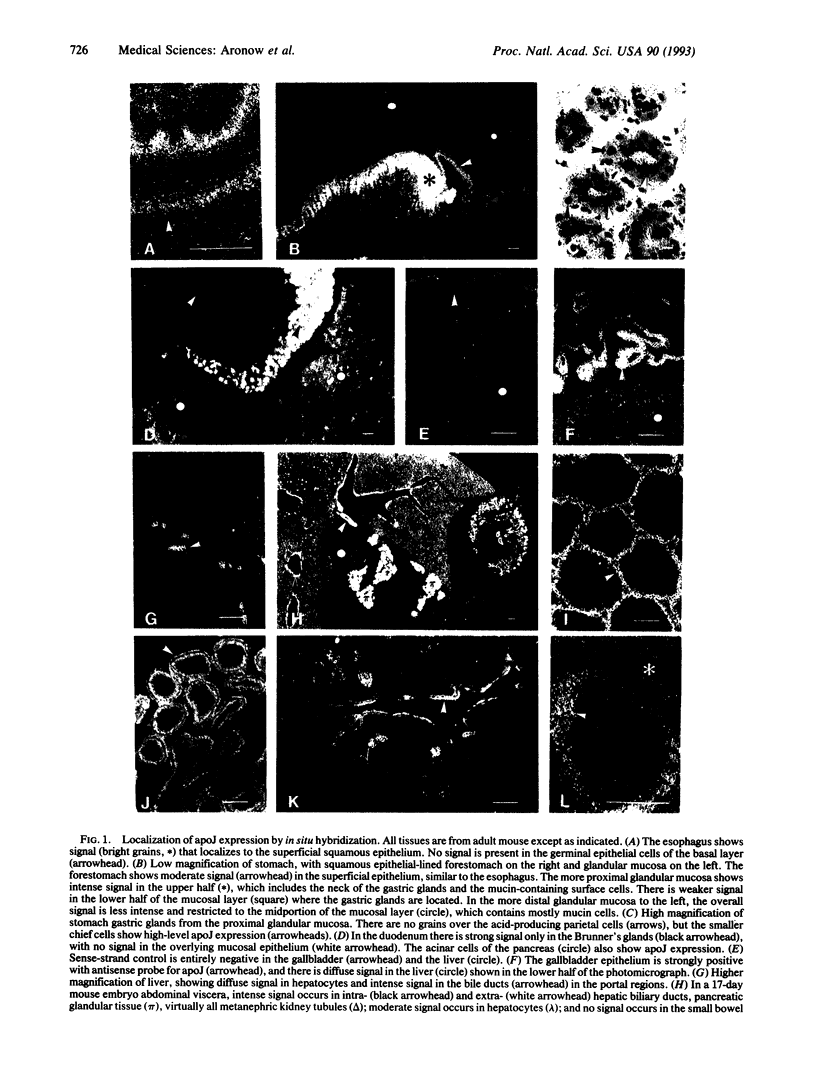
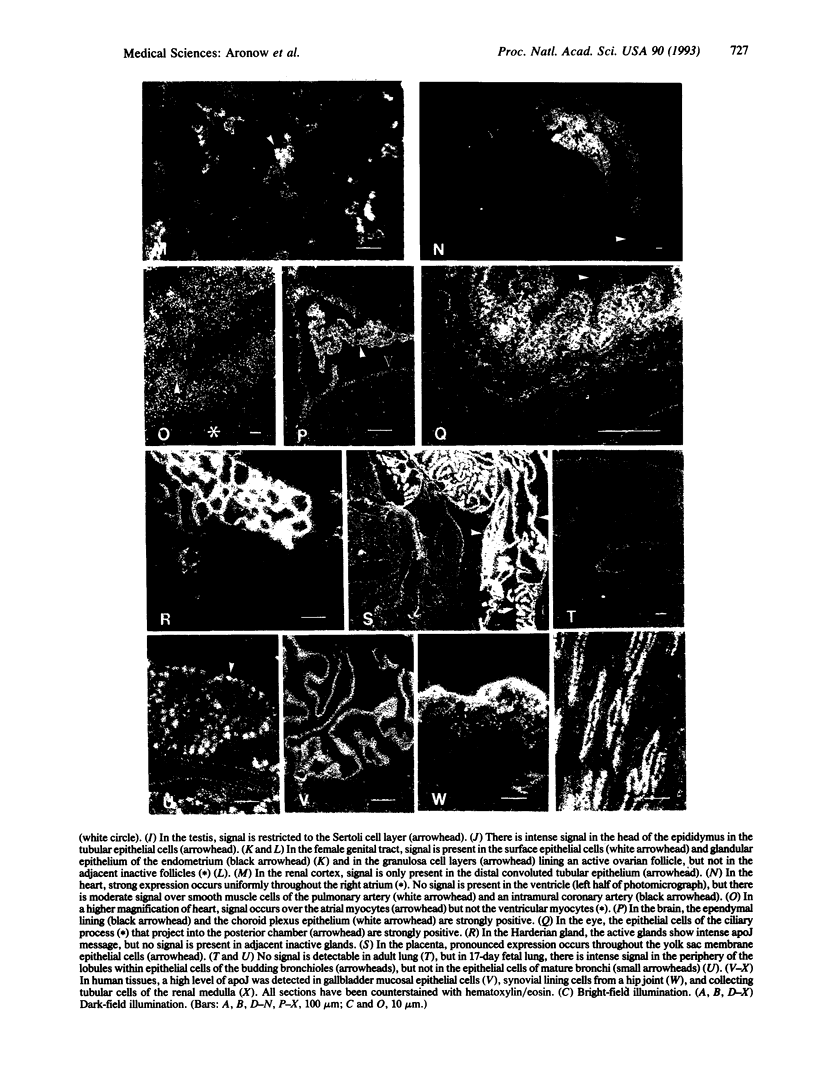
Abstract
Apolipoprotein J (apoJ) is a sulfated secreted glycoprotein that exhibits ubiquitous expression, evolutionary conservation, and diverse tissue inducibility. It has been proposed to have roles in programmed cell death, sperm maturation, complement regulation, and lipid transport. To identify cell types that synthesize apoJ and to aid evaluation of its function, we screened mouse and human tissues by in situ hybridization. ApoJ was expressed at high levels in an array of specialized cell types of adult and fetal mouse tissues and in similar cell types of human tissues. Most of these cell types are highly secretory and form the cellular interfaces of many fluid compartments. This group includes epithelial boundary cells of the esophagus, biliary ducts, gallbladder, urinary bladder, ureter, kidney distal convoluted tubules, gastric glands, Brunner's glands, choroid plexus, ependyma, ocular ciliary body, endometrium, cervix, vagina, testis, epididymus, and visceral yolk sac. Several nonepithelial secretory cell types that express high levels of apoJ also line fluid compartments, such as synovial lining cells and ovarian granulosa cells. In the context of its known biochemical properties, this expression pattern suggests that localized synthesis of apoJ serves to protect a variety of secretory, mucosal, and other barrier cells from surface-active components of the extracellular environment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpini G., Lenzi R., Zhai W. R., Slott P. A., Liu M. H., Sarkozi L., Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989 Jul;257(1 Pt 1):G124–G133. doi: 10.1152/ajpgi.1989.257.1.G124. [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., Ramalho M. J., Reis L. C., Menani J. V., Turrin M. Q., Gutkowska J., McCann S. M. Lesions of the hypothalamus and pituitary inhibit volume-expansion-induced release of atrial natriuretic peptide. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2956–2960. doi: 10.1073/pnas.88.7.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyk M. G., Sawczuk I. S., Olsson C. A., Katz A. E., Buttyan R. Characterization of the products of a gene expressed during androgen-programmed cell death and their potential use as a marker of urogenital injury. J Urol. 1990 Feb;143(2):407–413. doi: 10.1016/s0022-5347(17)39975-5. [DOI] [PubMed] [Google Scholar]
- Basbaum C. B., Mann J. K., Chow A. W., Finkbeiner W. E. Monoclonal antibodies as probes for unique antigens in secretory cells of mixed exocrine organs. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4419–4423. doi: 10.1073/pnas.81.14.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk O., Burdzy K., Fritz I. B. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983 Jun 25;258(12):7714–7720. [PubMed] [Google Scholar]
- Burkey B. F., deSilva H. V., Harmony J. A. Intracellular processing of apolipoprotein J precursor to the mature heterodimer. J Lipid Res. 1991 Jun;32(6):1039–1048. [PubMed] [Google Scholar]
- COHN S. A. Histochemical observations on the Harderian gland of the albino mouse. J Histochem Cytochem. 1955 Sep;3(5):342–353. doi: 10.1177/3.5.342. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Mazda T., Tomita M. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol. 1989 Sep;26(9):835–840. doi: 10.1016/0161-5890(89)90139-9. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Colten H. R., Gordon J. M., Rapp H. J., Borsos T. Synthesis of the first component of guinea pig complement by columnar epithelial cells of the small intestine. J Immunol. 1968 Apr;100(4):788–792. [PubMed] [Google Scholar]
- D'Cruz O. J., Haas G. G., Jr, Wang B. L., DeBault L. E. Activation of human complement by IgG antisperm antibody and the demonstration of C3 and C5b-9-mediated immune injury to human sperm. J Immunol. 1991 Jan 15;146(2):611–620. [PubMed] [Google Scholar]
- Danik M., Chabot J. G., Mercier C., Benabid A. L., Chauvin C., Quirion R., Suh M. Human gliomas and epileptic foci express high levels of a mRNA related to rat testicular sulfated glycoprotein 2, a purported marker of cell death. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8577–8581. doi: 10.1073/pnas.88.19.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Bohmont C. W., Liu N. G., Tourtellotte W. W. Changes in brain gene expression shared by scrapie and Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7260–7264. doi: 10.1073/pnas.86.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Littman B. H. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991 Sep;34(9):1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- Garden G. A., Bothwell M., Rubel E. W. Lack of correspondence between mRNA expression for a putative cell death molecule (SGP-2) and neuronal cell death in the central nervous system. J Neurobiol. 1991 Sep;22(6):590–604. doi: 10.1002/neu.480220605. [DOI] [PubMed] [Google Scholar]
- Genest J., Cantin M. The atrial natriuretic factor: its physiology and biochemistry. Rev Physiol Biochem Pharmacol. 1988;110:1–145. doi: 10.1007/BFb0027530. [DOI] [PubMed] [Google Scholar]
- Harding M. A., Chadwick L. J., Gattone V. H., 2nd, Calvet J. P. The SGP-2 gene is developmentally regulated in the mouse kidney and abnormally expressed in collecting duct cysts in polycystic kidney disease. Dev Biol. 1991 Aug;146(2):483–490. doi: 10.1016/0012-1606(91)90249-3. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Rauch J., Urban J., Parczyk K., Diel P., Pilarsky C., Appel D., Haase W., Mann K., Weller A. Molecular cloning of gp 80, a glycoprotein complex secreted by kidney cells in vitro and in vivo. A link to the reproductive system and to the complement cascade. J Biol Chem. 1991 May 25;266(15):9924–9931. [PubMed] [Google Scholar]
- James R. W., Hochstrasser A. C., Borghini I., Martin B., Pometta D., Hochstrasser D. Characterization of a human high density lipoprotein-associated protein, NA1/NA2. Identity with SP-40,40, an inhibitor of complement-mediated cytolysis. Arterioscler Thromb. 1991 May-Jun;11(3):645–652. doi: 10.1161/01.atv.11.3.645. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Lowin B., Peitsch M. C., Böttcher A., Schmitz G., Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem. 1991 Jun 15;266(17):11030–11036. [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992 Apr;17(4):154–159. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7123–7127. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirszbaum L., Sharpe J. A., Murphy B., d'Apice A. J., Classon B., Hudson P., Walker I. D. Molecular cloning and characterization of the novel, human complement-associated protein, SP-40,40: a link between the complement and reproductive systems. EMBO J. 1989 Mar;8(3):711–718. doi: 10.1002/j.1460-2075.1989.tb03430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988 Feb;122(2):552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- May P. C., Lampert-Etchells M., Johnson S. A., Poirier J., Masters J. N., Finch C. E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990 Dec;5(6):831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- McNeill T. H., Masters J. N., Finch C. E. Effect of chronic adrenalectomy on neuron loss and distribution of sulfated glycoprotein-2 in the dentate gyrus of prepubertal rats. Exp Neurol. 1991 Jan;111(1):140–144. doi: 10.1016/0014-4886(91)90062-h. [DOI] [PubMed] [Google Scholar]
- Meri S., Waldmann H., Lachmann P. J. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991 Nov;65(5):532–537. [PubMed] [Google Scholar]
- Montpetit M. L., Lawless K. R., Tenniswood M. Androgen-repressed messages in the rat ventral prostate. Prostate. 1986;8(1):25–36. doi: 10.1002/pros.2990080105. [DOI] [PubMed] [Google Scholar]
- Morales C. R., Griswold M. D. Variations in the level of transferrin and SGP-2 mRNAs in Sertoli cells of vitamin A-deficient rats. Cell Tissue Res. 1991 Jan;263(1):125–130. doi: 10.1007/BF00318407. [DOI] [PubMed] [Google Scholar]
- Morris K. M., Colten H. R., Bing D. H. The first component of complement. A quantitative comparison of its biosynthesis in culture by human epithelial and mesenchymal cells. J Exp Med. 1978 Oct 1;148(4):1007–1019. doi: 10.1084/jem.148.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan M. K., Baker H. W., Saunders J. R., Kirszbaum L., Walker I. D., Hudson P., Liu D. Y., Glew M. D., d'Apice A. J., Murphy B. F. Human seminal clusterin (SP-40,40). Isolation and characterization. J Clin Invest. 1990 May;85(5):1477–1486. doi: 10.1172/JCI114594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese J., Wesche A. The Harderian gland. Comp Biochem Physiol A Comp Physiol. 1989;93(4):655–665. doi: 10.1016/0300-9629(89)90480-5. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Brakier-Gingras L. Increase in the relative abundance of preproenkephalin A messenger RNA in the ventricles of cardiomyopathic hamsters. Biochem Biophys Res Commun. 1988 Aug 30;155(1):449–454. doi: 10.1016/s0006-291x(88)81107-0. [DOI] [PubMed] [Google Scholar]
- Palmer D. J., Christie D. L. Identification of molecular aggregates containing glycoproteins III, J, K (carboxypeptidase H), and H (Kex2-related proteases) in the soluble and membrane fractions of adrenal medullary chromaffin granules. J Biol Chem. 1992 Oct 5;267(28):19806–19812. [PubMed] [Google Scholar]
- Sawczuk I. S., Hoke G., Olsson C. A., Connor J., Buttyan R. Gene expression in response to acute unilateral ureteral obstruction. Kidney Int. 1989 Jun;35(6):1315–1319. doi: 10.1038/ki.1989.128. [DOI] [PubMed] [Google Scholar]
- Sundstrom S. A., Komm B. S., Ponce-de-Leon H., Yi Z., Teuscher C., Lyttle C. R. Estrogen regulation of tissue-specific expression of complement C3. J Biol Chem. 1989 Oct 5;264(28):16941–16947. [PubMed] [Google Scholar]
- Sylvester S. R., Morales C., Oko R., Griswold M. D. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biol Reprod. 1991 Jul;45(1):195–207. doi: 10.1095/biolreprod45.1.195. [DOI] [PubMed] [Google Scholar]
- Sylvester S. R., Skinner M. K., Griswold M. D. A sulfated glycoprotein synthesized by Sertoli cells and by epididymal cells is a component of the sperm membrane. Biol Reprod. 1984 Dec;31(5):1087–1101. doi: 10.1095/biolreprod31.5.1087. [DOI] [PubMed] [Google Scholar]
- Urban J., Parczyk K., Leutz A., Kayne M., Kondor-Koch C. Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J Cell Biol. 1987 Dec;105(6 Pt 1):2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. An autoradiographic, biochemical, and morphological study of the harderian gland of the mouse. J Morphol. 1980 Mar;163(3):349–365. doi: 10.1002/jmor.1051630308. [DOI] [PubMed] [Google Scholar]
- Watts M. J., Dankert J. R., Morgan E. P. Isolation and characterization of a membrane-attack-complex-inhibiting protein present in human serum and other biological fluids. Biochem J. 1990 Jan 15;265(2):471–477. doi: 10.1042/bj2650471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R., Roeth P. J., Easterbrook-Smith S. B. Clusterin enhances the formation of insoluble immune complexes. Biochem Biophys Res Commun. 1991 Jun 28;177(3):985–990. doi: 10.1016/0006-291x(91)90635-k. [DOI] [PubMed] [Google Scholar]
- Witte D. P., Welch T. R., Beischel L. S. Detection and cellular localization of human C4 gene expression in the renal tubular epithelial cells and other extrahepatic epithelial sources. Am J Pathol. 1991 Oct;139(4):717–724. [PMC free article] [PubMed] [Google Scholar]
- Witte D. P., Wiginton D. A., Hutton J. J., Aronow B. J. Coordinate developmental regulation of purine catabolic enzyme expression in gastrointestinal and postimplantation reproductive tracts. J Cell Biol. 1991 Oct;115(1):179–190. doi: 10.1083/jcb.115.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva H. V., Harmony J. A., Stuart W. D., Gil C. M., Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990 Jun 5;29(22):5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Duvic C. R., Wetterau J. R., Ray M. J., Ferguson D. G., Albers H. W., Smith W. R., Harmony J. A. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990 Aug 5;265(22):13240–13247. [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Park Y. B., Mao S. J., Gil C. M., Wetterau J. R., Busch S. J., Harmony J. A. Purification and characterization of apolipoprotein J. J Biol Chem. 1990 Aug 25;265(24):14292–14297. [PubMed] [Google Scholar]