Abstract
Purpose
Physicians require information on the comparative benefits and harms of medications for optimal treatment decisions. However, this type of data is limited, especially for pediatric patients.
Objective
Our aim was to use observational data to measure and compare medication utilization patterns in a pediatric patient population.
Methods
Using pharmacy claims data from a large, national-scale insurance program in the US, we identified all patients with a diagnosis of epilepsy treated with a first-generation (carabamazepine, ethosuximide, phenobarbital, phenytoin, valproate) or second-generation (carbamazepine XR, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, tiagabine, topiramate, valproate XR, zonisamide) antiepileptic drug. Treatment periods were defined based on prescription fill dates and medication days supplied. Medication use was measured for individual antiepileptic drugs and for first-generation and second-generation drugs as groups.
Results
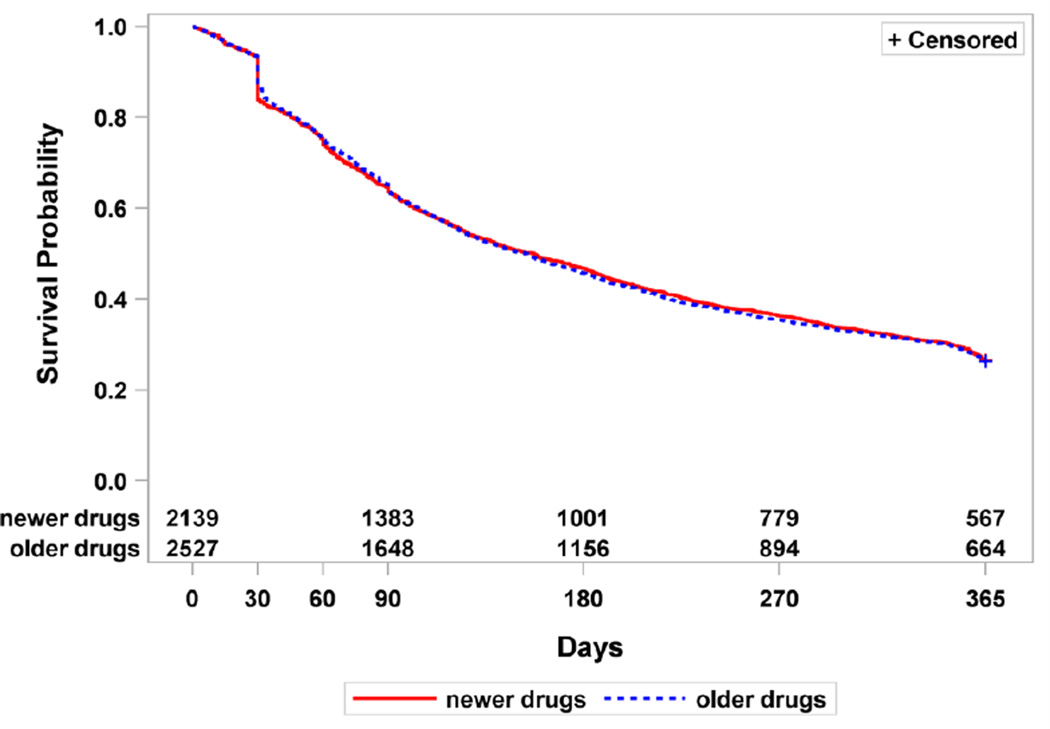
There were 2527 (54%) patients who initiated therapy with first-generation and 2139 (46%) with second-generation antiepileptics. First- and second-generation drugs had the same one-year retention rates (26% [95%CI 24–28] and 26% [95%CI 25–28], respectively). A total of 26% (95%CI 25–28) and 29% (95%CI 27–31) of patients who started on a first- or second-generation antiepileptic medication, respectively, resumed treatment with the initial drug after discontinuation. Overall, 73% (95%CI 71–74) of patients were treated with only one antiepileptic drug, with similar rates for patients started on first- and second-generation drugs (71% [95%CI 69–73] vs 74% [95%CI 72–76]).
Conclusions
Comparing drug utilization patterns in a pediatric population using observational data, we found similar rates of retention and therapeutic changes. These findings are consistent with available comparative data and demonstrate an approach that could be extended to other drug classes and conditions in pediatric populations to examine drug effectiveness.
INTRODUCTION
Physicians frequently lack comparative data when choosing from the growing armamentarium of pharmaceuticals and, as a result, there is tremendous variation in treatment practice.1,2 The lack of comparative effectiveness data is particularly pronounced for pediatric patients, given the small number of drug trials conducted in children compared with adults.3–7 Clinicians often show a preference for newer drugs, but not infrequently, newer drugs are later found to be less effective or safe than previously existing options.8–10 New drugs are generally approved based on demonstrated superiority to placebo and regulatory agencies—including the Food and Drug Administration (FDA) in the United States—typically do not require comparison to existing agents.1,11–13 If there were information available to clinicians and patients on the advantages and disadvantages of a medication compared to others in the same class, they might be less likely to opt for the newer and more costly agent and better able to select the drug that would yield the greatest health benefits for a specific patient.
There are many barriers to conducting large, randomized controlled drug trials in children and sole reliance on these trials to increase comparative drug data in this population may not be feasible. Therefore, we sought to leverage observational data to compare drug effectiveness based on drug utilization patterns, focusing here on first- and second-generation antiepileptic drugs in a pediatric patient population. For example, the length of time a patient continues therapy with a specific medicine may be reflective of the drug’s efficacy as well as safety and tolerability and these measures can be gleaned from prescription fill patterns in health insurance claims data.
Between 1994 and 2009, 10 new antiepileptic agents were approved by the FDA and readily adopted by many clinicians.14 However, there is limited data available describing how these newer, or second-generation, drugs compare to the older, first-generation agents.15 Observational data on drug utilization may provide the opportunity to compare the effectiveness of different treatment options in large patient populations over extended periods and augment data generated in controlled trials. The aim of this study was to use observational data to measure and compare medication utilization in a pediatric patient population treated with antiepileptic drugs.
METHODS
Data Source and Patient Population
The study population was 1,604,580 children between 0 and 18 years of age enrolled in a private national-scale insurance program in the United States. Members enrolled in this plan shared a common employer, were located throughout the United States, and were fully insured. Insurance claims for the program were available from January 1, 1999 through December 31, 2006 and included demographic data, information on medical encounters, and pharmacy prescription claims data. Prescription data consisted of National Drug Codes, date of prescription fill, and number of days supplied. Data on specific dosing regimens was not available. All patient information were de-identified. The Committee on Clinical Investigation at Boston Children’s Hospital deemed that the study did not represent human subjects research and granted a waiver of informed consent.
Patients were included in the study if they were enrolled in the insurance program for at least 120 days without filling a prescription for an antiepileptic drug, had at least one medical encounter associated with a diagnosis of epilepsy or seizures (as defined in the International Classification of Diseases, Ninth Review, codes 345.xx, 779.0, 780.3x), initiated antiepileptic therapy with a single antiepileptic medication.16–18 We considered diagnoses associated with both outpatient and inpatient visits. For patients treated with a specific antiepileptic drug for 30 days or less with no subsequent prescriptions for the same drug within 12 months of the initial treatment, we used the second antiepileptic drug (whenever a second one was prescribed) as the index drug for analysis. This was done in order to avoid analyzing drug utilization for medications used on a temporary basis, for example while the definitive medication is being titrated to the full dose. However, since this may also lead to the exclusion of patients who discontinue treatment due to safety or efficacy concerns within the first 30 days of treatment, we performed a sensitivity analysis in which patients treated with a medication for 30 days or less were included. The results were very similar to those of the main analysis in terms of comparative utilization patterns between first- and second-generation medications (Appendix Tables 1 and 2). Patients with less than a year of continuous insurance coverage after initiation of the index drug were dropped from further analysis.
Appendix Table 1.
Sensitivity Analysis for Measures of Medication Retention for First- and Second-Generation Antiepileptics
| First Treatment Medication |
1. Continuous treatment, mean days (95% CI) |
2. Continuous treatment including polytherapy, mean days (95% CI) |
3. Proportion treatment days with index drug, % (95% CI) |
4. One-year retention, % (95% CI) |
5. Treatment resumption, % (95% CI) |
|---|---|---|---|---|---|
| All antiepileptics | 160 (156, 163) | 171 (169, 176) | 84.5 (83.6, 85.3) | 21.5 (20.4, 22.6) | 22.9 (21.8, 24.1) |
| First-generation drugs | 156 (151, 161) | 168 (162, 173) | 82.9 (81.7, 84.0) | 20.9 (19.4, 22.4) | 21.4 (20.0, 23.0) |
| Second-generation drugs | 164 (158, 170) | 177 (171, 183) | 86.6 (85.4, 87.7) | 22.3 (20.6, 24.1) | 24.8 (23.1, 26.7) |
Appendix Table 2.
Sensitivity Analysis for Measures of Therapeutic Adjustment after Treatment Initiation with First- and Second-Generation Antiepileptics
| First Treatment Medication |
1. Proportion monotherapy (95% CI) |
2. Proportion polytherapy (95% CI) |
3. Number of antiepileptic drugs, mean (95% CI) |
4. Number of therapeutic adjustments, mean (95% CI) |
|---|---|---|---|---|
| All antiepileptics | 69.8 (68.8, 71.1) | 9.2 (8.4, 10.0) | 1.4 (1.4, 1.4) | 1.8 (1.8, 1.9) |
| First-generation drugs | 68.6 (66.8, 70.2) | 9.2 (8.2, 10.3) | 1.4 (1.4, 1.4) | 1.8 (1.7, 1.9) |
| Second-generation drugs | 71.5 (69.6, 73.4) | 9.1 (7.9, 10.3) | 1.4 (1.4, 1.4) | 1.8 (1.8, 1.9) |
Definition of Antiepileptic Drugs
Antiepileptic drugs were identified based on the Multum Drug Classification and included all medications listed in the category “anticonvulsants”.19 We excluded acetazolamide, magnesium sulfate, clonazepam, diazepam, and lorazepam for the purposes of this study since these are not considered primarily anticonvulsant agents. Ethotoin was also excluded because no patient in the study cohort was treated with this agent. First-generation drugs were those approved by the FDA pre-1993 and second-generation drugs those approved post-1993.20
Definition of Medication Treatment Periods
Medication treatment periods were defined as beginning on the day a prescription was filled and extending for the total number of medication days supplied. Gaps in medication treatment were defined as the total number of continuous days without medication supply.16 Gaps between prescriptions lasting 30 days or less were considered the result of poor medication adherence and the period analyzed as a continuous treatment period.16,21–23 This was done for consecutive prescriptions for the same drug as well as consecutive prescriptions for different antiepileptic agents. In these cases, the start date of the second prescription was considered the end date of the first prescription. Treatment gaps of 31 days or greater were defined as separate treatment periods.
Similarly, overlaps in treatment periods were not considered periods of combination therapy if they lasted 30 days or less. The treatment end date for the first drug was defined as the date the second agent was started. This was done for consecutive prescriptions of the same agent as well as for consecutive prescriptions of different antiepileptic drugs. Periods of combination drug treatment were defined as periods lasting longer than 30 days during which the subject was treated with more than one antiepileptic medication.
Drug Comparisons
We compared medication usage focusing on medication retention (the continuation of treatment after medication start) and therapeutic changes during the first year following treatment initiation. We examined 5 measures of medication retention: 1) Continuous treatment: days of continuous treatment with the index drug before any change in the antiepileptic drug regimen (including the discontinuation of the index drug or the addition of one or more antiepileptic drugs); 2) Continuous treatment including polytherapy: days of continuous treatment with the index drug including days of treatment with one or more other antiepileptic drugs; 3) Proportion treatment days with index drug: proportion of total treatment days over the course of the study period during which patients are treated with the index drug; 4) One-year retention: rate of index drug retention one year after initiation without any gaps in treatment or addition of other drugs; and 5) Treatment resumption: Proportion of patients who resume treatment with the index drug after it has been discontinued.
Four measures describe patterns of therapeutic changes following treatment initiation: 1) Proportion monotherapy: The proportion of patients treated with only the index drug throughout the study period; 2) Proportion polytherapy: the proportion of patients who require the addition of a second antiepileptic drug concurrent with the index drug during the first treatment period; 3) Number of antiepileptic drugs: the total number of anticonvulsants prescribed throughout the study period; and 4) Number of therapeutic adjustments: the number of changes in treatment regimen (including discontinuing, adding, and restarting an antiepileptic drug) throughout the study period.
Statistical Analysis
Medication use was measured for individual antiepileptic drugs and for first-generation and second-generation drugs as groups. Means and proportions were calculated with 95% confidence intervals (CI). Kaplan-Meier survival analyses were used to calculate retention rates at one year and log-rank tests performed to compare the retention curves. Post-hoc analyses used the Sidak adjustment for multiple comparisons. All data were analyzed with SAS statistical software (version 9.3, SAS Institute Inc., Cary, North Carolina).24
RESULTS
Study Population
We identified 10,083 patients 0 to 18 years of age who had a medical encounter associated with the diagnosis of epilepsy or seizure and were treated with an antiepileptic agent. Of these, 6,033 had a 120-day period without prescriptions for an antiepileptic drug and 6,158 initiated treatment with a single agent. Of these, 879 patients were excluded because they were not continuously enrolled for one year after the initiation of the index drug. Another 478 patients were excluded because they were treated with an antiepileptic drug for 30 days or less without a second antiepileptic drug prescription. Five of the 19 antiepileptic drugs identified in the dataset were dropped because few patients initiated treatment with them (felbamate N=2, mephobarbital N=2, methsuximide N=1, pregabalin N=1, and primidone N=4). The final study cohort consisted of 4666 patients.
There were 2527 (54%) patients in our study sample who initiated therapy with first-generation antiepileptic drugs and 2139 (46%) with second-generation drugs. Characteristics of patients initiated on first- and second-generation drugs were slightly different with patients initiating first-generation drugs slightly younger and more likely to be male. Patients in the two groups had similar numbers of medical encounters, though patients initiated on first-generation drugs had a slightly lower mean number of total drug prescriptions.
Medication Use
The most frequently prescribed antiepileptic drugs at treatment initiation were valproate (N=1158) and carabamazepine (N=724) among the first-generation drugs and oxcarbazepine (N=601) and topiramate (N=338) among the second-generation drugs (Table 2). The same relative distribution held over the course of the first year of treatment, with valproate (N=1439) and carbamazepine (N=867) the most frequently prescribed first-generation drugs and oxcarbazepine (N=780) and topiramate (N=568) the most commonly used second-generation drugs.
Table 2.
Utilization Rates for First- and Second-Generation Antiepileptics
| Medication | Year of regulatory approval in the U.S. |
Approved indicationsa | Number of patients initiating therapy, N (%) |
Number treated during N (%) |
|---|---|---|---|---|
| First-generation drugs | 2527 (54.2) | 2772 | ||
| Carbamazepine | 1968 | Partial seizures with complex symptomotology Generalized tonic-clonic seizures Mixed seizures or other partial or generalized seizures |
724 (28.7) | 867 |
| Ethosuximide | 1960 | Absence seizures | 113 (4.5) | 153 |
| Phenobarbitalc | N/A | Partial seizures Generalized tonic-clonic seizures |
212 (8.4) | 242 |
| Phenytoin | 1956 | Partial seizures Generalized tonic-clonic seizures |
320 (12.7) | 369 |
| Valproate | 1978 | Partial seizures Absence seziures |
1158 (46.8) | 1439 |
| Second-generation drugs | 2139 (45.8) | 2690 | ||
| Carbamazepine XR | 1996 | Partial seizures with complex symptomatology Generalized tonic-clonic seizures Mixed seizures or other partial or generalized seizures |
163 (7.6) | 235 |
| Gabapentin | 1994 | Partial seizures | 240 (11.2) | 329 |
| Lamotrigine | 1994 | Partial seizures Primary generalized tonic-clonic seizures Generalized seizures of Lennox-Gastaut syndrome |
312 (14.6) | 504 |
| Levetiracetam | 1999 | Partial seizures Myoclonic seizures |
162 (7.6) | 324 |
| Oxcarbazepine | 2000 | Partial seizures | 601 (28.1) | 780 |
| Tiagabine | 1997 | Partial seizures | 15 (0.7) | 35 |
| Topiramate | 1996 | Generalized tonic-clonic seizures | 338 (15.8) | 568 |
| Valproate XR | 2002 | Partial seizures Absence seziures |
239 (11.2) | 401 |
| Zonisamide | 2000 | Partial seizures | 69 (3.2) | 129 |
Abbreviations: XR, extended release
Indications related to epilepsy approved by the FDA in the United States as of December 31, 2006
Sum of patients for first- and second-generation drugs adds up to >100% due to some patients receiving treatment with drugs in both groups during the first year of treatment. Similarly, some patients were treated with more than one drug from either of the two drug groups.
Phenobarbital was never officially evaluated and approved by the FDA and the listed indication represents typical clinical use.
Medication Retention
Measures of medication retention showed very similar utilization patterns for first- and second-generation drugs during the first year of treatment (Table 3). The mean duration of continuous treatment with an antiepileptic drug before any treatment change was 189 days (95% CI 185, 193) and the mean duration of continuous treatment including concurrent treatment with another antiepileptic drug was 203 days (95% CI 199, 207). First- and second-generation drugs had the same mean continuous treatment durations before any treatment change (189 days [95% CI 183, 194] vs. 190 days [95% CI 184, 195]) as well as similar mean continuous treatment when including concurrent treatment (202 days [95% CI 197, 208] vs. 204 days [95% CI 199, 210]). The overall proportion of treatment days with the index drug was slightly higher for second-generation drugs (90.4% [95% CI 89.4, 91.3]) compared to first-generation drugs (87.4% [95% CI 86.4, 88.4]). A total of 26.4% (95% CI 26.4, 29.0) and 29.2% (95% CI 27.3, 31.2) of patients started on a first- or second-generation antiepileptic medication, respectively, resumed treatment with the initial drug after a period of discontinuation. The average period of discontinuation was 77 days (95% CI 73, 82) for older drugs and 79 days (95% CI 68, 76) for newer drugs.
Table 3.
Measures of Medication Retention for First- and Second-Generation Antiepileptics
| First Treatment Medication |
1. Continuous treatment, mean days (95% CI) |
2. Continuous treatment including polytherapy, mean days (95% CI) |
3. Proportion treatment days with index drug, % (95% CI) |
4. One-year retention, % (95% CI) |
5. Treatment resumption, % (95% CI) |
|---|---|---|---|---|---|
| All antiepileptics | 189 (185, 193) | 203 (199, 207) | 88.8 (88.1, 89.5) | 26.3 (25.0, 27.6) | 27.7 (26.4, 29.0) |
| First-generation drugs | 189 (183, 194) | 202 (197, 208) | 87.4 (86.4, 88.4) | 26.1 (24.4, 27.9) | 26.4 (24.7, 28.2) |
| Carabamazepine | 203 (192, 213) | 217 (207, 227) | 87.6 (85.6, 89.5) | 31.4 (28.0, 34.9) | 27.5 (24.3, 30.9) |
| Ethosuximide | 203 (178, 228) | 211 (185, 236) | 86.7 (81.3, 92.0) | 25.7 (17.9, 34.7) | 36.3 (27.4, 45.9) |
| Phenobarbital | 177 (159, 195) | 191 (173, 209) | 90.5 (87.4, 93.6) | 21.2 (15.9, 27.4) | 22.6 (17.2, 28.9) |
| Phenytoin | 147 (133, 161) | 167 (153, 181) | 79.2 (75.6, 82.8) | 17.2 (13.2, 21.8) | 17.2 (13.2, 21.8) |
| Valproate | 192 (184, 200) | 204 (197, 212) | 89.1 (87.7, 90.5) | 26.2 (23.7, 28.9) | 28.1 (25.5, 30.8) |
| Second-generation drugs | 190 (184, 195) | 204 (199, 210) | 90.4 (89.4, 91.3) | 26.5 (24.6, 28.4) | 29.2 (27.3, 31.2) |
| Carbamazepine XR | 202 (181, 224) | 218 (196, 239) | 90.4 (86.9, 93.9) | 29.4 (22.6, 37.1) | 30.1 (23.1, 37.7) |
| Gabapentin | 162 (145, 178) | 172 (156, 189) | 87.6 (84.4, 90.8) | 17.9 (13.3, 23.4) | 27.9 (22.3, 34.0) |
| Lamotrigine | 204 (188, 220) | 223 (208, 239) | 94.9 (93.0, 96.8) | 32.4 (27.2, 37.9) | 33.6 (28.4, 39.2) |
| Levetiracetam | 194 (172, 216) | 215 (193, 237) | 89.8 (86.0, 93.6) | 30.2 (23.3, 38.0) | 27.2 (20.5, 34.7) |
| Oxcarbazepine | 212 (200, 223) | 221 (210, 232) | 90.6 (88.7, 92.4) | 33.3 (30.0, 37.2) | 26.6 (23.1, 30.4) |
| Tiagabine | 127 (67, 187) | 150 (86, 214) | 94.1 (87.2, 100) | 6.7 (0.2, 32.0) | 33.3 (11.8, 61.6) |
| Topiramate | 166 (153, 180) | 183 (169, 197) | 91.2 (88.8, 93.5) | 16.6 (12.8, 21.0) | 34.9 (29.8, 40.3) |
| Valproate XR | 169 (152, 186) | 190 (173, 207) | 87.5 (84.2, 90.7) | 21.8 (16.7, 27.5) | 27.2 (21.7, 33.3) |
| Zonisamide | 184 (152, 216) | 195 (162, 229) | 84.6 (77.6, 91.5) | 24.6 (15.0, 36.5) | 17.4 (9.3, 28.4) |
Abbreviations: XR, extended release
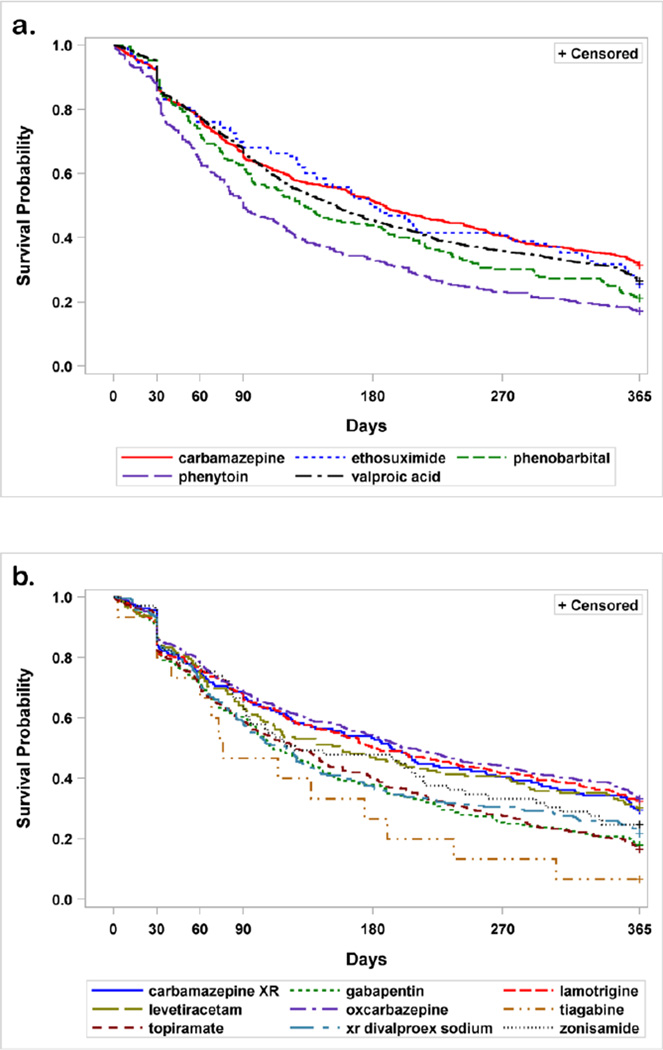
Retention rates were similar for first- and second-generation drugs (26.1% [95% CI 24.4, 27.9] and 26.5% [95% CI 24.6,28.4], respectively) but varied for individual medications. One-year survival rates for first- and second-generation agents combined and for the individual drugs are shown in Figures 1 and 2. When analyzed by category, there was no difference in mean survival rates for first- versus second-generations drugs (p-value=0.87). However, within category, individual drugs did differ. For first generation drugs, phenytoin had a lower survival rate than any of the other drugs (carbamazepine and ethosuximide p <0.001, valproate p=0.007, phenobarbital p=0.017). Cabamazepine also had a longer survival rate than phenobarbital (p=0.003) and ethosiximide (p=0.041). Among the second-generation drugs, oxcarbazepine had a longer survival rate than zonisamide, valproate XR, topiramate, gabapentin, tiagabine (p <0.001 for all) and levetiracetam (p=0.027). Lamotrigine had a longer survival rate than topiramate (p <0.001) and gabapentin (p=0.005). Carabamzepine XR had a longer retention than gabapentin (p=0.048) and topiramate (p=0.004). And finally, topiramate had a longer retention than tiagabine (p=0.006), but a shorter retention than levetiracetam (p=0.008) and zonisamide (p=0.011).
Figure 1. Kaplan-Meier survival analysis for first- and second-generation antiepileptic drugs by patients during the first year of treatment.
There was no difference in survival rates for first- and second-generation drugs (p-value=0.87)
Figure 2. Kaplan-Meier survival analysis for individual antiepileptic drugs by patients during the first year of therapy.
Panel A depicts the five first-generation antiepileptic drugs examined. Phenytoin had significantly lower survival rates compared to all other drugs (carbamazepine and ethosuximide p <0.001, valproate p=0.007, phenobarbital p=0.017). Cabamazepine had a longer survival rate than phenobarbital (p =0.003) and ethosiximide (p=0.041). Panel B depicts the 9 second-generation drugs examined. Oxcarbazepine had a longer survival rate than zonisamide, valproate XR, topiramate, gabapentin, tiagabine (p <0.001 for all) and levetiracetam (p=0.027). Lamotrigine had a longer survival rate than topiramate (p <0.001) and gabapentin (p=0.005). Carabamzepine XR had a longer retention than gabapentin (p=0.048) and topiramate (p=0.004). And finally, topiramate had a longer retention than tiagabine (p=0.006), but a shorter retention than levetiracetam (p=0.008) and zonisamide (p=0.011).
Medication changes
As shown in Table 4, overall, 72.5% (95% CI 71.2, 73.8) of patients were treated with only one antiepileptic drug throughout the study period, with similar rates for patients started on first- and second-generation antiepileptics (71.1% [95% CI 69.3, 72.9] vs 74.2% [95% CI 72.3, 76.0]). The overall mean number of antiepileptics prescribed to each patient was 1.4 (95% CI 1.4, 1.4) and the mean number of therapeutic changes for each patient after starting with the index drug was 1.8 (95% CI 1.8, 1.9). Values for these two measures were similar for patients started on first- and second-generation antiepileptics.
Table 4.
Measures of Therapeutic Adjustment after Treatment Initiation with First- and Second-Generation Antiepileptics
| First Treatment Medication |
1. Proportion monotherapy (95% CI) |
2. Proportion polytherapy (95% CI) |
3. Number of antiepileptic drugs, mean (95% CI) |
4. Number of therapeutic adjustments, mean (95% CI) |
|---|---|---|---|---|
| All antiepileptics | 72.5 (71.2, 73.8) | 10.4 (9.6. 11.4) | 1.4 (1.4, 1.4) | 1.8 (1.8, 1.9) |
| First-generation drugs | 71.1 (69.3, 72.9) | 10.8 (9.6, 12.0) | 1.4 (1.4, 1.4) | 1.8 (1.7, 1.9) |
| Carabamazepine | 71.6 (68.1, 74.8) | 9.8 (7.7, 12.2) | 1.4 (1.3, 1.4) | 1.7 (1.6, 1.8) |
| Ethosuximide | 72.6 (63.4, 80.5) | 8.0 (3.7, 14.6) | 1.4 (1.2, 1.5) | 2.0 (1.6, 2.4) |
| Phenobarbital | 76.9 (70.6, 82.4) | 13.2 (9.0, 18.5) | 1.3 (1.2, 1.4) | 1.8 (1.5, 2.0) |
| Phenytoin | 60.3 (54.7, 65.7) | 20.0 (15.8, 24.8) | 1.5 (1.4, 1.6) | 1.9 (1.7, 2.1) |
| Valproate | 72.6 (70.0, 75.2) | 8.6 (7.1, 10.4) | 1.4 (1.3, 1.4) | 1.8 (1.7, 1.9) |
| Second-generation drugs | 74.2 (72.3, 76.0) | 10.0 (8.8, 11.4) | 1.4 (1.3, 1.4) | 1.8 (1.8, 1.9) |
| Carbamazepine XR | 69.9 (62.3, 76.9) | 10.4 (6.2, 16.2) | 1.4 (1.3, 1.5) | 1.9 (1.6, 2.2) |
| Gabapentin | 71.7 (65.5, 77.3) | 8.3 (5.2, 12.6) | 1.4 (1.3, 1.5) | 1.9 (1.7, 2.1) |
| Lamotrigine | 79.2 (74.2, 83.5) | 11.9 (8.5, 16.0) | 1.3 (1.2, 1.4) | 1.8 (1.6, 2.0) |
| Levetiracetam | 72.8 (65.3, 79.5) | 14.8 (9.7, 21.2) | 1.4 (1.2, 1.4) | 1.8 (1.5, 2.1) |
| Oxcarbazepine | 77.5 (74.0, 80.8) | 8.5 (6.4, 11.0) | 1.3 (1.3, 1.4) | 1.7 (1.5, 1.8) |
| Tiagabine | 73.3 (44.9, 92.2) | 6.7 (0.2, 32.0) | 1.4 (0.9, 1.9) | 2.2 (1.2, 3.2) |
| Topiramate | 75.4 (70.5, 79.9) | 7.7 (5.1, 11.1) | 1.3 (1.3, 1.4) | 2.1 (1.9, 2.3) |
| Valproate XR | 65.7 (59.3, 71.7) | 13.8 (9.7, 18.8) | 1.5 (1.4, 1.6) | 2.0 (1.8, 2.2) |
| Zonisamide | 68.1 (55.8, 78.8) | 8.7 (3.3, 18.0) | 1.4 (1.2, 1.5) | 1.6 (1.2, 1.9) |
Abbreviations: XR, extended release
DISCUSSION
Comparing drug use in a pediatric population using observational data, we found that first- and second-generation antiepileptic medications had very similar utilization patterns in terms of medication retention and therapeutic changes. In particular, patients initiating first-time therapy with a drug in either group had similar lengths of continuous treatment, rates of treatment resumption, one-year retention rates, and rates of remaining on monotherapy with the index drug. We demonstrates the use of pharmacy claims data for the investigation of drug prescription and utilization patterns and our findings provide additional information to complement prior studies showing that the two drug classes are similar in terms of effectiveness.15,25–28
This approach may be especially useful in augmenting information on drug utilization in pediatric populations, given the low rate of clinical trial research that has been performed in children compared to adults.3,5,29 A number of reports have highlighted the paucity of evidence derived from clinical trials in children and have also identified alarming deficiencies in the quality of pediatric studies.6,7,30 As a result, physicians frequently lack pediatric-specific data to guide clinical care around pharmacotherapy and are forced to extrapolate data from adult studies.31,32 Observational data on the clinical management and outcome of children treated with specific medications could be leveraged to fill in the gaps and complement data derived from controlled trials.33,34
With the widespread and continuing adoption of electronic medical records, investigators have access to increasing amounts of high-quality observational data on clinical care. These data allow for retrospective or prospective examination of clinical data collected during routine clinical practice. Traditional randomized controlled studies measure the efficacy of a treatment using highly controlled experimental environments, volunteer patients, atypical physicians and healthcare settings, and protocolized care.17,35–38 The generalizability of their findings to clinical practice ultimately depends on how closely the trial protocols resemble real-world patient populations and treatment settings. Observational data, by contrast, are more likely to depict diverse patient populations and routine clinical care and are a better measure of a treatments’ effectiveness and of patients’ behaviors in standard practice.39,40 Other advantages of observational data include the ability to study large patient populations, rare diseases, multiple treatment paradigms simultaneously, and extended treatment periods.40,41 This can be done at fairly low cost and high speed compared to clinical trials.36 However, careful consideration must be given to controlling for confounding since the patient populations are not randomized.36,40,41 Additionally, observational data may not be suitable to assessing drugs newly introduced to the market as data on their routine clinical use will not yet be available.42
Medication retention has multiple determinants, including a patient’s willingness to continue a treatment, and is a composite measure of a drug’s efficacy, tolerability, safety, and patient preferences.43–46 It represents a measure of a drug’s overall performance in the real-world or, in other words, its effectiveness. As such, medication retention is considered one of the most relevant measures of the overall performance of an antiepileptic drug.47–49 While medication retention has historically been measured as the proportion of patients continuing therapy at a set time point following treatment initiation, examining more detailed components of drug retention as we did here, may provide insights into specific aspects of a drug’s performance. For example, we found similar proportions of patients resuming therapy after treatment discontinuation, indicating that the tolerability of the two drug groups are likely comparable. Prior studies have suggested that a drug’s tolerability may have greater impact in determining a drug’s long-term retention than its efficacy.43,44,50
Along the same lines, changes in therapeutic regimens may be reflective of a patient’s experience with a drug, including its tolerability and safety, when used in combination with other agents.44 Measuring changes in therapy provides additional detail to complement drug retention rates, focusing on a drug’s performance once its tolerability and safety in monotherapy has been established or identifying drug combinations that may increase a drug’s retention.
Measuring real-world use of medications provides the opportunity to measure the comparative effectiveness of specific agents within drug classes as well as the different treatment modalities available for a given condition. This may be particularly useful for conditions in which clinicians lack high-quality comparative data for the available drugs and other treatments.51–53 Many trials testing new drugs use placebo controls as opposed to active comparators and additionally may suffer from the sub-optimal choice of comparators.12,36 For example, a comparator drug may be unsuitable because it does not reflect the current standard of care for a condition or because it is administered at an inappropriate dose.11 Comparative metrics based on observational data allow investigators to evaluate a treatment of interest in relation to any number of other therapies and, furthermore, incorporate patient behaviors and the conditions of administration in real-world practice.
The limitations of this study are those inherent to the use of retrospective claims data. There may be miscoding of information or missing information due to patients discontinuing coverage with the insurance program. However, we do not expect these factors to be related to the use of specific antiepileptic drugs and it is unlikely that they affect our results comparing patients taking different drugs. The data also did not include additional information on the specific types of seizures that were being treated, the rational behind medication choices, which may have enabled more selective inclusion of medication treatments for analysis. Similarly, no outcomes data is available precluding specific analysis of the effectiveness of the medications. Given the observational nature of the data, we cannot exclude potential confounding by indication, although close examination of the two treatment groups using the data available to us indicated that they were very similar in terms of demographics and disease severity as measured by medical encounters and overall drug use. Finally, while we employed a national dataset covering an average of 900,343 children annually (range 738,766 to 1,058,723), we cannot ascertain the generalizability of our findings to other patient populations.
CONCLUSION
Few clinical trials have compared first- and second-generation antiepileptic drugs in children, with the available data indicating that the two drug classes are similar in terms of efficacy and safety.15,25–28 Here, we compare drug utilization patterns in an observational pharmacy claims data set and identify similar rates of medication retention and changes. This approach generates information that may be useful in conjunction with traditional trial evidence. The methodology could readily be leveraged to other drug classes and conditions and may be particularly relevant to pediatric populations given the limited data frequently available on medication utilization in children.
Table 1.
Characteristics of Patients on First- and Second-Generation Antiepileptics
| Characteristic | First-generation drug (N=2527) |
Second-generation drug (N=2139) |
P-value |
|---|---|---|---|
| Mean age, years | 9.8 | 11.5 | <0.001 |
| Female, N (%) | 42 | 51 | <0.001 |
| Mean total number of medical encountersa, N | 21.3 | 20.7 | 0.34 |
| Mean number of outpatient visitsb | 20.4 | 21.0 | 0.34 |
| Mean number of hospitalizations | 0.3 | 0.3 | 0.56 |
| Mean total number of drugs prescribeda,c, N | 5.9 | 6.5 | <0.001 |
During first year of therapy
Excludes emergency department visits
Includes non-antiepileptic agents
Key points.
Observational data on mediation utilization may offer the opportunity to measure and compare medication use to augment available trial data.
Comparing drug utilization patterns in an observational claims data set, we identified similar rates of medication retention and adjustment for first- and second-generation drugs, consistent with prior findings.
This approach could readily be leveraged to other drug classes and conditions to inform pharmacotherapeutic options and may be particularly relevant to pediatric populations given the limited data frequently available to guide drug selection in children.
Acknowledgments
Funding Support: Drs. Bourgeois and Mandl were supported by a grant from the National Institute of General Medical Sciences (1R01GM104303-01). Dr. Bourgeois was supported by a training grant (5T32HD040128) from the National Institute of Child Health and Human Development, and Dr. Mandl by a grant from the National Library of Medicine (5G08LM009778), National Institutes of Health. No funding was specifically received for this study.
Footnotes
Conflicts of Interest: Drs. Bourgeois, Olson, Poduri, and Mandl do not have any conflicts of interest to disclose.
REFERENCES
- 1.Zhang Y, Baicker K, Newhouse JP. Geographic variation in the quality of prescribing. N Engl J Med. 2010 Nov 18;363(21):1985–1988. doi: 10.1056/NEJMp1010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittler JN, Landon BE, Fisher ES, Cleary PD, Zaslavsky AM. Market variations in intensity of Medicare service use and beneficiary experiences with care. Health Serv Res. 2010 Jun;45(3):647–669. doi: 10.1111/j.1475-6773.2010.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JP, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012 Aug;130(2):285–292. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen E, Goldman RD, Ragone A, et al. Child vs adult randomized controlled trials in specialist journals: a citation analysis of trends, 1985–2005. Arch Pediatr Adolesc Med. 2010 Mar;164(3):283–288. doi: 10.1001/archpediatrics.2009.291. [DOI] [PubMed] [Google Scholar]
- 5.Cohen E, Uleryk E, Jasuja M, Parkin PC. An absence of pediatric randomized controlled trials in general medical journals, 1985–2004. J Clin Epidemiol. 2007 Feb;60(2):118–123. doi: 10.1016/j.jclinepi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Hamm MP, Hartling L, Milne A, et al. A descriptive analysis of a representative sample of pediatric randomized controlled trials published in 2007. BMC Pediatr. 2010;10:96. doi: 10.1186/1471-2431-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: the gap in high-quality study design. Pediatrics. 2008 Jul;122(1):52–57. doi: 10.1542/peds.2007-2849. [DOI] [PubMed] [Google Scholar]
- 8.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002 Dec 18;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 9.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009 Feb 28;373(9665):746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 10.Tsai AC, Rosenlicht NZ, Jureidini JN, Parry PI, Spielmans GI, Healy D. Aripiprazole in the maintenance treatment of bipolar disorder: a critical review of the evidence and its dissemination into the scientific literature. PLoS Med. 2011 May;8(5):e1000434. doi: 10.1371/journal.pmed.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chokshi DA, Avorn J, Kesselheim AS. Designing comparative effectiveness research on prescription drugs: lessons from the clinical trial literature. Health Aff (Millwood) 2010 Oct;29(10):1842–1848. doi: 10.1377/hlthaff.2010.0843. [DOI] [PubMed] [Google Scholar]
- 12.Stafford RS, Wagner TH, Lavori PW. New, but not improved? Incorporating comparative-effectiveness information into FDA labeling. N Engl J Med. 2009 Sep 24;361(13):1230–1233. doi: 10.1056/NEJMp0906490. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor AB. Building comparative efficacy and tolerability into the FDA approval process. JAMA. 2010 Mar 10;303(10):979–980. doi: 10.1001/jama.2010.257. [DOI] [PubMed] [Google Scholar]
- 14.Hollingworth SA, Eadie MJ. Antiepileptic drugs in Australia: 2002–2007. Pharmacoepidemiol Drug Saf. 2010 Jan;19(1):82–89. doi: 10.1002/pds.1871. [DOI] [PubMed] [Google Scholar]
- 15.Weijenberg A, Offringa M, Brouwer OF, Callenbach PM. RCTs with new antiepileptic drugs in children: a systematic review of monotherapy studies and their methodology. Epilepsy Res. 2010 Sep;91(1):1–9. doi: 10.1016/j.eplepsyres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Hansen RA, Dusetzina SB, Dominik RC, Gaynes BN. Prescription refill records as a screening tool to identify antidepressant non-adherence. Pharmacoepidemiol Drug Saf. 2010 Jan;19(1):33–37. doi: 10.1002/pds.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norris SL, Atkins D, Bruening W, et al. Observational studies in systemic reviews of comparative effectiveness: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011 Nov;64(11):1178–1186. doi: 10.1016/j.jclinepi.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Shcherbakova N, Rascati K, Brown C, et al. Factors Associated with Seizure Recurrence in Epilepsy Patients Treated with Antiepileptic Monotherapy: A Retrospective Observational Cohort Study using US Administrative Insurance Claims. CNS Drugs. 2014 Aug 3; doi: 10.1007/s40263-014-0191-1. [DOI] [PubMed] [Google Scholar]
- 19.Cerner, editor. Multum Drug Products. [Accessed October 31, 2011]; http://www.multum.com/Products.htm. [Google Scholar]
- 20.Pellock JM, Bourgeois BFD, Dodson WE, editors. Pediatric Epilepsy: Diagnosis and Therapy. 3rd ed. New York: Demos Medical Publishing; 2008. [Google Scholar]
- 21.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006 Aug;15(8):565–574. doi: 10.1002/pds.1230. discussion 575-567. [DOI] [PubMed] [Google Scholar]
- 22.Vink NM, Klungel OH, Stolk RP, Denig P. Comparison of various measures for assessing medication refill adherence using prescription data. Pharmacoepidemiol Drug Saf. 2009 Feb;18(2):159–165. doi: 10.1002/pds.1698. [DOI] [PubMed] [Google Scholar]
- 23.Hudson M, Rahme E, Richard H, Pilote L. Comparison of measures of medication persistency using a prescription drug database. Am Heart J. 2007 Jan;153(1):59–65. doi: 10.1016/j.ahj.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 24.SAS Institute Inc. SAS/STAT 9.3 User's Guide. [Accessed October 21, 2014];2011 http://support.sas.com/documentation/cdl/en/statug/63962/PDF/default/statug.pdf. [Google Scholar]
- 25.Guerreiro MM, Vigonius U, Pohlmann H, et al. A double-blind controlled clinical trial of oxcarbazepine versus phenytoin in children and adolescents with epilepsy. Epilepsy Res. 1997 Jun;27(3):205–213. doi: 10.1016/s0920-1211(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 26.Nieto-Barrera M, Brozmanova M, Capovilla G, et al. A comparison of monotherapy with lamotrigine or carbamazepine in patients with newly diagnosed partial epilepsy. Epilepsy Res. 2001 Aug;46(2):145–155. doi: 10.1016/s0920-1211(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 27.Resendiz-Aparicio JC, Rodriguez-Rodriguez E, Contreras-Bernal J, et al. A randomised open trial comparing monotherapy with topiramate versus carbamazepine in the treatment of paediatric patients with recently diagnosed epilepsy. Rev Neurol. 2004 Aug 1–15;39(3):201–204. [PubMed] [Google Scholar]
- 28.Wheless JW, Neto W, Wang S. Topiramate, carbamazepine, and valproate monotherapy: double-blind comparison in children with newly diagnosed epilepsy. J Child Neurol. 2004 Feb;19(2):135–141. doi: 10.1177/08830738040190020901. [DOI] [PubMed] [Google Scholar]
- 29.Thomson D, Hartling L, Cohen E, Vandermeer B, Tjosvold L, Klassen TP. Controlled trials in children: quantity, methodological quality and descriptive characteristics of pediatric controlled trials published 1948–2006. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demauro SB, Giaccone A, Kirpalani H, Schmidt B. Quality of reporting of neonatal and infant trials in high-impact journals. Pediatrics. 2011 Sep;128(3):e639–e644. doi: 10.1542/peds.2011-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindkvist J, Airaksinen M, Kaukonen AM, Klaukka T, Hoppu K. Evolution of paediatric off-label use after new significant medicines become available for adults: a study on triptans in Finnish children 1994–2007. Br J Clin Pharmacol. 2011 Jun;71(6):929–935. doi: 10.1111/j.1365-2125.2010.03881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waller DG. Off-label and unlicensed prescribing for children: have we made any progress? Br J Clin Pharmacol. 2007 Jul;64(1):1–2. doi: 10.1111/j.1365-2125.2007.02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viner RM, Hsia Y, Tomsic T, Wong IC. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010 Aug;11(8):593–602. doi: 10.1111/j.1467-789X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- 34.Murray ML, de Vries CS, Wong IC. A drug utilisation study of antidepressants in children and adolescents using the General Practice Research Database. Archives of disease in childhood. 2004 Dec;89(12):1098–1102. doi: 10.1136/adc.2004.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. A simple and valid tool distinguished efficacy from effectiveness studies. J Clin Epidemiol. 2006 Oct;59(10):1040–1048. doi: 10.1016/j.jclinepi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Sorenson C, Naci H, Cylus J, Mossialos E. Evidence of comparative efficacy should have a formal role in European drug approvals. BMJ. 2011;343:d4849. doi: 10.1136/bmj.d4849. [DOI] [PubMed] [Google Scholar]
- 37.Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007 Mar;62(3):219–223. doi: 10.1136/thx.2006.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003 Sep 24;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 39.Smith B. Comparative-effectiveness research as it affects clinical pharmacology. Clin Pharmacol Ther. 2011 Dec;90(6):751–754. doi: 10.1038/clpt.2011.260. [DOI] [PubMed] [Google Scholar]
- 40.Dreyer NA, Tunis SR, Berger M, Ollendorf D, Mattox P, Gliklich R. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff (Millwood) 2010 Oct;29(10):1818–1825. doi: 10.1377/hlthaff.2010.0666. [DOI] [PubMed] [Google Scholar]
- 41.Concato J, Lawler EV, Lew RA, Gaziano JM, Aslan M, Huang GD. Observational methods in comparative effectiveness research. Am J Med. 2010 Dec;123(12) Suppl 1:e16–e23. doi: 10.1016/j.amjmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. 2011 Dec;90(6):777–790. doi: 10.1038/clpt.2011.235. [DOI] [PubMed] [Google Scholar]
- 43.Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure. 2007 Jun;16(4):296–304. doi: 10.1016/j.seizure.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Wong IC, Mawer GE, Sander JW, Lhatoo SD. A pharmacoepidemiologic study of factors influencing the outcome of treatment with lamotrigine in chronic epilepsy. Epilepsia. 2001 Oct;42(10):1354–1358. doi: 10.1046/j.1528-1157.2001.02101.x. [DOI] [PubMed] [Google Scholar]
- 45.Bootsma HP, Ricker L, Diepman L, et al. Long-term effects of levetiracetam and topiramate in clinical practice: A head-to-head comparison. Seizure. 2008 Jan;17(1):19–26. doi: 10.1016/j.seizure.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Menachem E, Sander JW, Privitera M, Gilliam F. Measuring outcomes of treatment with antiepileptic drugs in clinical trials. Epilepsy Behav. 2010 May;18(1–2):24–30. doi: 10.1016/j.yebeh.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Lhatoo SD, Wong IC, Polizzi G, Sander JW. Long-term retention rates of lamotrigine, gabapentin, and topiramate in chronic epilepsy. Epilepsia. 2000 Dec;41(12):1592–1596. doi: 10.1111/j.1499-1654.2000.001592.x. [DOI] [PubMed] [Google Scholar]
- 48.Sander JW. New antiepileptic drugs in practice--how do they perform in the real world? Acta Neurol Scand Suppl. 2005;181:26–29. doi: 10.1111/j.1600-0404.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 49.Simister RJ, Sander JW, Koepp MJ. Long-term retention rates of new antiepileptic drugs in adults with chronic epilepsy and learning disability. Epilepsy Behav. 2007 Mar;10(2):336–339. doi: 10.1016/j.yebeh.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Bootsma HP, Ricker L, Hekster YA, et al. The impact of side effects on long-term retention in three new antiepileptic drugs. Seizure. 2009 Jun;18(5):327–331. doi: 10.1016/j.seizure.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Bourgeois FT, Murthy S, Mandl KD. Comparative effectiveness research: an empirical study of trials registered in ClinicalTrials.gov. PLoS One. 2012;7(1):e28820. doi: 10.1371/journal.pone.0028820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunn AG, Bourgeois FT, Murthy S, Mandl KD, Day RO, Coiera E. The Role and Impact of Research Agendas on the Comparative-Effectiveness Research Among Antihyperlipidemics. Clin Pharmacol Ther. 2012 Feb 29; doi: 10.1038/clpt.2011.279. [DOI] [PubMed] [Google Scholar]
- 53.van Luijn JC, Stolk P, Gribnau FW, Leufkens HG. Gap in publication of comparative information on new medicines. Br J Clin Pharmacol. 2008 May;65(5):716–722. doi: 10.1111/j.1365-2125.2007.03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]