Abstract
PURPOSE
Sunitinib and sorafenib are used widely in the treatment of renal cell carcinoma (RCC). These agents are associated with a significant incidence of cardiovascular (CV) dysfunction and left ventricular ejection fraction (LVEF) declines, observed largely in the metastatic setting. However, in the adjuvant population, the CV effects of these agents remain unknown. We prospectively defined the incidence of cardiotoxicity amongst resected, high-risk RCC patients treated with these agents.
METHODS
Sunitinib, sorafenib, or placebo was administered for up to 12 months in patients with high-risk, resected RCC. LVEF was measured by multi-gated acquisition (MUGA) scans at standard intervals. Additional CV adverse events were reported according to NCI Common Terminology Criteria for Adverse Events (CTCAE).
RESULTS
Among 1,943 patients randomized, 1,599 had at least 1 post-baseline MUGA. Within 6 months, 21 patients (1.3%) experienced a cardiac event, defined as an LVEF decline from baseline that was >15% and below the institutional lower limit of normal. Nine of 513 (1.8%) patients were on sunitinib; 7 of 508 (1.4%) on sorafenib; and 5 of 578 (0.9%) on placebo (p=0.28 and 0.56 comparing sunitinib and sorafenib to placebo, respectively). With dose interruption or adjustment, 16 of the 21 recovered their LVEF to >50%. The incidence of symptomatic heart failure, arrhythmia, or myocardial ischemia did not differ among groups.
CONCLUSION
In the adjuvant setting, we prospectively define low incidence of cardiotoxicity with sunitinib and sorafenib. These findings may be related to close CV monitoring, or potentially to fewer CV comorbidities in our non-metastatic population.
Keywords: cardiac safety, renal cell cancer, sorafenib, sunitinib, tyrosine kinase inhibitors
Introduction
Vascular endothelial growth factor receptor (VEGFR) inhibitors improve overall survival and/or progression free survival in metastatic renal cell and other carcinomas (1–7). However, a major concern with the use of the VEGFR tyrosine kinase inhibitors (VEGFR-TKIs) is the unintended adverse cardiovascular (CV) toxicities (8). Sunitinib and sorafenib have each been associated with hypertension, left ventricular systolic and diastolic dysfunction, heart failure (HF) and myocardial ischemia (9 –13). A recent meta-analysis suggests an incidence of symptomatic HF of 4.1% with sunitinib in the metastatic setting (14). The incidence of asymptomatic LVEF decline may be even greater, resulting in a growing population of patients with HF, and Stage B disease (15).
These agents inhibit a number of kinases, and have important “off-target” effects. Sunitinib inhibits VEGFR 1,2,3, platelet derived growth factor (PDGF), stem cell factor receptor (c-kit), receptor-type tyrosine-protein kinase 3 (FLT-3), colony stimulating factor-1 and (Flt-1), and sorafenib, also inhibits VEGFR 2,3, and RAF kinases C-raf and B-raf (16,17). Many of these pathways play fundamental roles in the maintenance of CV function, and response to CV stress. (9,10). As such, there have been clear associations with LVEF decline and subsequent Stage B HF. However, all previous reports of cardiotoxicity with VEGFR-TKIs have been in the setting of metastatic disease, limiting differentiation of treatment from disease related events, and the residual effects of previous anticancer therapy. There is a paucity of data in the adjuvant setting.
ECOG 2805 is a randomized, double blind phase III trial of one year of adjuvant sunitinib, sorafenib or placebo in previously untreated patients with completely resected RCC at high risk for recurrence. (18) Within E2805, we implemented a prospective cardiac monitoring protocol to determine if patients treated with sorafenib or sunitinib in the adjuvant setting experienced clinically significant decreases in LVEF. We also broadly captured additional CV adverse events such as arrhythmia and cardiac ischemia were reported according to NCI Common Terminology Criteria for Adverse Events (CTCAE) at each study visit. We report here the results of a detailed analysis from this substudy detailing the incidence, severity, and reversibility of cardiotoxicity.
Patients and Methods
The E2805 trial (18), led by ECOG (now ECOG-ACRIN) with participation from the Southwest Oncology Group (SWOG), Cancer and Leukemia Group B (now the Alliance), and the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG), accrued 1,943 patients between April 2006 and September 2010. All patients have completed therapy with the full assessment of the prospective cardiac safety sub-study.
Eligibility
Eligible patients had histologically proven, completely resected clear or non-clear cell RCC at high risk for recurrence (clinicaltrials.gov NCT00326898). Patients were treatment-naive for kidney cancer, had ECOG performance status 0 or 1, and normal organ function. Eligible patients had a normal LVEF of at least 50% by MUGA scan, no cardiac dysfunction or cardiac event (myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, symptomatic congestive heart failure, cerebrovascular accident or transient ischemic attack, or pulmonary embolism) in the 6 months prior to study drug administration, no significant ventricular or atrial arrhythmias, a QTC interval of less than 500 ms, and blood pressure of ≤ 130/90 mm Hg. We designed the trial to include a very 6-month criteria for major cardiac events, in comparison to prior metastatic renal cell cancer trials, which used a 12-month time period (19) but were not published at the time of the design of this study.
Treatment and Disease Evaluation
Patients were randomly assigned to receive nine 6-week cycles of either sunitinib 50 mg daily for 28 of 42 days per cycle, sorafenib 400 mg twice daily, or placebo. In 2009, to address toxicity issues, the starting doses were amended to 37.5 mg (sunitinib/placebo) or 400 mg once daily (sorafenib/placebo) for the first 1–2 cycles of therapy. Patients experiencing no > grade 1 or tolerable grade 2 side effects were escalated to full doses for subsequent cycles. Dose reductions occurred for grade 3 – 4 toxicities (NCI – CTCAE version 3.0). Patients were assessed every 6 weeks for toxicity, and imaged at regular intervals until disease recurrence or through 10 years.
Cardiac Assessments
All patients had LVEF measured by MUGA at baseline, 3, 6, 12 months, or at end of treatment; if cardiac symptoms developed; and 3 months after the last abnormal assessment. MUGA results were based on institutional reporting. Dose modifications for decline in LVEF (Table 1) were derived from prior published algorithms for cardiotoxic agents (20). If dosing was held due to a decline in LVEF, the MUGA was repeated in 2–4 weeks. Agents were resumed at the same dose level if the LVEF improved to the institutional lower limit of normal (ILN). If the LVEF improved to within 1–5% of ILN, the agents were resumed at one dose level reduction. If the LVEF failed to return to these levels, then agents were held an additional 2 weeks and the MUGA was again repeated. After holding agents for at least 4 weeks, agents were resumed if the LVEF had normalized, or the patient came off study.
Table 1.
Dose modification plan
| LVEF Decline | ||||
|---|---|---|---|---|
| Resulting LVEF | None | <10% | 10–15% | ≥ 16% |
| 0% < ILN | Continue | Continue | Continue | Discontinue treatment |
| 1–5% < ILN | Continue and repeat MUGA | Continue and repeat MUGA | Hold drug/repeat MUGA | |
| ≥ 6% Below ILN | Continue and repeat MUGA | Hold drug/repeat MUGA | Hold drug/repeat MUGA | |
Cardiac Sub-study Statistical Design
The primary objective of the cardiac sub-study was to determine if patients treated with sunitinib or sorafenib experienced significant decreases in LVEF within 6 months relative to placebo, defined according to protocol as an LVEF < ILN, with a decrease of > 15 absolute percentage points from baseline (per protocol event definition). Delayed LVEF events were defined as an absolute decline in LVEF of > 15% occurring after 6 months. Event rates on each treatment arm were calculated with 90% exact binomial confidence intervals (CI). A sample size of 200 patients per arm (600 total patients) was planned in order to distinguish the following rate differences in LVEF decline: 0.5% vs. 4%, 1% vs. 5%, or 2% vs. 6%. However, to comprehensively characterize changes in cardiac function, we collected MUGA scan information for all patients participating in this adjuvant trial.
In addition, an early safety evaluation was to be conducted if ≥4 of the first 100 patients experienced clinical HF. Clinically significant HF was defined using CTCAE version 3.0 adverse event criteria as left ventricular systolic or diastolic dysfunction: severe symptoms with any activity or from drop in LVEF responsive (Grade 3) or refractory to therapy (Grade 4). Restrictive cardiomyopathy ≥ Grade 3 was included as part of the definition. Based upon this decision rule, this early analysis had a high probability (74%) of detecting a true clinical heart failure rate of ≥ 5%.
Secondary cardiac outcomes also included cardiac ischemia or myocardial infarction (MI), arrhythmia. Additional objectives of this cardiac substudy were to describe the natural history of the primary cardiac events over the duration of follow-up, the association between primary cardiac events and clinical risk factors, and delayed declines in LVEF occurring after 6 months.
Hypertension, another secondary outcome, will be reported separately.
Statistical Analysis
Descriptive statistics were used to characterize patients at baseline. In addition to the 90% exact binomial confidence intervals used to describe event rates, cumulative incidence curves were used to portray the rate at which events occurred over time.
Because there is no single consensus definition for cardiac dysfunction in the setting of VEGFR-TKI therapy, sensitivity analyses were conducted using these other published definitions:
LVEF decline ≥15% to below ILN occurring at any time (Per protocol at any time)
LVEF decline as above, or any grade 2 or higher cardiac toxicity reported as an adverse event regardless of LVEF measurement. CTCAE Version 3 events classified as “Cardiac, General” or “Cardiac, Arrhythmia” other than hypertension, valvular heart disease, and cor pulmonale were included (Per protocol, including other)
Absolute reduction in LVEF of ≥10% from baseline to <50% +/− symptoms (20,21,22)
Absolute reduction of LVEF of ≥5% to <55% with symptoms of HF or an asymptomatic reduction of LVEF of ≥10% to <55% from baseline (Cardiac Review and Evaluation Committee [CREC]) (23)
Absolute reduction of LVEF ≥10% (24)
Fisher’s exact test was used to compare event rates on each experimental arm to the control arm, with a two-sided p-value of 0.025 considered significant (to control the overall Type I error for two comparisons at 0.05. Analyses were performed with an intent-to-treat comparison, including each patient on the arm to which they were randomized, and regardless of the time on treatment. We also examined the extent to which the point estimate of the percent of patients with events on each experimental arm fell outside the 90% confidence interval on the control arm. A sensitivity analysis examined events per person-year of follow-up; confidence intervals and comparisons were done using exact poisson methods.
Multivariable logistic regression models were used to identify factors associated with development of cardiac dysfunction. Differences between patients included and excluded from the analysis were examined using two-sided t-tests for continuous variables and Fisher’s exact test for categorical factors. Analyses were done using SAS version 9.2, Stata version 12.1, and R.
Results
Patient Characteristics of the Entire Analysis Population
As shown in the CONSORT diagram (supplemental figure), 1603 patients with at least 1 follow-up MUGA scan formed the primary analysis population. Of these 1315 were considered adequately assessed, as defined above. Patient characteristics are detailed in Table 2. At baseline, 1 patient was ineligible due to uncontrolled hypertension and 3 due to persistent atrial fibrillation. Overall, the baseline prevalence of CV risk factors was low.
Table 2.
Patient and Disease Characteristics
| Adequately Assessed (n=1599) |
Had Primary Event (n=21) |
No Primary Event (n=1578) |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Very High RCC Recurrence Risk | 808 | 50.6 | 14 | 66.7 | 794 | 50.4 |
| Clear Cell | 1341 | 84.0 | 19 | 90.5 | 1322 | 83.9 |
| PS 0 | 1260 | 78.9 | 14 | 66.7 | 1246 | 79.1 |
| Male | 1108 | 69.4 | 12 | 76.2 | 1092 | 69.3 |
| Prior CV History* | 363 | 22.7 | 8 | 38.1 | 355 | 22.5 |
| Hypertension developed in wk 1 | 494 | 32.1 | 6 | 30.0 | 488 | 32.1 |
| Age, Median (range) | 56 | (19 – 84) | 61 | (37–71) | 56 | (19 – 84) |
| Age > 55 | 872 | 54.4 | 17 | 81.0 | 855 | 54.1 |
| Weeks from Surgery to Baseline MUGA, Median (range) | 7.4 | (0 – 20.1) | 7.6 | (−0.6 – 12.1) | 7.7 | (1.3 – 12.7) |
| Treatment Duration (months), Median (range) | 12.7 | (0.3 – 16.2) | 12.6 | (1.0 – 14.4) | 12.7 | (0.3 – 16.2) |
| Baseline LVEF, Median (Range) | 61% | (46 – 91%) | 60% | (51 – 67%) | 61% | (46 – 91%) |
distant history of transient ischemic event, myocardial infarction or ischemia, history of arrhythmia, or similar event occurring more than 6 months prior to registration
Patients excluded due to the absence of follow-up scans were slightly older (median age 57 versus 56, p=0.04) and more likely to be female (42% versus 31%, p<0.001). Excluded patients did not differ with respect to baseline performance status (p=0.61) or baseline LVEF (p=0.35). While exact reasons for not having a follow-up scan were not known, these patients either withdrew before treatment (n=49) or had short treatment duration (median 1.2 months). About half of these patients discontinued due to patient-elected withdrawal.
Per-protocol Cardiac Safety Analyses
The protocol-specified sub-study analysis was conducted in August 2009, when 200 patients per arm completed at least one follow-up MUGA after 6 months or an event within 6 months. Fifteen of the 672 patients in this analysis population experienced primary LVEF events, and the minor observed differences in rates among treatment groups were not statistically significant. Furthermore, the safety analysis of clinically significant heart failure among the first 100 patients was not triggered as only 2 patients experienced LVEF declines and 1 patient experienced grade 3 restrictive cardiomyopathy.
Primary Cardiac Event Rate as Defined by Decline in LVEF
As of August 2013, over a maximum follow-up time of 54 months, 21 of the 1603 patients had experienced a primary cardiac event, defined as LVEF below the ILN, where the decrease was >15% absolute percentage points from baseline within 6 months (Table 3). Of these, 9 of 513 (1.7%) patients were on sunitinib (386.4 person-years), 7 of 510 (1.3%) patients were on sorafenib (402.3 person-years) and 5 of 580 (0.8%) patients were on placebo (519.9 person years). These rates were not significantly different (Fisher’s exact p=0.28 and 0.56 comparing sunitinib and sorafenib to placebo, respectively). As shown in Supplemental Table 1, a sensitivity analysis comparing events per person-year of follow-up also did not detect differences based on the primary endpoint definition (poisson exact p=0.17 and 0.46 comparing sunitinib and sorafenib to placebo, respectively).
Table 3.
Cardiac Events by Treatment Arm Including Per Protocol and Alternative Definitions
| Treatment Arm | P-values from Pairwise Comparisons |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sunitinib (n=513) | Sorafenib (n=510) | Placebo (n=580) | Sunitinib vs Placebo |
Sorafenib vs Placebo |
|||||||
| N | % | 90% CI | N | % | 90% CI | N | % | 90% CI | |||
| Per Protocol | 9 | 1.7 | 0.9 – 3.0 | 7 | 1.3 | 0.6 – 2.6 | 5 | 8 | 0.3 – 1.8 | 0.28 | 0.56 |
| Per protocol at any time | 14 | 2.7 | 1.7 – 4.2 | 10 | 1.9 | 1.1 – 3.3 | 8 | 1.3 | 0.7 – 2.5 | 0.13 | 0.48 |
| Per Protocol, including Other | 30 | 5.8 | 4.2 – 7.9 | 26 | 5.0 | 3.6 – 7.0 | 28 | 4.8 | 3.5 – 6.6 | 0.50 | 0.89 |
| CREC Criteria | 59 | 11.5 | 9.3 – 14.1 | 56 | 10.9 | 8.8 – 13.5 | 48 | 8.2 | 6.5 – 10.4 | 0.08 | 0.15 |
| Absolute reduction in LVEF of ≥10% from baseline to <50% | 27 | 5.2 | 3.7 – 7.2 | 24 | 4.7 | 3.3 – 6.6 | 17 | 2.9 | 1.9 – 4.4 | 0.06 | 0.15 |
| Absolute reduction in LVEF ≥10% | 84 | 16.3 | 13.7 – 19.3 | 80 | 15.6 | 13.1 – 18.6 | 87 | 15.0 | 12.6 – 17.7 | 0.56 | 0.80 |
| Any Criteria | 99 | 19.2 | 16.5 – 22.4 | 92 | 18.0 | 15.3 – 21.1 | 105 | 18.1 | 15.5 – 20.9 | 0.64 | 1.00 |
Cardiac Event Rates Using Alternative Definitions
Table 3 also details event rates when considering multiple definitions of cardiotoxicity. (21–25) Proportions are shown with 90% exact binomial confidence intervals and p-values from pairwise comparisons of arms. There was a trend toward higher event rates with sunitinib based on the CREC definition or using an LVEF decline of ≥10% to < 50% (p=0.08 and p=0.06, respectively). This was more pronounced in the analysis based on person-years (p=0.01 and p=0.02, respectively).
When we compared event rates between patients who began treatment at full dose and those who started at the lower dose, there were no statistically significant differences (Supplemental Table 2). We also explored whether patients who discontinued treatment due to adverse events might have had a lower PS or lower baseline LVEF and therefore more susceptible to cardiac events, or experienced substantial LVEF declines and thus prematurely discontinued therapy. However, there were also no differences in event rates between these two groups (Supplemental Tables 3a and 3b)
Clinical Factors Associated with an Increased Risk of Any LVEF Declines
Next, we sought to define those clinical variables associated with a cardiac event, defined by any of the criteria listed in Table 3: treatment arm, sex, performance status (0 vs. 1), RCC risk category and histology, method of ascertainment of kidney cancer (incidental vs. symptomatic), baseline systolic and diastolic blood pressure, treatment duration, and baseline LVEF (Table 4). In our multivariable model, male sex, longer treatment duration, and higher LVEF at baseline were associated with increased risk of an event. Although females had higher baseline LVEF than males, there was no statistically significant interaction between gender and LVEF. Baseline blood pressure was not associated with development of cardiac events.
Table 4.
Multivariable Model – Factors Associated with Occurrence of Event (Any Criteria)
| Parameter | Levels | OR | 95% CI | Wald p |
|---|---|---|---|---|
| Arm | Sunitinib vs. Placebo | 1.23 | 0.89 – 1.69 | 0.21 |
| Sorafenib vs. Placebo | 1.05 | 0.76 – 1.46 | 0.76 | |
| Sex | Male vs. Female | 1.40 | 1.04 – 1.88 | 0.03 |
| Treatment Duration (months) | 1.04 | 1.01 – 1.08 | 0.024 | |
| Baseline LVEF | 1.10 | 1.08 – 1.12 | <0.0001 |
The relationship among baseline LVEF, treatment duration, and likelihood of a cardiac event is complex (Supplemental Tables 4 and 5 and Supplemental Figures 1 and 2), and perhaps related to our outcome definition. Supplemental Table 5 shows a similar breakdown for a more constrained event definition, and the interactions are clearly less pronounced.
Reversibility in LVEF Declines Over Time
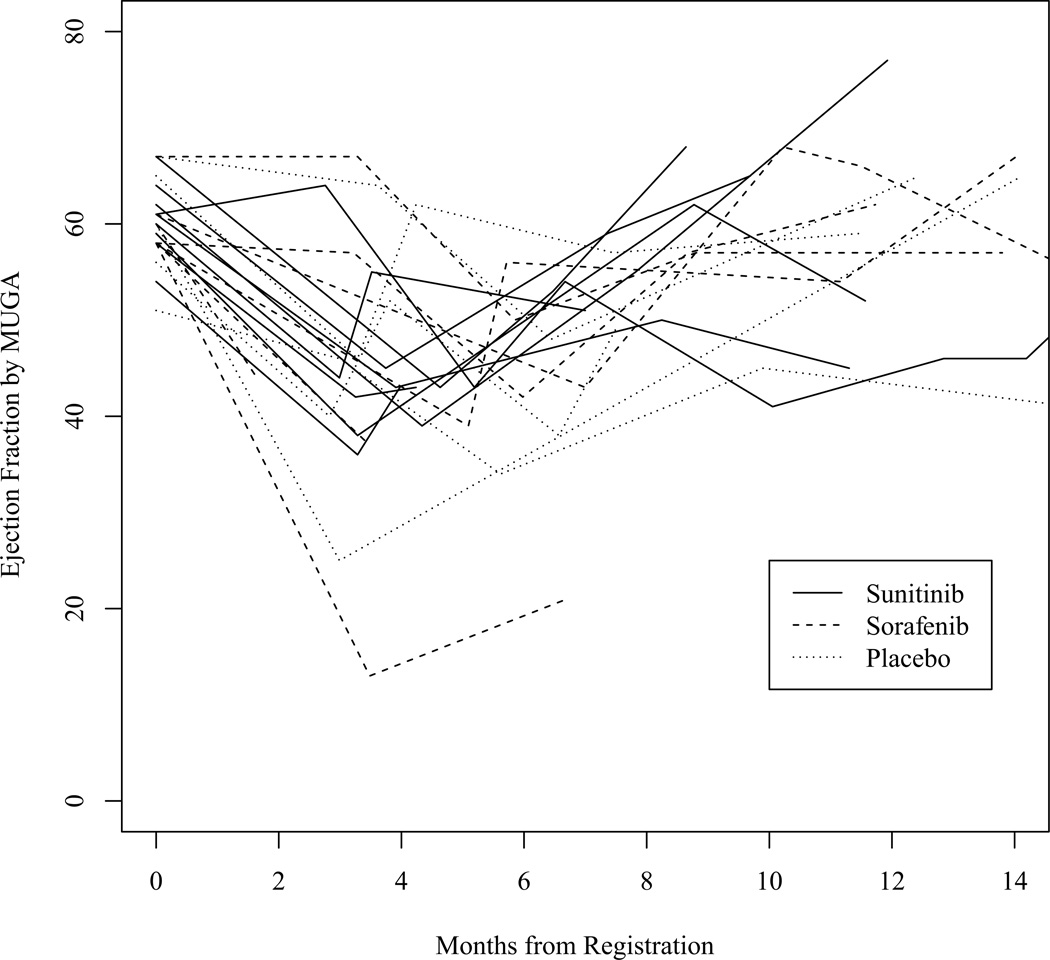
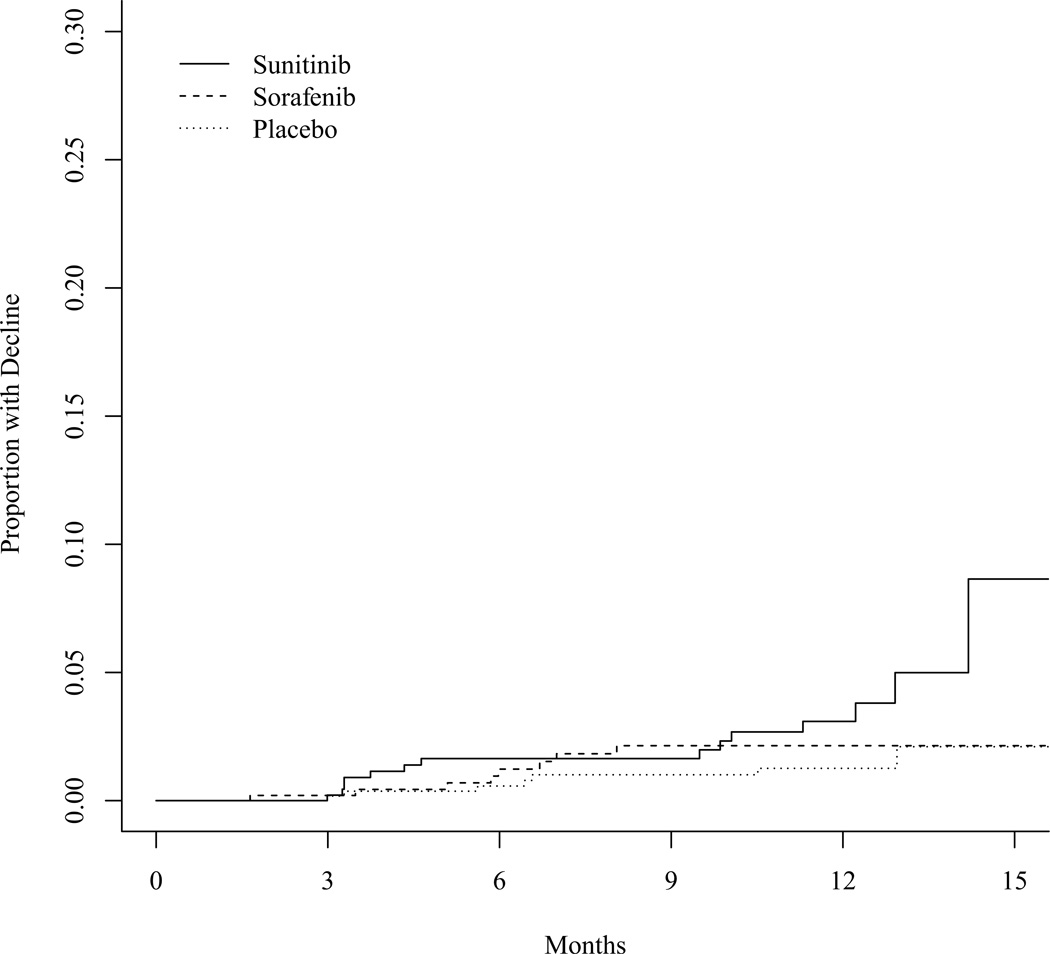
Figure 1 demonstrates the trajectories of LVEF among 21 patients with primary cardiac events. All but 2 patients with events reported at least one post-event MUGA LVEF value higher than the nadir. Sixteen of 21 patients had a recovery MUGA scan with an LVEF of ≥ 50%. Figure 2 shows the cumulative incidence of LVEF declines ≥ 16%.
Figure 1.
Changes in ejection fraction over time among patients with primary endpoint events, as assessed by MUGA.
Figure 2.
Cumulative incidence of LVEF declines of ≥16%.
Symptomatic Heart Failure
As previously indicated, the rate of symptomatic heart failure, defined as a grade 3 or 4 left ventricular systolic or diastolic dysfunction, or restrictive cardiomyopathy, was very low. Symptomatic left ventricular systolic dysfunction occurred in 5 patients in each treatment arm and in 2 patients on placebo (less than 1% per arm). Six of these patients had protocol-specified events. One report of restrictive cardiomyopathy was documented in a patient treated with sorafenib.
Grade 3 or Higher Adverse Cardiac Events
There was an overall low incidence of Grade 3 or 4 arrhythmias or cardiac ischemia in the treated groups- 5 for sunitinib, 1 for sorafenib, and 4 for placebo. There were 5 patients with Grade 3–5 cardiac ischemia, within each of the treatment arms, including placebo.
Discussion
Our study is the largest prospective placebo controlled study of the cardiac effects of VEGFR-TKIs and the first in a non-cancer-bearing population. As such, our population presents a unique opportunity to study patients naïve to the effects of prior cardiotoxic regimens and with a relatively low baseline prevalence of CV disease. We found the incidence of significant LVEF decline occurring in the first 6 months of treatment in patients treated with sunitinib or sorafenib to be low. In sensitivity analyses using alternative definitions of cardiac dysfunction, this remained low, with only very modest differences when we considered person-years in our analyses. The number of late declines in LVEF was also not statistically different amongst the 3 groups.
It is critical to note that in E2805, patients were carefully screened and those with baseline cardiovascular comorbidities were excluded from study participation. As such, these patients may have a lower prevalence of CV disease compared to metastatic populations exposed to these agents, although this comparison is limited (13,26). Further study patients were monitored for asymptomatic declines in LVEF and CV risk factors were also aggressively managed. Patients underwent dose interruptions and adjustments when these initial declines in LVEF were detected. Furthermore, an algorithm for blood pressure management, which can exacerbate LV dysfunction, was closely followed for all patients on the study (Supplemental Figure 2). The impact of these interventions in lowering the rate of symptomatic events is unknown, and impossible to discern without a concurrent control group receiving continued treatment and no CV monitoring. It is certainly possible that the incidence of cardiac dysfunction could have been higher if intervention had not occurred in asymptomatic patients. Therefore our study provides insight into the potential benefits of close CV monitoring and prompt HTN treatment in patients with RCC receiving sunitinib or sorafenib and at any stage.
Patients on this trial who experienced primary cardiac events had more advanced RCC prior to resection, tended to be older, and had a slightly higher incidence of cardiovascular risk factors. Furthermore, longer treatment duration, male sex, and higher baseline LVEF were associated with the risk of any subsequent cardiac events. The treatment duration effect suggests that longer-term exposure results in a stronger cardiotoxic signal, which has important implications for the need for continued CV monitoring through therapy. We saw no difference in event rate according to starting dose suggesting that a lead-in lower dose could not reduce the already low incidence of CV toxicity. The relationship between baseline LVEF and cardiac events is admittedly counterintuitive, but may be due to our outcome definition and in particular the variability of LVEF results within the normal range. Importantly, patients with decline in LVEF generally demonstrated some recovery in LVEF with dose interruption.
Limitations
There are limitations worth noting. The trial eligibility criteria excluded patients possibly more susceptible to effects on LVEF. The potential interaction between incident hypertension and LV dysfunction will be elucidated in a planned future study. Furthermore, cardiotoxicity assessment by LVEF alone is limited in its ability to detect subclinical damage to the myocardium and does not provide insight into alterations in diastolic function. Patients on sunitinib may have experienced declines in LVEF on therapy, which reverted during the two weeks off-therapy. The impact of such reversible declines remains unknown, and the long-term cardiovascular effects of transient, possibly repeated declines in LVEF need to be elucidated in longer-term studies, perhaps in other oncologic populations.
Finally, we note that patients who did not stay on therapy long enough to participate in 6-month MUGA scans due to treatment limiting toxicity may comprise a population more at risk for LVEF decline.
Implications for current and future use of these agents
Our study has a number of important implications. First, adjuvant sunitinib or sorafenib was associated with a low incidence of cardiotoxicity in the non-metastatic, treatment naive RCC clinical trial population. With the growing use of these agents and potential for use of these agents adjuvantly in patients with high risk primary or completely resected metastatic solid cancers, this finding alone is noteworthy. Furthermore, in our experience, LV dysfunction was largely reversible with the institution of dose interruption or modification in a population where cardiac adverse effects were very carefully monitored. However, treatment duration was associated with cardiac events, suggesting an important need for continued monitoring while on therapy. Applying these findings to the use of VEGF-TKIs in all settings imply that low toxicity rates may be achievable with careful CV monitoring.
Supplementary Material
Statement of Relevance.
Anti-angiogenic tyrosine kinase inhibitors have a significant risk of cardiotoxicity in patients with metastatic disease. In a Phase III adjuvant placebo-controlled, double blind study of adjuvant sorafenib versus sunitinib in high-risk patients with resected renal cell carcinoma, we performed detailed, prospective cardiovascular monitoring with serial assessment of left ventricular ejection fraction (LVEF), with dose interruptions and adjustments when LVEF declines were detected. A detailed blood pressure management algorithm was also incorporated for all patients. Adjuvant sunitinib or sorafenib was associated with a low incidence of cardiotoxicity in this population, and LVEF declines were largely reversible with dose interruptions or modification. Treatment duration was associated with cardiac events, suggesting an important need for continued monitoring while on therapy. Overall, low toxicity rates may be achievable with careful cardiovascular monitoring in the adjuvant setting, with potential implications for patients with other solid tumor malignancies treated with anti-angiogenic tyrosine kinase inhibitors.
Acknowledgement
We thank Dr. Michael S. Ewer as a paid consultant to Pfizer, and Dr. Subramanian Hariharan of Pfizer for their expert contributions to the design of this sub-study.
Financial support:
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA180867, CA180821, CA31946, CA077202, CA180863, CCSRI021039, CA32102, CA105409, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The cardiac sub-study was supported by Pfizer.
Footnotes
Potential conflicts: Dr Flaherty is an editor for Clinical Cancer Research
Portions of these results were presented at ASCO 2012 (abstract 4500).
REFERENCES
- 1.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012 Sep;13(9):897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 5.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012 Jun 10;30(17):2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear cell renal cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007 May;7(5):332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 9.Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, et al. Heart failure associated with sunitinib maleate. Cancer. 2008;26:5204–5212. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- 10.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008 Sep;19:1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 11.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet Dec. 2007;15(370):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, et al. Incidence and risk of congestive heart failure in patients with renal and non-renal cell carcinoma treated with sunitinib. J Clin Oncol. 2011 Sep 1;29(25):3450–3456. doi: 10.1200/JCO.2010.34.4309. [DOI] [PubMed] [Google Scholar]
- 13.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008 Nov 10;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 14.Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, Choueiri TK, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011 Sep 1;29(25):3450–3456. doi: 10.1200/JCO.2010.34.4309. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Oct 15;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Sutent (sunitinib maleate) capsules [package insert] New York, NY: Pfizer Labs; 2008. [Google Scholar]
- 17.Nexavar (sorafenib)tablets [package insert] Wayne: NJ Bayer Healthcare Pharmaceuticals Inc; 2008. [Google Scholar]
- 18. http://ecog.dfci.harvard.edu/ecoginst/prot/pdf/GU/E2805DOC.PDF. [Google Scholar]
- 19.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 20.Perez EA, Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J Clin Oncol. 2004 Jan 15;22(2):322–329. doi: 10.1200/JCO.2004.01.120. [DOI] [PubMed] [Google Scholar]
- 21.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006 Dec 5;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 22.Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007 Sep 1;25(25):3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 23.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010 Jul 20;28(21):3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 24.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002 Mar 1;20(5):1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 25.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011 Oct 6;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013 Feb;1(1):72–78. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.