Abstract
Bovine mastitis results in billion dollar losses annually in the United States alone. Streptococci are among the most relevant causative agents of this disease. Conventional antibiotic therapy is often unsuccessful and contributes to development of antibiotic resistance. Bacteriophage endolysins represent a new class of antimicrobials against these bacteria. In this work, we characterized the endolysins (lysins) of the streptococcal phages λSA2 and B30 and evaluated their potential as anti-mastitis agents. When tested in vitro against live streptococci, both enzymes exhibited near-optimum lytic activities at ionic strengths, pH, and Ca2+ concentrations consistent with cow milk. When tested in combination in a checkerboard assay, the lysins were found to exhibit strong synergy. The λSA2 lysin displayed high activity in milk against Streptococcus dysgalactiae (reduction of CFU/ml by 3.5 log units at 100 μg/ml), Streptococcus agalactiae (2 log), and Streptococcus uberis (4 log), whereas the B30 lysin was less effective. In a mouse model of bovine mastitis, both enzymes significantly reduced intramammary concentrations of all three streptococcal species (except for B30 vs. S. dysgalactiae), and the effects on mammary gland wet weights and TNFα concentrations were consistent with these findings. Unexpectedly, the synergistic effect determined for the two enzymes in vitro was not observed in the mouse model. Overall, our results illustrate the potential of endolysins for treatment of Streptococcus-induced bovine mastitis.
Keywords: Peptidoglycan hydrolase, endolysin, bacteriophage, antimicrobial, mastitis, Streptococcus
Introduction
Bovine mastitis is an infection of the mammary gland that results in annual losses of up to $2 billion in the United States alone, making it the most costly disease in the dairy industry (Kerr et al. 2001; Sordillo and Streicher 2002; Yancey 1999). Besides staphylococci (Staphylococcus aureus and coagulase-negative Staphylococcus), which are the most prevalent mastitis-causing pathogens, also streptococci play an important role as causative agents of this disease, with the species S. agalactiae (group B Streptococcus, GBS), S. dysgalactiae (group C Streptococcus, GCS), and S. uberis being most relevant (Lammers et al. 2001; Tenhagen et al. 2006; Wilson et al. 1997). While S. agalactiae is a contagious agent mainly transmitted from cow to cow through the milking process, S. uberis is an environmental pathogen, and S. dysgalactiae shows both routes of transmission (Calvinho et al. 1998; Leigh 1999). To date, the conventional method of treatment for bovine mastitis is the use of antibiotics (usually administered through intramammary infusion (Gehring and Smith 2006; Gruet et al. 2001)), which is often less than 50% effective and leads to premature culling in many cases (Cattell et al. 2001; Deluyker et al. 2005). Furthermore, the use of broad range antibiotics can contribute to development and spread of resistance in both mastitis pathogens and nonrelated commensal bacteria. For these reasons, there is high interest in alternative methods for treatment of mastitis that are more pathogen-specific and refractory to resistant strain formation.
Bacteriophage endolysins (peptidoglycan hydrolases, PGH) have been suggested as promising alternatives to antibiotics for treatment of bacterial infections (Donovan 2007; Fischetti 2005; Schmelcher et al. 2012a; Walsh 2003). These enzymes are used by bacteriophages to lyse infected host cells at the end of the lytic multiplication cycle, resulting in liberation of progeny virions. They access the cell wall ‘from within’ and degrade the peptidoglycan (PG) by cleavage of specific bonds. In the case of Gram-positive bacteria, which lack an outer membrane, endolysins are also able to lyse the cells when added externally, making them potential antimicrobials against Gram-positive pathogens. Endolysins usually exhibit specificity at least at the genus level (Schmelcher et al. 2012a), and development of bacterial resistance is believed unlikely because (i) phage and host have co-evolved (Fischetti 2005); (ii) endolysins target highly conserved bonds of the PG (Schleifer and Kandler 1972; Schmelcher et al. 2012a); and (iii) using an externally applied agent acting on the cell envelope avoids intracellular resistance mechanisms (e.g. modification of agent) (Donovan et al. 2009). To date, no endolysin-resistant strains have been reported, despite repeated efforts to identify or generate them (Fischetti 2005; Gilmer et al. 2013; Loeffler et al. 2001; Pastagia et al. 2011; Schuch et al. 2002). Endolysins from a Gram-positive background show a modular architecture, consisting of (i) cell wall binding domains (CBD), which specifically direct the enzymes to their substrate in the cell wall, and (ii) enzymatically active domains (EAD), which catalyze specific bond cleavage (Borysowski et al. 2006; Fischetti 2005; Hermoso et al. 2007; Schmelcher et al. 2012a). Previous studies have shown that two PGHs cleaving different PG bonds may act synergistically against target bacteria when used in combination, yielding increased treatment efficacy and further reducing the chance of resistance development (Becker et al. 2008; Loeffler and Fischetti 2003; Schmelcher et al. 2012b). The endolysins of the streptococcal bacteriophages λSA2 (Pritchard et al. 2007) and B30 (Pritchard et al. 2004) each consist of 2 different EADs in addition to either one or two CBDs. The λSA2 lysin features an N-terminal endopeptidase domain, which cleaves the bond between the D-glutamine and the L-lysine of the streptococcal PG stem peptide, and a C-terminal N-acetylglucosaminidase domain, which cleaves within the sugar backbone of the PG (Pritchard et al. 2007). The EADs are separated by a mid-protein region harboring two Cpl-7 CBDs (Garcia et al. 1990; Lopez and Garcia 2004). The B30 lysin consists of an N-terminal CHAP endopeptidase (Bateman and Rawlings 2003) cleaving the D-Ala-L-Ala bond between the stem peptide and the interpeptide bridge, a glycosidase (putative N-acetylmuramidase) domain in the center of the protein cutting the sugar backbone (Pritchard et al. 2004), and a C-terminal SH3b CBD (Donovan et al. 2006b; Ponting et al. 1999; Whisstock and Lesk 1999).
When evaluating PGHs for use in the treatment of bovine mastitis by intramammary infusion, it is important that these enzymes maintain their lytic activity in a milk environment. However, ex vivo experiments in cow milk alone inadequately mimic the complex situation within a cow’s udder, and studies in cows are often prohibitive due to the high costs associated. The mouse model of bovine mastitis was introduced in the 1970s as a relatively inexpensive alternative (Brouillette and Malouin 2005; Chandler 1970a; Notebaert and Meyer 2006), and numerous studies have used this model since for exploring the effect of various antimicrobials (Anderson and Craven 1984; Bramley and Foster 1990; Brouillette et al. 2004; Chandler 1971; Diarra et al. 2003; Sanchez et al. 1994).
Our lab recently reported activity of chimeric endolysins consisting of the λSA2 endopeptidase domain and Staphylococcus-specific SH3b CBDs against mastitis-causing S. aureus ex vivo in cow milk and in a mouse model of bovine mastitis, in synergy with the bacteriocin lysostaphin (Schmelcher et al. 2012b). Building on this work, here we evaluated the potential of the λSA2 and B30 endolysins as therapeutics for treatment of Streptococcus-induced bovine mastitis. To this end, we biochemically characterized the two endolysins, demonstrated synergistic antimicrobial activity in vitro, determined their activity ex vivo in whole cow milk, and evaluated their efficacy in a mouse model of bovine mastitis against three streptococcal species.
Materials and Methods
Plasmids and strains
The λSA2 and B30 coding sequences in pET21a (EMD Biosciences, San Diego, CA) were obtained as gifts from David Pritchard (University of Alabama, Birmingham, AL) (Pritchard et al. 2004; Pritchard et al. 2007). These inducible plasmid constructs introduce 6xHis-tags at the C-termini of the proteins to facilitate purification. Protein expression was performed in E. coli BL21 (DE3) (Invitrogen, Carlsbad, CA), which was cultured in modified Luria-Bertani medium (Schmelcher et al. 2010) (mLB; 15 g/l Tryptone, 8 g/l yeast extract, 5 g/l NaCl), supplemented with 150 μg/ml ampicillin for plasmid selection.
The three strains Streptococcus dysgalactiae NRRL B-65273, S. agalactiae NRRL B-65272, and S. uberis NRRL B-65274 used in this study are mastitis isolates originally obtained from M. Paape (USDA, Beltsville, MD). They were grown in Tryptic soy broth (TSB; BD, Sparks, MD) at 37°C.
Protein expression and purification
Protein expression in E. coli and purification of 6x His-tagged recombinant proteins was carried out essentially as described earlier (Donovan and Foster-Frey 2008), with the following modifications. Cells harvested from IPTG (1 mM)-induced cultures were resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 30% glycerol, pH 8.0) and disrupted by sonication prior to purification of proteins from the centrifugation-cleared lysate by nickel affinity chromatography using Nickel-NTA Superflow resin packed into empty polypropylene columns (QIAGEN, Valencia, CA). The matrix was washed with 20 mL lysis buffer and 10 mL wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 30% glycerol, pH 8.0). Target proteins were eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, 30% glycerol, pH 8.0), the buffer was exchanged to storage buffer (20 mM NaH2PO4, 120 mM NaCl, 30% glycerol, pH 7.5) using 5 ml ZEBA desalting columns (Thermo Fisher Scientific, Rockford, IL). When proteins were prepared for in vivo experiments, buffers as indicated above with 1% instead of 30% glycerol were used, and 0.1% Triton X-114 was included in the lysis buffer for removal of endotoxins as previously described (Reichelt et al. 2006; Schmelcher et al. 2012b). Proteins were dialyzed against 50 mM NaH2PO4, 150 mM NaCl, 1% glycerol, pH 7.5 instead of using ZEBA columns. All samples were filter sterilized (0.22 μm) and stored on ice at 4°C until used. The purity of each preparation was determined via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and protein concentrations were measured spectrophotometrically using a NanoDrop ND-1000 device (NanoDrop Technologies, Wilmington, DE). Endotoxin concentrations were determined using the Limulus Amoebocyte Lysate (LAL) assay (Lonza, Walkersville, MD), and preparations with concentrations < 5 EU/mg were used for in vivo studies.
Turbidity reduction assays
Turbidity reduction assays modified from Donovan et al. (2006a) were performed and analyzed in a plate reader as described previously (Donovan and Foster-Frey 2008). Frozen S. dysgalactiae NRRL B-65273 substrate cells were produced as described by Becker et al. (2009), by growing the bacteria in TSB to OD600nm = 0.7, harvesting, washing and resuspending them in 1/60 culture volume of 10 mM Tris, 150 mM NaCl, 25% glycerol, pH 7.5, and freezing aliquots at −80°C. For the assay, 100 μl of thawed and diluted substrate cells were added to each well of a 96-well dish containing 100 μl of enzyme in the desired buffer so that the initial OD600nm was 1.0, and the OD600nm was measured at 20 s intervals for 20 min. The steepest slopes of the resulting lysis curves correspond to the lytic activities (ΔOD600nm min−1 μg−1). A control (‘no enzyme’) value was subtracted from each experimental value. All results represent the average of at least three independent experiments.
To determine the influence of NaCl concentration on endolysin lytic activity, assays were performed in 10 mM Tris buffers at pH 7.5 with varying NaCl concentrations (0 mM, 50 mM, 100 mM, 150 mM, 200 mM, 300 mM, and 500 mM). The enzyme concentrations used were 25 μg/ml for λSA2 and 100 μg/ml for B30. Enzymatic activities were expressed as relative lytic activities, normalized to the maximum activity for each enzyme. The optimum pH for lytic activity was determined accordingly, by using a series of different buffers with pH values ranging from 3.5 to 10.0. Citrate buffers were used for pH 3.5, 4.5, 5.5, and 6.0, MOPS buffers for pH 6.5, 7.0, and 7.5, Tris buffers for pH 8.0 and 9.0, and carbonate/bicarbonate buffer for pH 10.0. The concentration of the buffering agent in all buffers used was 20 mM, and the NaCl concentration was 100 mM. For investigating the influence of divalent metal cations, assays were performed in 10 mM Tris, 100 mM NaCl, pH 7.5 with varying concentrations of MgCl2, MnCl2, or CaCl2 (0 mM, 0.1 mM, 1 mM, and 10 mM). Activities were normalized to the activity without metal ions.
Plate lysis assays
Plate lysis assays were performed as described previously (Donovan and Foster-Frey 2008) by spreading a lawn of mid-log phase (OD600nm = 0.4 – 0.6) cells of S. dysgalactiae NRRL B-65273, S. agalactiae NRRL B-65272, or S. uberis NRRL B-65274 on tryptic soy agar (TSA) plates, air-drying the plates for 15 min in a laminar flow hood, and then spotting 10 μl of buffer (10 mM Tris, 150 mM NaCl, pH 7.5) containing the desired amount of purified protein onto the lawn. Controls were included by spotting 10 μl of buffer alone. After air-drying, plates were incubated at 37°C overnight, and cleared spots were scored within 20 hours of plating the cells. For determination of the minimum inhibitory amount (MIA) (Schmelcher et al. 2012b), a 2-fold dilution series of the protein was spotted. Assays were performed at least in triplicate.
Determination of synergy
Checkerboard assays in a plate lysis format as previously described (Schmelcher et al. 2012b) were performed for determination of synergy between the λSA2 and B30 lysins. In brief, linear or 2-fold dilution series of both proteins were mixed in two dimensions in a 96-well dish, and the mixtures were spotted onto a lawn of S. dysgalactiae NRRL B-65273 on gridded (6 x 6 squares) TSA plate as described above. Plates were evaluated by densitometry using the software Alpha Imager (Alpha Innotech, San Leandro, CA) as previously described (Schmelcher et al. 2012b). For each lysis zone along the inhibitory line on the plate, the sum of the fractional inhibitory amounts (FIAs) of both proteins (ΣFIA = FIAA + FIAB) was calculated. An isobologram was created as described by Loeffler and Fischetti (2003) from the results of four experiments.
Determination of lytic activity in milk
Activity in milk was tested as described by Obeso et al. (2008). Commercial whole-fat Ultra High Temperature sterilized (UHT) milk (Parmalat) at 37°C was spiked with exponentially growing (OD600nm = 0.4 – 0.6) cells of S. dysgalactiae NRRL B-65273, S. agalactiae NRRL B-65272, or S. uberis NRRL B-65274 at a concentration of 1 x 103 or 5 x 106 cfu/ml. Immediately after inoculation, purified enzyme was added at concentrations between 1 μg/ml and 200 μg/ml in the same volume of buffer and the milk was incubated at 37°C without shaking for 3 hours. Samples were taken immediately before and immediately after addition of enzyme, as well as 1, 2, and 3 h after inoculation, and the number of CFUs was determined by serial dilution plating on TSA plates in triplicate. A sample with storage buffer added instead of enzyme served as a control. The absence of CFUs in non-inoculated milk was verified by direct plating on TSA plates.
Mouse model of Streptococcus-induced bovine mastitis
To evaluate the efficacy of purified endolysins applied individually or in combination against three different streptococcal species in a mouse model of bovine mastitis, female C57BL6/SJL mice were challenged intramammarily with S. agalactiae NRRL B-65272, S. dysgalactiae NRRL B-65273, or S. uberis NRRL B-65274, followed by intramammary infusions of proteins or buffer as control essentially as described earlier (Schmelcher et al. 2012b). In brief, mammary glands of experimental dams between days 7 and 15 of lactation were depleted from milk by allowing pups to nurse for 1 h. Animals were anesthesized by IP injection of Avertin (375 μg/g body weight), and glands R3, R4, L3, L4 were infused with 102 CFU of exponential phase bacteria in 50 μl of 0.4% Trypan Blue solution (Sigma, St. Louis, MO). Proteins in 50 μl of buffer (50 mM NaH2PO4, 150 mM NaCl, 1% glycerol, pH 7.5, supplemented with 1 mM CaCl2), or buffer alone as control were infused into inoculated glands in a randomized fashion 45 min post inoculation. Concentrations used were 25 μg/gland for λSA2, 250 μg/gland for B30, and 12.5 (λSA2) + 125 (B30) μg/gland for the combination treatment. Following enzyme administration, the teats were sealed with Vetbond surgical glue (3M Animal Care Products, St. Paul, MN) to prevent leakage and cross-contamination between animals. 24 hours after bacterial challenge, mice were euthanized, mammary glands dissected, weighed, homogenized in phosphate-buffered saline (100 mg/mL) using a Polytron (Kinematica, Lucerne, Switzerland), and serial dilution plated on TSA to determine intramammary bacterial concentrations. Aliquots of homogenized glands were centrifuged (15,800 x g, 15 min, 5°C), and 50 μl of the supernatants used for determination of TNF-α concentrations using The Quantikine Mouse TNF-α/TNFSF1A Immunoassay (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical analysis
For the mouse experiments with S. agalactiae, S. dysgalactiae, and S. uberis, the variables wet weight, Log10 CFU and TNF-α were analyzed as one-factor repeated measures models using PROC MIXED (SAS Institute, Cary, NC) with treatment as the factor and mouse ID as the repeated factor. The assumptions of the model were checked. One probable outlier for S. agalactiae, one for S. dysgalactiae, and two probable outliers for S. uberis were excluded from the analysis. To model the within mouse correlation, the best fitting variance-covariance structure was used for each variable-streptococci combination. These were wet weight: compound symmetric (S. agalactiae, S. dysgalactiae) and unstructured (S. uberis); Log10 CFU: unstructured (S. agalactiae, S. dysgalactiae) and compound symmetric (S. uberis); TNF-α: compound symmetric heterogeneous (S. agalactiae) and unstructured (S. dysgalactiae, S. uberis). When the F-test was statistically significant, means comparisons were done with Sidak adjusted p-values to hold the experiment-wise error at 0.05.
Results
λSA2 and B30 endolysins show near-optimum activity at physico-chemical conditions present in cow milk
The C-terminally 6xHis-tagged versions of the λSA2 and B30 lysins (Fig. 1) were produced in E. coli and isolated at >95% purity, as published previously from this lab (Donovan and Foster-Frey 2008; Donovan et al. 2006b). Depending on the preparation, protein concentrations ranging from 1.1 to 2.2 mg/mL for λSA2 and 10.3 mg/mL to 22.8 mg/mL for B30 were obtained (data not shown). When purified proteins were spotted on freshly plated lawns of S. dysgalactiae or S. uberis, 0.12 to 0.26 μg of λSA2 were sufficient to produce visible zones of lysis in the lawns, whereas approximately ten-fold more of B30 was required. The S. agalactiae strain tested was considerably less susceptible to both lysins in this assay (Table 1).
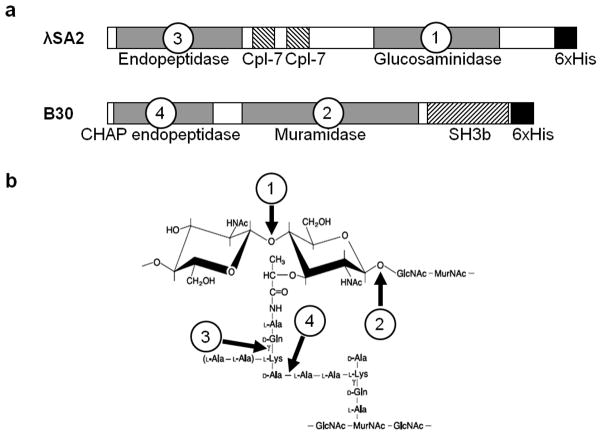
Fig. 1.
a Domain structure of the λSA2 and B30 endolysins. Cpl-7 and SH3b are cell wall binding domains. b Structure of a portion of the S. agalactiae peptidoglycan with the bonds cleaved by the enzymatically active domains of the λSA2 and B30 endolysins indicated by numbered arrows. Numbers correspond to those of the respective domains in panel a (modified from (Pritchard et al. 2007))
Table 1.
Minimum inhibitory amounts (MIAs) of λSA2 and B30 lysins (in a volume of 10 μl) as determined by plate lysis on bacterial lawns of strains from different streptococcal species. Mean values and standard deviations from at least three independent experiments are shown.
| S. dysgalactiae | S. agalactiae | S. uberis | |
|---|---|---|---|
| λSA2 | 0.12 ± 0.01 μg | 1.72 ± 0.58 μg | 0.26 ± 0.01 μg |
| B30 | 1.12 ± 0.26 μg | 4.69 ± 1.12 μg | 2.39 ± 0.53 μg |
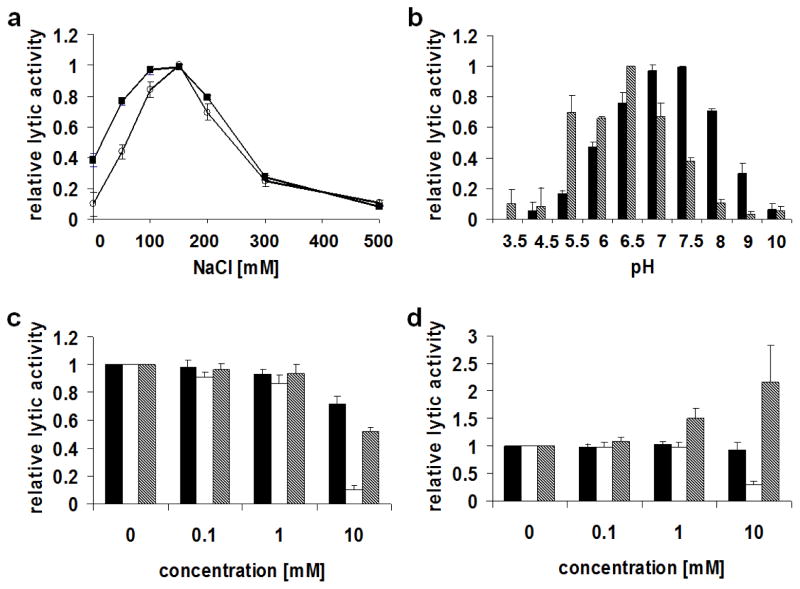
In view of their potential antimicrobial application in a milk environment against mastitis causing streptococci, both lysins were biochemically characterized in this study, investigating the influence of salt concentration, pH, and certain metal cations on their activity against streptococci (S. dysgalactiae). The ionic strength in bovine milk has been reported to be around 80 mM; the physiological pH of milk ranges from 6.5 to 6.7 and can be >7 in mastitic cows; and the concentration of free Ca2+, which is the most abundant divalent metal cation in cow milk, is ~3 mM (Blowey and Edmondson 2010; Donovan et al. 2006c; Gaucheron 2005; Webb et al. 1974). Under the conditions tested, both the λSA2 and B30 lysins displayed their highest activity at NaCl concentrations between 100 and 150 mM and retained ~77% and ~44% of their optimum activity, respectively, when the salt concentration was reduced to 50 mM (Fig. 2a). λSA2 displayed its pH optimum between pH 7.0 and 7.5, and its activity was reduced to ~76% at pH 6.5. B30 showed a more pronounced pH optimum at pH 6.5, with ~66% residual activity at pH 6.0 and pH 7.0 (Fig. 2b). Regarding the effect of metal ions, Ca2+ was slightly inhibitory for λSA2 (~52% residual activity at a concentration of 10 mM; Fig. 2c), whereas it enhanced the activity of B30 ~1.5-fold and more than 2-fold at concentrations of 1 mM and 10 mM, respectively (Fig. 2d). Mn2+ was shown to greatly inhibit lytic activity of both lysins at a concentration of 10 mM, and also 10 mM Mg2+ slightly reduced the activity of λSA2 to ~72%. However, the effects of Mg2+ and Mn2+ at physiologically relevant concentrations (0.81 mM and 0.36 – 0.91 μM, respectively) (Gaucheron 2005; Lonnerdal et al. 1981) are likely negligible.
Fig. 2.
Biochemical characterization of λSA2 and B30 lysins by turbidity reduction assays. All assays were performed with log phase S. dysgalactiae and depict relative lytic activity (for explanation see text). a Effect of NaCl concentration on lytic activity of λSA2 (■) and B30 (○). b Effect of pH on lytic activity of λSA2 (black bars) and B30 (striped bars). c, d Effect of the divalent metal cations Mg2+ (black bars), Mn2+ (white bars), and Ca2+ (striped bars) on lytic activity of λSA2 (c) and B30 (d). Error bars represent standard deviations from three trials, each composed of three replicates
The λSA2 and B30 lysins act synergistically against streptococci in vitro
The finding that the enzymatically active domains of the λSA2 and B30 endolysins attack different bonds within the streptococcal peptidoglycan gives rise to the possibility that these enzymes act synergistically against their target bacteria when applied in combination. When linear concentration series of λSA2 and B30 and combinations of both were spotted on a lawn of S. dysgalactiae on a gridded tryptic soy agar plate, the concave line connecting spots with reduced or inhibited growth (lysis zones) on the plate resembled a curve characteristic for a synergistic effect in the checkerboard assay (Loeffler and Fischetti 2003) (Fig. 3a), as opposed to a diagonal line produced by a strictly additive effect, as previously demonstrated (Schmelcher et al. 2012b). In order to quantify the synergistic effect, checkerboard plates with 2-fold serial dilutions of both proteins were scored by densitometry. The calculated ΣFIA from four independent experiments was 0.42 ± 0.09, suggesting strong synergy (Schmelcher et al. 2012b). The enzyme concentrations along the inhibitory lines of the checkerboard plates were transcribed into an isobologram, which revealed a curve characteristic for synergy (Fig. 3b).
Fig. 3.
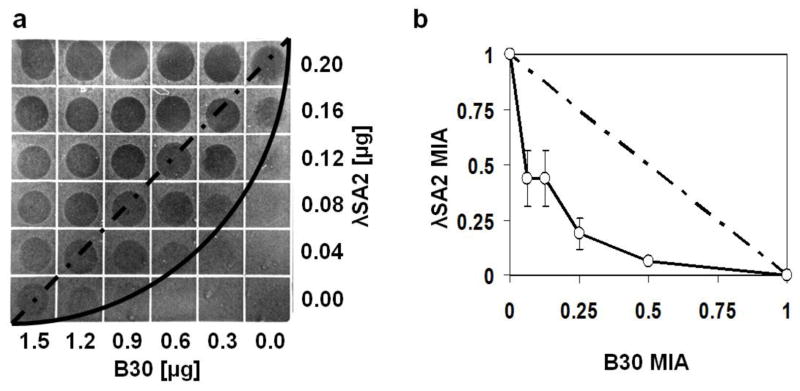
Synergistic effect of λSA2 and B30 lysins against S. dysgalactiae. a Linear dilution series of λSA2, B30 and combinations of both proteins spotted on a bacterial lawn on tryptic soy agar. Proteins were spotted in a volume of 10 μl per square of the gridded plate. The dashed and solid lines illustrate theoretical shapes of the inhibitory line in case of an additive effect and a synergistic effect, respectively. b Isobologram of the checkerboard synergy testing method in a plate lysis format with 2-fold serial dilutions of both proteins spotted. Plates were evaluated by densitometry. For each square of the plate along the inhibitory line, enzyme concentrations (as fractions of the enzymes’ MIAs) were entered in an x/y plot. Error bars represent standard deviations from four independent experiments. The dashed line is the theoretical curve in case of an additive effect. The calculated ΣFIA for the four experiments is 0.42 ± 0.09, indicating strong synergy
The λSA2 and B30 lysins kill streptococcal species in whole milk
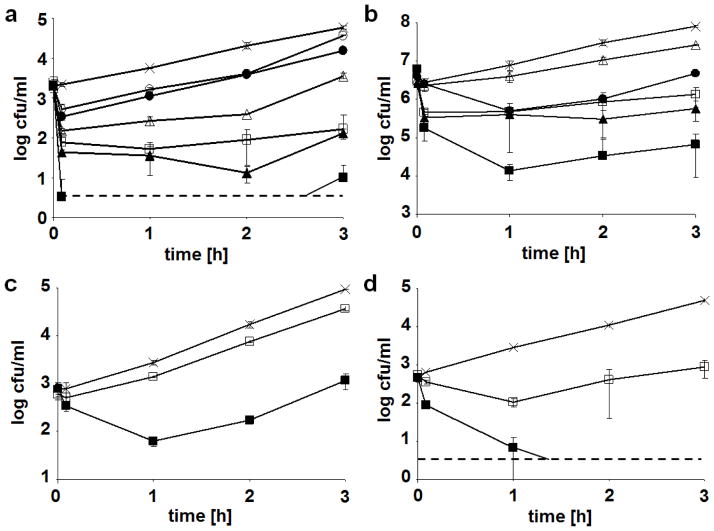
Activity of λSA2 and B30 lysins against streptococci in sterile homogenized whole milk was evaluated by adding different concentrations of the purified enzymes to milk at 37°C inoculated with ~103 cfu/ml of exponentially growing S. dysgalactiae, S. agalactiae, or S. uberis cells. In the case of S. dysgalactiae, also a higher inoculation level (~5 × 106) was tested. Addition of enzyme to milk spiked with S. dysgalactiae resulted in immediate decrease in cell numbers in a concentration-dependent manner (Fig. 4a, b). Consistent with previously determined MIAs (Table 1), the B30 lysin had a considerably weaker effect than λSA2 in this assay. While 100 μg/ml of λSA2 reduced bacterial concentrations (inoculum 103 cfu/ml) by more than 3.5 log units compared to the buffer control within 3 hours, the difference was only 2.5 log units for B30 (Fig. 4a). When the same B30 concentration was used against a higher inoculum, only a ~0.5 log decrease was achieved, whereas 200 μg/ml was required for a reduction of ~2 log units. In contrast, the efficacy of λSA2 against high inocula was similar to that against lower bacterial concentrations (e.g., > 3 log reduction by 50 μg/ml; Fig. 4b). S. agalactiae appeared less susceptible to the action of both enzymes than S. dysgalactiae (consistent with MIAs shown in Table 1). With 100 μg/ml λSA2 added to milk inoculated with ~103 cfu/ml of S. agalactiae, cell counts remained ~2 logs below those in the ‘no enzyme’ control after 3 h, whereas 100 μg/ml B30 resulted in a reduction of less than 0.5 log (Fig. 4c). In the case of S. uberis, 100 μg/ml λSA2 caused a decrease in bacteria from initially ~103 cfu/ml to undetectable after 2 hours, and no more cells were detected until the end of the experiment. For B30, 100 μg/ml caused a reduction of more than 1.5 log units compared to the control after 3 hours (Fig. 4d). It is important to note that in many of the experiments, bacteria resumed growth towards the end of the assay after an initial drop in cell numbers.
Fig. 4.
Effect of λSA2 and B30 lysins on cell survival of streptococcal species at different inoculation levels in UHT whole milk at 37°C. a S. dysgalactiae with 1 μg/ml (●), 10 μg/ml (▲), and 100 μg/ml (■) of λSA2, or 20 μg/ml (○), 60 μg/ml (△), and 100 μg/ml (□) of B30, or buffer as control (X). b S. dysgalactiae with 2 μg/ml (●), 12.5 μg/ml (▲), and 50 μg/ml (■) of λSA2, or 100 μg/ml (△) and 200 μg/ml (□) of B30, or buffer as control (X). c S. agalactiae with 100 μg/ml of λSA2 (■) or B30 (□) or buffer as control (X). d S. uberis with 100 μg/ml of λSA2 (■) or B30 (□) or buffer as control (X). Dashed horizontal lines indicate detection limits. Values are means of triplicate platings with standard deviations indicated by error bars
The λSA2 and B30 lysins reduce streptococcal concentrations and inflammation in infected murine mammary glands
To further elucidate the potential of the λSA2 and B30 lysins as therapeutics against Streptococcus-induced bovine mastitis, their efficacy at reducing intramammary bacterial numbers and their effect on inflammatory indicators in a mouse model of bovine mastitis were determined. Female C57BL6/SJL mice intramammarily infected with bacteria from either of the three species S. dysgalactiae, S. agalactiae, and S. uberis (102 CFU/gland) were treated by infusing λSA2 (25 μg/gland), B30 (250 μg/gland), or a mixture of both enzymes (12.5 + 125 μg/gland) into the teat canal 45 min post infection. The tenfold higher concentration of B30 compared to λSA2 used in these experiments was chosen based on the previously observed lower activity of B30 in plate lysis and milk assays (see Tab. 1, Fig. 4). For analysis of intramammary bacterial numbers and indicators of inflammation (gland wet weight and TNFα concentrations), mice were euthanized 24 hours after infection and mammary glands dissected (only glands that had been successfully infused, as visualized by trypan blue penetration, were used). For all three streptococcal species, bacterial concentrations in the mammary glands were significantly reduced (P < 0.05) by treatment with the individual enzymes (Tables 2, 3, 4), with the exception of B30 used against S. dysgalactiae, where no difference compared to the buffer control was observed (Table 2). This finding was unexpected, as B30 had been shown to be most effective against the same strain in vitro in both plate lysis and milk experiments (see Table 1, Fig. 4). In contrast, both λSA2 and B30 showed surprisingly high efficacy against S. agalactiae (reduction in CFU/mg by 2 and 4.5 log units, respectively; Table 3), even though the tested strain had been demonstrated to be clearly less susceptible in vitro to the action of both enzymes than the S. dysgalactiae and S. uberis strains. Overall, reduction in CFU/mg by individual enzyme applications compared to the buffer control ranged from 1.5 logs (λSA2 vs. S. uberis, Table 4) to 4.5 logs (B30 vs. S. agalactiae, Table 3). Another unexpected finding was that the synergistic effect demonstrated in vitro for the combination of the two endolysins did not hold true for the mouse model experiments. For all three species, the reduction in bacterial numbers caused by the combined application of enzymes was between or lower than those caused by the individual endolysin treatments (Tables 2, 3, 4). Overall, the results obtained for mammary gland wet weights and intramammary TNFα concentrations were consistent with the observed reduction in bacterial numbers. For all species and enzyme treatments, the mean values for the gland wet weights were lower than those of the respective buffer controls, suggesting lower inflammation levels due to decreased edema formation (Wall et al. 2009), even though statistical significance at the 0.05 level could be demonstrated only for λSA2 vs. S. uberis, λSA2 vs. S. agalactiae, and B30 vs. S. agalactiae. A similar trend was shown for mean concentrations of the inflammatory cytokine TNFα, which were lower for all enzyme treatments compared to those of the controls (statistical significance was demonstrated for λSA2 vs. S. dysgalactiae and B30 vs. S. uberis; Tables 2, 3, 4).
Table 2.
Effect of endolysin treatment on mean intramammary bacterial concentrations (Log10 CFU/mg), gland wet weights, and TNFα concentrations of murine mammary glands infected with S. dysgalactiae.
| Treatmenta | Log10 CFU/mg | Wet weight [g] | TNFα [pg/ml] |
|---|---|---|---|
| Buffer | 5.320 ± 0.159 a (12)b | 1.783 ± 0.049 a (9) | 119.82 ± 17.87 a (12) |
| λSA2 | 3.152 ± 0.525 b (10) | 1.621 ± 0.049 a (8) | 45.28 ± 8.55 b (11) |
| B30 | 5.418 ± 0.357 a (7) | 1.698 ± 0.052 a (7) | 83.81 ± 16.10 ab (7) |
| λSA2 + B30 | 4.360 ± 0.410 ab (9) | 1.625 ± 0.046 a (9) | 98.10 ± 19.40 a (9) |
Enzyme concentrations used for the treatment were 25 μg/gland for λSA2, 250 μg/gland for B30, and 12.5 μg/gland (λSA2) + 125 μg/gland (B30) for the combination treatment.
Means and standard errors are shown. Values followed by different letters are significantly different (P < 0.05). The number of glands used for each treatment in the analysis is given in parentheses.
Table 3.
Effect of endolysin treatment on mean intramammary bacterial concentrations (Log10 CFU/mg), gland wet weights, and TNFα concentrations of murine mammary glands infected with S. agalactiae.
| Treatmenta | Log10 CFU/mg | Wet weight [g] | TNFα [pg/ml] |
|---|---|---|---|
| Buffer | 5.627 ± 0.117 a (20)b | 1.709 ± 0.025 a (20) | 182.35 ± 43.45 a (20) |
| λSA2 | 3.637 ± 0.295 b (20) | 1.625 ± 0.025 b (20) | 98.30 ± 18.20 a (20) |
| B30 | 1.076 ± 1.104 b (7) | 1.616 ± 0.031 b (9) | 95.53 ± 12.43 a (10) |
| λSA2 + B30 | 2.558 ± 0.622 b (11) | 1.647 ± 0.028 ab (12) | 93.94 ± 21.59 a (12) |
Enzyme concentrations used for the treatment were 25 μg/gland for λSA2, 250 μg/gland for B30, and 12.5 μg/gland (λSA2) + 125 μg/gland (B30) for the combination treatment.
Means and standard errors are shown. Values followed by different letters are significantly different (P < 0.05). The number of glands used for each treatment in the analysis is given in parentheses.
Table 4.
Effect of endolysin treatment on mean intramammary bacterial concentrations (Log10 CFU/mg), gland wet weights, and TNFα concentrations of murine mammary glands infected with S. uberis.
| Treatmenta | Log10 CFU/mg | Wet weight [g] | TNFα [pg/ml] |
|---|---|---|---|
| Buffer | 5.913 ± 0.340 a (11)b | 1.780 ± 0.034 a (11) | 186.40 ± 32.80 a (11) |
| λSA2 | 4.432 ± 0.368 b (10) | 1.690 ± 0.035 b (10) | 164.20 ± 29.01 ab (10) |
| B30 | 3.816 ± 0.340 b (11) | 1.743 ± 0.033 ab (11) | 78.20 ± 18.64 b (11) |
| λSA2 + B30 | 4.559 ± 0.403 ab (8) | 1.694 ± 0.035 ab (10) | 109.00 ± 24.39 ab (10) |
Enzyme concentrations used for the treatment were 25 μg/gland for λSA2, 250 μg/gland for B30, and 12.5 μg/gland (λSA2) + 125 μg/gland (B30) for the combination treatment.
Means and standard errors are shown. Values followed by different letters are significantly different (P < 0.05). The number of glands used for each treatment in the analysis is given in parentheses.
Discussion
Although streptococcal species currently show higher susceptibility to antibiotic mastitis therapy than S. aureus (Notebaert and Meyer 2006), antibiotic resistance among streptococcal mastitis isolates has been reported (Gao et al. 2012). Furthermore, the effect of conventional broad-range antibiotics on commensal bacteria and the added risk they create for resistant strain development among both pathogenic and commensal populations make the isolation and characterization of novel antimicrobials that are refractory to resistance formation highly desirable. In view of a potential application as anti-mastitis agents, in this work the streptococcal phage λSA2 and B30 endolysins were compared for their biochemical properties and demonstrated to be active in cow milk and in murine mammary glands against representative strains from the three most relevant mastitis-causing streptococcal species. To our knowledge, this study represents the first time that efficacy of phage endolysins against Streptococcus-induced mastitis has been demonstrated. Both endolysins used here have previously been partially characterized in vitro, and their activity against multiple streptococcal species and strains has been demonstrated (Donovan et al. 2006a; Donovan and Foster-Frey 2008; Pritchard et al. 2004). It should be pointed out that the B30 lysin exhibited considerably weaker activity than λSA2 against all three species used in this study when applied at similar concentrations. Therefore, the vastly higher concentrations required of this enzyme to achieve effects comparable to those of λSA2 bring into question its suitability for practical application as an anti-mastitis agent.
Various previous studies have used the mouse model of bovine mastitis for investigating Streptococcus-induced mammary gland infections and the efficacy of different agents for preventing or treating them (Anderson and Craven 1984; Chandler 1970a; Demon et al. 2013; Rowson et al. 2011), even though the body of literature dealing with streptococcal infections is still relatively small compared to the multitude of publications available on mouse models of S. aureus-induced bovine mastitis (reviewed in Brouillette and Malouin 2005). Similar parameters reported in previous studies (with both streptococcal and staphylococcal species) were also applied in this work, such as the number of bacteria used as inoculum (~102 CFU per gland)(Chandler 1970b; Demon et al. 2013; Rowson et al. 2011). Similar to what has been reported for S. aureus infections (Brouillette and Malouin 2005; Schmelcher et al. 2012b), also in Streptococcus-induced mastitis, the level of infection is largely independent of the bacterial concentration of the inoculum, and maximal infection with bacterial numbers larger than 108 CFU per gland can be reached even with relatively low-concentrated inocula (Chandler 1970a). This is consistent with our finding that for all three species, bacterial numbers in the buffer controls exceeded 105 CFU/mg (corresponding to > 108 CFU/gland) at 24 hours after infection (see Tables 2, 3, 4). Even though mice infected with streptococci tend to show fewer clinical signs compared to S. aureus-infected animals at comparable bacterial loads (Chandler 1970a)(an observation also made in this study compared to our previous work with S. aureus (Schmelcher et al. 2012b)), the consequences of bacterial infection at the mammary gland level have been described to be very similar between these two genera, as evident by enlargement of glands, accumulation of cellular debris, and tissue infiltration with neutrophils and lymphoid cells (Chandler 1970a). Given these similarities, it is encouraging that the λSA2 endolysin showed similar efficacy against streptococcal infections (~2 log reduction) as the strong peptidoglycan hydrolase antimicrobial lysostaphin (Schindler and Schuhardt 1964; Wall et al. 2005) against S. aureus in a similar model and at identical concentrations (25 μg/gland) (Schmelcher et al. 2012b).
In view of the results of the checkerboard assay with the λSA2 and B30 lysins (Fig. 3) and the previously demonstrated synergy of staphylococcal peptidoglycan hydrolases against S. aureus both in vitro (in a similar assay) and in vivo (Schmelcher et al. 2012b), it was unexpected that no synergistic effect was observed between the two streptococcal lysins in the mouse model in this study. The ΣFIA of 0.42 obtained for the λSA2 and B30 lysins in vitro corresponds well with ΣFIC and ΣFIA values reported previously for other peptidoglycan hydrolases (Becker et al. 2008; Loeffler and Fischetti 2003; Schmelcher et al. 2012b) and indicates a strong synergistic effect, which was not surprising given the different peptidoglycan cleavage sites of the two enzymes (Fig. 1)(Pritchard et al. 2004; Pritchard et al. 2007). In general, such synergistic interactions can be explained by increased destructive effects on the three-dimensional peptidoglycan network when multiple unique bonds are attacked simultaneously, or by enhanced access to the cleavage site of one enzyme as a consequence of the catalytic action of the other (Loeffler and Fischetti 2003; Schmelcher et al. 2012b). However, it is not new that different methodologies can lead to vastly different results when analyzing interactions between two antimicrobial compounds (Lewis et al. 2002; Odds 2003), which might explain the seemingly contradictory results of this study, and underlines the necessity of using more than one method when characterizing such interactions. Nevertheless, the finding that synergy between the λSA2 and B30 lysins cannot be observed in a mastitis treatment situation does not necessarily preclude the possibility of capitalizing on the positive interaction of these two enzymes for other applications.
Similarly, the observation that in vitro activities of antimicrobials do not always correlate with their efficacy inside the mammary gland has been reported previously by several groups (Apparao et al. 2009; Demon et al. 2013; Demon et al. 2012; Pyorala 2009). Multiple factors influenced by the complex environment inside a mammary gland, such as altered growth characteristics of the pathogens, interaction of bacteria and host immune cells, and pharmacokinetic parameters of the drug, are known to affect the efficacy of antimicrobials administered intramammarily (Demon et al. 2013). Both the results of the aforementioned studies and the findings of our work (e.g., the inefficacy of the B30 lysin against S. dysgalactiae in vivo despite activity against this strain in several in vitro/ex vivo assays; the unexpectedly high efficacy of both lysins against S. agalactiae in vivo despite low susceptibility of the same strain in vitro; and the absence of synergy in vivo) corroborate the importance of in vivo experiments for evaluating the therapeutic potential of new anti-mastitis drugs.
Nevertheless, ex vivo cow milk assays can provide valuable information in addition to in vivo studies in the mouse, since the compositions of mouse and cow milk are not identical. Even though anti-mastitis therapy is frequently carried out on dried-off or milk-depleted udders, residual milk components may still interfere with enzymatic activity (Schmelcher et al. 2012b). The results of both in vivo and ex vivo experiments taken together may allow a more sophisticated evaluation of the potential of an antimicrobial as anti-mastitis therapeutic than either of them alone. The high activity of the λSA2 lysin in cow milk (as opposed to B30, whose activity had previously been shown to be reduced by half or more even in the presence of whey (Donovan et al. 2006a)) corroborates its potential for application in cows. It should be noted, however, that heat-treated, homogenized milk was used in this study in order to enhance reproducibility and avoid the potentially complex background flora present in raw milk. The activity of the lysins may be different in raw milk, since heat-treatment is known to affect the interaction of bacterial cells with certain milk components (Kuang et al. 2009; O’Flaherty et al. 2005). Despite a several-log reduction of bacterial numbers in milk by addition of λSA2, subsequent resumption of bacterial growth was observed during the course of most of the milk experiments (Fig. 4). Since a 3 h experiment is most likely too short for outgrowth of an endolysin-resistant subpopulation of streptococci, the most plausible explanation for this effect is rapid inactivation or reduction in availability of the endolysins within the complex environment of whole milk during the time period of the assay. Binding to milk components has been shown or suggested for both bacteriophage particles and endolysins (Celia et al. 2007; Donovan et al. 2006a; O’Flaherty et al. 2005), and this may also explain why relatively high enzyme concentrations are required in milk compared to buffer (Donovan and Foster-Frey 2008) for effective reduction of bacterial concentrations. Possible solutions for this problem include administration of a single high dose in order to reduce the bacterial load to concentrations low enough to be controlled by the cow’s immune system; or repeated application of the enzyme.
Besides therapy of bovine mastitis, streptococcal phage endolysins may find various other applications in the fields of medicine and biotechnology (Fischetti 2005; Schmelcher et al. 2012a), and the comparative biochemical characterization performed in this study with the λSA2 and B30 lysins may provide valuable preliminary information for further research. The results obtained here are in good agreement with previously published data (Donovan and Foster-Frey 2008; Pritchard et al. 2004), with the exception of the pH-dependence of λSA2 activity. In the earlier study (performed in a different buffer and with S. agalactiae instead of S. dysgalactiae), λSA2 had maintained its activity across a broad pH range (pH 5.5 – 9.5)(Donovan and Foster-Frey 2008), whereas in this work we saw optimum activity at pH 7 – 7.5, with considerably lower activity at pH 5.5 (< 20%), 9 (< 40%), and 10 (< 10%) (Fig. 2b). These differences may be explained not only by effects of the buffer on the enzyme itself, but parameters such as pH or ionic strength might also change conformation of bacterial surface molecules in a species-specific manner, thereby increasing or restricting access of the lytic protein to its cell wall binding domain ligand and/or its cut site in the peptidoglycan.
Overall, the results of this work suggest that bacteriophage endolysins hold promise as a novel class of antimicrobials for intramammary therapy of Streptococcus-induced mastitis in cows. It should be pointed out that mouse models and ex vivo experiments in cow milk cannot replace studies in cows. However, as previously discussed (Demon et al. 2012; Schmelcher et al. 2012b), they provide valuable intermediate data and help predict the efficacy of novel antimicrobials in the target species. In this regard, the λSA2 endolysin characterized here may be a promising candidate for experiments in cows.
Acknowledgments
Mentioning of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture (USDA). The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. USDA is an equal opportunity provider and employer.
Funding
This work was supported in part by National Institutes of Health grant 1RO1AI075077-01A1; National Research Initiative grant 2007-35204-18395 and United States State Department funds supporting United States-Pakistani and United States-Russian collaborations.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Statement regarding research on animals: All animal experiments were conducted in accordance with ARS Institutional Animal Care and Use Committee regulations and national animal care guidelines.
References
- Anderson JC, Craven N. Assessment in the mouse of cefoperazone as a treatment for mastitis. Vet Rec. 1984;114:607–12. doi: 10.1136/vr.114.25.607. [DOI] [PubMed] [Google Scholar]
- Apparao MD, Ruegg PL, Lago A, Godden S, Bey R, Leslie K. Relationship between in vitro susceptibility test results and treatment outcomes for gram-positive mastitis pathogens following treatment with cephapirin sodium. J Dairy Sci. 2009;92:2589–97. doi: 10.3168/jds.2008-1693. [DOI] [PubMed] [Google Scholar]
- Bateman A, Rawlings ND. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends BiochemSci. 2003;28:234–237. doi: 10.1016/S0968-0004(03)00061-6. [DOI] [PubMed] [Google Scholar]
- Becker SC, Foster-Frey J, Donovan DM. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol Lett. 2008;287:185–191. doi: 10.1111/j.1574-6968.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- Becker SC, Foster-Frey J, Stodola AJ, Anacker D, Donovan DM. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene. 2009;443:32–41. doi: 10.1016/j.gene.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Blowey RW, Edmondson P. Mastitis Control in Dairy Herds. CABI; Cambridge: 2010. [Google Scholar]
- Borysowski J, Weber-Dabrowska B, Gorski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med (Maywood) 2006;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- Bramley AJ, Foster R. Effects of lysostaphin on Staphylococcus aureus infections of the mouse mammary gland. Res Vet Sci. 1990;49:120–121. [PubMed] [Google Scholar]
- Brouillette E, Grondin G, Lefebvre C, Talbot BG, Malouin F. Mouse mastitis model of infection for antimicrobial compound efficacy studies against intracellular and extracellular forms of Staphylococcus aureus. Vet Microbiol. 2004;101:253–262. doi: 10.1016/j.vetmic.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brouillette E, Malouin F. The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect. 2005;7:560–568. doi: 10.1016/j.micinf.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Calvinho LF, Almeida RA, Oliver SP. Potential virulence factors of Streptococcus dysgalactiae associated with bovine mastitis. Vet Microbiol. 1998;61:93–110. doi: 10.1016/s0378-1135(98)00172-2. [DOI] [PubMed] [Google Scholar]
- Cattell MB, Dinsmore RP, Belschner AP, Carmen J, Goodell G. Environmental gram-positive mastitis treatment: in vitro sensitivity and bacteriologic cure. J Dairy Sci. 2001;84:2036–2043. doi: 10.3168/jds.S0022-0302(01)74647-4. [DOI] [PubMed] [Google Scholar]
- Celia LK, Nelson D, Kerr DE. Characterization of a bacteriophage lysin (Ply700) from Streptococcus uberis. Vet Microbiol. 2008;130:107–117. doi: 10.1016/j.vetmic.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Chandler RL. Experimental bacterial mastitis in the mouse. J Med Microbiol. 1970a;3:273–282. doi: 10.1099/00222615-3-2-273. [DOI] [PubMed] [Google Scholar]
- Chandler RL. Ultrastructural pathology of mastitis in the mouse. A study of experimental staphylococcal and streptococcal infections. Br J Exp Pathol. 1970b;51:639–45. [PMC free article] [PubMed] [Google Scholar]
- Chandler RL. Studies on experimental mouse mastitis relative to the assessment of pharmaceutical substances. J Comp Pathol. 1971;81:507–514. doi: 10.1016/0021-9975(71)90078-8. [DOI] [PubMed] [Google Scholar]
- Deluyker HA, Van Oye SN, Boucher JF. Factors affecting cure and somatic cell count after pirlimycin treatment of subclinical mastitis in lactating cows. J Dairy Sci. 2005;88:604–614. doi: 10.3168/jds.S0022-0302(05)72724-7. [DOI] [PubMed] [Google Scholar]
- Demon D, Breyne K, Schiffer G, Meyer E. Short communication: Antimicrobial efficacy of intramammary treatment with a novel biphenomycin compound against Staphylococcus aureus, Streptococcus uberis, and Escherichia coli-induced mouse mastitis. J Dairy Sci. 2013;96:7082–7. doi: 10.3168/jds.2013-7011. [DOI] [PubMed] [Google Scholar]
- Demon D, Ludwig C, Breyne K, Guede D, Dorner JC, Froyman R, Meyer E. The intramammary efficacy of first generation cephalosporins against Staphylococcus aureus mastitis in mice. Vet Microbiol. 2012;160:141–50. doi: 10.1016/j.vetmic.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Diarra MS, Petitclerc D, Deschenes E, Lessard N, Grondin G, Talbot BG, Lacasse P. Lactoferrin against Staphylococcus aureus mastitis. Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet Immunol and Immunopathol. 2003;95:33–42. doi: 10.1016/s0165-2427(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Donovan DM. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat Biotechnol. 2007;1:113–122. doi: 10.2174/187220807780809463. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Becker SC, Dong S, Baker JR, Foster-Frey J, Pritchard DG. Peptidoglycan hydrolase enzyme fusions for treating multi-drug resistant pathogens. Biotech International. 2009;21:6–10. [Google Scholar]
- Donovan DM, Dong S, Garrett W, Rousseau GM, Moineau S, Pritchard DG. Peptidoglycan hydrolase fusions maintain their parental specificities. Appl Environ Microbiol. 2006a;72:2988–2996. doi: 10.1128/AEM.72.4.2988-2996.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Foster-Frey J. LambdaSa2 prophage endolysin requires Cpl-7-binding domains and amidase-5 domain for antimicrobial lysis of streptococci. FEMS Microbiol Lett. 2008;287:22–33. doi: 10.1111/j.1574-6968.2008.01287.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Foster-Frey J, Dong S, Rousseau GM, Moineau S, Pritchard DG. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl Environ Microbiol. 2006b;72:5108–5112. doi: 10.1128/AEM.03065-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Lardeo M, Foster-Frey J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett. 2006c;265:133–139. doi: 10.1111/j.1574-6968.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Fischetti VA. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 2005;13:491–496. doi: 10.1016/j.tim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Gao J, Yu FQ, Luo LP, He JZ, Hou RG, Zhang HQ, Li SM, Su JL, Han B. Antibiotic resistance of Streptococcus agalactiae from cows with mastitis. Vet J. 2012;194:423–4. doi: 10.1016/j.tvjl.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Garcia P, Garcia JL, Garcia E, Sanchez-Puelles JM, Lopez R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990;86:81–88. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- Gaucheron F. The minerals of milk. Reprod Nutr Dev. 2005;45:473–83. doi: 10.1051/rnd:2005030. [DOI] [PubMed] [Google Scholar]
- Gehring R, Smith GW. An overview of factors affecting the disposition of intramammary preparations used to treat bovine mastitis. J Vet Pharmacol Ther. 2006;29:237–241. doi: 10.1111/j.1365-2885.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:2743–50. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruet P, Maincent P, Berthelot X, Kaltsatos V. Bovine mastitis and intramammary drug delivery: review and perspectives. Adv Drug Deliv Rev. 2001;50:245–259. doi: 10.1016/s0169-409x(01)00160-0. [DOI] [PubMed] [Google Scholar]
- Hermoso JA, Garcia JL, Garcia P. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol. 2007;10:461–472. doi: 10.1016/j.mib.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Kerr DE, Plaut K, Bramley AJ, Williamson CM, Lax AJ, Moore K, Wells KD, Wall RJ. Lysostaphin expression in mammary glands confers protection against staphylococcal infection in transgenic mice. Nat Biotechnol. 2001;19:66–70. doi: 10.1038/83540. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Jia H, Miyanaga K, Tanji Y. Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl Microbiol Biotechnol. 2009;84:135–142. doi: 10.1007/s00253-009-2008-6. [DOI] [PubMed] [Google Scholar]
- Lammers A, van Vorstenbosch CJ, Erkens JH, Smith HE. The major bovine mastitis pathogens have different cell tropisms in cultures of bovine mammary gland cells. Vet Microbiol. 2001;80:255–265. doi: 10.1016/s0378-1135(01)00305-4. [DOI] [PubMed] [Google Scholar]
- Leigh JA. Streptococcus uberis: a permanent barrier to the control of bovine mastitis? Vet J. 1999;157:225–38. doi: 10.1053/tvjl.1998.0298. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Diekema DJ, Messer SA, Pfaller MA, Klepser ME. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J Antimicrob Chemother. 2002;49:345–51. doi: 10.1093/jac/49.2.345. [DOI] [PubMed] [Google Scholar]
- Loeffler JM, Fischetti VA. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother. 2003;47:375–377. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler JM, Nelson D, Fischetti VA. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B, Keen CL, Hurley LS. Iron, copper, zinc, and manganese in milk. Annu Rev Nutr. 1981;1:149–74. doi: 10.1146/annurev.nu.01.070181.001053. [DOI] [PubMed] [Google Scholar]
- Lopez R, Garcia E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol Rev. 2004;28:553–580. doi: 10.1016/j.femsre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Notebaert S, Meyer E. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet Q. 2006;28:2–13. doi: 10.1080/01652176.2006.9695201. [DOI] [PubMed] [Google Scholar]
- O’Flaherty S, Coffey A, Meaney WJ, Fitzgerald GF, Ross RP. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett Appl Microbiol. 2005;41:274–279. doi: 10.1111/j.1472-765X.2005.01762.x. [DOI] [PubMed] [Google Scholar]
- Obeso JM, Martinez B, Rodriguez A, Garcia P. Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int J Food Microbiol. 2008;128:212–218. doi: 10.1016/j.ijfoodmicro.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother. 2011;55:738–44. doi: 10.1128/AAC.00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Aravind L, Schultz J, Bork P, Koonin EV. Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J Mol Biol. 1999;289:729–745. doi: 10.1006/jmbi.1999.2827. [DOI] [PubMed] [Google Scholar]
- Pritchard DG, Dong S, Baker JR, Engler JA. The bifunctional peptidoglycan lysin of Streptococcus agalactieae bacteriophage B30. Microbiology. 2004;150:2079–2087. doi: 10.1099/mic.0.27063-0. [DOI] [PubMed] [Google Scholar]
- Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl Environ Microbiol. 2007;73:7150–7154. doi: 10.1128/AEM.01783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyorala S. Treatment of mastitis during lactation. Ir Vet J. 2009;62(Suppl 4):S40–44. doi: 10.1186/2046-0481-62-S4-S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt P, Schwarz C, Donzeau M. Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr Purif. 2006;46:483–488. doi: 10.1016/j.pep.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Rowson AD, Wang YQ, Aalseth E, Forsberg NE, Puntenney SB. Effects of an immunomodulatory feed additive on the development of mastitis in a mouse infection model using four bovine-origin isolates. Animal. 2011;5(2):220–9. doi: 10.1017/S1751731110001850. [DOI] [PubMed] [Google Scholar]
- Sanchez MS, Ford CW, Yancey RJ., Jr Effect of tumor necrosis factor-alpha, interleukin-1 beta, and antibiotics on the killing of intracellular Staphylococcus aureus. J Dairy Sci. 1994;77:1251–1258. doi: 10.3168/jds.S0022-0302(94)77064-8. [DOI] [PubMed] [Google Scholar]
- Schindler CA, Schuhardt VT. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc Natl Acad Sci U S A. 1964;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012a;7:1147–71. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M, Powell AM, Becker SC, Camp MJ, Donovan DM. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl Environ Microbiol. 2012b;78:2297–305. doi: 10.1128/AEM.07050-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M, Shabarova T, Eugster MR, Eichenseher F, Tchang VS, Banz M, Loessner MJ. Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl Environ Microbiol. 2010;76:5745–5756. doi: 10.1128/AEM.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- Sordillo LM, Streicher KL. Mammary gland immunity and mastitis susceptibility. J Mammary Gland Biol Neoplasia. 2002;7:135–146. doi: 10.1023/a:1020347818725. [DOI] [PubMed] [Google Scholar]
- Tenhagen BA, Koster G, Wallmann J, Heuwieser W. Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Brandenburg, Germany. J Dairy Sci. 2006;89:2542–51. doi: 10.3168/jds.S0022-0302(06)72330-X. [DOI] [PubMed] [Google Scholar]
- Wall R, Powell A, Sohn E, Foster-Frey J, Bannerman D, Paape M. Enhanced host immune recognition of mastitis causing Escherchia coli in CD-14 transgenic mice. Anim Biotechnol. 2009;20:1–14. doi: 10.1080/10495390802594206. [DOI] [PubMed] [Google Scholar]
- Wall RJ, Powell A, Paape MJ, Kerr DE, Bannerman DD, Pursel VG, Wells KD, Talbot N, Hawk HW. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat Biotechnol. 2005;23:445–451. doi: 10.1038/nbt1078. [DOI] [PubMed] [Google Scholar]
- Walsh C. Where will new antibiotics come from? Nat Rev Microbiol. 2003;1:65–70. doi: 10.1038/nrmicro727. [DOI] [PubMed] [Google Scholar]
- Webb BH, Johnson AH, Alford JA. Fundamentals of dairy chemistry. AVI Publishing Co; Westport: 1974. [Google Scholar]
- Whisstock JC, Lesk AM. SH3 domains in prokaryotes. Trends Biochem Sci. 1999;24:132–133. doi: 10.1016/s0968-0004(99)01366-3. [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Gonzalez RN, Das HH. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J Dairy Sci. 1997;80:2592–2598. doi: 10.3168/jds.S0022-0302(97)76215-5. [DOI] [PubMed] [Google Scholar]
- Yancey RJ., Jr Vaccines and diagnostic methods for bovine mastitis: fact and fiction. Adv Vet Med. 1999;41:257–273. doi: 10.1016/s0065-3519(99)80020-2. [DOI] [PubMed] [Google Scholar]