Abstract
Over the last decade, zebrafish (Danio rerio) have become valuable as a complementary model in behavioral pharmacology, opening a new avenue for understanding the relationships between drug action and behavior. This species offers a useful intermediate approach bridging the gap between in vitro studies and traditional mammalian models. Zebrafish offer great advantages of economy compared to their rodent counterparts, their complex brains and behavioral repertoire offer great translational potential relative to in vitro models. The development and validation of a variety of tests to measure behavior, including cognition, in zebrafish has set the stage for the use of this animal for behavioral pharmacology studies. This has led to research into the basic mechanisms of cognitive function as well as screening for potential cognition-improving drug therapies, among other lines of research. As with all models, zebrafish have limitations, which spans pharmacokinetic challenges to difficulties quantifying behavior. The use, efficacy and limitations associated with a zebrafish model of cognitive function are discussed in this review, within the context of behavioral pharmacology.
Keywords: Zebrafish, pharmacology, learning, memory
I. Introduction
The small teleost zebrafish (Danio rerio) has expanded the landscape of behavioral pharmacology by allowing for more economic and higher-throughput assays of behavioral function, including cognition. Zebrafish provide a key intermediate model between high throughput in vitro screens and classic mammalian models as they have the accessibility of in vitro models and the complex functional capabilities of mammalian models. The clear chorion and embryo during development of the zebrafish allows for continuous visualization of the anatomical changes associated with development, which, along with short maturation times and the capability for complex behavior beginning at just 3 days post fertilization (dpf), makes this model particularly useful for measuring changes to the developing nervous system. Moreover, the rich array of developmental, behavioral, and molecular benefits offered by the zebrafish have contributed to an increasing demand for the use of zebrafish in behavioral pharmacology. Essential for this endeavor has been the development of tests to evaluate a spectrum of behavior and drug-related effects in zebrafish.
Learning and memory function has been quantified in this species, either via the use of procedures with clear rodent analogs or the development of novel, zebrafish-specific assays. As such, zebrafish have been used to 1) characterize the behavioral consequences following exposure to a variety of psychoactive drugs (e.g. therapeutic drugs, drugs of abuse, etc.), 2) to understand mechanistic pathways, and 3) have emerged as an important tool in drug discovery. Zebrafish have been shown to be useful in behavioral toxicology (see Bailey et al., 2013) and behavioral genetics (Norton & Bally-Cuif, 2010) as well as being an excellent model for neurobehavioral disorders (Kalueff et al., 2014). In this review, we present and discuss the use of zebrafish in behavioral pharmacology studies investigating cognition (including effects of drugs of abuse as well as therapeutic drugs). We cover the experimental procedures used to characterize cognitive function in zebrafish and the drawbacks posed by a zebrafish model.
Borrowing largely from a robust mammalian literature, procedures that assess Pavlovian and operant conditioning have been developed for the zebrafish. Any meaningful understanding of the functional effects of a drug includes an understanding of the drug's capacity to affect one's ability to make associations among stimuli or be sensitive to the consequences of one's actions. Every species tested has been shown to be capable of at least simple associative learning, including Drosophila and Aplysia. Zebrafish, despite their relatively small size and non-mammalian status, are capable of far more than simple CS:US associations. These animals display a wide-range of cognitive function, which can be indexed via tests of habituation learning, conditioned place preference (CPP), passive and active avoidance learning, spatial and visual discrimination, timing (of an interval), and self-control/impulsivity. The procedures described here are limited to those that are thought to measure learning and memory processes; other aspects of cognition (e.g. fear, anxiety, social behavior, models of brain disorders, etc.) are also accessible via a zebrafish model (see Iturriaga-Vasquez et al., 2012) but fall outside the scope of the present review. Associative learning in the zebrafish in general has already been reviewed elsewhere (see Gerlai, 2011) as have other cognitive endpoints (see Jesuthasan, 2012), and the present review is meant to complement these works.
Here, we selected the tools used to measure cognition that are common in the literature or which show particular value in characterizing drug effects. It is important to note that many of the experimental procedures described in this review share common features. These common features may ultimately measure similar behavioral processes that have historically been studied under the rubric of stimulus control in Pavlovian and operant learning preparations. Associating different stimuli with one another and responding differently in the presence of certain stimuli, or “stimulus control” (Catania, 1992), are measured by all the experimental procedures discussed here. So, the degree to which the tasks described here are meant to describe or tap discrete aspects of cognition should not be over-stated. Rather, a continued appreciation that learning and memory are apical processes, involving multiple systems and levels of information integration, is meant to be emphasized here.
II. Tools used to assess executive function in zebrafish
Habituation learning
Habituation refers to the situation in which a response (often a reflexive response) becomes less likely or less vigorous following repeated stimulation (Thompson & Spencer, 1966; Rankin et al., 2009) and can be understood within the context that animals tend to acclimate to a stimulus that is not particularly harmful, conserving resources for potentially dangerous stimuli. Although superficially quite simple, this process of habituation is indeed a form of learning, a reasonable distinction considering that memory of preceding stimuli mediates responsiveness to later stimuli. A range of behavioral tests for habituation learning have appeared in the zebrafish literature, many modeled after those used with rodents. A tap-elicited startle reflex has been used in adolescent and adult zebrafish to characterize drug effects (e.g. Eddins et al., 2010), and touch- or auditory-elicited reflexes in larvae or young fish are also common (Liu et al., 2012; Mann et al., 2010). In the tap-elicited startle habituation, the stimulus is delivered at a standard interval (e.g. 1 min) for 10 or more stimulus presentations, this way within-session learning curves are generated for each subject (See Figure 1 for an example of the testing apparatus).
Figure 1.
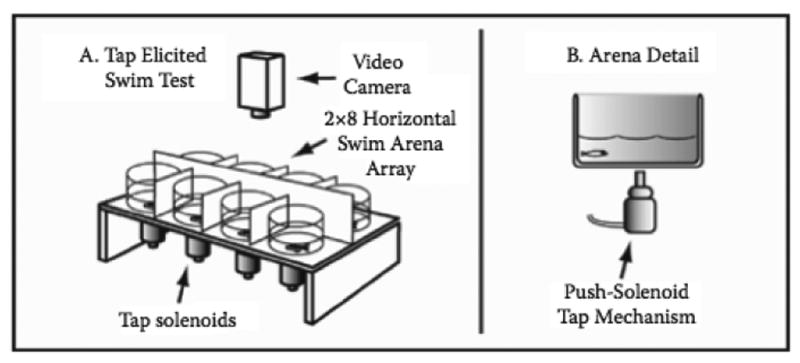
Diagram of apparatus used to measure startle response. Fish were tested in 8 cylindrical chambers separated by opaque dividers. A pushsolenoid delivered a tap as a startle stimulus at 1 min intervals (10 total trials). A camera mounted overhead acquired video, which was imported into EthoVision tracking software to measure swimming distance.
Conditioned Place Preference
A procedure sometimes conflated with selfadministration, although a distinctly different tool for assessing the appetitive characteristics of a drug, is conditioned place preference. CPP describes the selection by the zebrafish of an environment where it previously experienced the effects of a drug (Bardo & Bevins, 2000; Winger et al., 2005). This is an arrangement anchored theoretically in associative mechanisms, and ultimately measures an organism's capacity to learn. Although, because presumably what is learned in CPP is an association between environmental cues and the rewarding/hedonic effects of a drug, CPP is often described simply as a tool to assess the rewarding value of a drug.
A typical CPP arrangement in zebrafish, as in rodents, involves the differential pairing of two distinct environments (termed the CS) with the drug stimulus of interest (termed the US). The environment that has been paired, often simultaneously, with the drug acquires secondary rewarding effects. Stimuli associated with rewarding effects often elicit approach behavior in animals and it is this response that is captured with CPP; typically duration spent in the drug-paired environment or latency to enter the environment is the primary dependent measure. CPP has been used in zebrafish to characterize the appetitive characteristics of cocaine (Darland & Dowling, 2001), nicotine (Kedikian et al., 2013), opiates (Lau et al., 2006), ethanol (Parker et al., 2014), MK801 (Swain et al., 2004), salvia (Braida et al., 2007) and others (e.g. Collier & Echevarria, 2013), described below. It should be emphasized here that CPP performance is not analogous to “drug abuse”.
Avoidance Learning
Both active avoidance (i.e. the animal learns that a discrete response will result in the avoidance of an aversive stimulus being delivered) and passive avoidance (i.e. the animal learns to avoid an environment previously paired with an aversive stimulus) learning have been established in zebrafish (Kim et al., 2010; Ng et al., 2012; Richetti et al., 2011; Seibt et al., 2011; Truong et al., 2014). In these tests, avoidance learning is typically inferred by the amount of time spent outside the compartment previously associated with an aversive stimulus. Truong et al. (2014) have described an active avoidance procedure where zebrafish must shuttle from one side to the other side of a tank to avoid the delivery of a shock. To establish passive avoidance, methods are employed similar to those used in CPP although the US is aversive rather than appetitive. Here again, the dependent measure is time spent outside the USpaired environment. This sort of passive avoidance learning is frequently used to characterize associative learning and short- and long-term memory in zebrafish and has been used to characterize the effects on learning of antipsychotics (Seibt et al., 2011), scopolamine (Kim et al., 2010; Richetti et al., 2011), MK801 (Ng et al., 2012; Seibt et al., 2011) and others, described below.
Memory
Several tasks used in the zebrafish literature explicitly target memorial processes, though many (if not all) other tasks described in this review tap memory to some degree. Maze learning, for example, which is described below in the context of spatial discrimination learning certainly relies on memory. The many and important distinctions between acquisition (i.e. learning) and performance (i.e. memory) endpoints are not belabored here. Rather, two prominent tasks that explicitly tap memory are discussed, while other tasks more often used to characterize other aspects of cognition are described elsewhere.
Adapted from the rodent and primate literatures, the novel object recognition tasks can be used to quantify memorial function in zebrafish. Typically this test is arranged such that memory is tested in a single trial, with no prerequisite training phase. First, a zebrafish is allowed to explore one or two (identical) objects, such as small plastic balls or boxes. After this exploration condition, the zebrafish is presented with the previously encountered object and a novel object. Time spent exploring the novel object, over the familiar one, is characterized as an index of memory (Antunes & Biala, 2012). Often probe trials are introduced following varying delays (usually on the order of hours to days) (Lucon-Xiccato & Dadda, 2014). This arrangement has been used in zebrafish to characterize drug-induced memory impairment as well as models of pathological conditions like Alzheimer's disease. Recently, Braida et al. (2014a) have reported a modified version of novel object recognition in which virtual objects are displayed on monitors located along a tank wall, perhaps offering a higher throughput option for the implementation of this procedure among aquatic species.
The latent learning paradigm has also been used to characterize memory function in zebrafish, and is based largely on the existing rodent literature. Latent learning describes the situation in which an organism is allowed to passively explore a test environment (e.g. a maze), without any programmed consequences. Later, on a probe trial, this organism navigates the environment more efficiently than a one without prior history in the environment (Tolman & Honzik, 1930). Gomez-Laplaza and Gerlai (2010) demonstrated latent learning when zebrafish, who had previously experienced open or blocked maze arms during exploration, exhibited appropriate side bias during a consequated trial.
Spatial and Visual Discrimination
Spatial (e.g. T-maze, hole-board maze, 3-chamber test or vertical plus maze) and visual (e.g. color/pattern) discrimination procedures have been used to characterize operant learning in zebrafish, that is, sensitivity to the processes of reinforcement and punishment. T- and Y-mazes are simple methods for arranging an operant contingency, and require the acquisition of a spatial discrimination. As a brief example, if the animal makes a “correct” response (e.g. swimming down a central alley, turning left and entering a small compartment), the delivery of food or the image of conspecifics (or some other appetitive stimulus) is initiated. Then, accuracy and latency to engage in the correct response can be measured, and conclusions about learning drawn. Such T- and Y-maze arrangements likely tap similar spatial discrimination as a hole-board or plus maze.
The hole-board arrangement consists of an open arena tank with a centrally located board or box containing several holes, one of which is baited. The location of the baited hole can change across trials, requiring repeated acquisition of the baited arm or remain the same across trials, which more explicitly taps a memory component (for review see van der Staay et al., 2012). Similarly, the vertical column plus maze requires the placement of a single zebrafish into a tank with four compartments stacked 2×2 in the water column – creating bottom left and right and top left and right compartments (Pittman & Lott, 2014). As with other arrangements, one compartment is baited with an appetitive stimulus, and acquisition of that spatial discrimination is measured.
Arthur and Levin (2001) developed a three-chambered spatial discrimination test (Figure 2) in which zebrafish must acquire a spatial discrimination, a left or right turn out of a starting compartment, within a single session. An incorrect choice results in the application of an aversive stimulus (restricted swim space) while a correct choice results in an increased tank space (see Arthur & Levin, 2001; Eddins et al., 2009; Levin et al., 2011; Powers et al., 2011). Choice accuracy and latency to respond are used to characterize acute or long-term drug effects.
Figure 2. Diagram of the zebrafish 3-chamber task for assessing learning and memory. (Eddins et al., 2009).
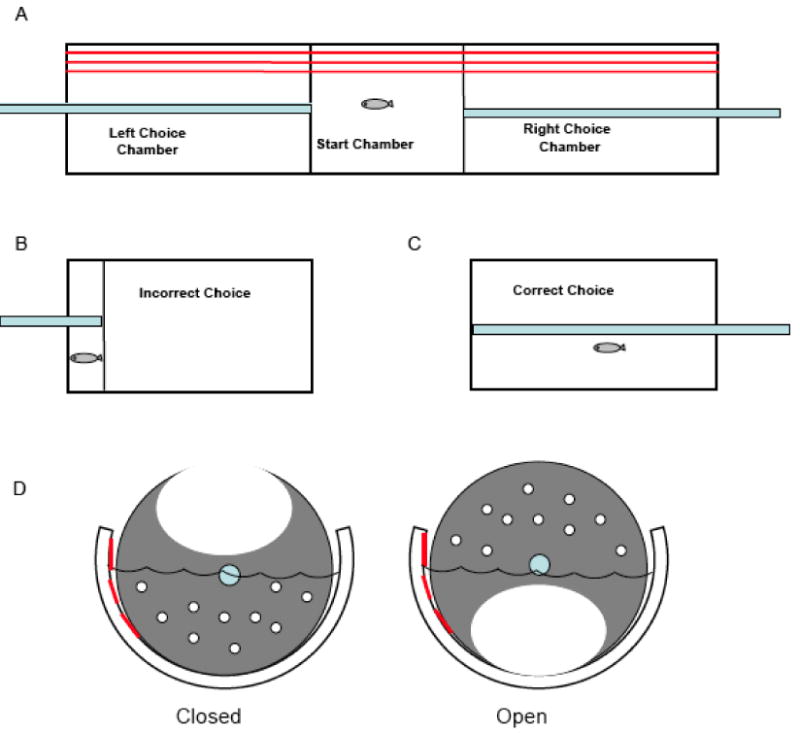
Similarly, visual discrimination tasks require the zebrafish to engage in a discrete response (e.g. swim into red or blue compartment) that initiates the delivery of an appetitive stimulus (e.g. food or image of conspecifics) (Colwill et al., 2005). For example, upon approach or entry to a visually distinct zone food is delivered (termed the S+) while approach to an alternate zone does not initiate food delivery (termed the S-). Acquisition of the discrimination, and subsequent memory probes, can be used to characterize both learning and memorial processes, but do so along a qualitatively different dimension than the spatial discrimination arrangement. The three-chambered apparatus described above can also be used to measure qualitative properties of stimuli, e.g. color discrimination (see Arthur & Levin, 2001).
Self-control/Impulsivity
Particularly relevant to questions about substance abuse are paradigms that tap self-control and impulsivity (Stevens et al., 2014; Winstanley et al., 2010). Such tasks are beginning to emerge in the zebrafish literature, following extensive use in mammals (human, non-human primate and rodent). Parker et al. (2012) provides one nice example of this, via the use of a three-choice serial reaction time task in which premature responding was probed using an extended inter-trial interval. Briefly, zebrafish were presented with three stimuli randomly following a fixed-time inter-trial interval. Correct responding (entry into an illuminated response aperture) within a limited hold period following stimulus presentation resulted in the delivery of food. Entry before stimulus presentation resulted in a timeout period. Here, premature responding is characterized as “impulsive” (Parker et al., 2012). Importantly, however, this arrangement and ones like it, have the added benefit of more directly assessing attentional processes. For a review on assessing attention in zebrafish see Echevarria et al. (2011).
Timing
At least nominally, one of the least characterized forms of learning in zebrafish is interval timing, or learning to accurately track the passage of time – interval timing has proven useful for characterizing drug or toxicant effects in other animals including humans (Paule et al., 1999). Cerutti et al. (2013), has, however, reported accurate interval timing in zebrafish using a delayed conditioning arrangement common in the associative learning literature. Here, zebrafish were found to have response latencies proportional to food delays, across several trials. Similarly, other reports have demonstrated that fish can acquire interval timing – although that behavior is often overshadowed by another behavioral endpoint (of primary interest), like shoaling. For example, in an investigation into the social behavior of zebrafish, Jia et al. (2014) found that subjects could accurately predict the location of shoal image following training in which the shoal was presented at different locations after reliable intervals of time. Other studies mentioned in the above sections also capture a component of interval timing. In fact, the capacity to time an interval has proven to be a sensitive marker of cognitive change in humans and rodent models, and is an important “cognitive characteristic” of some diseases (e.g. Parkinson's disease, see Merchant et al., 2008).
III. Cognitive effects of psychoactive drugs in zebrafish
In the following sections we discuss the various uses of zebrafish in studies of behavioral pharmacology (see Table 1). These include using zebrafish to capture some of the apical effects that psychoactive drugs have on behavior and studies that use drugs to probe questions of behavior. Many other investigations, including those which focus on the exploration of molecular and cellular level pathways mediating drug effects and those which target aspects of behavioral effects outside of learning and memory per se (e.g. fear and anxiety, social or aggressive behavior, and sensorimotor ability), are numerous but fall outside the scope of the present review.
Table 1. Cognitive Effects of Psychoactive Drugs in Zebrafish.
| Authors | Compound | Effect | Assay |
|---|---|---|---|
| Braida et al., 2014b | Nicotine | Improved performance | T-maze |
| Braida et al., 2014b | Nicotine | Increased discrimination score | Object recognition test |
| Eddins et al., 2009; Levin & Chen, 2004; Levin et al., 2006 | Nicotine | Increased accuracy | Spatial discrimination test |
| Kedikian et al., 2013 | Nicotine | Enhanced place preference | Conditioned place preference (for drug) |
| Braida et al., 2014b | Cytisine (and cytisine-derived partial agonists) | Improved performance | T-maze |
| Darland & Dowling 2001; Darland et al., 2012 | Cocaine | Enhanced place preference | Conditioned place preference (for drug) |
| Webb et al., 2009; Ninkovic & Bally-Cuif, 2006 | Amphetamine | Enhanced place preference | Conditioned place preference (for drug) |
| Chacon & Luchiari, 2014; Mathur et al., 2011 | Ethanol | Enhanced place preference | Conditioned place preference (for drug) |
| Chacon & Luchiari, 2014 | Ethanol | Memory impairment | Associative learning |
| Pittman & Lott, 2014 | Ethanol | Impaired performance | Vertical column plus-maze |
| Lau et al., 2006 | Morphine | Enhanced place preference | Conditioned Place Preference (for drug) |
| Kim et al., 2010; Richetti et al., 2011 | Scopolamine | Memory impairment | Passive avoidance |
| Cognato et al., 2012 | Scopolamine | Reduced exploration (novel arm) | Y-maze |
| Sison & Gerlai, 2011 | MK-801 | Learning impairment | Associative learning |
| Swain et al., 2004 | MK-801 | Decreased place preference | Conditioned Place Preference (for food) |
| Cognato et al., 2012 | MK-801 | Memory impairment | Y-maze |
| Blank et al., 2009; Ng et al., 2012; Seibt et al., 2011 | MK-801 | Learning/memory impairment | Avoidance learning (various) |
| Cofiel & Mattiolo, 2009 | L-histidine | Facilitated learning | Associative learning |
| Pittman & Lott, 2014 | Fluoxetine | Impaired performance | Plus maze |
| Grossman et al., 2011 | Piracetam | Improved performance | Plus maze |
| Ruhl et al., 2014 | THC | Memory impairment | Spatial memory test |
| Grossman et al., 2010 | LSD | Improved performance | T-maze |
| Braida et al., 2007 | Salvinorin A | Enhanced place preference | Conditioned place preference (for drug) |
Stimulants
The cognitive effects of several stimulant drugs have been evaluated using the zebrafish model, most notably this list includes drugs of abuse (e.g. nicotine), although therapeutic medications also appear in this literature. Methylphenidate, a common stimulant medication, is used clinically for the treatment of attention deficit disorder and attention deficit hyperactivity disorder in children and adolescents with increasing use in adults. In zebrafish, developmental methylphenidate exposure (days 1-5 postfertilization) has been shown to cause reduced accuracy on the three-chamber spatial discrimination task when the fish were tested in young adulthood (Levin et al., 2011). The authors suggest that the developmental nature of this exposure may have altered behavioral plasticity in zebrafish, manifesting as impairments in adapting to environmental contingencies. Stimulant exposure after critical periods of development (as described below) is not generally associated with cognitive impairment of this kind – and later life methylphenidate exposure per se has not yet been evaluated. Nicotine, however, is the stimulant drug most widely studied in zebrafish.
Nicotine, a stimulant found in cigarette smoke, is widely classified as a cognitive enhancer and improves indices of learning in zebrafish across a range of tasks, which corresponds to effects seen in other species, including humans. Specifically, nicotine improves performance on a T-maze (Braida et al., 2014b) and increases accuracy during the three-chamber spatial discrimination procedure (Eddins et al., 2009; Levin & Chen, 2004; Levin et al., 2006).
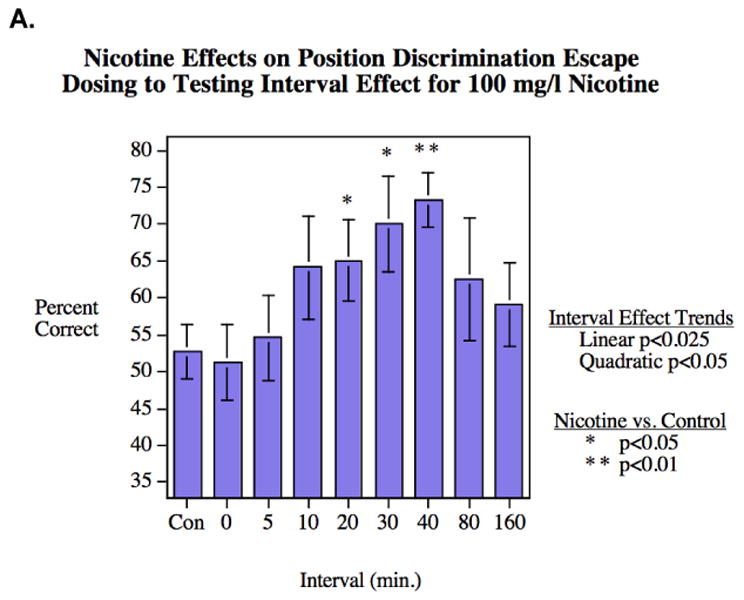
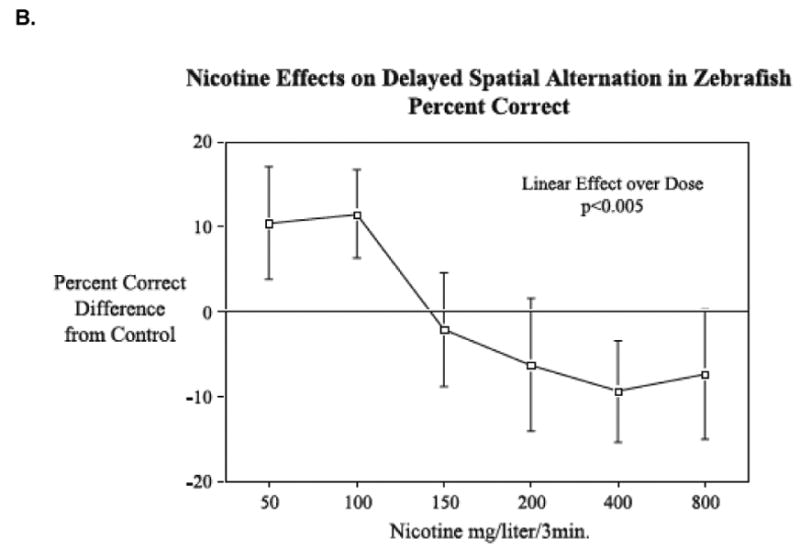
Braida et al. (2014b) demonstrated that cytisine and cytisine-derived partial agonists offered similar improvements as nicotine on T-maze performance, and nAChR antagonists were shown to block the enhancement by nicotine. Similarly, Eddins et al. (2009) showed that nicotine-induced learning enhancement in zebrafish followed increased brain levels of dihydroxyphenylacetic acid (DOPAC) (see Figure 3) and Levin et al. (2006) described the time-course of nicotine's effects on the acquisition of a spatial discrimination (see Figure 4a-b). Nicotine has also been shown to increase a discrimination score on the object recognition test, indicating enhanced visual attention (Braida et al., 2014a) (while scopolamine and mecamylamine decreased the discrimination score). These data are largely consistent with reports from the rodent and human literatures regarding the cognitive enhancement offered by nicotine and stimulant drugs more generally (for relevant reviews see Warburton, 1992; Levin, 2013; Levin & Rezvani, 2002).
Figure 3. Correlation of percent correct in the 3-chamber task with brain DOPAC concentration. (Eddins et al., 2009).
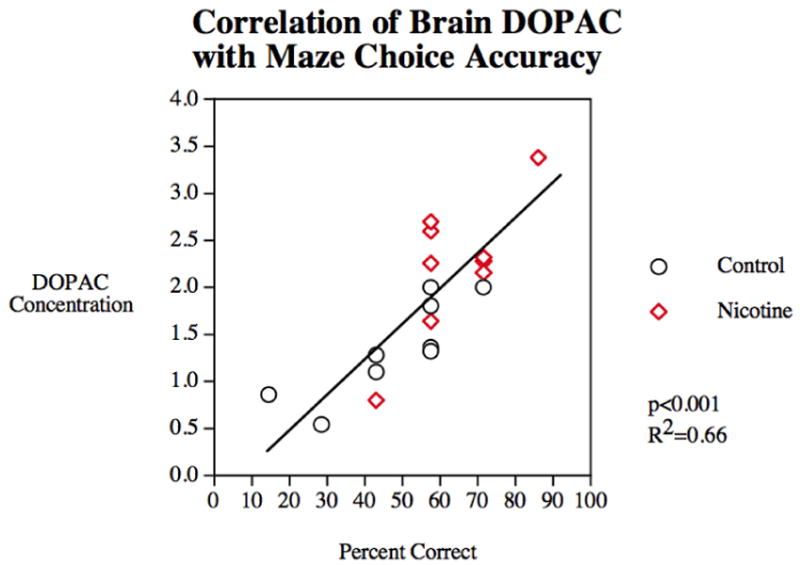
Figure 4. Nicotine effect improving A) spatial discrimination and B) spatial alternation in the 3-chamber task (mean±SEM). (Levin and Chen 2004, Levin et al., 2006).
As expected from the increased accuracy on the aforementioned operant arrangements, nicotine has also been shown to enhance a conditioned place preference response, an index of associative learning and measure of the rewarding properties of the drug (Kedikian et al., 2013). Cocaine (Darland & Dowling 2001; Darland et al., 2012) and amphetamine (Webb et al., 2009; Ninkovic & Bally-Cuif, 2006) also induce place preference learning in zebrafish. While most often the focus of these investigations involves understanding the genetic contributions to cocaine- and amphetamine-induced CPP (Darland & Dowling, 2001; Webb et al., 2009; Ninkovic & Bally-Cuif, 2006), the CPP data generated here are nearly identical to those described in rodent CPP arrangements and consistent with rodent self-administration assays. These consistencies help increase confidence in the use of zebrafish in behavioral pharmacology and also point to highly conserved behavioral mechanisms of drug action.
Depressants
A relatively robust literature on the teratogenic effects of developmental ethanol exposure as well as models of fetal alcohol syndrome has emerged using zebrafish in the last decade (e.g. Fernandes et al., 2014; Bailey et al., 2015; and for a review see Cole et al., 2012 or Ali et al., 2011). However, the literature describing the acute effects of ethanol exposure during adolescence or adulthood on learning and memory in zebrafish is more modest. As expected based on the rodent literature, acute and chronic exposure to moderate or high doses of ethanol during adulthood induces CPP in zebrafish (Chacon & Luchiari, 2014; Mathur et al., 2011) and exposure to ethanol during early development results in an exaggerated preference in ethanol-induced CPP during adulthood (Parker et al., 2014). These effects have largely been interpreted as corresponding primarily to the rewarding effects of ethanol, as memory impairment can be produced on simple associative learning tasks (like acquiring the association between a cue and food delivery) at comparable doses (Chacon & Luchiari, 2014) and impaired performance on a vertical column plusmaze has been reported with higher ethanol doses (Pittman & Lott, 2014). Moreover, these effects are consistent with those reported in other model species. Like ethanol, the opiate drug morphine induces a robust CPP response, and opioid receptor antagonists (i.e. naloxone) block this effect in zebrafish (Lau et al., 2006).
Experimental drugs
Several drugs, used as probes to understand mechanisms of action either at the level of behavior or at cellular and molecular levels fall outside of the categories of “therapeutic” or “drugs of abuse” and are therefore included here. Within the zebrafish cognition literature this category is so far primarily limited to the drugs scopolamine and MK-801. Scopolamine is a muscarinic acetylcholine receptor antagonist, and although it has been used therapeutically for motion sickness, it is frequently used experimentally to assess cholinergic-mediated behavioral processes, particularly learning and memory. As in other species, scopolamine has amnestic properties in the zebrafish and has been shown to impair memory as measured by performance on passive avoidance paradigms (Kim et al., 2010; Richetti et al., 2011). Scopolamine also alters Y-maze exploration in zebrafish, reducing exploration of the novel arm, (Cognato et al., 2012) similarly to rodents.
The NMDA glutamate receptor antagonist dizocilpine (MK-801) is used almost exclusively as an experimental drug to probe glutametergic-mediated behavioral processes. Associative learning procedures have been used to characterize the effects of MK-801 on learning and memory processes in a wide range of species, in which learning deficits or delays are routinely reported (for a review see Castellano et al., 2001). Comparable learning deficits have been demonstrated in MK-801 treated zebrafish where a visual cue was paired with the image of conspecifics (an appetitive unconditioned stimulus) (Sison & Gerlai, 2011). Similarly, deficits have been demonstrated with food-induced CPP (Swain et al., 2004) and on indices of memory during Y-maze performance (Cognato et al., 2012). Avoidance learning paradigms have been widely employed across many species to measure cognitive deficits associated with MK-801 administration, and a variety of avoidance arrangements have captured MK-801-induced learning or memory impairment in zebrafish (Blank et al., 2009; Ng et al., 2012; Seibt et al., 2011).
Other Psychoactive Drugs
A few other psychiatric and therapeutic drugs have been evaluated for cognitive effects using zebrafish, and are listed briefly here along with their effect on cognition. The histaminergic precursor L-histidine facilitated learning in a delay conditioning (associative learning) task (Cofiel & Mattiolo, 2009). Fluoxetine, the selective serotonin reuptake inhibitor (SSRI) used as an antidepressant, has been shown to impair zebrafish performance on a modified plus maze without otherwise affecting locomotion or activity levels (Pittman & Lott, 2014). The atypical antipsychotics sulpiride and olanzapine were shown to partially correct amnesia in an inhibitory avoidance test induced by the drug MK-801 (Seibt et al., 2011). Finally, piracetam, a nootropic investigated as treatment for mild cognitive impairment and whose mechanism is not fully understood, was found to improve zebrafish performance in a plus-maze test after chronic administration (Grossman et al., 2011).
Hallucinogens and Other Psychedelics
A number of psychedelic drugs of various classes have been administered to zebrafish, although the investigations into the effects of these drugs on zebrafish cognition are still quite sparse. THC, the primary psychoactive ingredient in marijuana and agonist at the endocannabinoid receptor CB1, significantly impaired the performance of a spatial memory task without affecting a color discrimination test or general locomotor activity levels (Ruhl et al., 2014). Lysergic acid diethylamide (LSD), an agonist at a number of dopaminergic, adrenergic, and serotinergic receptors, appears to improve zebrafish performance in the T-maze, reducing arm entry and freezing behaviors (Grossman et al., 2010). Salvinorin A, the hallucinogenic compound found in the Salvia plant, was found to be reinforcing for zebrafish, increasing the time the fish spent in the drug-associated compartment in a conditioned place preference test. Antagonists for kappa-opioid receptors and CB1 individually blocked the reinforcing properties of salvinorin A for zebrafish (Braida et al., 2007).
IV. Limitations of zebrafish in the pharmacology of cognition
Route of Administration
While zebrafish have undoubtedly become useful models of the cognitive effects of many drugs (as detailed above), they are not without their limitations. The ease of pharmaceutical administration to zebrafish via immersion is countered by the criticisms sometimes levied against the route, which span water solubility constraints and pharmacokinetic issues to concerns of translational validity. While oral administration (e.g. Kulkarni et al., 2014) and injection (e.g. Parker et al., 2012) have both been successfully used in zebrafish, the vast majority of neuropharmacological studies thus far have utilized aqueous immersion. Regarding translational validity, immersion may not directly correlate to common routes of administration for neuroactive compounds in humans, although the method can be bolstered by measuring drug levels directly in target tissues such as the zebrafish brain, allaying some pharmacokinetic concerns. Moreover, some drugs may have very different kinetic profiles in zebrafish compared to humans or rodents, or absorption may be impacted by the presence or absence of a chorion and egg washing protocols if exposure is developmental (e.g. ethanol exposure, see Fernandes & Gerlai, 2009; Zhang et al., 2013; Zhang et al., 2014). These points require careful consideration as does the selection of dose ranges. Moreover, it is important to recognize that, while zebrafish are seemingly reliable models for the behavioral effects of neuroactive drugs, due to differences in drug administration and metabolism in zebrafish, full pharmacokinetic testing and validation of prospective compounds may yet still require additional, complimentary model systems.
Biology
As described above, zebrafish are relatively good models of mammalian neurobiology (Del Bene & Wyart, 2012; Feierstein et al., 2014). There are a few differences between zebrafish and mammalian brains, though, which are important to take into consideration. Although zebrafish have no cortex or hippocampus (Panula et al., 2010), analogous structures exist in the zebrafish brain that appear to be sufficiently biochemically and functionally similar to the mammalian brain to permit the use of neuropharmacological manipulations, which are expected to produce behaviors that mirror those seen in mammals. Additionally, zebrafish lack midbrain dopaminergic populations such as the substantia nigra and ventral tegmental area (Panula et al., 2010), although again zebrafish are perfectly capable of displaying behaviors classically attributed to these areas and these behaviors are sensitive to dopamine-targeted pharmacological stimulation (Panula et al., 2006). Other neurotransmitter systems, such as those for histamine and nitric oxide, are not yet fully characterized in zebrafish, and thus the validity of using zebrafish as models for these systems is still unclear (Rico et al., 2011).
The assessment of learning
Finally, an important but under-discussed limitation to measuring learning or memory in zebrafish is the role of motor function, which is intrinsically intertwined, to varying degrees, in all of the aforementioned tasks. Not surprisingly, functional impairments or enhancements in motor ability are a significant hurdle when attempting to draw conclusions about learning or memory from tasks that require motoric responses from the organism. In the rodent model this issue has been dealt with via a number of interventions. For instance, the use of interval rather than ratio schedules of reinforcement can diminish the impact of motor function (e.g. Nevin et al., 2001). So too can employing specific experimental controls for motor function (e.g. Bailey et al., 2010) and by plotting both rate and accuracy-type measures, so that motor changes could be detected. Similar interventions should be emphasized within the context of zebrafish cognition as well, so that motoric effects do not masquerade as changes in cognition.
V. Future Directions
Considering that a zebrafish model of learning and memory is in its relative infancy compared to its rodent counterpart, a number of elegant and sophisticated tasks have been developed or adapted for this small aquatic species. Unsurprisingly, however, a number of gaps exist in this literature, which fall into two broad categories: un-investigated drug classes and sub-optimal behavioral paradigms. Of course, the zebrafish has not been utilized for the investigation of any number of particular drugs (or drug/dose combinations), but perhaps more surprisingly, entire drug classes still remained largely foreign to the zebrafish model of behavioral pharmacology. The investigations of many antipsychotic medications (e.g. haloperidol), other therapeutics (e.g. opiate drugs, anesthetics) and emerging drugs of abuse (e.g. “bath salts”) have not yet widely utilized the zebrafish model. Moreover, there is a relative dearth in paradigms that tap operant mechanisms of learning in zebrafish. As mentioned above, operant conditioning is thought to characterize a fundamental process via which organisms learn about and adapt to their environment. Procedures that quantify operant learning have proven indispensible in the rodent literature and would likely improve the characterization of drug effects in zebrafish similarly.
VI. Conclusions
Behavior is the primary means by which an organism interacts with its environment (Weiss & Cory-Slechta, 1994). To survive, organisms must be sensitive to events occurring in their environments and respond appropriately. To this end, it is important to emphasize drug effects at the level of behavior. In fact, the final criterion in any study of CNS insult or modification (e.g. drug use) are characteristics associated with the whole animal; characteristics like the development and longevity of the animal are important, but so too is the functional integrity and quality of performance that animal is capable of engaging in, as is the emotional reactivity of the animal (Weiss, 1978). And because the whole animal is our interest, it is necessary that behavior is our subject matter as only it reflects the summed and integrated capacity of an organism to handle the environment within which it exists (Weiss, 1978). The tasks described here, and the data generated from them, build upon many decades of work from Pavlovian conditioning and operant psychology where task development was driven by the desire to understand and predict behavior. Building on these literatures, contemporary scientists are tasked with adapting and developing procedures for the use in new animal models that will produce informative and reliable measures of behavior change so that the field of behavioral pharmacology may continue to make advancements in our understanding of drug effects, and endogenous systems.
Highlights.
Zebrafish can be used to assess neurobehavioral processes of learning and memory
Zebrafish provide a complementary model for screening the cognitive effects of drugs
Zebrafish can be used to help characterize neural systems underlying cognitive function
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali S, Champagne DL, Spaink HP, Richardson MK. Zebrafish embryos and larvae: a new generation of disease models and drug screens. Birth Defects Res C Embryo Today. 2011;93(2):115–133. doi: 10.1002/bdrc.20206. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012 May;13(2):93–110. doi: 10.1007/s10339-011-0430-z. Epub 2011 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur D, Levin ED. Spatial and non-spatial discrimination learning in zebrafish (Danio rerio) Animal Cognition. 2001;4:125–131. [Google Scholar]
- Bailey JM, Johnson JE, Newland MC. Mechanisms and performance measures in mastery-based incremental repeated acquisition: behavioral and pharmacological analyses. Psychopharmacology (Berl) 2010 May;209(4):331–41. doi: 10.1007/s00213-010-1801-3. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Oliveri AN, Zhang C, Frazier JM, Mackinnon S, Cole GJ, Levin ED. Longterm behavioral impairment following acute embryonic ethanol exposure in zebrafish. Neurotoxicol Teratol. 2015 Jan 16;:48C, 1–8. doi: 10.1016/j.ntt.2015.01.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Research Part C - Embryo Today. 2013;99:14–23. doi: 10.1002/bdrc.21027. Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Blank M, Guerim LD, Cordeiro RF, Vianna MR. A one-trial inhibitory avoidance task to zebrafish: rapid acquisition of an NMDA-dependent long-term memory. Neurobiol Learn Mem. 2009;92(4):529–534. doi: 10.1016/j.nlm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M. Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappaopioid and CB1-cannabinoid receptor involvement. Psychopharmacology (Berl) 2007;190(4):441–448. doi: 10.1007/s00213-006-0639-1. [DOI] [PubMed] [Google Scholar]
- Braida D, Ponzoni L, Martucci R, Sala M. A new model to study visual attention in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry. 2014a;55:80–86. doi: 10.1016/j.pnpbp.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Braida D, Ponzoni L, Martucci R, Sparatore F, Gotti C, Sala M. Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish. Psychopharmacology (Berl) 2014b;231(9):1975–1985. doi: 10.1007/s00213-013-3340-1. [DOI] [PubMed] [Google Scholar]
- Castellano CCV, Ciamei A. NMDA receptors and learning and memory processes. Curr Drug Targets. 2001;2(3):273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- Catania AC. Learning. 3rd. Englewood Cliffs, NJ: Prentice Hall; 1992. [Google Scholar]
- Cerutti DT, Jozefowiez J, Staddon JE. Rapid, accurate time estimation in zebrafish (Danio rerio) Behav Processes. 2013;99:21–25. doi: 10.1016/j.beproc.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Chacon DM, Luchiari AC. A dose for the wiser is enough: the alcohol benefits for associative learning in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:109–115. doi: 10.1016/j.pnpbp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Cofiel LP, Mattiolo R. L-histidine enhances learning in stressed zebrafish. Braz J Med Biol Res. 2009;42(1):128–134. doi: 10.1590/s0100-879x2009000100018. [DOI] [PubMed] [Google Scholar]
- Cognato GP, Bortolotto JW, Blazina AR, Christoff RR, Lara DR, Vianna MR, Bonan CD. Y-Maze memory task in zebrafish (Danio rerio): the role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol Learn Mem. 2012;98(4):321–328. doi: 10.1016/j.nlm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Cole GJ, Zhang C, Ojiaku P, Bell V, Devkota S, Mukhopadhyay S. Effects of ethanol exposure on nervous system development in zebrafish. Int Rev Cell Mol Biol. 2012;299:255–315. doi: 10.1016/B978-0-12-394310-1.00007-2. [DOI] [PubMed] [Google Scholar]
- Collier AD, Echevarria DJ. The utility of the zebrafish model in conditioned place preference to assess the rewarding effects of drugs. Behav Pharmacol. 2013;24(5-6):375–383. doi: 10.1097/FBP.0b013e328363d14a. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav Processes. 2005;70(1):19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci U S A. 2001;98(20):11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Mauch JT, Meier EM, Hagan SJ, Dowling JE, Darland DC. Sulpiride, but not SCH23390, modifies cocaine-induced conditioned place preference and expression of tyrosine hydroxylase and elongation factor 1alpha in zebrafish. Pharmacol Biochem Behav. 2012;103(2):157–167. doi: 10.1016/j.pbb.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene F, Wyart C. Optogenetics: a new enlightenment age for zebrafish neurobiology. Dev Neurobiol. 2012 Mar;72(3):404–14. doi: 10.1002/dneu.20914. [DOI] [PubMed] [Google Scholar]
- Echevarria DJ, Jouandot DJ, Toms CN. Assessing attention in the zebrafish: Are we there yet? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(6):1416–1420. doi: 10.1016/j.pnpbp.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32(1):99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology (Berl) 2009;202(1-3):103–109. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Portugues R, Orger MB. Seeing the whole picture: A comprehensive imaging approach to functional mapping of circuits in behaving zebrafish. Neuroscience. 2014 Nov 27;S0306-4522(14):01014–8. doi: 10.1016/j.neuroscience.2014.11.046. pii. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009 Apr;33(4):601–9. doi: 10.1111/j.1530-0277.2008.00874.x. Epub 2009 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Tran S, Abraham E, Gerlai R. Embryonic alcohol exposure impairs associative learning performance in adult zebrafish. Behav Brain Res. 2014 May 15;:265, 181–7. doi: 10.1016/j.bbr.2014.02.035. Epub 2014 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Associative learning in zebrafish (Danio rerio) Methods Cell Biol. 2011;101:249–270. doi: 10.1016/B978-0-12-387036-0.00012-8. [DOI] [PubMed] [Google Scholar]
- Gomez-Laplaza LM, Gerlai R. Latent learning in zebrafish (Danio rerio) Behav Brain Res. 2010;208(2):509–515. doi: 10.1016/j.bbr.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L, Stewart A, Gaikwad S, Utterback E, Wu N, Dileo J, Kalueff AV. Effects of piracetam on behavior and memory in adult zebrafish. Brain Res Bull. 2011;85(1-2):58–63. doi: 10.1016/j.brainresbull.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Grossman L, Utterback E, Stewart A, Gaikwad S, Chung KM, Suciu C, Kalueff AV. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res. 2010;214(2):277–284. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Iturriaga-Vasquez P, Osorio F, Riquelme S, Catro S, Herzog R. Zebrafish: A model for behavioral pharmacology. Rev Farmacol Chile. 2012;5(1):27–32. [Google Scholar]
- Jesuthasan S. Fear, anxiety, and control in the zebrafish. Dev Neurobiol. 2012;72(3):395–403. doi: 10.1002/dneu.20873. [DOI] [PubMed] [Google Scholar]
- Jia J, Fernandes Y, Gerlai R. Short-term memory in zebrafish (Danio rerio) Behav Brain Res. 2014;270:29–36. doi: 10.1016/j.bbr.2014.04.046. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci. 2014;35(2):63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedikian X, Faillace MP, Bernabeu R. Behavioral and molecular analysis of nicotine-conditioned place preference in zebrafish. PLoS One. 2013;8(7):e69453. doi: 10.1371/journal.pone.0069453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Lee Y, Kim D, Jung MW, Lee CJ. Scopolamine-induced learning impairment reversed by physostigmine in zebrafish. Neurosci Res. 2010;67(2):156–161. doi: 10.1016/j.neures.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Kulkarni P, Chaudhari GH, Sripuram V, Banote RK, Kirla KT, Sultana R, Chatti K. Oral dosing in adult zebrafish: proof-of-concept using pharmacokinetics and pharmacological evaluation of carbamazepine. Pharmacol Rep. 2014;66(1):179–183. doi: 10.1016/j.pharep.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Lau B, Bretaud S, Huang Y, Lin E, Guo S. Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav. 2006;5(7):497–505. doi: 10.1111/j.1601-183X.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- Levin ED. Complex relationships of nicotinic receptor actions and cognitive functions. Biochem Pharmacol. 2013;86(8):1145–1152. doi: 10.1016/j.bcp.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr Drug Targets CNS Disord. 2002;1(4):423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol Teratol. 2004;26(6):731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology (Berl) 2006;184(3-4):547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Sledge D, Roach S, Petro A, Donerly S, Linney E. Persistent behavioral impairment caused by embryonic methylphenidate exposure in zebrafish. Neurotoxicol Teratol. 2011;33(6):668–673. doi: 10.1016/j.ntt.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Bailey I, Hale ME. Alternative startle motor patterns and behaviors in the larval zebrafish (Danio rerio) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198(1):11–24. doi: 10.1007/s00359-011-0682-1. [DOI] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Dadda M. Assessing memory in zebrafish using the onetrial test. Behav Processes. 2014;106:1–4. doi: 10.1016/j.beproc.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Mann KDHC, Feldman S, Blunt L, Raymond A, Page-McCaw PS. Cardiac response to startle stimuli in larval zebrafish: sympathetic and parasympathetic components. Am J Physiol Integr Comp Physiol. 2010;298(5):R1288–1297. doi: 10.1152/ajpregu.00302.2009. -Central. [DOI] [PubMed] [Google Scholar]
- Mathur P, Lau B, Guo S. Conditioned place preference behavior in zebrafish. Nat Protoc. 2011;6(3):338–345. doi: 10.1038/nprot.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Luciana M, Hooper C, Majestic S, Tuite P. Interval timing and Parkinson's disease: heterogeneity in temporal performance. Exp Brain Res. 2008;184(2):233–248. doi: 10.1007/s00221-007-1097-7. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Randolph, Holland S, McLean AP. Variable-ratio versus variable-interval schedules: response rate, resistance to change, and preference. J Exp Anal Behav. 2001 Jul;76(1):43–74. doi: 10.1901/jeab.2001.76-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MC, Hsu CP, Wu YJ, Wu SY, Yang YL, Lu KT. Effect of MK-801-induced impairment of inhibitory avoidance learning in zebrafish via inactivation of extracellular signal-regulated kinase (ERK) in telencephalon. Fish Physiol Biochem. 2012;38(4):1099–1106. doi: 10.1007/s10695-011-9595-8. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39(3):262–274. doi: 10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula SSV, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, Moshnyakov M, Podlasz P. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3(2):235–247. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- Parker MO, Evans AM, Brock AJ, Combe FJ, Teh MT, Brennan CH. Moderate alcohol exposure during early brain development increases stimulusresponse habits in adulthood. Addiction Biology. 2014 doi: 10.1111/adb.12176. doi:10.1111. [DOI] [PubMed] [Google Scholar]
- Parker MO, Millington ME, Combe FJ, Brennan CH. Development and implementation of a three-choice serial reaction time task for zebrafish (Danio rerio) Behav Brain Res. 2012;227(1):73–80. doi: 10.1016/j.bbr.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG, Meck WH, McMillan DE, McClure GY, Bateson M, Popke EJ, Chelonis JJ, Hinton SC. The use of timing behaviors in animals and humans to detect drug and/or toxicant effects. Neurotoxicol Teratol. 1999 Sep-Oct;21(5):491–502. doi: 10.1016/s0892-0362(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Pittman JT, Lott CS. Startle response memory and hippocampal changes in adult zebrafish pharmacologically-induced to exhibit anxiety/depression-like behaviors. Physiol Behav. 2014;123:174–179. doi: 10.1016/j.physbeh.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Powers CM, Levin ED, Seidler FJ, Slotkin TA. Silver exposure in developing zebrafish produces persistent synaptic and behavioral changes. Neurotoxicol Teratol. 2011;33(2):329–332. doi: 10.1016/j.ntt.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetti SK, Blank M, Capiotti KM, Piato AL, Bogo MR, Vianna MR, Bonan CD. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav Brain Res. 2011;217(1):10–15. doi: 10.1016/j.bbr.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Rico EP, Rosemberg DB, Seibt KJ, Capiotti KM, Da Silva RS, Bonan CD. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol Teratol. 2011;33(6):608–617. doi: 10.1016/j.ntt.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Ruhl T, Prinz N, Oellers N, Seidel NI, Jonas A, Albayram O, von der Emde G. Acute administration of THC impairs spatial but not associative memory function in zebrafish. Psychopharmacology (Berl) 2014;231(19):3829–3842. doi: 10.1007/s00213-014-3522-5. [DOI] [PubMed] [Google Scholar]
- Seibt KJ, Piato AL, da Luz Oliveira R, Capiotti KM, Vianna MR, Bonan CD. Antipsychotic drugs reverse MK-801-induced cognitive and social interaction deficits in zebrafish (Danio rerio) Behav Brain Res. 2011;224(1):135–139. doi: 10.1016/j.bbr.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Sison M, Gerlai R. Associative learning performance is impaired in zebrafish (Danio rerio) by the NMDA-R antagonist MK-801. Neurobiol Learn Mem. 2011;96(2):230–237. doi: 10.1016/j.nlm.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Subst Abuse Treat. 2014 Jul;47(1):58–72. doi: 10.1016/j.jsat.2014.01.008. Epub 2014 Feb 10. [DOI] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26(6):725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Tolman EC, Honzik CH. Introduction and removal of reward, and maze performance in rats. University of California Publications in Psychology. 1930;4:257–275. [Google Scholar]
- Truong L, Mandrell D, Mandrell R, Simonich M, Tanguay RL. A rapid throughput approach identifies cognitive deficits in adult zebrafish from developmental exposure to polybrominated flame retardants. Neurotoxicology. 2014;43:134–142. doi: 10.1016/j.neuro.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Staay FJ, Gieling ET, Pinzon NE, Nordquist RE, Ohl F. The appetitively motivated “cognitive” holeboard: a family of complex spatial discrimination tasks for assessing learning and memory. Neurosci Biobehav Rev. 2012;36(1):379–403. doi: 10.1016/j.neubiorev.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Warburton DM. Nicotine as a cognitive enhancer. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16(2):181–191. doi: 10.1016/0278-5846(92)90069-q. [DOI] [PubMed] [Google Scholar]
- Webb KJ, Norton WH, Trumbach D, Meijer AH, Ninkovic J, Topp S, Bally-Cuif L. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biol. 2009;10(7):R81. doi: 10.1186/gb-2009-10-7-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Cory-Slechta DA. Assessment of behavioral toxicity. In: Hayes AW, editor. Principles and Methods of Toxicology. 3rd. New York: Raven Press; 1994. pp. 1091–1155. [Google Scholar]
- Weiss B. The whole animal as an assay system. In: Toribara TY, Coleman JR, Dahneke BE, Feldman J, editors. Environmental Pollutants. New York: Plenum; 1978. pp. 53–66. [Google Scholar]
- Winger G, Woods JH, Galuska CM, Wade-Galuska T. Behavioral Perspectives on the Neuroscience of Drug Addiction. Journal of the Experimental Analysis of Behavior. 2005;84(3):667–681. doi: 10.1901/jeab.2005.101-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010 Aug;34(8):1306–18. doi: 10.1111/j.1530-0277.2010.01215.x. Epub 2010 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Frazier JM, Chen H, Liu Y, Lee JA, Cole GJ. Molecular and morphological changes in zebrafish following transient ethanol exposure during defined developmental stages. Neurotoxicol Teratol. 2014 Jul-Aug;44:70–80. doi: 10.1016/j.ntt.2014.06.001. Epub 2014 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ojiaku P, Cole GJ. Forebrain and hindbrain development in zebrafish is sensitive to ethanol exposure involving agrin, Fgf, and sonic hedgehog function. Birth Defects Res A Clin Mol Teratol. 2013 Jan;97(1):8–27. doi: 10.1002/bdra.23099. Epub 2012 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]


