouse models and humans has also been associated with enhanced fear extinction and decreased post-traumatic stress disorder (PTSD) symptom severity, respectively. The mechanisms mediating these effects remain unknown, but may involve alterations in the activities of corticotropin-releasing factor (CRF)-expressing cells, which are known to be involved in fear regulation. To test the hypothesis that AT1aR signaling in CRFergic neurons is involved in conditioned fear expression, we generated and characterized a conditional knockout mouse strain with a deletion of the AT1aR gene from its CRF-releasing cells (CRF-AT1aR(−/−)). These mice exhibit normal baseline heart rate, blood pressure, anxiety, and locomotion, and freeze at normal levels during acquisition of auditory fear conditioning. However, CRF-AT1aR(−/−) mice exhibit less freezing than wild type mice during tests of conditioned fear expression—an effect that may be caused by a decrease in the consolidation of fear memory. These results suggest that central AT1R activity in CRF-expressing cells plays a role in the expression of conditioned fear, and identify CRFergic cells as a population on which AT1R antagonists may act to modulate fear extinction.
Introduction
Angiotensin II, a component of the renin-angiotensin system, and its receptor, the angiotensin II receptor type 1a (AT1aR), have been studied extensively in the context of blood pressure regulation (Mehta and Griendling, 2007, Castrop, 2015, Chen and Coffman, 2015) and the stress response (Chen et al., 2012, Krause et al., 2011, Armando et al., 2007). However, recent studies in humans and mice have suggested roles for AT1aR activation in post-traumatic stress disorder (PTSD) and auditory fear conditioning, respectively. For example, treatment of humans with angiotensin receptor blockers (ARBs) has been associated with a decrease in the hyperarousal and intrusive symptoms of PTSD (Khoury et al., 2012, Nylocks et al., 2015). Similarly, either acute or chronic administration of the ARB losartan in mice has been found to enhance the extinction of fear memory, but not fear acquisition or extinction training (Marvar et al., 2013). However, these previous investigations involved systemic administration of ARBs, and therefore did not address whether ARBs are acting centrally and which cell types they are acting on to create these behavioral effects.
To address this, we investigated whether AT1aR deletion from a genetically defined neural population—corticotropin-releasing factor (CRF)-expressing cells—affected the expression of conditioned fear. CRF is a hormone secreted by the paraventricular nucleus of the hypothalamus (PVN) during times of stress, and is a component of the hypothalamic-pituitary-adrenal axis (Rivier and Plotsky, 1986). CRF mRNA is also enriched in the central amygdala (CeA) (Pitts et al., 2009) and bed nucleus of the stria terminalis (BNST) (Beckerman et al., 2013)—regions implicated in the expression of conditioned fear (Sullivan et al., 2004, Wilensky et al., 2006). In support of a functional link between AT1aR activity and central CRFergic tone, previous work has shown that chronic systemic ARB administration can prevent the decrease in PVN CRF release induced by isolation stress (Armando et al., 2007) and that whole-brain AT1aR knockout decreases CRF mRNA expression in the PVN (Yamamoto et al., 2011).
Based on these findings, we hypothesized that AT1aRs on CRFergic cells are involved in the expression of conditioned fear, and predicted that an AT1aR knockout confined to CRFergic cells would impair cued fear expression. Furthermore, because AT1aR and CRF are both expressed in moderate levels in the CeA (Ciccocioppo et al., 2014, Shekhar et al., 2005, Von Bohlen Und Halbach and Albrecht, 1998b) and PVN (Premer et al., 2013, Aguilera et al., 1995a), we expected that this knockout would be pronounced in these areas.
Materials and Methods
Animals
Mice with a floxed AT1aR gene (AT1aRflox/flox; JAX Stock #016211) (Rateri et al., 2011) were bred with mice that selectively express Cre recombinase in CRFergic cells (CRF::Cre; JAX Stock #011087) (Martin et al., 2010, Gafford et al., 2014, Gafford et al., 2012) to generate offspring with a conditional knockout of the AT1aR confined to CRF-expressing cells. Offspring were genotyped for the floxed and wild type AT1aR alleles and the Cre recombinase gene using PCR on DNA extracted from ear punches (Figure S1). Male littermates between 8 and 12 weeks that were positive for Cre recombinase and also homozygous for either the floxed (CRF-AT1aR(−/−)) or wild type (AT1aR(+/+)) AT1aR allele were used for the behavioral tests. The same mice were used for the behavioral and cardiovascular tests, but only 8 out of 15 AT1aR(+/+) and 10 out of 16 CRF-AT1aR(−/−) mice that underwent the behavioral tests also underwent the cardiovascular ones. Bacterial artificial chromosome mice that express GFP in AT1aR-expressing cells (AT1a-GFP) (MMRRC ID# 036905-UCD) (Gonzalez et al., 2012, Marques-Lopes et al., 2015) were used for immunohistochemical studies, but not for behavioral ones. All procedures were conducted by male experimenters (Sorge et al., 2014) and were approved by the Institutional Animal Care and Use Committee of Emory University.
Immunohistochemistry
AT1a-GFP mice were perfused transcardially with 4% paraformaldehyde in 0.1M phosphate buffer, and their brains were extracted and fixed overnight in the same fixative. The next day, 40 μm coronal sections were cut using a vibratome. Sections were permeablized using Triton X-100 and blocked with normal horse serum and bovine serum albumin. GFP and CRF staining was performed by simultaneously incubating sections in 1:2000 and 1:200 dilutions of chicken anti-GFP (Abcam, ab13970) and rabbit anti-CRF (Abcam, ab11133) antibodies, respectively, for 48 hours, followed by simultaneous incubation in 1:500 and 1:100 dilutions of goat anti-chicken IgY Alexa Fluor 488 (Abcam, ab150169) and goat anti-rabbit IgG Alexa Fluor 568 (Life Technologies, A-11011) antibodies, respectively, for 48 hours. Sections were mounted on Superfrost Plus slides (Fisher Scientific, 12-550-15) and air-dried before being coverslipped with Mowiol mounting medium (Sigma-Aldrich, 81381). Sections were imaged using a 20X oil immersion objective (Leica HC PL APO 20X/0.75 IMM) on a Leica SP8 confocal microscope.
In vitro receptor autoradiography
Mice were anesthetized using isoflurane and their brains were removed, quickly frozen, and stored at −80 °C. The brains were sectioned coronally at a thickness of 20 μm with a cryostat and thaw-mounted onto charged microscope slides in a sequential manner such that adjacent sections were mounted on different slides in repeating sets of 5. The sections were air-dried for 5–20 minutes and kept refrigerated for no more than 2 weeks. Receptor autoradiography was carried out as described previously (Speth et al., 1999) using 125I-sarcosine1, isoleucine8 angiotensin II (125I-SI Ang II), prepared as described previously (Speth and Harding, 2001) through the Peptide Radioiodination (Shared Resource at Georgetown University), with the following modifications. The concentration of 125I-SI Ang II was ~250 pM, PD123319 was present at a concentration of 10 μM to saturate all AT2Rs, non-specific binding in sections adjacent to the “total binding” sections was determined in the presence of an AT1R saturating concentration of losartan (10 μM), and the film images were scanned into MCID 7.0 at 2400 dpi resolution and quantified relative to brain paste standards with known concentrations of 125I. For each assay, an AT1aR(+/+) and a CRF-AT1aR(−/−) brain were run in parallel to control for any day-to-day variations in the radioligand or assay conditions. To control for the possibility that the area delineated as having high binding corresponding to the regions of interest differed in size, measurements of both the density of binding and the density times area of binding were determined for group comparisons. Another set of adjacent sections were stained with thionin to provide anatomical registration for the autoradiographic images so as to localize areas of high 125I-SI Ang II binding to specific brain regions.
Anxiety and locomotion tasks
Mice performed elevated plus maze and open field tasks to measure their baseline levels of anxiety (Pellow et al., 1985) and locomotion (Tatem et al., 2014), respectively. The elevated plus maze task involved allowing each mouse to explore a plus-shaped maze with two walled arms and two open arms elevated 50 cm from the ground for 5 minutes. As the automated scoring system often tracked the mouse’s position to locations outside the maze, data were scored manually using the center of the mouse’s body to determine its location in the maze. The open field task involved allowing each mouse to explore a 27.3 cm2 arena (Med Associates Inc.) for 10 minutes. Data were scored automatically using the Med Associates Activity Monitor software.
Blood pressure and heart rate measurements
Two weeks after the extinction test, a tail-cuff sphygmomanometer (Hatteras Instruments, MC4000) was used to collect baseline blood pressure and heart rate measurements as previously described (Marvar et al., 2010).
Auditory fear conditioning and extinction
During habituation, mice were pre-exposed to conditioning cages for 2 days before training. Mice were then trained with an auditory fear conditioning paradigm, as previously described (Choi et al., 2010), that consisted of one day of 5 tone/shock pairings (30 second, 6 kHz, 75 dB tones co-terminating with 500 ms, 1 mA footshocks; 60 second inter-trial interval; room light on). For extinction, 24 hours later mice received 30 tone presentations (30 second, 6 kHz, 75 dB tones; 60 second inter-trial interval) in a different context (room light off, red cage light on, plexiglass floor). Freezing data were scored using the Actimetrics FreezeFrame 3 software.
Statistical analyses
Data are expressed as mean ± SEM and values of p < 0.05 were considered statistically significant. Averaged freezing data were analyzed using an unpaired, two-tailed Student’s t-test, as these were the first experiments conducted, and it was necessary to test for effects in both directions. Binned freezing data were analyzed using a repeated measures ANOVA (Prism 6.0) with a Bonferroni post-hoc analysis. 125I-SI Ang II binding levels were analyzed using a paired, one-tailed Student’s t-test, as the anticipated direction of the effect had been determined a priori based on genetic information.
Results
AT1aR and CRF co-localize in the PVN and CeA
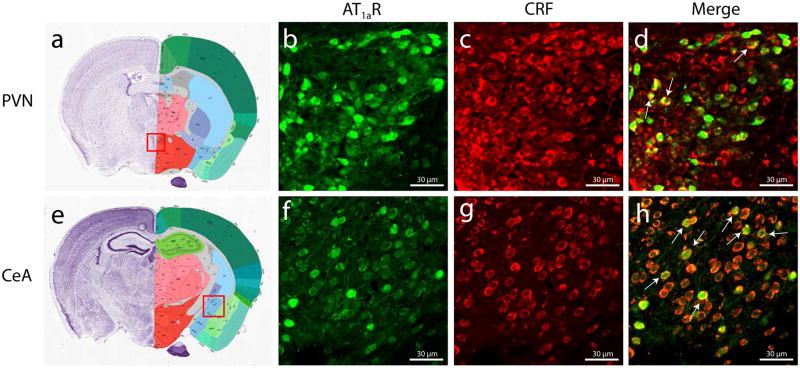
To determine whether AT1aR and CRF are co-expressed, we performed a dual-label IHC on the brains of AT1aR-GFP mice. Co-localization was observed in the PVN and CeA—areas heavily implicated in the stress response and fear expression, respectively (Figure 1). CRF cell-specific AT1aR knockout (CRF-AT1aR(−/−)) mice were then generated by crossing homozygous floxed AT1aR mice with CRF::Cre mice (see Methods).
Figure 1. AT1aR and CRF co-localize in subsets of PVN and CeA neurons.
Dense AT1aR and CRF expression is observed in the PVN and CeA of AT1aR-GFP mice. Arrows in (d) and (h) indicate co-localization.
AT1aR expression is reduced in the PVNs of CRF-AT1aR(−/−) mice
To assess whether CRF-AT1aR(−/−) mice express significantly less of the AT1R than wild type (AT1aR(+/+)) mice, we conducted an in vitro quantitative densitometric AT1R autoradiography analysis. Densitometric analysis of specific 125I-SI Ang II binding in the PVN indicated that the density of AT1Rs was significantly higher in AT1aR(+/+) brains (164±36 fmol/mg initial wet weight) than CRF-AT1aR(−/−) brains (121±28 fmol/mg) (Figure 2). A lower level of 125I-SI Ang II binding was observed in the basolateral amygdala (BLA) but no significant difference in binding density was observed; 21±7 fmol/mg for AT1aR(+/+)and 21±9 fmol/mg for CRF-AT1aR(−/−) brains.
Figure 2. Autoradiographic analysis of AT1R binding.
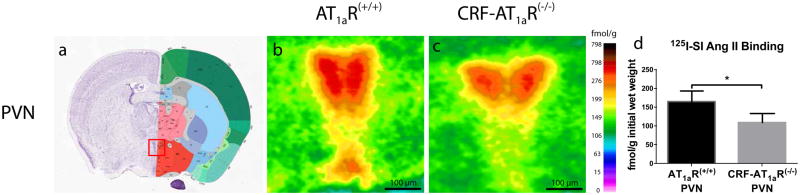
AT1R autoradiography revealed a decrease in AT1R expression in the PVNs of CRF-AT1aR(−/−) mice compared to controls. Data are presented as mean ± SEM. * p<0.05.
AT1aR knockout from CRFergic cells reduces the expression of conditioned fear
To test for an effect of CRFergic cell-specific AT1aR knockout on the acquisition, consolidation, or extinction of fear memory, we subjected mice to auditory fear conditioning and extinction protocols. AT1aR(+/+) and CRF-AT1aR(−/−) mice showed similar levels of freezing during auditory fear conditioning on a trial-by-trial basis (Figure 3A). In a test of fear expression 24 hours later, CRF-AT1aR(−/−) mice displayed significantly less overall freezing than AT1aR(+/+) (t(30) = 2.1; *p < 0.05) (Figure 3B). Furthermore, when analyzed over the course of the fear expression test (on a 10 CS bin-by-bin basis) using a repeated measures ANOVA, there was a significant main effect by group (F(1, 30)=4.31, p=0.046), but no interaction for the 3 bins of 10 CS presentations (F(2, 60)=0.02, p=0.974) (Figure 3C). Collectively, these data indicate a decrease in fear expression in CRF-AT1aR(−/−) mice.
Figure 3. AT1aR knockout from CRFergic cells has no effect on acquisition of conditioned fear, but leads to decreased fear expression and enhanced extinction retention.
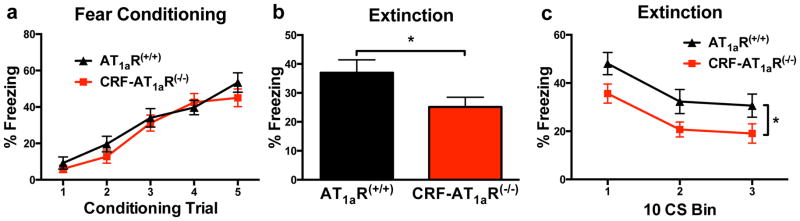
(a) AT1aR(+/+) and CRF-AT1aR(−/−) mice show similar levels of freezing during fear acquisition (n=15–17). (b and c) During extinction, CRF-AT1aR(−/−) mice show significantly less freezing than AT1aR(+/+) mice overall and during the last 10 CS presentations (n=15–17).
Baseline blood pressure, heart rate, anxiety, and locomotion are not affected by knockout of the AT1aR from CRFergic cells
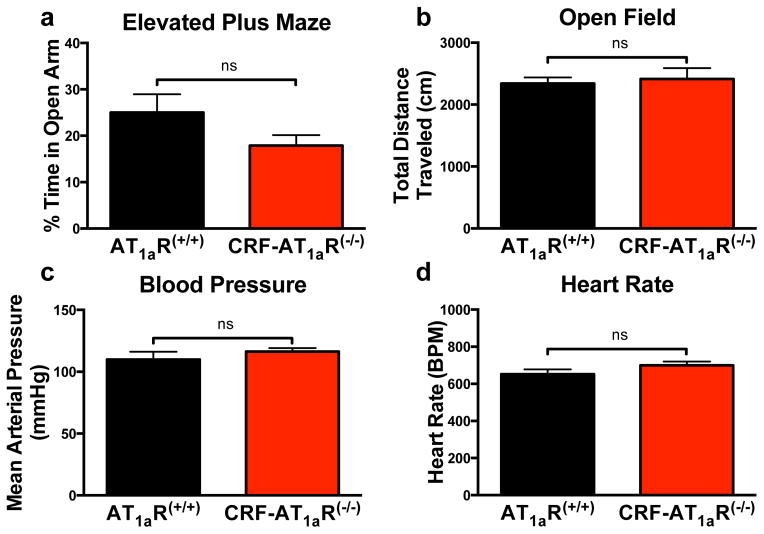
To evaluate whether this knockout affects baseline levels of anxiety, locomotion, and cardiovascular measures, we subjected AT1aR(+/+) and CRF-AT1aR(−/−) mice to elevated plus maze and open field tasks, and measured blood pressure and heart rate. AT1aR(+/+) and CRF-AT1aR(−/−) mice spent similar amounts of time in the open arms of the elevated plus maze (though there was a non-significant (p=0.13) trend toward reduced open arm exploration in the CRF-AT1aR(−/−) mice) (Figure 4A), traveled similar distances in the open field (Figure 4B), and showed no differences in baseline blood pressure or heart rate (Figure 4C–D).
Figure 4. AT1aR knockout from CRFergic cells does not affect baseline levels of anxiety, locomotion, blood pressure, or heart rate.
(a) AT1aR(+/+) and CRF-AT1aR(−/−) mice spend similar amounts of time on the open arms of an elevated plus maze (n=15–16) and (b) travel similar total distances during an open field test (n=15–17). Both groups also have similar (c) mean arterial pressure and (d) heart rate at baseline (n=8–10). Data are presented as mean ± SEM.
Discussion
The AT1aR is expressed in many organs, including the heart, kidney, blood vessels, and brain, and inhibition of this receptor is widely used for the treatment of hypertension and cardiovascular disease (Mehta and Griendling, 2007, Wright and Harding, 1995). However, previous studies by our lab (Khoury et al., 2012, Marvar et al., 2013, Nylocks et al., 2015) and others (Marinzalda Mde et al., 2014, Krause et al., 2011) suggest an important role for this receptor in fear-related pathologies such as PTSD. Our current data extend these findings by demonstrating for the first time that AT1aRs on CRF-expressing cells contribute to conditioned fear expression without affecting fear acquisition or baseline anxiety, locomotion, blood pressure, or heart rate.
These results contribute to a growing body of evidence implicating central AT1R activity in the expression of conditioned fear and anxiety (Khoury et al., 2012, Marvar et al., 2013, Marinzalda Mde et al., 2014, Shekhar, 2014, Shekhar et al., 2006, Johnson et al., 2013, Nylocks et al., 2015, Saavedra et al., 2006), and identify CRFergic cells as a population on which AT1R antagonists may act to create these effects. Further, they indicate that the fear expression-attenuating effects of AT1R inactivation may be caused by a decrease in fear memory consolidation (i.e., the transfer of a memory from a short-term to a long-term store in a transcription-dependent manner)—rather than an inability to acquire conditioning—as AT1aR(+/+) and CRF-AT1aR(−/−) mice showed similar levels of freezing during auditory fear conditioning, but 24 hours later, CRF-AT1aR(−/−) mice displayed significantly less overall freezing than wild types. Considering that both groups acquired similar amounts of fear, this trend may be attributable to an impairment in memory consolidation in CRF-AT1aR(−/−) mice. This interpretation is consistent with the previous finding that administration of an AT1R antagonist immediately before fear expression testing had no effect on expression (Marvar et al., 2013), indicating that this manipulation is not directly affecting conditioned responding.
The AT1aR is known to interact with multiple signaling pathways involved in memory consolidation (Higuchi et al., 2007, Guo et al., 2001) and the brain renin-angiotensin system has been implicated in both synaptic plasticity (Tchekalarova and Albrecht, 2007, Von Bohlen Und Halbach and Albrecht, 1998a) and some forms of memory consolidation (Kerr et al., 2005, Wright et al., 2002, Frenkel et al., 2005). The molecular mechanisms by which AT1/AT2/AT4R activation influences the long-term changes underlying memory consolidation remain poorly understood, but likely involve coupling of these G protein-coupled receptors to Gαq subunits, resulting in activation of PLC and subsequent intracellular Ca2+ release (Guo et al., 2001), which is known to facilitate synaptic plasticity (Sheng and Kim, 2002). Further, AT4R activation has been implicated in spatial memory formation (Wright et al., 1999) and may contribute to fear memory consolidation. Therefore, future studies aimed at elucidating the role of brain angiotensin II signaling in memory consolidation should use chemogenetic techniques (Sternson and Roth, 2014) to determine the role of Gαq activation in this process, and should focus on providing a more mechanistic understanding of how AT1 receptor activation influences the intracellular signaling cascades involved in memory consolidation and how this interaction differs for the consolidation of fear and extinction memories.
Additionally, the downstream effects of AT1aR knockout from CRFergic cells are not known. CRF receptor activation has been implicated in fear memory formation (Rainnie et al., 2004), but it is unclear how knockout of the AT1aR affects CRF release or if a change in CRF release is the primary mechanism by which this knockout reduces fear expression. Further, because there may be considerable functional heterogeneity in both the CRF-releasing (Valentino et al., 2001) and CRF-responsive (Kirby et al., 2000) populations, it is possible that AT1aR knockout affects various functionally, genetically, and spatially defined subsets of neurons differently (Lew et al., 2003, Hirawa et al., 1999).
It is worth noting that CRF-AT1aR(−/−) animals showed a trend toward less open arm exploration in the elevated plus maze test. This result, while not statistically significant, would seem to indicate slightly enhanced baseline anxiety levels in these mice, considering that their baseline locomotion levels (as measured by the total distance they traveled during the open field test) do not differ from AT1aR(+/+) animals. However, because the strains do not differ in baseline blood pressure or heart rate, it is likely that this trend does not indicate increased anxiety in CRF-AT1aR(−/−) mice; still, the technique used to collect these cardiovascular measurements suffers from low sensitivity. Future studies should use more sensitive tests of HPA axis activity such as plasma corticosterone measurements or PVN c-Fos quantification to determine whether this knockout confers a slight increase in baseline anxiety.
The receptor autoradiography analyses demonstrated an incomplete reduction in AT1R binding in the PVNs of CRF-AT1aR(−/−) mice, which may have occurred because AT1Rs are not exclusively expressed by CRFergic PVN neurons. It was not possible to demonstrate a reduction in AT1R binding in the CeAs of CRF-AT1aR(−/−) mice. AT1aR expression in the CeA is normally low, and a small subset of CeA cells express both AT1aR and CRF; as a result, the small differences in CeA AT1aR expression created by our conditional knockout model were likely not detectable with receptor autoradiography.
Given that the aim of our study was to investigate whether AT1aR deletion from a genetically defined neural population affects fear learning, our use of a constitutive knockout mouse model imposes certain limitations that merit mention. Because this knockout is present from birth, it may affect the animals’ normal development (Aguilera et al., 1994); however, because wild type and knockout animals showed similar baseline levels of blood pressure, heart rate, and anxiety, and acquired conditioned fear similarly, we feel that any effects of this knockout on development did not meaningfully affect the behaviors in which we were primarily interested (i.e., fear learning and expression). A further limitation of this model is its inability to provide conclusive evidence regarding which CRFergic populations are contributing to the observed reduction in fear expression. However, the finding that AT1aR and CRF co-localize in the CeA and PVN indicates that the CRFergic cells in these areas may be involved in creating this phenotype. CeA CRFergic cells have been implicated in fear memory retention (Gafford et al., 2014, Pitts and Takahashi, 2011, Pitts et al., 2009) and CeA AT1Rs have been implicated in anxiety (Marinzalda Mde et al., 2014). Additionally, PVN CRFergic cells are involved in conditioned fear expression (Otagiri et al., 2000) and anxiety (Bale et al., 2002), and are regulated by angiotensin II (Aguilera et al., 1995b).
Future studies involving site-specific AT1aR knockouts will be required in order to determine which brain areas (e.g., CeA, PVN, BNST) contain the AT1aR- and CRF-expressing cells most involved in creating this effect on conditioned fear memory. However, the identification of a genetically defined subset of neurons on which AT1R antagonists act to influence fear expression will allow for more targeted future studies and potential new treatments of normal and dysregulated fear memory.
Supplementary Material
Bands from WT AT1aR (AT1aR(WT/WT)), heterozygous floxed AT1aR (AT1aR(WT/Flox)), homozygous floxed AT1aR (AT1aR(Flox/Flox)), and Cre recombinase-positive mice are shown.
Acknowledgments
We would like to thank Donald Rainnie (Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine) for the anti-CRF antibody, Colin Young (Department of Pharmacology and Physiology, The George Washington University School of Medical and Health Sciences) for his comments on the manuscript, and Teresa A. Milner (Director of the Neuroanatomy EM Core, Feil Family Brain and Mind Research Institute, Weill Cornell Medical College) for the AT1a-GFP brain sections and her comments on figure design. This research project was supported by the Emory University Integrated Cellular Imaging Microscopy Core and the following sources of funding: NIH-NHLBI 113905 (R.C.S., A.L., L.C.), Nova Southeastern University President’s Faculty Development Grant (R.C.S., A.L., P.J.M.), Nova Southeastern University Cardiovascular Neuroscience Program (R.C.S., A.L., L.C.), Shared Resource Center supported by NIH-P30 CA51008 and by NCATS 8 UL1 TR000101 (R.C.S.) and NIH R00 HL107675-03 (P.J.M).
Footnotes
Author Contributions: R.C.H, O.P.K., R.C.S., K.J.R, and P.J.M designed the study; R.C.H., J.C.G., A.L., and L.C. performed experiments; R.C.H., O.P.K., A.L., L.C., R.C.S., K.J.R., and P.J.M. analyzed data; R.C.H. made the figures and wrote the paper; A.L., L.C., and R.C.S. reviewed and edited the paper.
Conflicts of Interest: The authors declare no competing financial interests.
References
- Aguilera G, Kapur S, Feuillan P, Sunar-Akbasak B, Bathia AJ. Developmental changes in angiotensin II receptor subtypes and AT1 receptor mRNA in rat kidney. Kidney Int. 1994;46:973–9. doi: 10.1038/ki.1994.356. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Kiss A, Luo X. Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol. 1995a;7:775–83. doi: 10.1111/j.1365-2826.1995.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology. 1995b;61:437–44. doi: 10.1159/000126866. [DOI] [PubMed] [Google Scholar]
- Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res. 2007;1142:92–9. doi: 10.1016/j.brainres.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Lee KF, Vale WW. The role of corticotropin-releasing factor receptors in stress and anxiety. Integr Comp Biol. 2002;42:552–5. doi: 10.1093/icb/42.3.552. [DOI] [PubMed] [Google Scholar]
- Beckerman MA, Van Kempen TA, Justice NJ, Milner TA, Glass MJ. Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Exp Neurol. 2013;239:120–32. doi: 10.1016/j.expneurol.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrop H. A role for AT1 receptor-associated proteins in blood pressure regulation. Curr Opin Pharmacol. 2015;21:43–47. doi: 10.1016/j.coph.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Chen D, Coffman TM. AT Angiotensin receptors-vascular and renal epithelial pathways for blood pressure regulation. Curr Opin Pharmacol. 2015;21:122–126. doi: 10.1016/j.coph.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Chen D, Jancovski N, Bassi JK, Nguyen-Huu TP, Choong YT, Palma-Rigo K, Davern PJ, Gurley SB, Thomas WG, Head GA, Allen AM. Angiotensin type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. J Neurosci. 2012;32:2051–61. doi: 10.1523/JNEUROSCI.5360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–80. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, De Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, Roberto M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci. 2014;34:363–72. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel L, Maldonado H, Delorenzi A. Memory strengthening by a real-life episode during reconsolidation: an outcome of water deprivation via brain angiotensin II. Eur J Neurosci. 2005;22:1757–66. doi: 10.1111/j.1460-9568.2005.04373.x. [DOI] [PubMed] [Google Scholar]
- Gafford G, Jasnow AM, Ressler KJ. Grin1 receptor deletion within CRF neurons enhances fear memory. PLoS One. 2014;9:e111009. doi: 10.1371/journal.pone.0111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109:16330–5. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, Marques-Lopes J, Young CN, Butler SD, Davisson RL, Iadecola C, Pickel VM, Pierce JP, Milner TA. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience. 2012;226:489–509. doi: 10.1016/j.neuroscience.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–80. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112:417–28. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- Hirawa N, Uehara Y, Kawabata Y, Numabe A, Gomi T, Ikeda T, Suzuki T, Goto A, Toyo-Oka T, Omata M. Long-term inhibition of renin-angiotensin system sustains memory function in aged Dahl rats. Hypertension. 1999;34:496–502. doi: 10.1161/01.hyp.34.3.496. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Sajdyk TJ, Fitz SD, Hale MW, Lowry CA, Hay-Schmidt A, Shekhar A. Angiotensin II’s role in sodium lactate-induced panic-like responses in rats with repeated urocortin 1 injections into the basolateral amygdala: amygdalar angiotensin receptors and panic. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:248–56. doi: 10.1016/j.pnpbp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS, Bevilaqua LR, Bonini JS, Rossato JI, Kohler CA, Medina JH, Izquierdo I, Cammarota M. Angiotensin II blocks memory consolidation through an AT2 receptor-dependent mechanism. Psychopharmacology (Berl) 2005;179:529–35. doi: 10.1007/s00213-004-2074-5. [DOI] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–55. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–62. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Krause EG, De Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci. 2011;31:15009–15. doi: 10.1523/JNEUROSCI.0892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RA, Mustafa T, Ye S, Mcdowall SG, Chai SY, Albiston AL. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP) J Neurochem. 2003;86:344–50. doi: 10.1046/j.1471-4159.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- de Marinzalda ML, Perez PA, Gargiulo PA, Casarsa BS, Bregonzio C, Baiardi G. Fear-potentiated behaviour is modulated by central amygdala angiotensin II AT1 receptors stimulation. Biomed Res Int. 2014;2014:183248. doi: 10.1155/2014/183248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Lynch MK, Van Kempen TA, Waters EM, Wang G, Iadecola C, Pickel VM, Milner TA. Female protection from slow-pressor effects of angiotensin II involves prevention of ROS production independent of NMDA receptor trafficking in hypothalamic neurons expressing angiotensin 1A receptors. Synapse. 2015;69:148–65. doi: 10.1002/syn.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG, Nemeroff CB, Owens MJ. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry. 2010;67:1212–6. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Goodman J, Fuchs S, Choi DC, Banerjee S, Ressler KJ. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol Psychiatry. 2013;75:864–72. doi: 10.1016/j.biopsych.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Thabet SR, Guzik TJ, Lob HE, Mccann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–70. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Nylocks KM, Michopoulos V, Rothbaum AO, Almli L, Gillespie CF, Wingo A, Schwartz AC, Habib L, Gamwell KL, Marvar PJ, Bradley B, Ressler KJ. An angiotensin-converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin-pathway medications on posttraumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet. 2015 doi: 10.1002/ajmg.b.32313. [DOI] [PubMed] [Google Scholar]
- Otagiri A, Wakabayashi I, Shibasaki T. Selective corticotropin-releasing factor type 1 receptor antagonist blocks conditioned fear-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. J Neuroendocrinol. 2000;12:1022–6. doi: 10.1046/j.1365-2826.2000.00563.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol Learn Mem. 2011;95:86–91. doi: 10.1016/j.nlm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci. 2009;29:7379–88. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premer C, Lamondin C, Mitzey A, Speth RC, Brownfield MS. Immunohistochemical Localization of AT1a, AT1b, and AT2 Angiotensin II Receptor Subtypes in the Rat Adrenal, Pituitary, and Brain with a Perspective Commentary. Int J Hypertens. 2013;2013:175428. doi: 10.1155/2013/175428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ Res. 2011;108:574–81. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier CL, Plotsky PM. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol. 1986;48:475–94. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sanchez-Lemus E. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology. 2006;31:1123–34. doi: 10.1038/sj.npp.1300921. [DOI] [PubMed] [Google Scholar]
- Shekhar A. Angiotensin type 1 receptor antagonists-a novel approach to augmenting posttraumatic stress disorder and phobia therapies? Biol Psychiatry. 2014;75:836–7. doi: 10.1016/j.biopsych.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, Dimicco JA. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–15. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–19. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–80. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, Mcphail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–32. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Speth RC, Barry WT, Smith MS, Grove KL. A comparison of brain angiotensin II receptors during lactation and diestrus of the estrous cycle in the rat. Am J Physiol. 1999;277:R904–9. doi: 10.1152/ajpregu.1999.277.3.R904. [DOI] [PubMed] [Google Scholar]
- Speth RC, Harding JW. Radiolabeling of Angiotensin peptides. Methods Mol Med. 2001;51:275–95. doi: 10.1385/1-59259-087-X:275. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tatem KS, Quinn JL, Phadke A, Yu Q, Gordish-Dressman H, Nagaraju K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J Vis Exp. 2014:51785. doi: 10.3791/51785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchekalarova J, Albrecht D. Angiotensin II suppresses long-term depression in the lateral amygdala of mice via L-type calcium channels. Neurosci Lett. 2007;415:68–72. doi: 10.1016/j.neulet.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–63. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- Von Bohlen Und Halbach O, Albrecht D. Angiotensin II inhibits long-term potentiation within the lateral nucleus of the amygdala through AT1 receptors. Peptides. 1998a;19:1031–6. doi: 10.1016/s0196-9781(98)00044-8. [DOI] [PubMed] [Google Scholar]
- Von Bohlen Und Halbach O, Albrecht D. Visualization of specific angiotensin II binding sites in the rat limbic system. Neuropeptides. 1998b;32:241–5. doi: 10.1016/s0143-4179(98)90043-9. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, Ledoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–96. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Brain angiotensin receptor subtypes AT1, AT2, and AT4 and their functions. Regul Pept. 1995;59:269–95. doi: 10.1016/0167-0115(95)00084-o. [DOI] [PubMed] [Google Scholar]
- Wright JW, Kramar EA, Meighan SE, Harding JW. Extracellular matrix molecules, long-term potentiation, memory consolidation and the brain angiotensin system. Peptides. 2002;23:221–46. doi: 10.1016/s0196-9781(01)00599-x. [DOI] [PubMed] [Google Scholar]
- Wright JW, Stubley L, Pederson ES, Kramar EA, Hanesworth JM, Harding JW. Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J Neurosci. 1999;19:3952–61. doi: 10.1523/JNEUROSCI.19-10-03952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Akazawa H, Fujihara H, Ozasa Y, Yasuda N, Ito K, Kudo Y, Qin Y, Ueta Y, Komuro I. Angiotensin II type 1 receptor signaling regulates feeding behavior through anorexigenic corticotropin-releasing hormone in hypothalamus. J Biol Chem. 2011;286:21458–65. doi: 10.1074/jbc.M110.192260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bands from WT AT1aR (AT1aR(WT/WT)), heterozygous floxed AT1aR (AT1aR(WT/Flox)), homozygous floxed AT1aR (AT1aR(Flox/Flox)), and Cre recombinase-positive mice are shown.