Abstract
PGG beta glucan is a Saccharomyces cerevisiae derived 1,3/1,6 glucose polymer with innate immune system activation potential. This phase I/II clinical trial enrolled 20 eligible CLL patients with high-risk biological markers for early initial treatment with alemtuzumab, rituximab, and PGG beta glucan (1-2-4 mg/kg/dose) over 31 days. PGG beta glucan at 4 mg/kg was well tolerated and used for the phase II study. There were three grade 3-4 toxicities at least possibly attributed to treatment. Nineteen (95%) patients responded to treatment with 13 (65%) complete responses. All patients were alive at a median follow-up of 24.4 months (range: 9.5–37). Eleven patients had progressive disease (median 17.6 months, 95% CI: 9.7-32.1) and eight patients were retreated (median 35.3 months, 95% CI: 17.9– not reached). We conclude that PGG beta glucan, alemtuzumab, and rituximab treatment is tolerable and results in a high complete response rate.
Keywords: Chronic lymphocytic leukemia/small lymphocytic lymphoma, CLL, high risk, PGG beta glucan, alemtuzumab, rituximab
Introduction
Unconjugated monoclonal antibodies (mAb) have a well-established role in the management of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL). However, monotherapy therapy with either rituximab or alemtuzumab rarely achieves a complete or sustained response and is not curative. Combination therapy with alemtuzumab and rituximab is effective and tolerable resulting in high complete response rates in phase II studies [1-5]. Although these response rates appear to be better than those reported previously for monotherapy with either mAb [1-5], there are no reported randomized studies comparing therapy with alemtuzumab alone with the combination of alemtuzumab and rituximab. In addition, the outcomes for both of these regimens are suboptimal, and even patients who achieve a complete response (CR) to therapy have a relatively short duration of response. Novel drug combinations that improve mAb efficacy without increasing toxicity could be highly beneficial in the treatment of patients with CLL.
The cytotoxic effects of alemtuzumab and rituximab are primary mediated by the innate immune system [6,7]. Treatment efficacy could thus be improved by interventions that increase the cytotoxic capacity of the innate immune system. We have previously reported that adding GM-CSF to alemtuzumab and rituximab for early therapy of patients with high risk CLL in a phase II was not sufficiently promising to support further testing of this combination [4]. We now report the results of a phase I/II clinical trial testing the efficacy and safety of combining PGG beta glucan with alemtuzumab and rituximab in the early treatment of patients with high risk CLL.
The human innate immune system has evolved to recognize and respond to fungal antigens [8]. Yeast cell wall carbohydrate beta glucan can activate the innate immune system as reviewed by Chan et al [8]. Larger and insoluble beta glucan molecules activate mediators of the innate immune system by ligating Toll like receptor 2 (TLR2), TLR6, and Dectin-1. In contrast, the smaller soluble beta glucan molecules produced by macrophage digestion of larger beta glucan molecules, bind complement receptor 3 (CR3) increasing its affinity for the complement component 3 (C3) metabolite iC3b [8-10]. Binding of soluble low molecular weight beta glucan with CR3 has been shown to increase monoclonal antibody mediated complement dependent cellular cytotoxicity of tumor cells in animal models of malignancy [11]. Use of soluble low molecular weigh beta glucan could thus potentially increase the efficacy of mAb therapy in patients with CLL.
PGG beta glucan (Imprime PPG, Biothera, Eagan MN) is an intravenous (IV) formulation of a 1,3/1,6 glucose polymer prepared from a strain of Saccharomyces cerevisiae. This low molecular weight soluble beta glucan has been developed as an adjunct to complement activating mAb (Biothera Investigator's Brochure, Eagan MN, January, 2010). Two phase I studies in healthy subjects and one phase I/II study with PGG beta glucan in combination with G-CSF showed no serious adverse events (Imprime PPG Investigators Brochure, Biothera, Eagan, MN). PGG beta glucan at doses up to 6 mg/kg was tolerated in clinical trials in combination regimens including cetuximab or bevacizumab in treatment of colon and non-small cell lung cancer (Imprime PPG Investigators Brochure). At the time that this study was planned, there was no published evidence to suggest that beta glucan could stimulate the growth of CLL cells. However, a recently published study showed that CLL cells from a small subset of patients (~0.3%) expressing a somatically hypermutated IGHV utilizing VH3-7 have a B cell receptor that ligates yeast derived beta glucans resulting in increased proliferation in culture [12]. The ability of PGG beta glucan to interact with these cells has not been tested.
We now report that the addition of PGG beta glucan to therapy with alemtuzumab and rituximab was well tolerated and resulted in high CR rates.
Methods
This phase I/II study (LS1084) was conducted at Mayo Clinic Rochester and the University of Iowa with the approval of both Institutional Review Boards according to the principles of the Declaration of Helsinki and was registered with ClinicalTrials.gov (NCT01269385). Rituximab and alemtuzumab were used for early treatment of patients with high risk CLL as previously described [3,4]. The primary aims of this study were to determine the maximum tolerated dose of PGG beta glucan in combination with alemtuzumab and rituximab (phase I) and to assess the CR rate of patients with high risk early-intermediate stage CLL treated with alemtuzumab, rituximab, and PGG beta glucan before meeting standard NCI-IWCLL criteria for treatment [13] (phase II). The secondary aims were to evaluate treatment toxicity, measure the overall response rate (ORR) and duration of response using time to disease progression, time to next treatment (TTT), and overall survival (OS).
All qualifying sequential consenting patients were enrolled into this clinical trial at the Mayo Clinic Rochester. Inclusion criteria included no prior treatment for CLL, age ≥ 18 years, a diagnosis of CLL based on standard immunophenotypic criteria [13] with an absolute blood lymphocyte count of > 5 × 109/L, and an interphase fluorescent in situ hybridization (FISH) analysis negative for IGH/CCND1 and/or immunostaining negative for cyclin D1 expression. High-risk status was defined as at least one of the following poor prognostic factors determined in a hierarchical manner: 17p13 deletion; 11q22.3 deletion; unmutated IGHV (< 2%) or VH3-21 gene segment usage (irrespective of IGHV mutation status) together with either CD38 expression (≥30%) and/or ZAP70 expression (≥20%). Early treatment was defined as therapy of patients with Rai [14] stage 0-II CLL that did not meet standard NCI-IWCLL criteria for therapy of their disease [13] and had limited clinical disease burden (no lymph nodes > 5 cm in any diameter, splenomegaly < 6 cm below the left costal margin in the midclavicular line at rest on clinical examination). Patients required adequate organ function (creatinine <1.5 x upper limit of normal (UNL), bilirubin <3.0 x UNL) and ECOG performance status of 0-2. Exclusion criteria included New York Heart Association Class III or IV heart disease, recent myocardial infarction (<1 month), pregnancy, uncontrolled infection, and infection with the human immunodeficiency virus (HIV/AIDS), serological evidence of active hepatitis B or C infection, active autoimmune complications, or other active primary malignancy requiring treatment or limiting survival to <2 years.
Therapy
PGG beta glucan was given IV on days 1, 5, 10, 17, 24, and 31. The starting dose (dose level 0) in the phase I study was 1 mg/kg, dose level 1 was 2mg/kg/dose and dose level 2 was 4 mg/kg/dose. For the first dose of PGG beta glucan patients were premedicated with 1000 mg acetaminophen by mouth (po), 50 mg diphenhydramine po, and 100 mg hydrocortisone IV. Based on the standard phase I trial design, the study was designed to treat a minimum of three and maximum of six patients at each dose level. There was no planned dose escalation in each patient. The same previously described short duration alemtuzumab and rituximab regimen was used for all patients [4]. In brief, patients initiated therapy with subcutaneous (SQ) alemtuzumab therapy daily for a dose escalation from 3-10-30 mg/day on days 3-5 of treatment if tolerated. Subsequent therapy was alemtuzumab 30 mg SQ starting on day 8 and given 3 times a week (Monday-Wednesday-Friday) for 4 weeks. During alemtuzumab dose escalation patients were premedicated with acetaminophen (1000 mg po) and diphenhydramine (50 mg po) and subsequent premedication was used only as required. Competent patients who were tolerating alemtuzumab therapy could be trained to self-administer the drug from the second week of therapy. Rituximab therapy was given at 375 mg/m2/week IV for four doses starting on day 10 of treatment with standard premedication. All patients received herpes virus and Pneumocystis jiroveci prophylaxis during treatment and then for an additional 6 months. All patients had blood testing for cytomegalovirus (CMV) DNA by PCR weekly during therapy and then monthly for 3 months. Patients with detectable circulating CMV DNA were evaluated for clinical evidence of CMV infection. Asymptomatic or mildly symptomatic patients were treated with oral valganciclovir for a minimum of 2 weeks and therapy was continued until weekly CMV DNA testing by polymerase chain reaction was negative on two consecutive occasions. Patients with more severe CMV infections were managed with appropriate anti-CMV therapy and CLL therapy was suspended until the CMV infection had resolved.
Response Evaluation
Patients were evaluated by physical examination and blood testing weekly during treatment, then monthly for 3 months, and then at 6, 9 and 12 months after completing therapy followed by event monitoring every 6 months for up to 4 more years. Treatment toxicity was evaluated using NCI Common Terminology Criteria for Adverse Events v4.0 except for anemia, thrombocytopenia, and neutropenia which were graded according to the grading scale for hematologic toxicity in CLL studies [13]. Increased white blood cell counts due to CLL related lymphocytosis and treatment induced lymphocytopenia were not reported as treatment toxicity.
Dose-limiting toxicity (DLT) was defined as an adverse event not clearly related to disease progression or intercurrent illness and meeting at least one of the following criteria: 1. Absolute neutrophil count (ANC) ≤ 0.5 × 109/L for more than 5 days; 2. Febrile neutropenia of any duration (ANC <1.0 ×109/L and fever ≥ 38.5°C); 3. Grade 4 thrombocytopenia or grade 3 thrombocytopenia with bleeding or any requirement for platelet transfusion; 4. Grade 4 anemia; 5. Any non-hematologic grade 3 as per NCI Common Terminology Criteria for Adverse Events v4.0.
Response evaluation used the NCI-IWCLL criteria of 2008 [13] with no imaging for initial CLL staging or evaluation of response to treatment. CR required all of the following for a period of at least 2 months: 1. Resolution of palpable lymphadenopathy (lymph nodes >1.5 cm) and hepatosplenomegaly on physical examination; 2. No constitutional symptoms; 3. Complete blood count (CBC) with neutrophils > 1.5 × 109/L, platelets >100 ×109/L, hemoglobin >11.0 g/dL, and absolute lymphocyte count < 4 × 109/L. In addition, a bone marrow biopsy study 3 months after completion of treatment was required to be at least normocellular with <30% lymphocytes and no nodules comprising CLL cells. Patients who fulfilled all criteria for a CR but who had persistent cytopenia not attributable to residual CLL were classified as CR with incomplete marrow recovery (CRi) [13]. A stringent CR (sCR) was defined as a CR with no evidence of residual CLL on a bone marrow study that includes immunohistochemical staining for minimal residual disease sensitive to ~ 1% CLL cells as previously described [4]. Patients who met all the criteria for a CR except for having nodules of CLL cells in the bone marrow were defined as having a nodular partial remission (nPR). Partial response (PR) was defined as a ≥ 50% decrease in ALC and ≥ 50% reduction in the sum of the products of the maximal perpendicular diameters of palpable lymph nodes and size of liver and/or spleen as measured by physical examination as well as a 50% improvement in ANC, platelet counts, and hemoglobin above baseline or ANC > 1.5 × 109/L, platelet count > 100 × 109/L and hemoglobin > 11 g/dL. Progressive disease (PD) was defined as a ≥ 50% increase in the sum of the products of at least 2 lymph nodes on 2 consecutive determinations 2 weeks apart or the appearance of new palpable lymph nodes >1.5 cm or a ≥ 50% increase in the size of the liver and/or spleen as determined by measurement below the respective costal margin on 2 consecutive determinations 2 weeks apart; or appearance of hepatomegaly or splenomegaly which was not previously present at baseline or a ≥ 50% increase in the ALC above the lowest ALC recorded since the start of treatment and with an ALC of at least 5 × 109/L. For patients who achieved a CR or nodular PR, progression was defined as recurrence of a circulating leukemia cell clone with an ALC > 5 × 109/L or recurrence of adenopathy >1.5 cm not due to a tumor flare. Stable disease (SD) was defined as not meeting any of the criteria for response or PD.
Statistical Analysis
The maximum tolerated dose (MTD) of PGG beta glucan was defined as the dose level below the lowest dose that induced DLT in at least one-third of patients (at least 2 of a maximum of 6 new patients). A total of 6 patients treated at the MTD was considered sufficient to identify common toxicities at the MTD. A standard 3+3 phase I study design was used.
The Phase II study was designed to evaluate the safety and efficacy of the treatment regimen. Based on the results of a previous study of this antibody regimen in a comparable patient population in which 37% of patients achieved a complete response [3], we proposed that in the phase II component of the study, a CR rate of 30% would not be of interest and a CR rate of 50% would be considered promising. Using a one-stage three-outcome design with an interim analysis [15] we calculated that a minimum of 17 and a maximum of 39 evaluable patients would be required to test the null hypothesis that the true success proportion is at most 30%.
Time to disease progression was defined as the time from registration to the earliest date of documentation of disease progression. Time to subsequent treatment was defined as the time from registration to the time subsequent therapy was initiated. The distribution of all time-to-event endpoints were estimated using the method of Kaplan-Meier [16].
Results
Twenty-two patients (4 at dose level 0, 4 at dose level 1, and 14 at dose level 2) were enrolled between February 2011 and May 2013 and data was frozen on September 23, 2014. Two patients enrolled in the phase I component of the trial were replaced. One patient on dose level 0 developed neutropenia mandating holding of therapy which did not occur as required per protocol, and one patient on dose level 1 had an extensive cutaneous hypersensitivity reaction attributed to alemtuzumab, and all therapy was stopped. The 20 evaluable patients are included in this analysis and their characteristics and treatment are summarized in Tables I and II. Patients were considered to have high risk CLL because of 17p13 deletion in 5 (one of these patients also had 11q22 deletion), 11q22 deletion in 7, unmutated IGHV + ZAP70 positive in 7 (all negative for expression of CD38), and VH3-21 (mutated) + ZAP70 positive (CD38 negative) in 1.
Table I.
Patient Demographics
| Factor | N (%) |
|---|---|
| Age, median (Min, Max) | 61 (47, 77) |
| Male | 14 (70%) |
| Rai Stage | |
| 0 | 7 (35%) |
| 1 | 10 (50%) |
| 2 | 3 (15%) |
| CD38 | |
| Positive | 3 (15%) |
| Negative | 17 (85%) |
| ZAP70 | |
| Positive | 17 (85%) |
| Negative | 3 (15%) |
| IGHV | |
| Unmutated | 14 (70%) |
| Mutated | 5 (25%) |
| Indeterminate | 1 (5%) |
| FISH* | |
| 17p- | 5 (25%) |
| 11q- | 7 (35%) |
| Trisomy 12 | 3 (15%) |
| 13q- | 3 (15%) |
| Negative | 2 (10%) |
Hierarchical classification[18]
Table II.
Treatment Summary
| Phase | Dose Level | # Patients | Beta Glucan Dose | DLTs | Responses |
|---|---|---|---|---|---|
| I | 0 | 3 | 1 mg/kg | 0/3 | 2 CR, 1 PR |
| I | 1 | 3 | 2 mg/kg | 0/3 | 2 CR, 1 nPR |
| I | 2 | 6 | 4 mg/kg | 1/6 | 3 CR, 1 CRi, 2 PR |
| II | 2 | 8 | 4 mg/kg | NA | 1 sCR, 3 CR, 1 CRi, 1 nPR, 1 PR, 1 SD |
CR complete response, sCR stringent complete response, CRi complete response with incomplete marrow recovery, PR partial response, nPR nodular partial response, SD stable disease, NA not applicable, DLT dose limiting toxicity.
Toxicity
Of the 12 evaluable patients for determining the MTD on the phase I component of the study, one dose limiting toxicity was seen at dose level 2 (Table II). This patient had a grade 3 diarrhea possibly related to treatment. Dose level 2 (PGG beta glucan 4mg/kg/dose) was thus used for the phase II component of the study and 8 additional evaluable patients were accrued at this dose level. Three grade 3-4 toxicities at least possibly related to treatment occurred in the 20 evaluable patients (grade 3 diarrhea, grade 4 neutropenia, grade 4 febrile neutropenia). CMV reactivation was detected in 4 (20%) patients at low viral load (< 10,000 copies/ml) and responded well to oral valganciclovir therapy.
Seven patients had therapy interruptions: Four had rituximab first dose reactions, two had PGG beta glucan first dose reactions (infusion reaction n=1, grade 1 chest pressure n=1), and one had an interruption in administration of all three drugs because of grade 3 diarrhea possibly related to treatment. This last patient missed one dose of alemtuzumab. No other doses were missed in any patients. There were no dose reductions for any drugs.
Treatment Response
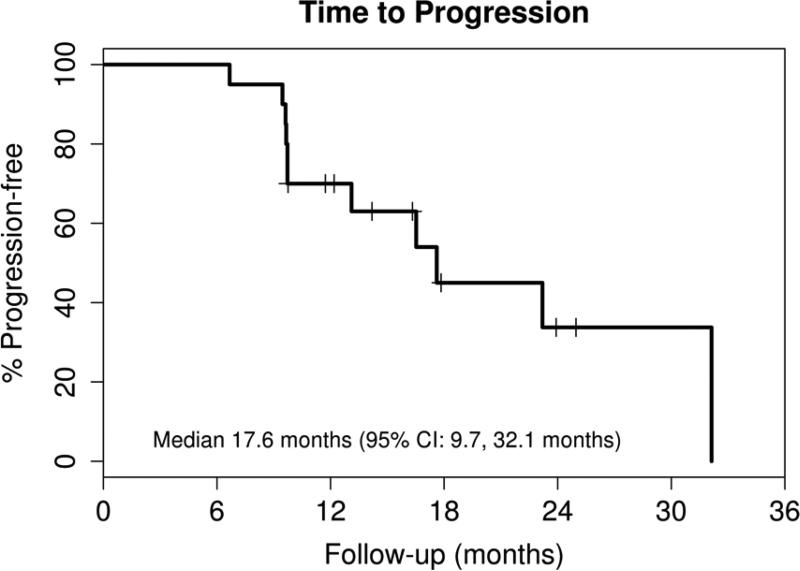
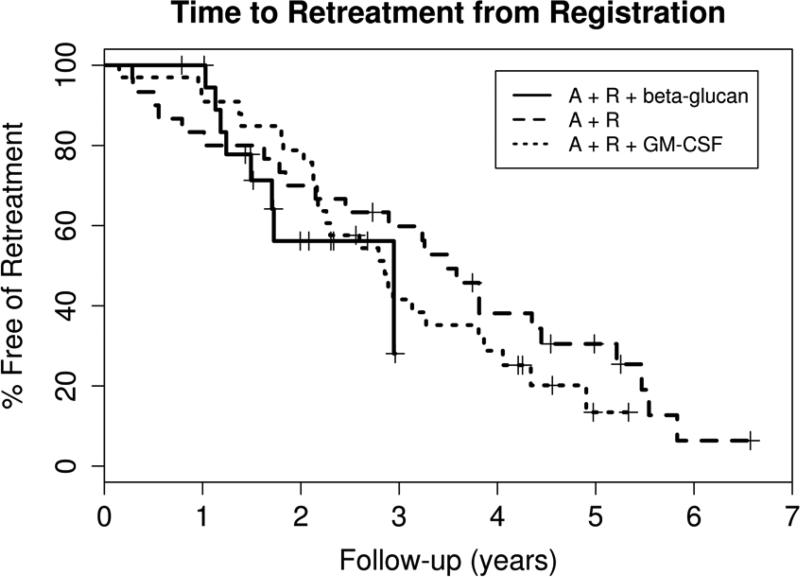
Nineteen patients responded to therapy resulting in an overall response rate of 95% (95% CI: 75, 100) with 13 (65%) CR (CR n=11/CRi n=2)(95% CI: 41, 85) and 2 (10%) nPR (Table II). Only one of the thirteen patients with a CR was negative for minimal residual disease by immunohistochemical staining of the bone marrow (sCR). All patients were alive at the time of this report with a median follow-up of 24.4 months (range: 9.5 – 37 months). Eleven patients have had progressive disease with a median time to progression (TTP) of 17.6 months (95% CI: 9.7-32.1) (Figure 1). Eight patients have been retreated (one before meeting the criteria for progressive disease) with a median time to retreatment of 35.3 months (95% CI: 17.9 – not reached) (Figure 2). The retreatment regimens were fludarabine, cyclophosphamide and rituximab (FCR, n=4), pentostatin, cyclophosphamide and rituximab (PCR, n=2), cyclophosphamide (n=1), and high dose methylprednisolone and rituximab (n=1).
Figure 1. Time to Progression.
Nineteen patients responded to treatment and at a median follow-up duration of 24.4 months (range: 9.5 – 37), 11 have progressive disease as shown by this Kaplan-Meier survival curve.
Figure 2. Time to Retreatment.
The Kaplan-Meier survival curves for time to treatment from three sequential phase II clinical trials using the same 5-week regimen of alemtuzumab and rituximab for early treatment of high risk CLL are plotted for comparison. MCO38G [3] used only alemtuzumab and rituximab (A+R) and enrolled 30 patients with a median time of follow up of 5.2 years (range 2.7–8.2). Twenty-four (80%) patients required retreatment for progressive CLL at a median time of 3.5 years (95% CI 2.1, 4.4). The subsequent trial MC0785 [4] added GM-CSF to the regimen (A+R+GM-CSF) and enrolled 33 patients with a median follow up of 5.0 years (range 3.9-5.6). Twenty-six (79%) of these patients have required retreatment at a median time of 2.9 years (95% CI 2.2, 3.8). The LS1084 study reported in this paper (A+R+ beta-glucan) enrolled 20 eligible patients with a median follow up of 2.0 years (range 0.8 – 3.1). Eight (40%) of these patients have required retreatment with a median time to retreatment of 2.9 years (range 1.5 to not reached).
Discussion
This phase I study shows that PGG beta glucan can be safely combined with alemtuzumab and rituximab for the early therapy of patients with high risk CLL. The limited data set suggests that this combination is effective at achieving a high rate of complete responses. However, because of the limited accrual to the phase II component of the study, these data remain preliminary.
PGG beta glucan was generally well tolerated and the 3 grade 3-4 toxicities at least possibly attributable to the study drug were manageable with no long-term adverse consequences. There were two PGG beta glucan first dose reactions requiring interruptions in drug administration, but both these patients did subsequently receive the full planned dose of therapy. There was no observed increase in the rate of first dose reactions to IV rituximab or SQ alemtuzumab. These data suggest that PGG beta glucan can be safely administered in combination with rituximab and alemtuzumab.
This study was designed to test the null hypothesis that the CR rate is ≤ 30% in 37 patients accrued to the phase II component of the study. The lower limit of the 95% confidence level for the CR rate in all 20 eligible patients was 41%. However, because only 14 patients were treated at the phase II dose level of 4 mg/kg of PGG beta glucan, no conclusions can be made about the efficacy of this regimen.
The major limitation of this study was the low accrual to the phase II component of the trial. Accrual was affected by several factors not directly related to the design or conduct of the study. The interest in alemtuzumab containing clinic trials among treating physicians and CLL patients was substantially decreased following the removal of alemtuzumab from the market in the US by the manufacturers in 2012 for commercial reasons unrelated to the treatment of CLL. Interest in this trial was further decreased by the introduction of highly effective novel targeted B cell receptor pathway targeting drugs, which are considerably less immunosuppressive than alemtuzumab. However, rituximab and later generation anti-CD20 mAb remain of considerable interest in the management of CLL and other B cell malignancies. The data generated by this trial are important for developing combination therapy regimens designed to test if PGG beta glucan can improve the efficacy of anti-CD20 mAb in the treatment of CLL and other B cell malignancies.
The short treatment regimen used in this study achieved a high CR rate according to standard criteria. However, bone marrow biopsy examination using IHC (sensitive to ~ 1% residual CLL) showed that 12 of the 13 (92%) CR patients had residual bone marrow disease. Despite the early treatment of patients with a lower CLL disease burden, the treatment regimen rarely achieved a minimal residual disease negative status. Minimal residual disease negative status evaluation using a more sensitive flow cytometry assay (sensitive to ~ 0.01% residual CLL) has previously been shown to predict a durable response to treatment with alemtuzumab based therapy [17]. In our study, the high rate of detection of minimal residual disease in the patients with CR together with adverse disease biology of the patients enrolled in this study, is likely to contribute to the short duration of response to treatment for most patients. This study was too small to provide any preliminary data on whether the addition of PGG beta glucan improved the duration of response to therapy with alemtuzumab and rituximab. However, the similar time to next treatment for the patients treated in the current study compared to historical data (figure 2) suggests that a major improvement is unlikely.
In conclusion, the phase I study determined that PGG beta glucan (4 mg/kg) can be safely used in combination with mAb in the treatment of patients with CLL and the limited phase II study data could be useful for designing future studies of novel combinations including PGG beta glucan for the treatment of B cell malignancies.
Acknowledgement
Funded by the University of Iowa/Mayo Clinic Lymphoma SPORE (CA097274), The Gateway for Cancer Research, and Biothera.
References
- 1.Faderl S, Thomas DA, O'Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415. doi: 10.1182/blood-2002-07-1952. [DOI] [PubMed] [Google Scholar]
- 2.Nabhan C, Patton D, Gordon L, et al. A Pilot Trial of Rituximab and Alemtuzumab Combination Therapy in Patients with Relapsed and/or Refractory Chronic Lymphocytic Leukemia (CLL). Leukemia & lymphoma. 2004;45:2269–2273. doi: 10.1080/10428190412331286096. [DOI] [PubMed] [Google Scholar]
- 3.Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008;113:2110–2118. doi: 10.1002/cncr.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zent CS, Wu W, Bowen DA, et al. Addition of GM-CSF does not improve response to early treatment of high risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Leukemia & lymphoma. 2013;54:476–482. doi: 10.3109/10428194.2012.717276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankfurt O, Ma S, Gordon L, et al. Phase II study of alemtuzumabrituximab therapy in previously untreated patients with chronic lymphocytic leukemia: short- and long-term outcomes. Leukemia & lymphoma. 2014:1–9. doi: 10.3109/10428194.2014.910654. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RP, Lindorfer MA. Analyses of CD20 Monoclonal Antibody-Mediated Tumor Cell Killing Mechanisms: Rational Design of Dosing Strategies. Mol Pharmacol. 2014 doi: 10.1124/mol.114.092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golay J, Introna M. Mechanism of action of therapeutic monoclonal antibodies: Promises and pitfalls of in vitro and in vivo assays. Arch Biochem Biophys. 2012;526:146–153. doi: 10.1016/j.abb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Allendorf DJ, Hansen R, et al. Yeast beta-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J Immunol. 2006;177:1661–1669. doi: 10.4049/jimmunol.177.3.1661. [DOI] [PubMed] [Google Scholar]
- 10.Hong F, Hansen RD, Yan J, et al. Beta-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 2003;63:9023–9031. [PubMed] [Google Scholar]
- 11.Li B, Allendorf DJ, Hansen R, et al. Combined yeast {beta}-glucan and antitumor monoclonal antibody therapy requires C5a-mediated neutrophil chemotaxis via regulation of decay-accelerating factor CD55. Cancer Res. 2007;67:7421–7430. doi: 10.1158/0008-5472.CAN-07-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogeboom R, van Kessel KP, Hochstenbach F, et al. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J Exp Med. 2013;210:59–70. doi: 10.1084/jem.20121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 15.Sargent DJ, Chan V, Goldberg RM. A three-outcome design for phase II clinical trials. Control Clin Trials. 2001;22:117–125. doi: 10.1016/s0197-2456(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Rawstron AC, Kennedy B, Evans PA, et al. Quantitation of minimal disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood. 2001;98:29–35. doi: 10.1182/blood.v98.1.29. [DOI] [PubMed] [Google Scholar]
- 18.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]