Abstract
Background
Staphylococcal aureus (SA) colonization in early infancy is common, but the pattern and factors affecting its acquisition and persistence in the first few months of life are not well studied.
Objectives
To study the rate of SA nasopharyngeal (NP) colonization at monthly intervals in the first six months of life, and its association with environmental and host factors, and other pathogenic NP bacteria.
Methods
Data from a prospective study were analyzed on bacterial cultures of 1765 NP swabs from 367 infants who were followed from birth to 6 months of age. Demographic, breastfeeding, cigarette smoke exposure and daycare attendance data were collected at each monthly visit.
Results
The rate of infants colonized with SA was highest at age 1 month (25%) and declined to lowest rate by age 6 months (12%). The proportion of SA strains that was methicillin-resistant (MRSA) was also highest at age 1 month and declined rapidly by age 4 months (18% vs 6%, P = 0.05). Colonization with Streptococcus pneumoniae (SP), nontypeable Haemophilus influenzae (HI) and Moraxella catarrhalis (MC) increased at different rates up to age 6 months. Univariate analysis showed that SA colonization rate was significantly lower with increasing age, black race, day care attendance, and colonization with NTHI, MC and SP (P <0.05). Multivariate analysis showed that this effect was independently associated only with increasing age and MC colonization (P ≤0.05). Furthermore, the time to first acquisition of SA from one month of age onwards was significantly associated with day care attendance, and NTHI and MC colonization. None of the infants colonized with SA developed SA infections through age 6 months.
Conclusions
SA colonization of NP begins very early in life and declines quickly. MRSA has lower ability to maintain prolonged colonization status than methicillin-susceptible strains in the first 6 months of life. As the NP is colonized with other respiratory bacterial pathogens, the colonization with SA declines; however, this effect is stronger with Gram negative bacteria such as NTHI and MC.
Keywords: Staphylococcus aureus, nasopharyngeal colonization, infants
Introduction
Staphylococcus aureus (SA) infections have shown a dramatic increase in the past decade. The burden of infection due to methicillin-resistant strains of SA (MRSA) is significantly more evident in children compared with other age groups [1]. Children are also an important reservoir of SA and play a central role in disseminating SA in the community and hospital settings.
In the past few years, a large number of studies have been conducted to assess MRSA nasal colonization in children, both in health care centers and in the community. Children and adolescents under 20 year of age have higher persistent carriage rates than adults [1-2]. Infants are known to be colonized with SA soon after birth [3-6]. The known risk factors for infant SA colonization include breastfeeding, number of household members, low birth weight, early gestational age at birth, indwelling catheters, and duration of antibiotic or ventilator days.
Previous studies have shown that Streptococcus pneumoniae (SP) colonization is negatively associated with SA colonization [6-14]. However, some of these studies have been performed in older children (more than 6 months of age) who are typically immunized with protein-conjugate pneumococcal vaccine. Furthermore, there are limited number of published studies in infants in the first few months of life with respect to interaction between SA and Gram negative bacterial otopathogens colonized in NP, specifically nontypeable Haemophilus influenzae (NTHI) or Moraxella catarrhalis (MC). Indeed, there is no published report of MC interaction with SA in infants less than 6 months of age.
In this report, data on monthly NP bacterial cultures in the first six month of life from a prospective cohort of infants were analyzed to determine the pattern of acquisition of SA and its relationship with host and environmental factor, as well as interaction with SP, NTHI and MC.
Methods
i. Study design and subjects
The study was part of a prospective, longitudinal study of infants in the first year of life to evaluate the prevalence and risks for viral upper respiratory viral infections (URIs) and acute otitis media (AOM) development [7]. Between October 2008 and April 2013, 367 subjects were enrolled. The study was approved by the University of Texas Medical Branch (UTMB) Institutional Review Board. Written informed consent was obtained for all subjects. Study subjects were recruited from the newborn nursery or the primary care pediatrics clinics at UTMB before the first month of age. The infants were otherwise healthy; preterm infants and those with major medical problems or anatomical/physiological defects of the ear or NP were excluded.
All of recruited infants resided on Galveston Island. UTMB is the only primary healthcare and emergency care provider for infants and children on the Galveston Island, and uses the same electronic medical record system across all practice sites. Thus, documented episodes of infection were easily available for review.
At enrollment, demographic information including gender, race/ethnicity, use of breast milk or formula feeding, passive cigarette smoke exposure, day care attendance, NP swabs are collected at 1, 2, 3, 4, 5, and 6 months mostly through home visits by the study staff. Data related to monthly NP bacterial cultures and host demographic and environmental factors are presented in this report. At the time of these monthly encounters, data related to symptoms of upper respiratory infection (URI) were collected, and the NP swabs were processed for viral pathogens as described previously [15].
ii. Specimen collection
NP swabs were collected by trained study personnel by introducing Mini-Tip Culturette® (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) through the nostrils until resistance was met and then rotated gently 180 degrees. The specimens were submitted to the UTMB Clinical Microbiology Laboratories for bacterial identification and antimicrobial susceptibility testing according to standard laboratory methods.
iii. Statistical Analysis
The study outcome variables of rates of co-presence of various pathogens and rates of MRSA and MSSA were analyzed by Chi-Square test. To analyze the time to first SA colonization, we used Cox proportional hazards modeling with recurrent events. To analyze the risk for SA colonization, we used a generalized logistic model. Both of these models took into account a child with multiple positive SA samples. Within each model, we used a Type II model which indicated that the covariates existed any time before SA sample positivity. Finally, within each model, the marginal and multivariate P values are listed. The marginal P values do not take into account the effects of other variables, whereas the multivariate P values takes into account the effects of other variables. All statistical procedures were run using libraries in the R programming environment (http://cran.r-project.org/).
Results
i. Demographic and clinical characteristics
As shown in Table 1, among 367 enrolled subjects, there were slightly more males (54%) than females. The majority of infants were exclusively formula fed (63%), the remainder being breastfed in a mixed fashion for a variable duration during 6 months of follow up. Most infants were not exposed to cigarette smoke (80%) and did not attend daycare (83%) during the study period.
Table 1. Demographic and clinical characteristics of infants (n=367).
| Characteristics | No. | % |
|---|---|---|
|
| ||
| Gender | ||
| Male | 199 | 54 |
|
| ||
| Race/ethnicity | ||
| Hispanic | 184 | 50 |
| White* | 94 | 26 |
| Black | 84 | 23 |
| Asian | 5 | 1 |
|
| ||
| Feeding pattern | ||
| Breastfed< 3 Months | 71 | 19 |
| Breastfed> 3 Months | 66 | 18 |
| Formula fed only | 230 | 63 |
|
| ||
| Cigarette smoke exposure** | ||
| No | 294 | 80 |
| Yes | 73 | 20 |
|
| ||
| Daycare attendance** | ||
| No | 304 | 83 |
| Yes | 63 | 17 |
|
| ||
| Diagnosed with URI** | ||
| Yes | 287 | 78 |
| No | 80 | 22 |
Non-Hispanic white
During any period of study follow up
ii. Rates of SA and other respiratory bacteria colonization by monthly increment of age
Figure 1 shows the rates of colonization of SA, MC and NTHI by each month of age. SA colonization was highest at 1st month of age with of 25% colonization rate of all tested infants, and declined gradually to 12% by 6 months of age. On the other hand, SP, MC and NTHI colonization rates were the lowest at 1st month of age. MC colonization rate increased quickly, peaking to 39% rate by 5 months of age, while SP rate increased gradually to 22% by 6 months of age. NTHI rate increase was even slower, rising to 15% by 6 months of age.
Fig 1. Nasopharyngeal colonization rates for various bacterial pathogens at monthly intervals (n = 367 infants).
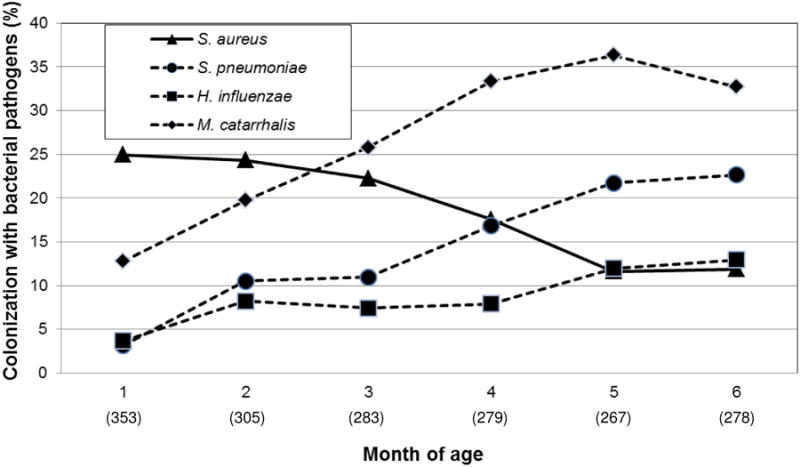
Figures in parentheses reflect the number total number children tested at each month of age. Not all infants had nasopharyngeal bacterial cultures done at each monthly interval.
When the rate of MRSA among all SA isolates was evaluated by month of age (Figure 2), the MRSA rate was highest at 1st of age (18% of all SA strains) and declined to lowest rate of 6% by 4 months of age (P = 0.05), but rose to 12.1% by 6 months of age.
Fig 2. Nasopharyngeal colonization rates of MRSA and MSSA by month of age (n=367 infants).
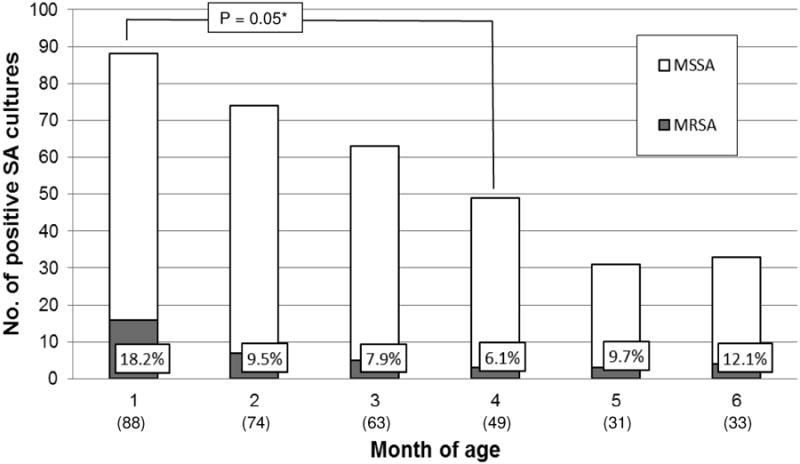
SA = S. aureus, MRSA = methicillin-resistant SA, MSSA = methicillin-sensitive SA. Figures in parentheses are number of children who tested positive for SA at each month of age. Figures in boxes represent percentage of MRSA of the total positive SA cultures. *P value were calculated by Chi-Square test. Not all infants had nasopharyngeal bacterial cultures done at each monthly interval.
iii. Factors affecting SA colonization
Table 2 shows the factors predicting the risk for SA colonization anytime during the entire study period. Using univariate analysis, without respect to other factors, older age, black race, daycare attendance, SP, NTHI and MC colonization decreased the risk for SA colonization. However, multivariate analysis showed that only older age and MC colonization were independently associated with lower risk for SA colonization. We found no relationship between presence of viral URI and colonization risk for SA. Furthermore, there was no relationship between specific viral etiology of URI and SA colonization (data not shown).
Table 2. Factors predicting SA colonization of the nasopharynx at any time during monthly evaluation (n =1765 cultures from 367 infants).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
| ||||
| Factor | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value |
|
| ||||
| Age | -0.011 (-0.014, -0.0079) | <0.001 | -0.0086 (-0.012, -0.0050) | <0.001 |
|
| ||||
| Gender (ref. female) | -0.29 (-0.81, 0.23) | 0.27 | -0.34 (-0.90, 0.22) | 0.24 |
|
| ||||
| Race/ethnicity | ||||
| White* | 0.013 (-0.57, 0.59) | 0.96 | Comparator | |
| Hispanic | 0.40 (-0.12, 0.93) | 0.13 | 0.16 (-0.52, 0.84) | 0.64 |
| Black | -0.62 (-1.28, 0.0049) | 0.056 | -0.78 (-1.63, 0.081) | 0.076 |
| Asian | 0.26 (-2.04, 2.44) | 0.81 | 0.24 (-2.20, 2.68) | 0.85 |
|
| ||||
| Breastfeeding | -0.35 (-0.86, 0.16) | 0.18 | -0.51 (-1.07, 0.054) | 0.076 |
|
| ||||
| Cigarette smoke exposure | -0.27 (-0.88, 0.31) | 0.37 | -0.11 (-0.76, 0.54) | 0.74 |
|
| ||||
| Daycare attendance | -0.74 (-1.42, -0.094) | 0.028 | 0.10 (-0.61, 0.81) | 0.78 |
|
| ||||
| NTHI Colonization | -1.12 (-1.76, -0.53) | <0.001 | -0.41 (-1.09, 0.27) | 0.23 |
|
| ||||
| MC colonization | -1.09 (-1.52, -0.68) | <0.001 | -0.52 (-1.00, -0.034) | 0.036 |
|
| ||||
| SP colonization | -0.84 (-1.38, -0.33) | 0.002 | 0.011 (-0.59, 0.61) | 0.97 |
Non-Hispanic white
Above statistical analysis used logistic mixed model. This model took into account a child with multiple positive SA samples All of the factors were present prior to the positivity of SA colonization. NS = not significant; URI = upper respiratory infection; SA = S. aureus, SP = S. pneumoniae, NTHI = non-typeable H. influenzae, MC = M. catarrhalis; CI = confidence interval.
iv. Factors predicting time to first acquisition of SA
Table 3 shows the factors affecting the time to first acquisition of SA in the child from first month of age onwards. Using univariate analysis, without respect to other factors, prior presence of SP, NTHI and MC colonization increased the time to SA colonization. However, multivariate analysis showed that only day care attendance and prior presence of NTHI and MC colonization, independently increased the time to SA acquisition.
Table 3. Factors predicting time to SA colonization of the nasopharynx from age one month onwards (n=1765 cultures from 367 infants).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
| ||||
| Factor | Coefficient (95% CI) | P value | Effect | P value |
|
| ||||
| Gender | -0.13 (-0.44, 0.17) | 0.39 | -0.13 | 0.38 |
|
| ||||
| Race/ethnicity | 0.09 | 0.13 | ||
| White* | 0.028 (-0.31, 0.47) | Comparator | ||
| Hispanic | 0.24 (-0.064, 0.54) | 0.14 (-0.22, 0.49) | ||
| Black | -0.45 (-0.84, 0.065) | -0.37 (-0.84, 0.10) | ||
| Asian | 0.39 (-1.09, 1.87) | 0.38 (-1.10, 1.86) | ||
|
| ||||
| Breastfeeding | -0.11 (-0.42, 0.19) | 0.46 | -0.24 (-0.55, 0.065) | 0.12 |
|
| ||||
| Cigarette smoke exposure | 0.0024 (-0.36, 0.36) | 0.99 | 0.027 (-0.34, 0.40) | 0.89 |
|
| ||||
| Daycare attendance | -0.30 (-0.79, 0.18) | 0.22 | -0.075 (-0.24, -0.024) | 0.015 |
|
| ||||
| NTHI Colonization | -0.74 (-1.25, 0.23) | 0.004 | -0.60 (-1.12, -0.085) | 0.023 |
|
| ||||
| MC colonization | -0.53 (-0.84, -0.21) | 0.001 | -0.45 (-0.44, -0.12) | 0.007 |
|
| ||||
| SP colonization | -0.33 (-0.78, -0.04) | 0.009 | -0.071 (-0.48, 0.34) | 0.74 |
Non-Hispanic white
Above statistical analysis used Cox proportionate hazards model. This model took into account a child with multiple positive SA samples. All of the factors were present prior to the positivity of SA colonization. NS = not significant; SA = S. aureus, SP = S. pneumoniae, NTHI = non-typeable H. influenzae, MC = M. catarrhalis; CI = confidence interval.
Discussion
We studied NP cultures to investigate SA colonization and its interaction with other respiratory bacterial pathogens in the first six months of life. The study shows that the risk for SA colonization of infants is highest in the first month of life, and thereafter declines quickly as other respiratory bacterial pathogens are acquired.
The higher SA colonization rates in the period soon after birth has also been reported by Jimenez-Truque et al who studied SA colonization at birth, 2 and 4 months; the SA colonization rate peaked at 2 months of age [4]. Peacock et al performed NP cultures in the first 6 months of life at 2-week intervals during first 3 months and at 1-month intervals for next 3 months, and found 50% of infants colonized with SA by 2 months of age, and declining to 21% by 6 months of age [8]. The source of early SA colonization has been shown to be primarily of maternal NP site [4,8]; neonates can be colonized within 3-4 days of birth [5]. However, why the rate declines over next few months is not clear. It could be due to either age related maturity of local innate host defenses or due to environmental influences, including interference from other NP pathogenic and non-pathogenic flora.
Our present study reports for the first time the interaction between SA and three other important respiratory bacterial pathogens, namely, SP, NTHI and MC, in the first six months of life. We show that the decline in SA colonization is associated with increased colonization with other respiratory bacterial pathogens, the highest influence being MC, and then NTHI, and to a lesser extent with SP. Shiri et al conducted a longitudinal study in infants from 1 month to 12 months of age; there was a negative association between SA and SP or NTHI colonization, however, MC colonization was not studied [6]. Rodrigues et al who conducted a cross-sectional study of older children, also found a negative association between SA and NTHI colonization but not with SP, however, MC colonization was not studied [8]. Xu et al evaluated NP colonization in infants older than 6 months of age; while there was negative association between SP and SA colonization, there was no association between SA and NTHI or MC [10]. On the other hand, van der Berth et al, who studied children 6 to 24 months of age, reported a stronger negative association between SA and MC colonization than between SA and SP colonization [11]. Other investigators have exclusively focused on SA and SP interaction. Bogaert et al have shown that in older children, age 1-19 years, who had not been vaccinated against SP, there was a negative association between SA and SP colonization [7]. Regev-Yochav et al also showed a similar negative association in non-SP-vaccinated children who were of median age 1.3 years [12]. We did not perform serotyping of SP strains to evaluate the relationship between vaccine serotypes of SP and SA.
The mechanisms by which various factors directly or indirectly influence SP colonization are complex and poorly understood. Several host genetic polymorphisms and local immunity factors that contribute to predisposition to SA colonization have been described [13]. The direct inhibition of SA colonization by SP could be due to H2O2 or pilus produced by SP [14]. However, there are no published studies of how Gram negative bacteria such as NTHI and MC can directly inhibit SA colonization. Additional studies of SA interaction with MC and NTHI are needed since we show that these two bacteria interfere with SA colonization much more than SP in the first 6 months of life.
We did not demonstrate any effect of breastfeeding, daycare attendance and cigarette smoke exposure on the overall risk for SA colonization; however, this study was not designed to investigate these factors. Other investigators have also observed contradictory effects of these factors on SA colonization. In our study, daycare attendance was shown to increase the time to SA colonization. The reasons for our observation may be that the daycare attendance rate was low in our study, and the attendance began towards the end of the study period which led to increased colonization risk at a later age in this group.
Viral URI was also not a risk for SA colonization in our study population. This lack of association may not be surprising in the first few months of life as the colonization rate of SA is highest soon after birth, which is primarily of maternal origin, and as the infant grows older, SA colonization rate continues to decline even though there is more community exposure to respiratory viruses. There are few studies investigating the role of URI in SA colonization in infants. Van der Bergh et al showed that in older infants and young children, influenza virus infection was positively associated with SA colonization [11]. Rodrigues et al observed falling rates of SA colonization even as rhinitis symptoms increased [8]. On the other hand, NTHI and SP colonization increased as rates of rhinitis increased. Xu et al also observed that the SA colonization rate at visits for acute otitis media, which typically occurs following URI, was not significantly different that at healthy visits [9].
Another observation in our study was that the proportion of SA strains that were MRSA was highest in the first month but the subsequent decline was proportionately greater for MRSA strains than MSSA. This observation is similar to those of Jimenez-Truque et al and Peacock et al who showed that after peaking at 2 months of age, MRSA colonization rate declines by 4 months of age [4,7]. The reasons for the faster decline of MRSA have not been explored, but it is possible that MRSA strains have lower colonizing advantage due to alterations in surface adhesins and biofilm formation than MSSA strains, or that MSSA strains acquired at later age occupy a competitive niche that directly inhibits MRSA colonization. In the present study, we did not perform the strain typing of SA isolates to show whether any specific strain, such as USA300, has any relationship to the colonization pattern in the nasopharynx of young infants. Further studies are needed to explore these associations.
The SA colonization rate in our young cohort was not associated with the risk for invasive infections. This low risk of infection despite high colonization rate is in keeping with the study of Chatzakis et al who showed that during one year of follow up of 128 infants from birth, infants with NP colonization with SA did not have any invasive infection [3]. Nonetheless, infrequent occurrence of infection has been documented among colonized young infants [4].
Our study limitations are that we did not analyze the source of various bacterial species, the genetic profile of bacteria to assess longitudinal persistence of the same species, and the effect of family size and antibiotic use during the study period. We also did not analyze the influence of SP vaccine as our study subjects were not immunized with SP vaccine in the first two months of life (as per vaccination schedule), they did not complete the primary series until 6 months of age, and the SP colonization rate rose even as immunization began after 2 months of age; therefore, the overall SP vaccination impact on SA colonization through indirect effect on SP colonization is likely to be limited in our study group.
In summary, the present study shows that SA colonization is negatively associated with other common respiratory bacterial pathogens in first six months of life, specifically with MC. Other than these bacteria, other environmental and host factors do not appear to be important. Additional studies are needed to identify direct and indirect factors that alter the dynamics of colonization of bacterial pathogens in NP of infants after birth.
Acknowledgments
We thank Linda Ede, Esther Valdivia, Rocio Trujillo, Ami Patel and Lilia Rodriguez for specimen and clinical data collection, data entry, and Xiong Ying for specimen processing.
Funding source: This work was supported by the National Institutes of Health grants R01DC005841 and UL1TR000071
Footnotes
Financial Disclosure Statement: none.
References
- 1.Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1–7. doi: 10.1097/INF.0b013e31815819bb. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong-Esther CA. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann Hum Biol. 1976;3:221–227. doi: 10.1080/03014467600001381. [DOI] [PubMed] [Google Scholar]
- 3.Chatzakis E, Scoulica E, Papageorgiou N, Maraki S, Samonis G, Galanakis E. Infant colonization by Staphylococcus aureus: role of maternal carriage. Eur J Clin Microbiol Infect Dis. 2011;30:1111–7. doi: 10.1007/s10096-011-1199-9. [DOI] [PubMed] [Google Scholar]
- 4.Jimenez-Truque N, Tedeschi S, Saye EJ, McKenna BD, Langdon W, Wright JP, et al. Relationship between maternal and neonatal Staphylococcus aureus colonization. Pediatrics. 2012;129:e1252–1259. doi: 10.1542/peds.2011-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leshem E, Maayan-Metzger A, Rahav G, Dolitzki M, Kuint J, Roytman Y, Goral A, Novikov I, Fluss R, Keller N, Regev-Yochay G. Transmission of Staphylococcus aureus from mothers to newborns. Pediatr Infect Dis J. 2012;31:360–3. doi: 10.1097/INF.0b013e318244020e. [DOI] [PubMed] [Google Scholar]
- 6.Shiri T, Nunes MC, Adrian PV, Van Niekerk N, Klugman KP, Madhi SA. Interrelationship of Streptococcus pneumoniae, Haemophilus influenza and Staphylococcus aureus colonization within and between pneumococcal-vaccine naïve mother-child dyads. BMC Infect Dis. 2013;13:483. doi: 10.1186/1471-2334-13-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, Verbrugh HA, Hermans PW. Colonisation by Streptococcus pneumonia and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–2. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 8.Peacock SJ, Justice A, Griffiths D, de Silva GD, Kantzanou MN, Crook D, Sleeman K, Day NP. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol. 2003;41:5718–25. doi: 10.1128/JCM.41.12.5718-5725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues F, Foster D, Nicoli E, Trotter C, Vipond B, Muir P, Gonçalves G, Januário L, Finn A. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenza and Staphylococcus aureus in children attending daycare. Pediatr Infect Dis J. 2013;32:227–32. doi: 10.1097/INF.0b013e31827687fc. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Almudervar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerg Infect Dis. 2012;18:1738–45. doi: 10.3201/eid1811.111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Bergh MR, Biesbroek G, Rossen JW, de Steenhuijsen Piters WA, Bosch AA, van Gils EJ, Wang X, Boonacker CW, Veenhoven RH, Bruin JP, Bogaert D, Sanders EA. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One. 2012;7:e47711. doi: 10.1371/journal.pone.0047711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, Derazne E, Rahav G, Rubinstein E. Association between carriage of Streptococcus pneumonia and Staphylococcus aureus in Children. JAMA. 2004;11:716–20. doi: 10.1001/jama.292.6.716. [DOI] [PubMed] [Google Scholar]
- 13.Weidenmaier C, Goerke C, Wolz C. Staphylococcus aureus determinants for nasal colonization. Trends Microbiol. 2012;20:243–50. doi: 10.1016/j.tim.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumonia and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol. 2006;188:4996–5001. doi: 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chonmaitree T, Alvarez-Fernandez P, Jennings K, Trujillo R, Marom T, Loeffelholz MJ, Miller AL, McCormick DP, Patel JA, Pyles RB. Symptomatic and asymptomatic respiratory viral infections in the first year of life: Association with acute otitis media development. Clin Infect Dis. 2015;60:1–9. doi: 10.1093/cid/ciu714. [DOI] [PMC free article] [PubMed] [Google Scholar]


