Abstract
Purpose
Anaplastic thyroid cancer (ATC) is a rare but lethal malignancy without any effective therapy. The aim of the present study was to use a high-throughput drug library screening to identify a novel therapeutic agent that targets dysregulated genes/pathways in ATC.
Experimental Design
We performed quantitative high-throughput screening (qHTS) in ATC cell lines using a compound library of 3,282 drugs. Dysregulated genes in ATC were analyzed using genome-wide expression analysis and immunohistochemistry in human ATC tissue samples and ATC cell lines. In vitro and in vivo studies were performed for determining drug activity, effectiveness of targeting, and the mechanism of action.
Results
qHTS identified 100 active compounds in three ATC cell lines. One of the most active agents was the first-in-class survivin inhibitor YM155. Genome-wide expression analysis and immunohistochemistry showed overexpression of survivin in human ATC tissue samples, and survivin was highly expressed in all ATC cell lines tested. YM155 significantly inhibited ATC cellular proliferation. Mechanistically, YM155 inhibited survivin expression in ATC cells. Furthermore, YM155 treatment reduced claspin expression, which was associated with S-phase arrest in ATC cells. In vivo, YM155 significantly inhibited growth and metastases and prolonged survival.
Conclusions
Our data show that YM155 is a promising anticancer agent for ATC and that its target, survivin, is overexpressed in ATC. Our findings support the use of YM155 in clinical trials as a therapeutic option in advanced and metastatic ATC.
Keywords: Anaplastic thyroid cancer, survivin, claspin, survivin inhibitor, YM155
Introduction
Anaplastic (undifferentiated) thyroid cancer (ATC) has an incidence of approximately 1 to 2 cases per million per year (1, 2). However, patients with ATC have a median survival time of less than 6 months, with fewer than 20% of patients surviving one year. Because ATC, although rare, is a lethal malignancy, accounting for approximately one-third of all thyroid cancer–related deaths (2–4), there is a pressing need for new effective therapies.
Traditional approaches to drug discovery require a significant investment of time and funding. The drug development process is further limited by the declining number of new active substances entering development phases, as well as by the persistently high rate of late-stage drug failures (5). An emerging approach to developing therapies for rare, or orphan, cancers such as ATC instead exploits the multitude of established compounds, which are already approved for clinical use or currently in clinical trials (6–8). Recycling these established agents for new indications is an attractive approach for several reasons. Given that repurposed compounds have known pharmacokinetic, pharmacodynamic, and toxicity profiles, promising drugs can be rapidly transitioned into phase II or phase III clinical trials to test efficacy. Furthermore, profiling drugs may also help to uncover new insights into the processes underlying carcinogenesis as well as aid in identifying novel cancer-specific targets for therapy.
In this study, we undertook a quantitative high-throughput screening (qHTS) of 3,282 clinically approved drugs in ATC cell lines. One of the most active agents was the first-in-class survivin inhibitor YM155. YM155 significantly inhibited ATC cellular proliferation. Survivin was overexpressed in human ATC and differentiated thyroid cancer, and YM155 reduced survivin in ATC cells. To understand the effect of YM155 on cellular proliferation, we performed an apoptosis protein array with YM155 treatment and discovered it also reduces claspin expression in ATC cells, which is associated with ATC cell arrest in S phase. The potent anticancer activity of YM155 in ATC was further confirmed as it significantly inhibited growth and metastases and prolonged survival in in vivo experiments.
Materials and Methods
Cell Lines and Animals
Human ATC cell line 8505C was purchased from the European Collection of Cell Cultures (United Kingdom), and cell lines C643 and SW1736 were purchased from CLS Cell Lines Service GmbH (Germany). The human thyroid cancer cell lines THJ-16T, THJ-21T and THJ-29T were a kind gift from Dr. John A. Copland (Mayo Clinic). The ATC cell lines were maintained in Dulbecco's Modified Eagle Medium (DMEM) with 4,500 mg/L D-glucose, 2 mM L-glutamine, and 110 mg/L sodium pyruvate, supplemented with 10% fetal bovine serum (FBS), penicillin (10,000 U/mL), streptomycin (10,000 U/mL), and fungizone (250 mg/mL), in a standard humidified incubator at 37°C, in a 5% CO2 atmosphere. The cell lines were authenticated using short tandem repeat profiling. Six- to eight-week-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ breeder mice were purchased from the Jackson Laboratory. The mice were maintained and bred according to the guidelines of the NIH Animal Research Advisory Committee (ARAC).
National Institutes of Health Chemical Genomics Center pharmaceutical library screening
The National Institutes of Health Chemical Genomics Center Pharmaceutical Collection (NPC) and the Mechanism Interrogation PlatE (MIPE) collection consists of 3,282 small-molecule compounds and drugs approved for human or animal use. Test compounds from the library were prepared as previously described (9).
Quantitative high-throughput proliferation assay
Cell viability after compound treatment was measured using a luciferase-coupled ATP quantitation assay (CellTiter-Glo) in 8505C, C643, and SW1736 cells. The change of intracellular ATP content represents the number of metabolically competent cells after compound treatment. The final concentration of the compounds in the assay ranged from 0.5 nM to 46 μM.
Survivin expression analysis in thyroid tissue
The dataset GSE29265, with 49 thyroid samples, was downloaded from the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database (GSE29265). There were 20 normal samples, 20 papillary thyroid cancers (PTCs), and 9 ATCs in this dataset. Normalized intensity values for survivin, also known as BIRC5 (probeset ID X202094_at, X202095_s_at, and X210334_x_at), were extracted for analysis and adjusted for multiple comparison.
Independent thyroid tissue samples for immunohistochemistry were used to evaluate survivin protein expression. Tissue microarrays were purchased from US Biomax (#TH641). This array contained duplicates of 6 follicular adenomas, 6 follicular thyroid carcinomas, 6 PTCs, and 6 ATCs, as well as 16 normal tissues from lungs, thyroid, and testis.
Cell proliferation
Cell proliferation assays were performed in quadruplicates, with 8505C, SW1736, C643, THJ-16T, THJ-21T, and THJ-29T cells plated in 96-well black plates at a concentration of 2 × 103 to 4 × 103 cells per well, depending on the cell line, in 100 μl culture medium. After 24 hours (day 0), 100 μl of culture media containing YM155 or corresponding vehicle was added to each well. CyQuant (Invitrogen) proliferation assays were performed at days 0, 2, 4, and 6 according to the manufacturer's instructions. The cell densities were determined using a 96-well fluorescence microplate reader (Molecular Devices) at 485 nm/538 nm.
Western blot
The total protein lysate was analyzed by SDS/PAGE, transferred to a nitrocellulose membrane, and immunostained overnight at 4°C with the following antibodies: anti-survivin (1:250, Cell Signaling Technology); anti-GAPDH (1:3000, Santa Cruz Biotechnology); anti-claspin (1 μg/mL, R&D Systems, Inc); anti-β-actin (1:3000, Santa Cruz Biotechnology). GAPDH and β-actin were used as a loading control. The membranes were incubated with the appropriate horseradish peroxidase–conjugated IgG (anti-rabbit 1:3000, Cell Signaling Technology, or anti-mouse 1:10000, Santa Cruz Biotechnology), and proteins were detected by enhanced chemiluminescence (ECL; Thermo Scientific).
Immunohistochemistry
To examine the protein expression of survivin, immunohistochemistry was performed using a tissue array TH641 (US Biomax, Inc) with a rabbit polyclonal antibody (Novus Biologicals, NB500-201) at 1:500 dilution. Briefly, slides were deparaffinized in xylene and graded alcohols, and subjected to antigen retrieval in a pressure cooker with citrate buffer (pH6) for 20 minutes. Endogenous enzyme activity was blocked with 3% hydrogen peroxide in methanol with additional serum-free protein blocking to reduce nonspecific reactions. Subsequently, slides were incubated with primary antibody for 60 minutes at room temperature, and antigen–antibody reaction was detected with Dako Envision+ Dual Link system-HRP (Cat.K4061, Dako), visualized in 3,3'-diaminobenzidine, counterstained with hematoxylin, dehydrated, and coverslipped. The staining result was reviewed by a pathologist (A.R.).
Apoptosis array
A human apoptosis array to detect the expression level of 35 apoptosis-related proteins was purchased from R&D Systems (Catalog # ARY009). The experiments were performed according to the manufacturer's instructions. Signal intensity for proteins showing differences in intensity was quantified with ImageJ and averaged for each protein.
Cell cycle assay
Cells were plated in 6-well plates at 6 × 104 to 12 × 104 cells per well, depending on the cell line, in 2 mL culture medium. After 24 hours (day 0), fresh culture medium of YM155 or corresponding vehicle was added to each well. Following treatment for 24 hours and 48 hours, the cells were harvested, fixed with cold 70% ethanol for 30 minutes at 4°C, and incubated in the dark with RNase (100 mg/mL) and propidium iodide (50 mg/mL) for 30 minutes at 37°C. A total of 20,000 nuclei were examined by flow cytometry using a Calibur flow cytometer (Becton/Dickinson), and data were analyzed using ModFit software (Verity Software House).
Small interfering (si)RNA transfection
The siRNA for human Claspin (siRNA ID: s34330 and s34331) and scrambled negative control (Part#: 4390843) were purchased from Applied Biosystems. 8505c, C643, and SW1736 cells were reverse transfected with each individual siRNA at a concentration of 80 nmol/l using Lipofectamine RNAiMAX (Invitrogen). Total RNA was isolated and the level of Claspin mRNA was determined by quantitative RT-PCR.
In vivo mouse studies
Animal studies were approved by the National Cancer Institute Animal Care and Use Committee. The mice were maintained according to the guidelines of the NIH ARAC. A metastatic mouse model was utilized for in vivo studies of YM155 (10). 8505C cells were first stably transfected with linearized pGL4.51[luc2/CMV/Neo] vector luciferase reporter (8505C/luc2 cells). NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice were then injected via tail vein with 8505C-luc2 cells (30,000 cells per mouse). Lung tumor development was confirmed at one week after tumor cell implantation, using bioluminescence imaging and the Xenogen in vivo imaging system. The images were analyzed using IVIS Living Image software (Caliper Life Sciences Inc.). Changes in tumor burden were subsequently reassessed using the same bioluminescence imaging protocol.
YM155 (Astellas Pharma, Inc.) was first dissolved in dimethyl sulfoxide (DMSO) according to the manufacturer's directions. Beginning on day 7 after tumor cell injection, the mice received a subcutaneous implant of a continuous infusion, microosmotic pump (DURECT Corporation) loaded with either YM155 (2 mg/kg) or DMSO vehicle solution. Pumps provided continuous infusion of YM155 or vehicle solution for 28 days following implantation. Survival endpoints were defined as either death or reaching humane euthanasia criteria endpoints. Mice were euthanized by CO2 inhalation.
Statistical analysis
To determine compound activity in the qHTS assay, raw plate reads for each titration point were first normalized to a positive control (tetraoctylammonium bromide, a toxin, 100% inhibition) and DMSO-only wells (0% inhibition). The titration-response data for each sample were plotted and modeled by a 4-parameter logistic fit, yielding IC50 (concentration of half-maximal inhibition) and efficacy (maximal response) values.
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software). Parametric and nonparametric data were analyzed using a two-tailed t-test and the Mann/Whitney U test, respectively. Difference in survival for in vivo studies was assessed using Kaplan–Meier survival curves. A p value < 0.05 was considered statistically significant. Data are presented as mean ± SD or mean ± SEM.
Results
Identification of YM155 as an active agent in ATC cells
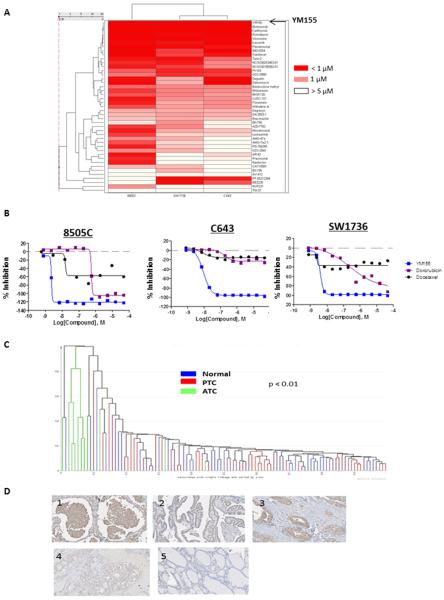
We identified 100 active compounds in the three ATC cell lines (8505C, C643, and SW1736). One of the most active compounds with high efficacy and low IC50 was YM155 (Fig. 1A, Table 1). YM155 had an efficacy greater than 90% in all three ATC cell lines and an IC50 in the nanomolar range. The IC50 in the ATC cell lines was well below that achieved in humans (11). The ATC cells had greater sensitivity to YM155 than to the therapeutic agents previously used in the clinic (Fig. 1B) (12–14).
Figure 1.
Quantitative high-throughput screening (qHTS) of the NPC compound library in anaplastic thyroid cancer (ATC) cells and surviving expression. A, Activity profile of the top-ranking active agents in the 8505C, C643, and SW1736 cell lines. Drugs included in the heat map were pan-active against the three ATC cell lines, had an efficacy ≥ 50%, and had an IC50 below 10 μM. Drug names are shown on the right of each row and are listed from lowest to highest IC50 in the heat map. The spectrum of colors in the heat map represents the range of IC50 values in each cell line. B, Comparison of YM155 activity to doxorubicin and docetaxel in 3 ATC cell lines. Survivin expression in thyroid tissue samples and ATC cell lines. C, Supervised hierarchical cluster analysis of survivin mRNA expression in 9 ATC, 20 PTC, and 20 normal thyroid samples. Normalized intensity values for survivin were extracted from the publically available GEO database (GSE29265). D, Representative images of survivin immunohistochemistry. 1: ATC; 2: PTC; 3: follicular thyroid cancer; 4: follicular adenoma; and 5: normal thyroid tissue.
Table 1.
Pharmacokinetic analysis of YM155 in 3 ATC cell lines.
| Cell Line | Curve Class* | IC50 (μM) | Maximum Attainable Response (%) |
|---|---|---|---|
| 8505C | −1.1 | 0.002 | −116.0 |
| C643 | −1.1 | 0.009 | −99.0 |
| SW1736 | −1.1 | 0.004 | −93.2 |
Dose response curve to YM155 treatment
Survivin is overexpressed in human thyroid cancer (including ATC) and ATC cell lines
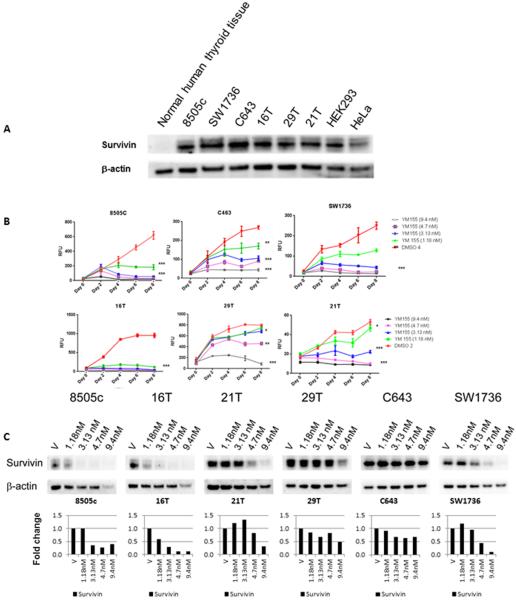
YM155 is a first-in-class suppressant of survivin. Consequently, we sought to determine whether survivin is in fact overexpressed in thyroid cancer. We first used samples from the publically available GEO database (GSE29265) to examine mRNA expression levels of survivin in ATC, PTC, and normal thyroid tissue. Survivin was significantly upregulated in ATC as compared to PTC and normal thyroid tissue (p < 0.01), and the ATC samples clustered together (Fig. 1C). We next evaluated survivin protein expression in an independent set of thyroid tissue samples (Fig. 1D). Survivin protein expression was positive in all ATC and PTC samples, in 3 of 6 follicular thyroid cancer samples, and no follicular adenomas or normal thyroid tissue samples. Two of the 6 ATC samples had nuclear staining in addition to cytoplasmic staining (Fig. 1D). Western blot analysis of survivin expression in ATC cell lines revealed abundant survivin expression levels (Fig. 2A).
Figure 2.
YM155 effect on cellular proliferation and survivin and claspin expression. A, Survivin protein expression in ATC cell lines, HEK293 and HeLa cell lines, and normal thyroid tissue. β-actin protein expression was used as a loading control. B, YM155 inhibits ATC cellular proliferation in a dose- and time-dependent fashion. * P < 0.05; ** P < 0.01, ***P < 0.001. Error bars are ± SD
C, YM155 reduces survivin protein expression in 6 ATC cell lines. Cells were treated at concentrations within the clinically achievable range (1.18 nM–9.4 nM). Lower panel shows band densitometry of surviving level normalized to β-actin and vehicle control = 1.
YM155 inhibits cell proliferation, reduces survivin and claspin expression, and arrests cells in S phase
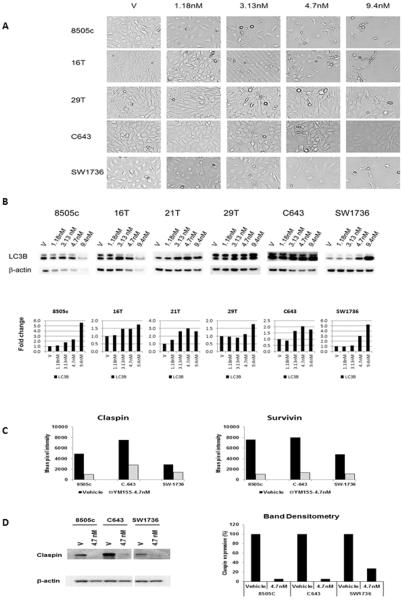
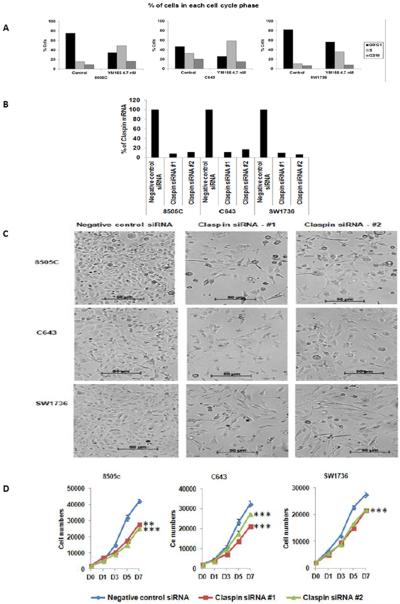
Given the activity of YM155 in the qHTS assay and the overexpression of its target in thyroid cancer, especially ATC, we next validated the antiproliferative effect of YM155 in 6 ATC cell lines. Treatment with YM155 inhibited cellular proliferation in all ATC cell lines tested, and this response was found to be dose- and time-dependent (Fig. 2B). YM155 treatment reduced survivin expression in all ATC cell lines (Fig. 2C). Interestingly, YM155 treatment in 6 of the ATC cell lines had no significant effect on caspase 3/7 activity (3–8 nM vs. vehicle for 12–72 hours, data not shown). Thus, we evaluated cell morphology with YM155 treatment and also found increased LC3B expression, a marker of autophagy, with YM155 treatment (Fig. 3A and 3B) (15, 16). To further determine the mechanism by which YM155 inhibited cellular proliferation, we analyzed key proteins involved in regulating apoptosis using a human apoptosis array. In addition to inhibiting survivin expression and increasing LC3B expression, YM155 reduced claspin expression in ATC cells (Fig. 3C). The reduced expression of claspin was confirmed by Western blot analysis (Fig. 3D). Because claspin regulates DNA replication in normal cells, we performed cell cycle analysis on the ATC cells after YM155 treatment (3–8 nM vs. vehicle) (17, 18). YM155 treatment increased the number of cells in S phase (by 14–34%) and decreased the number of cells in G0G1 in the ATC cell lines tested (8505C, C643, and SW1736) (Fig. 4A). To determine if the effect of YM155 on reduced claspin expression may mediate the effect of YM155 on cellular proliferation and cell cycle, we directly knocked down the expression of claspin in ATC cell lines (8505C, C643, and SW1736) (Fig. 4B). Claspin knockdown in ATC cells reduced cellular proliferation, and increased the number of cells in S phase (4.4–8.9%) and G2M (5.2–7.9%) while reducing the number of cells in G0G1(14.1–15.9%) (Fig. 4C–D).
Figure 3.
YM155 treatment effects on cell morphology, LC3B and apoptosis related proteins.
A, Cell morphology with YM155 treatment in ATC cells. Images show cells at 2 days after treatment using a phase contrast microscope at 10X magnification.
B, YM155 treatment increased LC3B expression in ATC cells. ATC cells were treated for 48 hours at indicated concentrations. Lower graph indicates band densitometry normalized to β-actin levels and vehicle control = 1.
C, Densitometry measurements from apoptosis protein array with YM155 treatment in ATC cells (8505C, C643, SW1736) shows reduced survivin and claspin expression. ATC cells were treated with YM155 (4.7 nM) for 48 – 53 hours, and cell lysates were incubated with apoptosis protein array (see Materials and Methods).
D, Claspin expression is reduced with YM155 treatment in ATC cells. ATC cells were treated with YM155 (4.7 nM) for 48 (8505C) to 53 (C643 and SW1736) hours, and the cell lysates were used for Western blot to detect claspin expression. The claspin protein expression levels were quantified by band densitometry normalized to β-actin level with control cells considered as 100% (right panel).
Figure 4.
YM155 effect on cell cycle analysis and claspin knockdown effect on cell morphology and proliferation.
A, Representative FACS data with YM155 treatment (4.7 nM for 48 hour treatment) compared to vehicle shows increased number of cells in S phase and decreased number of cells in G0/G1.
B, Claspin knockdown in ATC cells. Four days after transfection, total RNA was isolated, and the claspin mRNA expression was determined using GAPDH as an internal control.
C, Cell morphology with claspin knockdown. Bright field microscope images were taken at day 7 of siRNA transfection. The results shown are representative of three separate experiments.
JD Claspin knockdown decreases cellular proliferation. Two days after transfection, cells were plated into 96 well black plates (Day 0). Cell proliferation was determined using CyQuant proliferation assays. Error bars are ± SD. ** for p < 0.01; *** for p < 0.001. The results shown are representative of four separate experiments.
YM155 induces significant regression of metastatic tumor burden and prolongs survival in vivo
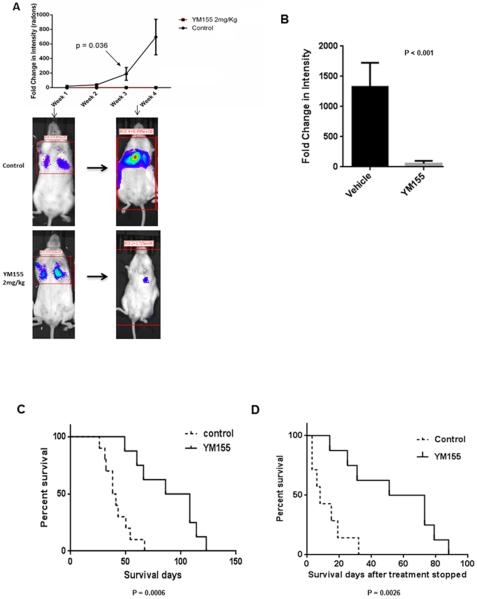
We further validated the antitumor activity of YM155 in an in vivo ATC metastases mouse model, which is a clinically relevant model for human ATC. Following 4 weeks of treatment, mice receiving YM155 at 2 mg/kg showed tumor growth inhibition, and, in some mice, regression of metastatic tumor burden was observed (Fig. 5A). This significant antitumor effect was observed after only 1 week of treatment, with sustained activity over the remaining treatment period. We then validated these results in an independent experiment at 5 weeks, 1 week after treatment was stopped, and observed significant growth inhibition (Fig. 5B). There was no significant difference between the treated and untreated mice in weight change, and no serious adverse effects were observed during the treatment period. The overall survival of mice treated with YM155 was significantly longer, as compared to the control, with a mean survival of 42 days for the control group and 89 days for YM155-treated mice (Fig. 5C). As shown in Fig. 5D, the animal life spans were significantly increased in the treatment group, even after stopping YM155 treatment.
Figure 5.
YM155 is active in mouse model of metastatic ATC. A, YM155 inhibits growth of 8505C metastases. Upper panel shows fold change in tumor intensity and lower panel shows representative images of mice at week 1 and at week 4. There were 5 mice in each group. B, Validation in vivo effect of YM155 at 5 weeks, 1 week after treatment withdrawal. There were 5 mice in each group. C, Survival is significantly increased with YM155 treatment. Significance was calculated using the log-rank (Mantel-Cox) test. D, Mice survival after stopping YM155 treatment. The first day after YM155 treatment stopped is day 1. Significance was calculated using the log–rank (Mantel-Cox) test.
Discussion
In this study, we used qHTS to identify YM155, the first-in-class agent that targets survivin, as an active agent against ATC cells. We found significant overexpression of survivin in ATC, PTC, and follicular thyroid cancer, as compared with benign thyroid tumors and normal thyroid tissue. We validated the potent dose- and time-dependent effect that YM155 has on growth in vitro, and on metastases and survival in an ATC metastatic mouse model. In addition to inhibiting survivin expression in ATC cells, YM155 also reduced claspin expression, which was associated with an increased number of cells in S phase. Together, these findings support the use of YM155 as a novel therapeutic option in locally advanced and metastatic ATC.
YM155 is a first-in-class survivin inhibitor currently in phase I/II clinical trials as monotherapy or combination therapy for a variety of human malignancies (11, 19–23). Our qHTS in ATC cell lines revealed that YM155 was one of the most active therapeutic agents, with an IC50 10-fold lower than the Cmax from phase I clinical trials. Furthermore, delivery of YM155 by continuous infusion (24) presented many additional advantages, widening the therapeutic window of the drug and avoiding the excessive fluctuations in plasma levels that either produce unwanted side effects or result in subtherapeutic levels (25, 26). Finally, screening data that compared the antiproliferative effects of YM155 with chemotherapeutic agents used in patients with ATC, doxorubicin and docetaxel, showed greater efficacy of YM155 at much lower drug concentrations.
We show, for the first time, that YM155 has potent anticancer activities in ATC cells, inducing dose- and time-dependent growth inhibition that is associated with suppression of survivin and claspin expression. While the specific mechanism by which YM155 reduces survivin expression is unclear, it is believed to target the promoter region of the survivin gene, BIRC5, suppressing expression at both the mRNA and protein levels (24). Furthermore, YM155 has been shown to selectively suppress survivin, with little effect on the expression levels of other apoptosis-related proteins (24). Using an apoptosis protein array, we also found YM155 treatment produced no significant variation in anti- and pro-apoptosis proteins. Survivin is known to associate with microtubules of the mitotic spindle at the beginning of mitosis, serving as an important regulator of cell cycle progression (27). Furthermore, disruption of survivin–microtubule interactions has been shown to induce cell cycle arrest, apoptosis, and subsequent cell death (28). Several studies have shown that survivin is upregulated in ATC and is associated with more aggressive thyroid cancer (29, 30). Claspin is required for efficient DNA replication during normal S phase and is highly expressed in more aggressive cancers (17, 31–33). YM155 treatment did affect cell morphology in ATC cells and increased LC3B expressing consistent with its effect on autophagy in other cancer cells (15, 16, 34). Mechanistically, then, by suppressing both survivin and claspin expression and increasing the expression of LC3B, YM155 inhibits growth and metastasis in vitro and in vivo. It is possible that the effects of YM155 may be mediated through yet undefined additional mechanisms in ATC cells.
When Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice with metastatic ATC were treated with YM155 at 2 mg/kg, a durable response was observed in widely metastatic tumor. This effect was both rapid in onset, appearing within 1 week of treatment, and sustained, continuing throughout the remaining treatment period and after withdrawal of treatment. Furthermore, there was no statistically significant difference between treated and control groups in the amount of weight lost, and no adverse effects were observed in the test group. A longer survival time was also observed in the treatment group, even after withdrawal of YM155 treatment. These findings suggest that YM155 would be an effective anticancer agent for locally advanced and metastatic ATC in humans.
In conclusion, we identified the first-in-class survivin suppressant, YM155, as an active agent in ATC cells, using qHTS. We confirmed that YM155 exhibits strong antitumor activity against human ATC cells in vitro and validated its antitumor activity in vivo, using a metastasis mouse model that recapitulates the clinical features of ATC. Moreover, we found that survivin was overexpressed in ATC, making it an attractive target for ATC therapy and one to be considered for clinical trials.
Translation Relevance.
Anaplastic thyroid cancer (ATC) is a rare malignancy but is one of the most fatal human cancers. There are no standard or effective therapies for locally advanced and metastatic ATC and most patients localized ATC develop recurrence. We performed an integrated genome-wide expression analysis and high-throughput drug screening in ATC. We show robust overexpression of survivin in ATC and that the first-in-class survivin suppressant YM155 induces durable anticancer activity in ATC in vitro and in vivo. YM155 inhibited survivin and claspin expression in ATC cells, which was associated with S phase arrest. Moreover, direct knockdown of claspin in ATC cells recapitulated the effect of YM155 treatment in vitro. Our findings provide a preclinical basis upon which to evaluate YM155 therapy for locally advanced and metastatic ATC in humans.
Acknowledgments
Financial Support: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH), and by the NIH Medical Research Scholars Program, a public–private partnership supported jointly by NIH; and by the generous contributions to the Foundation for NIH from Pfizer, the Doris Duke Charitable Foundation, Alexandria Real Estate Equities, Inc., Mr. and Mrs. Joel S. Marcus, the Howard Hughes Medical Institute, and other private donors. For a complete list of donors, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program
Footnotes
Conflicts of interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011:542358. doi: 10.1155/2011/542358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103:1330–5. doi: 10.1002/cncr.20936. [DOI] [PubMed] [Google Scholar]
- 3.Granata R, Locati L, Licitra L. Therapeutic strategies in the management of patients with metastatic anaplastic thyroid cancer: review of the current literature. Curr Opin Oncol. 2013;25:224–8. doi: 10.1097/CCO.0b013e32835ff44b. [DOI] [PubMed] [Google Scholar]
- 4.Nilubol N, Kebebew E. Should small papillary thyroid cancer be observed? A population-based study. Cancer. 2015;121:1017–24. doi: 10.1002/cncr.29123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–68. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- 6.Miller SC, Huang R, Sakamuru S, Shukla SJ, Attene-Ramos MS, Shinn P, et al. Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action. Biochem Pharmacol. 2010;79:1272–80. doi: 10.1016/j.bcp.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shum D, Smith JL, Hirsch AJ, Bhinder B, Radu C, Stein DA, et al. High-content assay to identify inhibitors of dengue virus infection. Assay Drug Dev Technol. 2010;8:553–70. doi: 10.1089/adt.2010.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahinas D, Liang M, Datti A, Pillai DR. A repurposing strategy identifies novel synergistic inhibitors of Plasmodium falciparum heat shock protein 90. J Med Chem. 2010;53:3552–7. doi: 10.1021/jm901796s. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, He M, Zhang Y, Nilubol N, Shen M, Kebebew E. Quantitative high-throughput drug screening identifies novel classes of drugs with anticancer activity in thyroid cancer cells: opportunities for repurposing. J Clin Endocrinol Metab. 2012;97:E319–28. doi: 10.1210/jc.2011-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Gaskins K, Yu Z, Xiong Y, Merino MJ, Kebebew E. An in vivo mouse model of metastatic human thyroid cancer. Thyroid. 2014;24:695–704. doi: 10.1089/thy.2013.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segerhammar I, Larsson C, Nilsson IL, Backdahl M, Hoog A, Wallin G, et al. Anaplastic carcinoma of the thyroid gland: treatment and outcome over 13 years at one institution. J Surg Oncol. 2012;106:981–6. doi: 10.1002/jso.23177. [DOI] [PubMed] [Google Scholar]
- 13.Onoda N, Kashiwagi S, Noda S, Kawajiri H, Takashima T, Ishikawa T, et al. High efficacy of chemoradiation therapy sensitized by weekly docetaxel for anaplastic thyroid cancer. Anticancer Res. 2013;33:3445–8. [PubMed] [Google Scholar]
- 14.Troch M, Koperek O, Scheuba C, Dieckmann K, Hoffmann M, Niederle B, et al. High efficacy of concomitant treatment of undifferentiated (anaplastic) thyroid cancer with radiation and docetaxel. J Clin Endocrinol Metab. 2010;95:E54–7. doi: 10.1210/jc.2009-2827. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Chen Z, Diao X, Huang S. Induction of autophagy-dependent apoptosis by the survivin suppressant YM155 in prostate cancer cells. Cancer Lett. 2011;302:29–36. doi: 10.1016/j.canlet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Wang YF, Zhang W, He KF, Liu B, Zhang L, Zhang WF, et al. Induction of autophagy-dependent cell death by the survivin suppressant YM155 in salivary adenoid cystic carcinoma. Apoptosis. 2014;19:748–58. doi: 10.1007/s10495-013-0960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petermann E, Helleday T, Caldecott KW. Claspin promotes normal replication fork rates in human cells. Mol Biol Cell. 2008;19:2373–8. doi: 10.1091/mbc.E07-10-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith-Roe SL, Patel SS, Zhou Y, Simpson DA, Rao S, Ibrahim JG, et al. Separation of intra-S checkpoint protein contributions to DNA replication fork protection and genomic stability in normal human fibroblasts. Cell Cycle. 2013;12:332–45. doi: 10.4161/cc.23177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Bartlett NL, Vose JM, Lopez-Hernandez A, Seiz AL, Keating AT, et al. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer. 2012;118:3128–34. doi: 10.1002/cncr.26510. [DOI] [PubMed] [Google Scholar]
- 20.Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, et al. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–6. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RJ, Thomas A, Rajan A, Chun G, Lopez-Chavez A, Szabo E, et al. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:2601–6. doi: 10.1093/annonc/mdt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y, et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–80. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- 23.Tolcher AW, Quinn DI, Ferrari A, Ahmann F, Giaccone G, Drake T, et al. A phase II study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancer. Ann Oncol. 2012;23:968–73. doi: 10.1093/annonc/mdr353. [DOI] [PubMed] [Google Scholar]
- 24.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–21. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 25.Fara J, Mitchell C. The value of infusion and injection regimens in assessing efficacy and toxicity of drugs. Trends Pharmacol Sci. 1986;5:21–5. [Google Scholar]
- 26.Sikic BI, Collins JM, Mimnaugh EG, Gram TE. Improved therapeutic index of bleomycin when administered by continuous infusion in mice. Cancer Treat Rep. 1978;62:2011–7. [PubMed] [Google Scholar]
- 27.Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994;6:74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–21. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 29.Ito Y, Yoshida H, Uruno T, Nakano K, Miya A, Kobayashi K, et al. Survivin expression is significantly linked to the dedifferentiation of thyroid carcinoma. Oncol Rep. 2003;10:1337–40. [PubMed] [Google Scholar]
- 30.Pannone G, Santoro A, Pasquali D, Zamparese R, Mattoni M, Russo G, et al. The role of survivin in thyroid tumors: differences of expression in well-differentiated, non-well-differentiated, and anaplastic thyroid cancers. Thyroid. 2014;24:511–9. doi: 10.1089/thy.2013.0196. [DOI] [PubMed] [Google Scholar]
- 31.Verlinden L, Vanden Bempt I, Eelen G, Drijkoningen M, Verlinden I, Marchal K, et al. The E2F-regulated gene Chk1 is highly expressed in triple-negative estrogen receptor/progesterone receptor/HER-2 breast carcinomas. Cancer Res. 2007;67:6574–81. doi: 10.1158/0008-5472.CAN-06-3545. [DOI] [PubMed] [Google Scholar]
- 32.Allera-Moreau C, Rouquette I, Lepage B, Oumouhou N, Walschaerts M, Leconte E, et al. DNA replication stress response involving PLK1, CDC6, POLQ, RAD51 and CLASPIN upregulation prognoses the outcome of early/mid-stage non-small cell lung cancer patients. Oncogenesis. 2012;1:e30. doi: 10.1038/oncsis.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benevolo M, Musio A, Vocaturo A, Dona MG, Rollo F, Terrenato I, et al. Claspin as a biomarker of human papillomavirus-related high grade lesions of uterine cervix. J Transl Med. 2012;10:132. doi: 10.1186/1479-5876-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng SM, Chang YC, Liu CY, Lee JY, Chan HH, Kuo CW, et al. YM155 down-regulates survivin and XIAP, modulates autophagy and induces autophagy-dependent DNA damage in breast cancer cells. Br J Pharmacol. 2015;172:214–34. doi: 10.1111/bph.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]