Abstract
The complex cross-talk of intricate inter-cellular signaling networks between the tumor and stromal cells promotes cancer progression. Hypoxia is one of the most common conditions encountered within the tumor microenvironment that drives tumor progression. Most responses to hypoxia are elicited by a family of transcription factors called hypoxia-inducible factors (HIFs), which induce expression of a diverse set of genes that assist cells to adapt to hypoxic environments. Among the three HIF protein family members, the role of HIF-1 is well established in cancer progression. HIF-1 functions as a signaling hub to coordinate the activities of many transcription factors and signaling molecules that impact tumorigenesis. This mini review discusses the complex role of HIF-1 and its context-dependent partners under various cancer-promoting events including inflammation and generation of cancer stem cells (CSCs), which are implicated in tumor metastasis and relapse. In addition, the review highlights the importance of therapeutic targeting of HIF-1 for cancer prevention.
Keywords: hypoxia, inflammation, HIF-1, microenvironment, cancer stem cells, drug resistance
Introduction
Low oxygen levels (hypoxia) play a key role in physiological conditions such as development and wound healing.1,2 However, deregulated hypoxia signaling results in the development and progression of a variety of diseases including cancer, ischemic heart disease, advanced atherosclerosis, stroke and chronic lung disease, which are responsible for the majority of deaths worldwide. 3
Hypoxic adaptation is largely mediated by a family of transcriptional regulators called hypoxia-inducible factors (HIFs), which induces a panel of specific target genes.3 HIFs act as heterodimers, which are composed of an oxygen-regulated α subunit, and an oxygen-independent β subunit (also called aryl hydrocarbon receptor nuclear translocator (ARNT)).4 There are three HIF-α family proteins identified in humans: HIF-1α, -2α and -3α. Under normal oxygen conditions, HIF-α subunits are tightly regulated by a set of enzymes called HIF prolyl hydroxylases (PHDs). PHDs are non-heme Fe (II)- and 2-oxoglutarate-dependent dioxygenases that hydroxylate HIF-α subunits at specific prolyl residues. The hydroxylated HIF-α subunits are recognized by the von-Hippel Lindau (VHL) tumor suppressor E3 ligase for degradation through the proteasome pathway.3,4 In addition, factor inhibiting HIF (FIH) hydroxylates HIFs at Asn803, leading to its decreased transcriptional activity. Diminished PHD and FIH activity during periods of hypoxia stabilizes HIF-α and results in its translocation to the nucleus where HIF-α heterodimerizes with HIF-β.3,4 The HIFα/β complex binds to the promoter regions of target genes containing hypoxia-responsive elements (HREs; 5′-RCGTG-3′, where R=A or G) and transactivates the expression of genes involved in diverse signaling pathways.1–4
Among the HIF-α proteins, the functions of HIF-1α and HIF-2α vary depending on the tumor and cell type.5 In contrast, HIF-3α, and its splice variant HIF-3α4, act as dominant negative regulators of both HIF-1-, and HIF-2-mediated transcriptional activity by competing for HIF-1β.6,7 However, recently HIF-3α was also shown to function as a transcriptional activator promoting a distinct transcriptional response to hypoxia in zebrafish embryos, suggesting the complexity in biological systems.8 Among HIF-α factors, the role of HIF-1 in cancer and inflammatory diseases is best understood.5,9 This review summarizes the role of HIF-1in cancer signaling and inflammation and highlights the key partners of HIF-1 in these contexts. Further, given the important role of HIF-1 in cancer progression, this article emphasizes inhibition of HIF-1 as a promising therapeutic strategy for the management of patients with cancer and cancer-associated inflammation.
HIF-1 levels are elevated in tumors
Hypoxia is one of the most common characteristics of the tumor microenvironment that drives aggressiveness of tumors.10,11 In solid tumors, about 60% of the tumors exhibit less than 1% O2 with a partial oxygen pressure (pO2) of less than 10 mm Hg compared to pO2 of ~40–65 mm Hg in adjacent normal tissues. Whereas transient or acute hypoxia occurs in tumors with inadequate blood perfusion, chronic hypoxia limiting oxygen diffusion occurs in enlarged tumors.10 Intratumoral hypoxia activates both HIF-1 and HIF-2, with overexpression of HIF-1α documented in several human cancers with strong association to increased metastasis and mortality.11,12 In particular, high HIF-1α expression is seen in glioblastoma multiforme, hemangioblastoma, colonic adenocarcinoma, and subtypes of lung, prostate and breast cancer.12 HIF-1α overexpression in these cancers may result from factors other than hypoxia. These include, insulin, insulin-like growth factor (IGF) -1 or IGF-2, v-Src, lactate, pyruvate, and genetic alterations such as oncogene activation or tumor suppressor gene inactivation 13–16 Upregulation of HIF-1α has a central role in tumor progression by activating various hallmarks of cancer such as angiogenesis, migration and invasion, pH regulation, and glucose metabolism (Figure 1).3–5,9–12
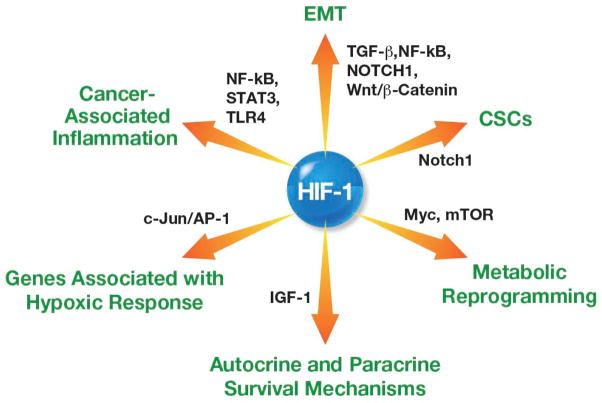
Figure 1. Hypoxia/HIF-1 links several pathways involved in tumor aggressiveness.
Scheme representing cellular functions regulated by HIF-1 and showing examples of direct target genes involved in various signaling pathways. Examples of existing inhibitors and/or FDA-approved drugs, which are specific to various HIF-1 regulated genes/pathways, are shown. CXCR4, Chemokine receptor 4; Nanog; Homeobox transcription factor; Oct-4, Octamer-binding transcription factor 4; Snail, Zinc finger transcriptional repressor; Sox-2, Sex determining region Y box-2; Twist, Basic helix-loop-helix transcription factor.
The role of HIF-1 in inflammatory responses
HIF-1 plays an essential role in execution of an optimal inflammatory response by immune cells. 9,17–20 Like tumor cells, infiltrating immune cells are also exposed to a hypoxic environment as they extravasate from the oxygen rich bloodstream to the site of inflammation, thus activating HIF-1 in immune cells.9,17 The deletion of HIF-1α in myeloid cells causes a reduction in the cellular ATP pool and severe impairment of myeloid cell aggregation, motility, invasiveness, anti-bacterial activity, and survival.17,18 Likewise, T cell-specific HIF-1α knockouts show severe colonic inflammation due to impairment of Th1 and Th17 responses.19,20 In dendritic cells (DCs), HIF-1α promotes their maturation and subsequent functions under inflammatory conditions.21 HIF-1α regulates lymphatic regeneration during wound repair and inflammatory lymphangiogenesis by regulating the expression of lymphangiogenic cytokines. 22 In addition, HIF-1 regulates immune checkpoint receptors by directly activating the expression of their ligands such as programmed death ligand 1 (PD-L1), which contributes to myeloid-derived suppressor cells (MDSC)-mediated T cell activation.23 Additionally, HIF-1 exacerbates experimental colitis through the regulation of macrophage migration inhibitory factor (MIF), which in turn facilitates inflammatory cell infiltration and generation of edema in the colon.24 Furthermore, conditional deletion of HIF-1α in the myeloid lineage protects mice against lipopolysaccharide (LPS)-induced mortality and blocks sepsis-associated hypotension and hypothermia.25 The diverse and complex roles of HIF-1 in inflammatory responses indicate that HIF-1 is not simply a bystander and should be expected to have a major impact on the progression of inflammatory-related diseases and cancer through its actions in both immune and non-immune cells.
The role of HIF-1 in linking inflammation and cancer
HIF-1 is involved in both intrinsic and extrinsic activation of tumor-associated inflammatory signaling.26,27 The growth of solid tumors eventually outpaces the supply of oxygen and nutrients, leading to necrosis.9,10,27 Hypoxic and necrotic areas of a tumor in turn produce proinflammatory mediators that recruit more immune cells resulting in suppression of the immune response at the tumor site as well as tumor cell proliferation, angiogenesis and metastasis.27,28 HIF-1 serves as a critical mediator in the signaling events culminating in lymphangiogenesis and lymphatic metastasis.29
Chronic inflammation results in the activation of the transcription factor nuclear factor-kappa B (NF-κB), a key coordinator of innate immunity and inflammation.30 A large body of evidence suggests that NF-κB and HIF-1 link inflammatory signaling to hypoxia.31 In response to inflammation, inhibitory IκB proteins are dissociated from NF-κB allowing its nuclear translocation and activation of tumor-promoting genes including interleukin-6 (IL-6), cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (NOS2), platelet endothelial cell adhesion molecule-1 (PECAM-1) and matrix metalloproteinase 9 (MMP9).30,31 NF-κB and HIF-1 coordinate the activation of these genes’ promoters. Furthermore, pro-survival genes such as BCL2, CXCR1, and CXCR2 are induced by NF-κB and HIF-1.32,33
In addition to NF-κB, the signal transducer and activator of transcription 3 (STAT3), one of the seven members of the STAT family of transcription factors, also serves as a partner for HIF-1 in cancer-associated inflammatory signaling.34 Phosphorylation of STAT3 by Janus kinases (JAKs) leads to its dimerization, nuclear translocation, DNA binding, and activation of genes involved in survival (e.g. survivin, mcl-1), proliferation (e.g. c-myc, cyclin D1), invasion (MMP2), and angiogenesis (vascular endothelial growth factor (VEGF)).35 STAT3 cooperates with HIF-1 and NF-κB in the regulation of these genes.36,37 In addition, STAT3 interacts physically with HIF-1α, which is essential for activation of HIF-1 target genes under hypoxic conditions as demonstrated in MDA-MB-231 human breast cancer and RCC4 renal carcinoma cells.38 Furthermore, a cooperative induction of HIF-1 and STAT3 contributes to hypoxia-mediated immunoresistance in lung cancer cells.39 Of note, STAT3 and NF-κB activation promotes chemoresistance and radioresistance in various cancer cell lines, which is akin to that of HIF-1.40–44
HIF-1 regulates tumor-associated inflammatory responses in part through its target gene Toll-like receptor 4 (TLR4).25,45 TLR4 belongs to the pattern recognition receptor family of proteins and recent studies have indicated its role in tumor progression and chemoresistance in addition to its function in innate immunity.46 In glioblastoma tumorigenesis, a feed-forward loop between TLR4-HIF-1α sustains inflammatory signaling, and both HIF-1 and TLR4 have synergistic functions in the development of pancreatic adenocarcinoma.47,48 Together, these findings provide compelling evidence for cooperative relationship between NF-κB, STAT3, TLR4 and HIF-1-dependent tumor-associated signaling events.
The transcription factors c-Jun and AP-1 cooperate with HIF-1 to allow fine-tuned regulation of gene expression during hypoxia.49,50 HIF-1 also mediates the activation of several genes in response to IGF-1 that promote cell survival and motility.51,52 In summary, these studies reiterate the fact that HIF-1 links both hypoxia and inflammation through context-dependent partners to drive tumor promoting signaling.
HIF-1: a key mediator of tumor metabolism
HIF-1 is an important mediator of the tumor-associated metabolic switch, Warburg effect, in which tumor cells generate energy mainly by non-oxidative breakdown of glucose rather than conventional oxidative phosphorylation.14,53,54 HIF-1 drives the expression and activity of isoforms of glycolytic enzymes that differ from those found in non-malignant cells, thereby potentiating energy production as well as macromolecular biosynthesis pathways to support the Warburg effect.53,54 For instance, the expression of pyruvate kinase isoform M2 (PKM2), but not PKM1, is necessary for the Warburg effect, because PKM2 physically interacts with HIF-1 and stimulates HIF-1 activity.55,56
The targets of HIF-1in the glycolytic pathway include glucose transporters 1 and 3 (GLUT1, GLUT3) and enzymes such as hexokinase 1 (HK1) and HK2, phosphofructokinase liver type (PFK-L), aldolase A and C (ALD-A, ALD-C), phosphoglycerate kinase 1 (PGK1), enolase alpha (ENO-alpha), PKM2, lactate dehydrogenase A (LDH-A) and fructose 2, 6 bisphosphatase (PFKFB-3).54,55 Some of these genes, including, Glut1, HK2, and LDH-A are also direct targets of the oncogenic MYC transcription factor.57 In addition, recent studies point to mammalian target of rapamycin (mTOR) as another important regulator of the Warburg effect, which may in part be due to its effect on HIF-1α.58,59 By analogy, both Myc and mTOR in co-operation with HIF-1“-serve the cancer cell’s metabolic needs-” by providing high glycolytic flux. Additionally, HIF-1 activates catabolite transporters such as monocarboxylate transporter 4 (MCT4) in cancer-associated fibroblasts (CAFs), a major cellular component of the tumor stroma.60–62 Such activated CAFs provide metabolic coupling, which enables anabolic tumor cells to survive in their hypoxic environment.60,61 HIF-1α also regulates the metabolic fate and multipotency of human mesenchymal stem cells (hMSCs).63
An important consequence of the glycolytic switch is acidosis of the tumor microenvironment.64 Enhanced glycolysis leads to an acidic microenvironment due to the increase in the levels of lactate and H+ that are actively expelled from tumor cells through the functions of many intracellular pH (pHi)-regulating proteins that include, MCT1, MCT4, and Na+/H+ exchanger 1 (NHE1).64 Hypoxia promotes acidosis because HIF-1 induces expression of NHE1, MCT4, and carbonic anhydrase IX (CAIX).62,65,66 NHE1 directly manages free intracellular H+ when the buffering capacity of intracellular proteins is exhausted.64 Tumor cell-specific expression of MCT1 and stromal cell-specific expression of MCT4 optimizes lactate utilization and is associated with the progression of prostate cancer.67 In addition to lactate, carbon dioxide production by CAIX also causes acidosis in the tumor environment.66,68 Acidosis also modulates the inflammatory response and immune cell functions to attenuate anti-tumor immunity and the efficacy of drug uptake by tumors.69,70 Together, the acidic microenvironment and shift in the metabolism provides many metabolic intermediates that drive progression and aggressiveness of the cancers.53,54,64,69
Like tumor cells, immune cells such as activated M1 macrophages or DCs generate ATP and essential components for survival by metabolic reprogramming through activation of HIF-1 regulated genes.71,72 Evidence is also emerging that the Warburg effect is important in adaptive immunity by regulating Th17 and Treg cells.73 A recent study demonstrated that mTOR-HIF-1α pathway is critical for the metabolic basis of epigenetically reprogrammed myeloid cells (also called trained immunity).74 These findings indicate that HIF-1 serves as a crucial mediator of Warburg metabolism, -which is not only relevant to cancer progression but also inflammation.
Tumor microenvironment and cancer progression: “HIF-1 in the Driver’s Seat”
The behavior of the tumorigenic cells is highly influenced by their microenvironment.53,75 Under hypoxia and nutrient-deprived conditions, cancer cells have to dramatically re-wire their metabolism to survive and proliferate.53,75 Tumors are heterogeneous, composed of numerous cell types such as tumor-associated endothelial cells (TAECs), CAFs, adipocytes, MDSCs, tumor-associated macrophages (TAMs) and other immune cells.75 Hypoxia is a critical parameter that modulates stromal and/or endothelial/tumor cell interactions.29,76 Under hypoxia, breast cancer cells secrete HIF-1-induced factors such as angiopoietin 2 (Ang2), angiopoietin-like 4 (ANGPTL4), L1 cell adhesion molecule (L1CAM), platelet-derived growth factor B (PDGFB), stem cell factor (SCF, or kit ligand), stromal-derived factor 1 (SDF1), and VEGF.77–81 These factors directly mediate functional interactions with blood endothelial cells (BECs), lymphatic endothelial cells (LECs), and other bone marrow-derived cells (BMDCs) that promote angiogenesis, lymphangiogenesis, and metastasis.77–81
The balance between suppressive and cytotoxic responses of the tumor immune microenvironment has a direct effect on the treatment and prognosis of cancers.26,28,75 Tumor immune suppression is due to impaired release of granular cytosolic content, decreased presentation of tumor-associated antigens as well as inhibition of T and B cell response.82–84 Intratumoral hypoxia leads to release of factors that recruit TAMs, MDSCs and other immune cells to the tumor site, and induce angiogenesis, and metastasis as well as immune suppression through the secretion of pro-inflammatory cytokines and other mediators.26,28,29,82–84 TAMs promote angiogenesis, and the presence of TAMs correlates with increased metastasis and mortality.85,86 TAMs require HIF-1 activity to promote angiogenesis, because most of the pro-angiogenic factors (VEGF, IL-6, TNFα, and tyrosine kinase receptor Tie2)85–88 and pro-angiogenic enzymes (iNOS, MMP-9, and Cox-2)86,89,90 expressed in TAMs are regulated by HIF-1. In addition, HIF-1-dependent production of colony stimulating factor-1 (CSF-1) in tumor cells recruits TAMs, which in turn provide epidermal growth factor (EGF) to the tumor cells, leading to a paracrine partnership that supports tumor metastasis.91,92 Furthermore, HIF-1α expression in the myeloid lineage promotes the differentiation of MDSCs, which contribute to tumor progression.93 A recent study points to a role of the HIF-1/CAIX system in the production of soluble mediators such as G-CSF, which are required for the recruitment of MDSCs to the lungs and thereby generate premetastatic niches.94 In a mouse model of triple negative breast cancer, HIF1-dependent secretory factors recruit MSCs, TAMs and MDSCs, which in turn potentiate the invasion and metastasis of tumor cells.92
HIF-1 also plays a key role in the activation of CAFs, which promotes persistent chronic inflammation within the tumor microenvironment.60,61 The markers of chronic inflammation in the tumor microenvironment include Cox-2, NF-κB, IL-6, IL-8, S100 calcium binding protein A8 (S100A8), and VEGF.26,27,29,37,86,89 In pancreatic cancer, tumor hypoxia triggers HIF-1-dependent and hedgehog (SHH)-mediated tumor-stromal interactions that amplify a desmoplastic reaction, leading to a dense fibroinflammatory microenvironment, which limits cancer drug delivery due to decreased blood perfusion.95
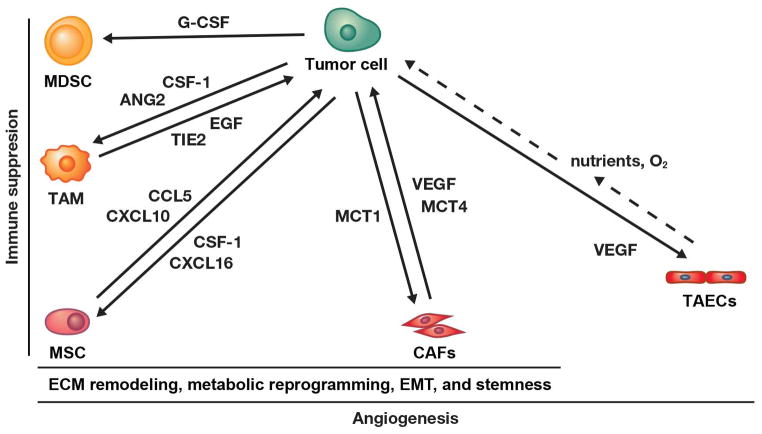
In addition, HIF-1 promotes changes in the extracellular matrix (ECM) that contribute to tumor cell invasion and metastasis.96,97 HIF-1 activated CAFs secrete ECM remodeling proteins such as collagen prolyl hydroxylases (P4HA1 and P4HA2) and lysyl hydroxylases (PLOD2), which promote cancer cell invasion and metastasis.96,97 As more pieces of the puzzle are put together, HIF-1-mediated activation of MDSCs, CAFs, MSCs, TAECs, TAMs and ECM remodeling takes on a central role in the modifications of the tumor microenvironment that promote tumor aggressiveness and immune suppression (Figure 2).
Figure 2. Simplified illustration showing tumor promoting cell-cell interactions within the tumor microenvironment supported by HIF-1 activity.
Hypoxia, inflammatory conditions and genetic alterations activate HIF-1 that mediates cross-talk between tumor and multiple stromal cell types through specific factors, only a few of which are shown (for details see text). Details about role of MSCs in immune suppression, EMT, and stemness can be found in this recent review.98
Role of HIF-1 in the epithelial to mesenchymal transition (EMT): a pre-requisite for metastasis
EMT, by which epithelial cells lose their polarity and acquire a mesenchymal phenotype, is a major facilitator of tumor metastasis, which is induced by the tumor microenvironment.99 Repression of epithelial-specific proteins such as E-cadherin, desmoplakin, plakoglobin and zona occludens-1 (ZO-1) in the tumor cells is a crucial step of EMT, which is accompanied by an increase in mesenchymal markers and cell motility.99–101 HIF-1 activates the expression and activity of several EMT-inducing factors including SNAIL, SLUG, TWIST and ZEB1, and inhibits the expression of E-cadherin.102–105 HIF-1 activation of CAFs and its target gene CAIX in CAFs leads to EMT-inducing conditions for tumor cells.106,107 Additionally, HIF-1 promotes EMT under inflammatory conditions.108,109
Members of the TGF-β family of growth factors are major inducers of EMT.99 Both HIF-1 and TGF-β promote each other’s expression and trigger hypoxia-induced EMT in many cancer cell types.110–113 In addition, Wnt/β-catenin signaling can play an important role in EMT.99,114 Some studies have shown that the Wnt/β-catenin signaling pathway has an important role in HIF-1-induced EMT in human prostate and hepatocellular carcinoma cell lines.115,116 In pancreatic cancer cells, HIF-1 mediated EMT requires NF-κB activity117. Furthermore, the Notch signaling pathway also plays a role in hypoxia/HIF-1-induced EMT of several cancer cell lines.118 These studies provide compelling evidence for the role of HIF-1 and its cross talk with other factors in EMT.
HIF-1 and Cancer Stem Cells (CSCs): “the core of the enemy”
EMT is associated with the emergence of stem-like characteristics in cancer cells, which in recent times have received great attention.119 The current CSC hypothesis suggests that a small subset of cancer cells possess extended self-renewal properties that drive tumorigenesis, promote metastasis, and contribute to treatment resistance.120 Dick and coworkers first demonstrated the stem cell concept in leukemia, which was later shown in breast cancer. 121–123
Microenvironmental factors of the CSC niche provide cues that are important for the maintenance of the CSC state.120,124 However, the mechanisms regulating CSC generation and maintenance are poorly understood. Notably, hypoxia and the HIF-1 signaling pathway are known to be important for the regulation and sustenance of CSCs and the EMT phenotype.125 HIF-1α-mediated EMT results in the enrichment of stem-like side population cells in thyroid and prostate cancer cells.126,127 The hypoxic and/or necrotic areas of tumor tissues, which are considered a niche for CSCs, promote the induction of HIF-1 regulated CSC-signature genes such as Oct4, Sox-2, Nanog, Myc, CD44, and CD133.128 The role of hypoxia and of HIF-1 in the control of the tumorigenic capacity of CSCs has been demonstrated in glioblastoma.129 In pancreatic cancer, gastric cancer, and neuroblastoma cells, intermittent hypoxia enhances stem-like characteristics in cancer cells along with the upregulation of HIF-1α. 130–132 HIF-1α also plays a key role in promoting mammary tumor growth and metastasis, in part through regulation of CSCs.133 The interplay between HIF-1 and Notch appears to be important for stem cell maintenance under hypoxia in a variety of cancer cell lines.127, 134,135 These studies highlight the important role of HIF-1 in CSC maintenance as one of the mechanisms by which HIF-1 promotes tumorigenesis and metastasis.
HIF-1 and reactive oxygen species (ROS): “an intimate relationship”
ROS are elevated in many cancer types and have emerged as critical signaling stimuli in tumorigenesis.136 ROS can drive the de-differentiation of tumor cells leading to EMT and metastasis.108,136 Chronic hypoxia causes an increase in the levels of intracellular ROS at the Q0 site of complex III of the mitochondrial electron transport chain.137 In addition to affecting the mitochondrial electron transport chain, hypoxic conditions also increase ROS levels through NADPH oxidase, xanthine oxidase, and eNOS.138 The increase in ROS stabilizes the redox sensitive factor HIF-1α and thereby activates its downstream pathways.137–139 For instance, the HIF-1 target lysyl oxidase enables tumor cells to acquire invasive competence.139 Additionally, the endotoxin LPS induces TLR4/myeloid differentiation factor (MyD) 88-dependent ROS generation and HIF-1 activation, which is required for monocyte–macrophage differentiation in inflamed tissues.140 A ROS/STAT3/HIF-1α/TWIST1/N-cadherin signaling cascade is involved in prostate cancer progression.141 Furthermore, ROS and HIF-1 enhance the development of resistance to chemotherapeutics such as Doxorubicin and etoposide in lung, cervical carcinoma and melanoma cell lines.142,143 However, attenuation of ROS by antioxidants suppresses hypoxia-induced EMT and metastasis in pancreatic cancers.144 In summary, these findings demonstrate that both ROS and HIF-1 mutually benefit from each other for their generation and activation, respectively, which heightens aggressiveness of the tumors.
Concluding remarks
“-Hitting several birds with one stone-”: combating cancer and cancer-associated inflammation through HIF-1
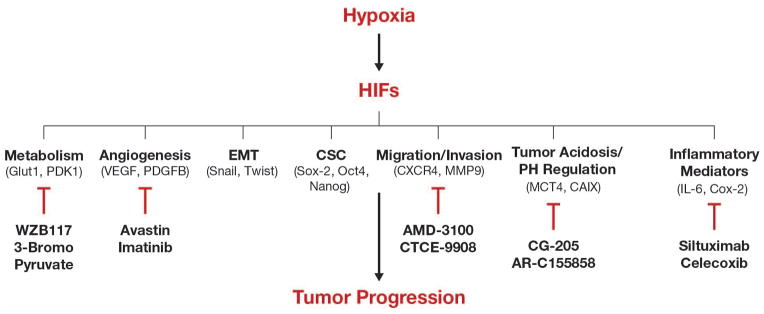
Understanding the precise mechanisms underlying tumor progression is key to effective therapeutic interventions. Among the pro-oncogenic factors, HIF-1 plays a pleiotropic role in augmenting many biological processes associated with tumorigenesis. HIF-1 exerts this effect through collaboration with many factors including, but not limited to, STAT3, NF-κB, TLR4, Myc, mTOR, AP-1, IGF-1, Wnt, TGF-β, and Notch1 (Figure 3). Besides the functions discussed in this review, in support of its central role in mediating cancer progression, HIF-1 also regulates other aspects of cancer biology such as miRNAs, epigenetic alterations and autophagy as discussed elsewhere.128,145,146 Given the critical role of HIF-1 in tumor progression including its role in CSC maintenance, it is apparent that HIF-1 signaling contributes significantly to metastasis and treatment resistance.12,80,92,144 Accordingly, efforts have been made to target various HIF-1-regulated pathways (see also Figure 1). For instance, the VEGF-neutralizing antibody Avastin (bevacizumab) is an approved anti-angiogenic cancer therapeutic. However, some reports show that anti-angiogenic agents indeed increase tumor invasiveness and metastasis in xenograft models due to increased tumor hypoxia and HIF-1α expression.147–149 These findings emphasize the need to explore the possibilities of targeting HIF-1 directly to solve the problem of resistance and relapse. Interestingly, the therapeutic effects of HIF-1 inhibition by small molecules have already been demonstrated in preclinical mouse models. Therefore, this area of research promises to lead to significant advances in the near future.134,150–154 Targeting HIF-1 directly could potentially resolve the primary hurdle associated with treatment-refractory cancers and simultaneously overcome the problems associated with cancer-related inflammation.
Figure 3. Partners in Crime.
Scheme summarizing the collaborators of HIF-1 in the signaling pathways associated with hypoxia adaptation, cancer progression and cancer-associated inflammation (see text for details).
Acknowledgments
I thank Esta Sterneck, Howard Young, Shyam Sharan, Chhavi Chauhan, Ira Daar, Namratha Sheshadri, Michael D Hall, Snehalata Pawar, Vijay Walia, Devikala Gurusamy, Matthew D Anderson, and Emilee Senkevitch, and the NIH Fellows Editorial Board for their critical comments and suggestions, and Allen Kane, and Joseph Meyer for preparation of the figures. Due to space limitations, I could not cite all of the important reports relevant to this review. The author is supported by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations
- AP-1
Activator protein-1
- BECs
Blood endothelial cells
- BMDCs
Bone marrow-derived cells
- CAIX
Carbonic anhydrase IX
- CAFs
Cancer-associated fibroblasts
- CSCs
Cancer Stem Cells
- COX-2
cyclooxygenase 2
- CTL
Cytotoxic T lymphocytes
- DCs
Dendritic cells
- EMT
Epithelial-mesenchymal transition
- FIH
Factor inhibiting HIF
- HIFs
Hypoxia-inducible factors
- HREs
Hypoxia-responsive elements
- IL-6
Interleukin-6
- LECs
Lymphatic endothelial cells
- MIF
Macrophage migration inhibitory factor
- mTOR
Mammalian target of rapamycin
- MMPs
Matrix metalloproteinases
- MSCs
Mesenchymal stem cells
- MCT4
Monocarboxylate transporter 4
- MDSCs
Myeloid-derived suppressor cells
- NHE1
Na+/H+ exchanger 1
- NOS2
inducible nitric oxide synthase
- NF-κB
Nuclear factor-κB
- PECAM-1
Platelet endothelial cell adhesion molecule-1
- PD-L1
Programmed death ligand 1
- PHDs
Prolyl hydroxylases
- ROS
Reactive Oxygen Species
- STAT3
Signal transducer and activator of transcription3
- TAECs
Tumor-associated endothelial cells
- TAMs
Tumor-associated macrophages
- TGF-β
Transforming growth factor-β
- TLR4
Toll-like receptor 4
- VEGF
Vascular endothelial growth factor
- VHL
von-Hippel Lindau
Footnotes
Conflict of interest: There are no conflicts of interest to disclose.
References
- 1.Lokmic Z, Musyoka J, Hewitson TD, et al. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol. 2012;296:139–85. doi: 10.1016/B978-0-12-394307-1.00003-5. [DOI] [PubMed] [Google Scholar]
- 2.Hong WX, Hu MS, Esquivel M, et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv Wound Care (New Rochelle) 2014;3:390–99. doi: 10.1089/wound.2013.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. Oxygen sensing, hypoxia-inducible factors and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 4.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;306:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 5.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara S, Hamada J, Kobayashi C, et al. Expression and Characterization of Hypoxia-Inducible Factor (HIF)-3a in Human Kidney: Suppression of HIF-Mediated Gene Expression by HIF-3a. Biochem Biophys Res Commun. 2001;287:808–13. doi: 10.1006/bbrc.2001.5659. [DOI] [PubMed] [Google Scholar]
- 7.Maynard MA, Evans AJ, Shi W, et al. Dominant-negative HIF-3 alpha 4 suppresses VHL-null renal cell carcinoma progression. Cell Cycle. 2007;15:2810–16. doi: 10.4161/cc.6.22.4947. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Yao Q, Lu L, et al. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014;6:1110–21. doi: 10.1016/j.celrep.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. 2013;13:646–53. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Vaupel P, Mayer A. Hypoxia in tumors: pathogenesis-related classification, characterization of hypoxia subtypes, and associated biological and clinical implications. Adv Exp Med Biol. 2014;812:19–24. doi: 10.1007/978-1-4939-0620-8_3. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Schmid T, Schnitzer S, et al. Tumor hypoxia and cancer progression. Cancer Lett. 2006;237:10–21. doi: 10.1016/j.canlet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–35. [PubMed] [Google Scholar]
- 13.Feldser D, Agani F, Iyer NV, et al. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 1999;59:3915–18. [PubMed] [Google Scholar]
- 14.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–15. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen dependent proteolysis. Nature. 1999;399:271–75. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 16.Jiang BH, Agani F, Passaniti A, et al. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328–35. [PubMed] [Google Scholar]
- 17.Cramer T, Yamanishi Y, Clausen BE, et al. Hif-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by hif-1alpha-dependent NF-kB activity. J Exp Med. 2005;201:105–15. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang EV, Barbi J, Yang HY, et al. Control of t(h)17/t(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higashiyama M, Hokari R, Hozumi H, et al. HIF-1 in T cells ameliorated dextran sodium sulfate-induced murine colitis. J Leukoc Biol. 2012;91:901–9. doi: 10.1189/jlb.1011518. [DOI] [PubMed] [Google Scholar]
- 21.Jantsch J, Chakravortty D, Turza N, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 22.Zampell JC, Yan A, Avraham T, et al. HIF-1α coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J. 2012;26:1027–39. doi: 10.1096/fj.11-195321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah YM, Ito S, Morimura K, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–48. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyssonnaux C, Cejudo-Martin P, Doedens A, et al. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–9. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 27.Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer. 2013;132:2721–29. doi: 10.1002/ijc.27901. [DOI] [PubMed] [Google Scholar]
- 28.Noman MZ, Messai Y, Carré T, et al. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol. 2011;31:357–77. doi: 10.1615/critrevimmunol.v31.i5.10. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32:4057–63. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruning U, Fitzpatrick SF, Frank T, et al. NFκB and HIF display synergistic behavior during hypoxic inflammation. Cell Mol Life Sci. 2012;69:1319–29. doi: 10.1007/s00018-011-0876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zong WX, Edelstein LC, Chen C, et al. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–87. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell PJ, Gallagher R, Seaton A, et al. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–45. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 34.Lavecchia A, Di Giovanni C, Cerchia C. Novel inhibitors of signal transducer and activator of transcription 3 signaling pathway: an update on the recent patent literature. Expert Opin Ther Pat. 2014;24:383–400. doi: 10.1517/13543776.2014.877443. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–98. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 37.Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2012;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulus MR, Wang L, Hu CJ. STAT3 and HIF1α cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene. 2014;33:1670–9. doi: 10.1038/onc.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noman MZ, Buart S, Van Pelt J, et al. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol. 2009;182:3510–21. doi: 10.4049/jimmunol.0800854. [DOI] [PubMed] [Google Scholar]
- 40.Tamatani T, Azuma M, Ashida Y, et al. Enhanced radiosensitization and chemosensitization in NF-kappaB-suppressed human oral cancer cells via the inhibition of gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int J Cancer. 2004;108:912–21. doi: 10.1002/ijc.11640. [DOI] [PubMed] [Google Scholar]
- 41.Yan HQ, Huang XB, Ke SZ, et al. Interleukin 6 augments lung cancer chemotherapeutic resistance via ataxia-telangiectasia mutated/NF-kappaB pathway activation. Cancer Sci. 2014;105:1220–7. doi: 10.1111/cas.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin Z, Zhang Y, Li Y, et al. Prognostic significance of STAT3 expression and its correlation with chemoresistance of non-small cell lung cancer cells. Acta Histochem. 2012;114:151–58. doi: 10.1016/j.acthis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Yang C, He L, He P, et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol. 2015;32:14. doi: 10.1007/s12032-014-0352-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C, Yang X, Zhang Q, et al. STAT3 inhibitor NSC74859 radiosensitizes esophageal cancer via the downregulation of HIF-1α. Tumour Biol. 2014;35:9793–99. doi: 10.1007/s13277-014-2207-3. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Choi YJ, Joung SM, et al. Hypoxic stress up-regulates the expression of toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology. 2009;4:516–24. doi: 10.1111/j.1365-2567.2009.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tewari R, Choudhury SR, Ghosh S, et al. Involvement of TNFα-induced TLR4-NF-κB and TLR4-HIF-1α feed-forward loops in the regulation of inflammatory responses in glioma. J Mol Med (Berl) 2012;90:67–80. doi: 10.1007/s00109-011-0807-6. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JJ, Wu HS, Wang L, et al. Expression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinoma. World J Gastroenterol. 2010;21:2881–88. doi: 10.3748/wjg.v16.i23.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alfranca A, Gutiérrez MD, Vara A, et al. c-Jun and hypoxia-inducible factor 1 functionally cooperate in hypoxia-induced gene transcription. Mol Cell Biol. 2002;22:12–22. doi: 10.1128/MCB.22.1.12-22.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salnikow K, Kluz T, Costa M, et al. The regulation of hypoxic genes by calcium involves c-Jun/AP-1, which cooperates with hypoxia-inducible factor 1 in response to hypoxia. Mol Cell Biol. 2002;22:1734–41. doi: 10.1128/MCB.22.6.1734-1741.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gariboldi MB, Ravizza R, Monti E. The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochem Pharmacol. 2013;80:455–62. doi: 10.1016/j.bcp.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Mancini M, Gariboldi MB, Taiana E, et al. Co-targeting the IGF system and HIF-1 inhibits migration and invasion by (triple-negative) breast cancer cells. Br J Cancer. 2014;110:2865–73. doi: 10.1038/bjc.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–64. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marín-Hernández A, Gallardo-Pérez JC, Ralph SJ, et al. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9:1084–101. doi: 10.2174/138955709788922610. [DOI] [PubMed] [Google Scholar]
- 55.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumor growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 56.Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–44. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dang CV, Kim J-W, Gao P, et al. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 58.Sun Q, Chen X, Ma J, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA. 2011;108:4129–34. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balamurugan K, Wang JM, Tsai HH, et al. The tumour suppressor C/EBPδ inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J. 2010;29:4106–17. doi: 10.1038/emboj.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–83. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281:9030–7. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 63.Palomäki S, Pietilä M, Laitinen S, et al. HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells. 2013;31:1902–9. doi: 10.1002/stem.1435. [DOI] [PubMed] [Google Scholar]
- 64.Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–23. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 65.Shimoda LA, Fallon M, Pisarcik S, et al. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;29:L941–9. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 66.Svastová E, Hulíková A, Rafajová M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–45. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 67.Sanità P, Capulli M, Teti A, et al. Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. BMC Cancer. 2014;14:154. doi: 10.1186/1471-2407-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamagata M, Hasuda K, Stamato T, et al. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer. 1998;77:1726–31. doi: 10.1038/bjc.1998.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Nadai TR, de Nadai MN, Albuquerque AA, et al. Metabolic acidosis treatment as part of a strategy to curb inflammation. Int J Inflam. 2013;2013:601424. doi: 10.1155/2013/601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojtkowiak JW, Verduzco D, Schramm KJ, et al. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8:2032–8. doi: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010;17:1540–50. doi: 10.1038/cdd.2010.27. [DOI] [PubMed] [Google Scholar]
- 72.Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–49. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi LZ, Wang R, Huang G, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–76. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 76.Chouaib S, Kieda C, Benlalam H, et al. Endothelial cells as key determinants of the tumor microenvironment: interaction with tumor cells, extracellular matrix and immune killer cells. Crit Rev Immunol. 2010;30:529–45. doi: 10.1615/critrevimmunol.v30.i6.30. [DOI] [PubMed] [Google Scholar]
- 77.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 79.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol. 2008;217:809–18. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 80.Schito L, Rey S, Tafani M, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc Natl Acad Sci USA. 2012;109:E2707–16. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang H, Wong CC, Wei H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–70. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Johnson BF, Clay TM, Hobeika AC, et al. Vascular endothelial growth factor and immunosuppression in cancer: current knowledge and potential for new therapy. Expert Opin Biol Ther. 2007;7:449–60. doi: 10.1517/14712598.7.4.449. [DOI] [PubMed] [Google Scholar]
- 83.Woo SR, Corrales L, Gajewski TF. Innate Immune Recognition of Cancer. Annu Rev Immunol. 2015;33:15.1–15.30. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 84.Solito S, Pinton L, Damuzzo V, et al. Highlights on molecular mechanisms of MDSC-mediated immune suppression: paving the way for new working hypotheses. Immunol Invest. 2012;41:722–37. doi: 10.3109/08820139.2012.678023. [DOI] [PubMed] [Google Scholar]
- 85.Medrek C, Pontén F, Jirström K, et al. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Werno C, Menrad H, Weigert A, et al. Knockout of HIF-1α in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis. 2010;31:1863–72. doi: 10.1093/carcin/bgq088. [DOI] [PubMed] [Google Scholar]
- 87.Tsutsui S, Yasuda K, Suzuki K, et al. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425–31. [PubMed] [Google Scholar]
- 88.Coffelt SB, Tal AO, Scholz A, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–80. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 89.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen WT, Hung WC, Kang WY, et al. Overexpression of cyclooxygenase-2 in urothelial carcinoma in conjunction with tumor-associated-macrophage infiltration, hypoxia-inducible factor-1alpha expression, and tumor angiogenesis. APMIS. 2009;117:176–84. doi: 10.1111/j.1600-0463.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 91.Hernandez L, Smirnova T, Kedrin D, et al. The EGF/CSF-1 paracrine invasion loop can be triggered by heregulin beta1 and CXCL12. Cancer Res. 2009;69:3221–7. doi: 10.1158/0008-5472.CAN-08-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaturvedi P, Gilkes DM, Takano N, et al. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci USA. 2014;111:E2120–9. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chafe SC, Lou Y, Sceneay J, et al. Carbonic Anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res. 2015;75:1–13. doi: 10.1158/0008-5472.CAN-14-3000. [DOI] [PubMed] [Google Scholar]
- 95.Hingorani SR, Palculict TB, Izzo J, et al. Hypoxia triggers hedgehog-mediated tumor-stromal interactions in pancreatic cancer. Cancer Res. 2013;73:3235–47. doi: 10.1158/0008-5472.CAN-11-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gilkes DM, Bajpai S, Chaturvedi P, et al. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288:10819–29. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Gilkes DM, Bajpai S, Wong CC, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11:456–66. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Poggi A, Musso A, Dapino I, et al. Mechanisms of tumor escape from immune system: role of mesenchymal stromal cells. Immunol Lett. 2014;159:55–72. doi: 10.1016/j.imlet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–36. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 101.Ikenouchi J, Matsuda M, Furuse M, et al. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 102.Krishnamachary B, Zagzag D, Nagasawa H, et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–31. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 103.Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J, Cheng Q, Zhou Y, et al. Slug is a key mediator of hypoxia induced cadherin switch in HNSCC: correlations with poor prognosis. Oral Oncol. 2013;49:1043–50. doi: 10.1016/j.oraloncology.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Zhu GH, Huang C, Feng ZZ, et al. Hypoxia-induced snail expression through transcriptional regulation by HIF-1α in pancreatic cancer cells. Dig Dis Sci. 2013;58:3503–15. doi: 10.1007/s10620-013-2841-4. [DOI] [PubMed] [Google Scholar]
- 106.Giannoni E, Bianchini F, Calorini L, et al. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 2011;14:2361–71. doi: 10.1089/ars.2010.3727. [DOI] [PubMed] [Google Scholar]
- 107.Fiaschi T, Giannoni E, Taddei ML, et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle. 2013;12:1791–801. doi: 10.4161/cc.24902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim HJ, Park JW, Cho YS, et al. Pathogenic role of HIF-1α in prostate hyperplasia in the presence of chronic inflammation. Biochim Biophys Acta. 2013;1832:183–94. doi: 10.1016/j.bbadis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Chuang MJ, Sun KH, Tang SJ, et al. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008;99:905–13. doi: 10.1111/j.1349-7006.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McMahon S, Charbonneau M, Grandmont S, et al. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–81. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 111.Nishi H, Nakada T, Hokamura M, et al. Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast. Endocrinology. 2004;145:4113–18. doi: 10.1210/en.2003-1639. [DOI] [PubMed] [Google Scholar]
- 112.Copple BL. Hypoxia stimulates hepatocyte epithelial to mesenchymal transition by hypoxia-inducible factor and transforming growth factor-beta-dependent mechanisms. Liver Int. 2010;30:669–82. doi: 10.1111/j.1478-3231.2010.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin G, Gai R, Chen Z, et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 prevents epithelial-mesenchymal transition induced by hypoxia and TGF-β1. Eur J Pharmacol. 2014;729:45–53. doi: 10.1016/j.ejphar.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 114.Kato S, Hayakawa Y, Sakurai H, et al. Mesenchymal-transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B. Cancer Sci. 2014;105:281–89. doi: 10.1111/cas.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang YG, Luo Y, He DL, et al. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol. 2007;14:1034–39. doi: 10.1111/j.1442-2042.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Q, Bai X, Chen W, et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013;34:962–73. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- 117.Cheng ZX, Sun B, Wang SJ, et al. Nuclear factor-κB-dependent epithelial to mesenchymal transition induced by HIF-1α activation in pancreatic cancer cells under hypoxic conditions. PLoS One. 2011;6:e23752. doi: 10.1371/journal.pone.0023752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sahlgren C, Gustafsson MV, Jin S, et al. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–7. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh AK, Arya RK, Maheshwari S, et al. Tumor heterogeneity and cancer stem cell paradigm: Updates in concept, controversies and clinical relevance. Int J Cancer. 2015;136:1991–2000. doi: 10.1002/ijc.28804. [DOI] [PubMed] [Google Scholar]
- 121.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–37. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 122.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–48. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 123.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–88. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cojoc M, Mäbert K, Muders MH, et al. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin Cancer Biol. 2014;31:16–27. doi: 10.1016/j.semcancer.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 125.Philip B, Ito K, Moreno-Sánchez R, et al. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013;34:1699–707. doi: 10.1093/carcin/bgt209. [DOI] [PubMed] [Google Scholar]
- 126.Lan L, Luo Y, Cui D, et al. Epithelial-mesenchymal transition triggers cancer stem cell generation in human thyroid cancer cells. Int J Oncol. 2013;43:113–20. doi: 10.3892/ijo.2013.1913. [DOI] [PubMed] [Google Scholar]
- 127.Luo Y, Cui X, Zhao J, et al. Cells susceptible to epithelial-mesenchymal transition are enriched in stem-like side population cells from prostate cancer. Oncol Rep. 2014;31:874–84. doi: 10.3892/or.2013.2905. [DOI] [PubMed] [Google Scholar]
- 128.Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17:30–54. doi: 10.1111/jcmm.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiang L, Wu T, Zhang HW, et al. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012;19:284–94. doi: 10.1038/cdd.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu H, Wang D, Zhang L, et al. Upregulation of autophagy by hypoxia-inducible factor-1α promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep. 2014;32:935–42. doi: 10.3892/or.2014.3298. [DOI] [PubMed] [Google Scholar]
- 131.Miao ZF, Zhao TT, Wang ZN, et al. Influence of different hypoxia models on metastatic potential of SGC-7901 gastric cancer cells. Tumour Biol. 2014;35:6801–8. doi: 10.1007/s13277-014-1928-7. [DOI] [PubMed] [Google Scholar]
- 132.Bhaskara VK, Mohanam I, Rao JS, et al. Intermittent hypoxia regulates stem-like characteristics and differentiation of neuroblastoma cells. PLoS One. 2012;7:e30905. doi: 10.1371/journal.pone.0030905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schwab LP, Peacock DL, Majumdar D, et al. Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res. 2012;14:R6. doi: 10.1186/bcr3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y, Liu Y, Malek SN, et al. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yeung TM, Gandhi SC, Bodmer WF. Hypoxia and lineage specification of cell line-derived colorectal cancer stem cells. Proc Natl Acad Sci USA. 2011;108:4382–87. doi: 10.1073/pnas.1014519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giannoni E, Parri M, Chiarugi P. EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal. 2012;16:1248–63. doi: 10.1089/ars.2011.4280. [DOI] [PubMed] [Google Scholar]
- 137.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–38. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 138.Callapina M, Zhou J, Schmid T, et al. NO restores HIF-1α hydroxylation during hypoxia: role of reactive oxygen species. Free Radic Biol Med. 2005;39:925–36. doi: 10.1016/j.freeradbiomed.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 139.Kirschmann DA, Seftor EA, Fong SF, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–83. [PubMed] [Google Scholar]
- 140.Nishi K, Oda T, Takabuchi S, et al. LPS induces hypoxia-inducible factor 1 activation in macrophage-differentiated cells in a reactive oxygen species-dependent manner. Antioxid Redox Signal. 2008;10:983–95. doi: 10.1089/ars.2007.1825. [DOI] [PubMed] [Google Scholar]
- 141.Cho KH, Choi MJ, Jeong KJ, et al. A ROS/STAT3/HIF-1α signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate. 2014;74:528–36. doi: 10.1002/pros.22776. [DOI] [PubMed] [Google Scholar]
- 142.Calvani M, Comito G, Giannoni E, et al. Time-dependent stabilization of hypoxia inducible factor-1α by different intracellular sources of reactive oxygen species. PLoS One. 2012;7:e38388. doi: 10.1371/journal.pone.0038388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seebacher NA, Richardson DR, Jansson PJ. Glucose Modulation Induces Reactive Oxygen Species and Increases Pgp-Mediated Multidrug-Resistance to Chemotherapeutics. Br J Pharmacol. 2015 Jan 14; doi: 10.1111/bph.13079. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shimojo Y, Akimoto M, Hisanaga T, et al. Attenuation of reactive oxygen species by antioxidants suppresses hypoxia-induced epithelial-mesenchymal transition and metastasis of pancreatic cancer cells. Clin Exp Metastasis. 2013;30:143–54. doi: 10.1007/s10585-012-9519-8. [DOI] [PubMed] [Google Scholar]
- 145.Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 146.Liang D, Kong X, Sang N. Effects of histone deacetylase inhibitors on HIF-1. Cell Cycle. 2006;5:2430–5. doi: 10.4161/cc.5.21.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Conley S, Gheordunescu E, Kakarala P, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci USA. 2012;109:2784–89. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang R, Cheng L, Xia J, et al. Gemcitabine resistance is associated with epithelial-mesenchymal transition and induction of HIF-1α in pancreatic cancer cells. Curr Cancer Drug Targets. 2014;14:407–17. doi: 10.2174/1568009614666140226114015. [DOI] [PubMed] [Google Scholar]
- 149.Miyazaki S, Kikuchi H, Iino I, et al. Anti-VEGF antibody therapy induces tumor hypoxia and stanniocalcin 2 expression and potentiates growth of human colon cancer xenografts. Int J Cancer. 2014;135:295–307. doi: 10.1002/ijc.28686. [DOI] [PubMed] [Google Scholar]
- 150.Kung AL, Wang S, Klco JM, et al. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–40. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 151.Rapisarda A, Zalek J, Hollingshead M, et al. Schedule-dependent inhibition of hypoxia-inducible factor-1alpha protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res. 2004;64:6845–8. doi: 10.1158/0008-5472.CAN-04-2116. [DOI] [PubMed] [Google Scholar]
- 152.Zhang H, Qian DZ, Tan YS, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–86. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lao BB, Grishagin I, Mesallati H, et al. In vivo modulation of hypoxia-inducible signaling by topographical helix mimetics. Proc Natl Acad Sci USA. 2014;111:7531–36. doi: 10.1073/pnas.1402393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shay JE, Imtiyaz HZ, Sivanand S, et al. Inhibition of hypoxia-inducible factors limits tumor progression in a mouse model of colorectal cancer. Carcinogenesis. 2014;35:1067–77. doi: 10.1093/carcin/bgu004. [DOI] [PMC free article] [PubMed] [Google Scholar]