Abstract
The primary aim of the current study was to examine whether physiological reactivity to depression-relevant stimuli, measured via pupil dilation, serves as a biomarker of depression risk among children of depressed mothers. Participants included 47 mother-child dyads. All mothers had a history of major depressive disorder (MDD). Pupil dilation was recorded while children viewed angry, happy, and sad faces. Follow-up assessments occurred 6, 12, 18, and 24 months after the initial assessment, during which structured interviews were used to assess for children’s levels of depressive symptoms as well as the onset of depressive diagnoses. Children exhibiting relatively greater pupil dilation to sad faces experienced elevated trajectories of depressive symptoms across the follow up as well as a shorter time to depression onset. These findings were not observed for children’s pupillary reactivity to angry or happy faces. The current findings suggest that physiological reactivity to sad stimuli, assessed using pupillometry, serves as one potential biomarker of depression risk among children of depressed mothers. Notably, pupillometry is an inexpensive tool that could be administered in clinical settings, such as pediatricians’ offices, to help identify which children of depressed mothers are at highest risk for developing depression themselves.
Keywords: intergenerational transmission, youth depression, pupillometry, emotion processing, vulnerability
Introduction
Depression is the leading cause of disability worldwide and a significant contributor to the global burden of disease (Ferrari et al., 2013). Onset of major depressive disorder (MDD) during childhood and adolescence is particularly deleterious and is associated with significant impairment in social and academic settings, greater risk for a chronic and recurrent course, and an increased risk for suicide (Birmaher et al., 2002; Naicker et al., 2013). One of the strongest risk factors for MDD in youth is a positive family history of the disorder. Specifically, children of depressed mothers are three to four times more likely to experience major depressive disorder (MDD) by early adulthood than are offspring of mothers with no history of MDD (for reviews, see Goodman 2007; Hammen, 2009). However, not all children of depressed mothers go on to develop depression, suggesting the presence of factors that may moderate, or exacerbate, this risk. A key area of research, therefore, is identifying specific factors that help to determine which offspring of depressed mothers are at greatest risk for depression. The identification of physiological or neural risk factors, in particular, for this population can aid clinicians and researchers in objectively measuring specific (bio)markers of risk early in development, even in the absence of overt behavioral signs. Biomarkers, in turn, have the potential to increase our ability to predict the development of new depressive episodes in at-risk children, and to identify specific targets for treatment.
One promising biological marker of risk may be disrupted physiological reactivity to negative stimuli. Specifically, according to cognitive models of depression, the way in which people process negative information plays a significant role in the onset and maintenance of the disorder (Ingram, Miranda, & Segal, 1998). Studies utilizing pupillometry, the assessment of changes in pupil dilation, have provided promising evidence for the role of disrupted reactivity in depression risk. Research suggests that the pupil becomes dilated in response to stimuli that require greater cognitive load or that have greater emotional intensity (e.g., Siegle, Steinhauer, Carter, Ramel, & Thase, 2003a). Importantly, researchers have linked changes in pupil dilation to brain function in areas associated with the regulation of emotions, including the dorsolateral PFC (Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003b) and the ACC (Critchley, Tang, Glaser, Butterworth, & Dylan, 2005; Urry et al., 2006). Thus, pupillometry is suggested to be a promising peripheral index for a range of brain mechanisms associated with the processing of emotionally-salient information (Steidtmann, Ingram, & Siegle, 2011).
Research has shown that currently depressed, compared to never depressed, adults exhibit greater pupil dilation to depression-relevant negative words (Siegle, Steinhauer, Carter, Ramel, & Thase, 2003). Importantly, similar results have also been observed among adults with remitted depression, a group known to be at high risk for developing future episodes of depression. Specifically, individuals with remitted MDD, compared to individuals with no prior history of MDD, exhibited an increased pupil response to negative words prior to a negative mood induction (Steidtmann, Ingram, & Siegle, 2011). These results suggested a sustained pupillary response to negative words among adults with remitted MDD in that the pupillary response peaked following the onset of the negative word and remained elevated for several seconds following the word presentation. Together, these studies suggest that pupillary reactivity to depression-relevant stimuli may be a trait-like marker that is demonstrated in adults experiencing a current depressive episode as well as those at high-risk for depression in the future.
In an effort to examine whether similar results would be observed in children at high risk for depression, we recently conducted a study examining differences in pupil dilation to emotional faces between children of mothers with a history of MDD during the children’s lives versus those with no lifetime history of depression (Burkhouse, Siegle, & Gibb, 2014). As predicted and consistent with the adult literature, we found that children at high risk for depression exhibited increased pupil dilation to sad stimuli relative to children at low risk for depression. This difference in reactivity was not found for happy or angry faces, and was only demonstrated at higher levels of stimulus emotional intensity. These results suggest that physiological reactivity to negative cues measured via pupil dilation may be one important mechanism involved in risk for the intergenerational transmission of depression. Consistent with this, there is evidence that children of depressed mothers who have survived depression free into adulthood have reduced pupil dilation for emotional words, which has been interpreted as a marker of resilience in these at-risk offspring (Bistricky, Ingram, Siegle, & Short, 2015). Taken together, these studies suggest that there is variability in pupil dilation among children of depressed mothers and this variability may help to predict which children of depressed mothers are at greatest risk for depression themselves.
The primary aim of the current study was to examine the predictive validity of children’s pupil dilation to emotional stimuli using a prospective design. In doing so, we followed up 47 of the children (ages 8–14) of depressed mothers reported in our previous study (Burkhouse, Siegle, & Gibb, 2014) over two years to determine if pupil dilation to sad faces assessed at baseline prospectively predicted trajectories of children’s depressive symptoms and the development of new depressive episodes. We chose to focus on this age range because rates of depression increase as much as sixfold between childhood and adolescence (for a review, see Avenevoli, Knight, Kessler, & Merikangas, 2008). Therefore, examining potential risk factors (i.e., heightened pupil dilation to sad faces) in children and early adolescents will help us to understand the specific factors involved in the rise of depression rates during this critical developmental window. In the current study, we predicted that children of depressed mothers exhibiting relatively higher pupil dilation to sad faces at the baseline assessment would experience (i) elevated trajectories of depressive symptoms over the two-year follow up and (ii) increased risk for the onset of depressive disorders among children of depressed mothers. Exploratory analyses were also conducted to determine whether our findings would be maintained when controlling for other known risk factors for child depression (i.e., children’s baseline levels of depressive symptoms, level of pubertal development, age, sex, family income). That is, to determine the utility of pupillary reactivity to sad stimuli as a biomarker of depression risk, it is important to know that it exhibits increased predictive validity beyond that accounted for by these other well-known risk factors.
Method
Participants
Participants in this study were 47 mothers and their children drawn from the community who were participating in a larger study of the intergenerational transmission of depression (Burkhouse, Siegle, & Gibb, 2014).1 To qualify for inclusion, mothers were required to meet criteria for MDD during the child’s lifetime according to the DSM-IV (American Psychiatric Association, 1994). Exclusion criteria included symptoms of schizophrenia, alcohol or substance abuse within the last six months, or history of bipolar disorder. Children’s participation was limited such that no more than one child per family could participate and all children were between the ages of 8–14 years at the initial assessment. If more than one child was available within this age range, one child was chosen at random for participation. The average age of mothers in our sample was 41.57 years (SD = 7.46, Range = 26–55) and 89% were Caucasian. The median family income was $35,001–40,000 and, in terms of education level, 25% of the mothers had graduated from college. For the children in our sample, the average age was 11.19 years (SD = 2.03) at baseline, 47% were girls, and 72% were Caucasian.
Measures
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1995) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, & Rao, 1997) were used to assess for current DSM-IV Axis I disorders in mothers and their children, respectively. Two separate trained interviewers administered the SCID-I and the K-SADS-PL to mothers and children, respectively. As noted above, 47 mothers met criteria for MDD during their child’s life. Of these 47 mothers, 13 met criteria for current MDD at the baseline assessment. The K-SADS-PL was used to assess for clinically significant episodes of major and minor depression in children.2 At the initial assessment, 8 children met criteria for a lifetime depressive diagnosis (2 past major depression; 1 current major depression; 5 past minor depression), 7 met criteria for a lifetime behavior disorder (attention-deficit hyperactivity disorder = 5, conduct disorder = 1, oppositional defiant disorder = 1), and 12 met criteria for a lifetime anxiety disorder (generalized anxiety disorder = 4, obsessive-compulsive disorder = 1, panic disorder = 1, post-traumatic stress disorder = 1, separation anxiety disorder = 3, social phobia = 5).3 The depression section of the K-SADS was also administered at the follow-up assessments to determine whether children met criteria for major or minor depression during the follow-up period and, if so, the date of onset. During the follow-up, 11 children met criteria for a new depressive episode (6 major depression and 5 minor depression; 8 with a first onset and 3 with a recurrence). A subset of 20 SADS-L and 20 K-SADS-PL interviews from this project were coded by a second interviewer and kappa coefficients for depressive diagnoses were excellent (κs = 1.00).
Children’s symptoms of depression were assessed using the Children’s Depression Rating Scale-Revised (CDRS-R; Poznanski & Mokros, 1996). The CDRS-R is a 17-item interviewer-administered measure and has demonstrated excellent reliability and validity in previous research (e.g., Mayes et al., 2010; Poznanski & Mokros, 1996). The CDRS-R was administered at each of the assessment points and exhibited good internal consistency throughout the study (αs = .70 – .80 across all time points).
Children’s levels of pubertal development were assessed using the Sexual Maturation Scale (SMS; Marshall & Tanner, 1969, 1970). The SMS consists of drawings of the five Tanner stages of pubertal development with separate drawings available for girls and boys. Written descriptions are provided for each stage and the respondent is asked to indicate which stage best represents the current level of pubertal development. Consistent with past research examining affective aspects of pubertal development (e.g., Forbes, Williamson, Ryan, & Dahl, 2004) participants were classified as pre-pubertal if they were in Tanner stages 1 or 2 and as pubertal/post-pubertal if they were in Tanner stages 3, 4, or 5.
Pupil dilation was assessed in a moderately lit room using Tobii T60 & T60XL eye-trackers while participants viewed facial displays of emotion. The stimuli were full-color pictures of actors taken from a standardized stimulus set (Matsumoto & Ekman, 1988) displaying a variety of emotions (e.g., sad, happy, angry, neutral). The stimuli consisted of emotional and neutral photographs from each actor, morphed to form a continuum of 10% increments between the two photographs. Each emotion is represented by 4 continua (2 male and 2 female actors), for a total of 12 continua. Eleven morphed images were used from each continuum, representing 10% increments of the two emotions ranging from 100% neutral (0% target emotion) to 100% target emotion (e.g., 90% Neutral, 10% Sad; 80% Neutral, 20% Sad; and so on). The pictures, measuring 8.0(w) × 6.5(h) inches, were presented, one at a time in the middle of the screen at a visual angle of 14.18° for a duration of three seconds, in random order in 2 blocks and the participant was asked to indicate which emotion was being presented (sad, happy, angry, neutral) by pressing a corresponding button on a keypad. A fixation cross was presented between stimuli for 500ms and the inter-trial interval varied randomly between 750ms and 1250ms. Participants completed 264 trials (88 trials per emotion). To provide an adequate number of trials for pupillary analyses within each morph level, images were binned into three separate morph conditions for analyses: low (10%, 20%, ad 30%), medium (40%, 50%, 60%, and 70%), and high (80%, 90%, and 100%). Because our previous study found that children of depressed mothers exhibit increased pupil dilation specifically to sad stimuli at the highest morph level (Burkhouse, Siegle, & Gibb, 2014), we chose to focus solely on images at the highest morph level for the current study. Table 1 provides descriptive statistics for participants’ responses to each facial emotion type at the highest morph level (i.e., accuracy, pupil dilation, and response times).
Table 1.
Descriptive statistics (means, standard deviations) for the emotional faces paradigm.
| Angry Faces | Happy Faces | Sad Faces | |
|---|---|---|---|
| Peak Pupil Dilation (mm) | .042 (.027) | .035 (.038) | .055 (.033) |
| Detection Accuracy | .89 (.10) | .93 (.06) | .87 (.12) |
| Response Time (ms) | 1666 (613) | 1551 (572) | 1775 (655) |
During this task, pupil size was recorded using the eye trackers, which took measurements every 16.7ms (60 Hz) for 3s following the onset of each facial stimulus. Data were cleaned using Siegle et al.’s (2008) standard procedures. Trials comprised of over 50% blinks were removed from consideration. This resulted in the exclusion of an average of 16% of trials per participant (Range = 0–26%; SD = 9%). Following standard procedures, linear interpolation was used to replaced blinks throughout the data set and the data were smoothed using a 10-point weighted average filter. The total number of rejected trials and the percentage of pupil data that was replaced with linear interpolations were not significantly correlated with children’s depressive symptoms at any time point or with their likelihood of developing a depressive episode during the follow up (lowest p = .10, r = .16). Effects associated with a light-reflex that were independent of stimulus type were removed by subtracting the mean waveform across all three valences from the average waveform for each valence (Franzen, Buysse, Dahl, Thompson, & Siegle, 2009). The average pupil diameter over the 333 ms preceding the onset of the stimulus was subtracted from pupil diameter after stimulus onset to produce stimulus-related pupil dilation. Peak stimulus-related pupil dilation was calculated by taking the maximum pupil response on average across all trials for each valence (angry, happy, and sad). No outliers were identified for peak pupil dilation to angry, happy, or sad faces. Figure 1 provides an illustration of children’s pupillary responses to angry, happy, and sad faces at the highest morph level across the three-second interval.
Figure 1.
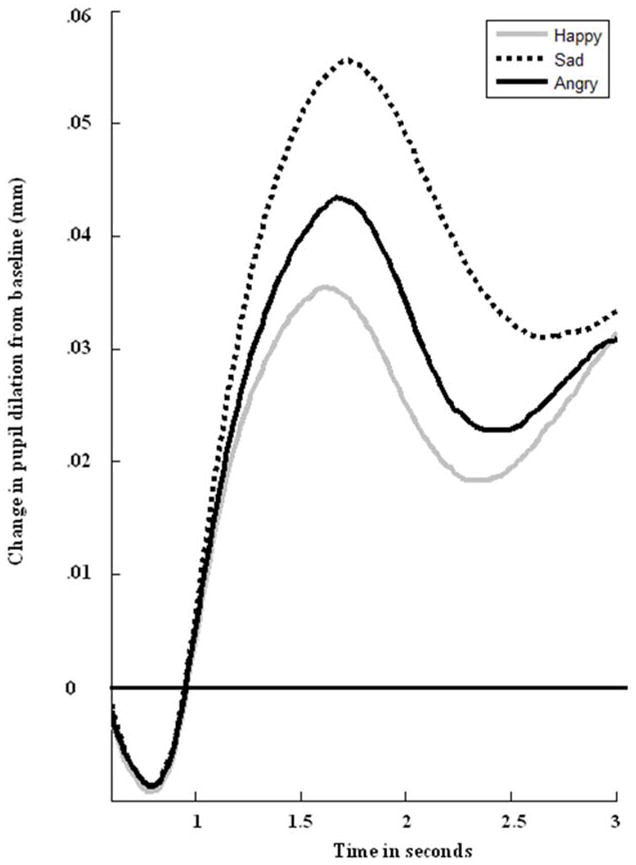
Participants’ change in pupil diameter from baseline in response to angry, happy, and sad faces.
Procedure
Potential participants were recruited from the community through a variety of means (e.g., newspaper and bus advertisements, flyers). Mothers responding to the recruitment advertisements were initially screened over the phone to determine potential eligibility. As part of the larger project, those reporting significant depressive symptoms during the child’s lifetime or no significant lifetime symptoms of depression were invited to participate in the study (cf. Burkhouse et al., 2014). Upon arrival at the laboratory, mothers were asked to provide informed consent and children were asked to provide assent to be in the study. Next, the mother was administered the K-SADS-PL and CDRS-R by a research assistant. During this time, the child completed the emotional faces paradigm. After completing the K-SADS-PL with the mother, the same interviewer then administered the K-SADS-PL and CDRS-R to the child. During this time, the mother was then administered the SCID-I by a separate interviewer. Follow-up assessments occurred 6, 12, 18, and 24 months after the initial assessment, during which mothers and children were administered the K-SADS-PL and the CDRS-R. Families were compensated $275 for their participation. All study procedures were approved by the University’s Institutional Review Board.
Results
Of the mother-child pairs participating in the initial assessment, 43, 41, 35, and 34 participated in the 6, 12, 18, and 24-month follow-ups, respectively. If a family missed a follow-up assessment, the following K-SADS assessment focused on the entire time since the previous completed assessment. For example, if a family missed the 6-month assessment, the 12-month assessment focused on any episodes since Time 1 (T1). Therefore, data were available for 47 families who completed at least one follow-up.
Does children’s peak pupil dilation to sad faces predict children’s depressive symptom trajectories over two years?
We used hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002; Raudenbush et al., 2004) to examine trajectories in children’s depressive symptoms across the follow-up. Specifically, we tested whether children’s peak pupil dilation to angry, happy, and sad faces assessed at the baseline assessment would predict children’s depressive symptom trajectories across the follow-up. The Level 1 model for these analyses was:
Where CDRS-Rij represents the CDRS-R score at month i for participant j, p0j is the CDRS-R intercept (CDRS-R score at the initial assessment), p1j is the slope of the linear change in CDRS-R scores over time (in months), p2j is the slope of the quadratic change in CDRS-R scores over time, and eij represents the error term.
The Level 2 Model was:
where β01 is the cross-level interaction term representing the effect of children’s peak pupil dilation (angry, happy, or sad) on the CDRS-R intercept. β11 and β21 are the cross-level interaction terms representing the effect of children’s peak pupil dilation on the slope of the relation between Time and CDRS-R scores (i.e., β11 = linear change in depressive symptom levels across the follow-up; β21 = quadratic change in depressive symptom levels across the follow-up). Finally, β00, β10, and β20 represent the intercepts of their respective equations, and r0j, r1j, and r2j represent the error terms.
We first examined whether children’s peak pupil dilation to sad faces assessed at baseline predicted changes in their depressive symptoms over the two-year follow up. Although children’s peak pupil dilation to sad faces was not significantly related to the CDRS-R intercept, t(44) = .60, p = .55, r effect size = .09, or the linear change in CDRS-R scores across the follow-up t(44) = 1.26, p = .22, r effect size = .19, it did predict a quadratic change in CDRS-R scores across the follow up, t(44) = 2.01, p = .05, r effect size = .29. As can be seen in Figure 2, children with higher peak dilation to sad faces maintained their depressive symptoms over the two-year follow-up period, whereas children with lower pupil dilation to sad faces exhibited a decline in their depressive symptoms. To more formally test this maintenance hypothesis, we re-centered the data at 24 months. Results indicated a significant effect of peak pupil dilation to sad faces at the intercept, (t(44) = 3.74, p = .001, r effect size = .49), suggesting that children with higher peak pupil dilation to sad faces differed significantly from children with lower peak pupil dilation in their depressive symptoms at the end of the follow-up period.
Figure 2.
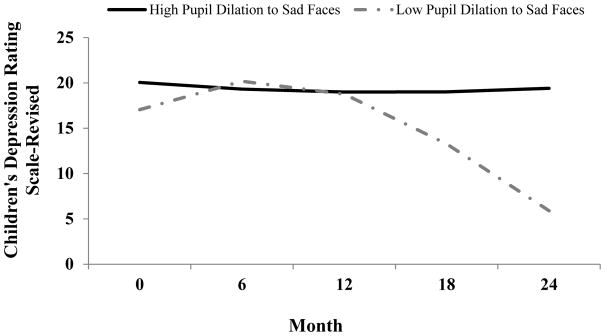
The effect of children’s peak pupil dilation to sad faces on children’s depressive symptoms using the Children’s Depression Rating Scale-Revised (CDRS-R) over a two-year follow-up period. High and low pupil dilation defined as 1 standard deviation above and below the mean, respectively.
Next, to determine the robustness of this effect, we examined whether the differences in depressive symptom levels at the end of the follow-up would be maintained when controlling for other known risk factors for depression (i.e., family income, children’s pubertal status, age, and sex). Importantly, our finding was maintained when controlling for these variables, (t(40) = 3.54, p = .001, r effect size = 49). Last, to examine the specificity of our findings, we tested whether children’s peak pupil dilation to happy and angry faces assessed at baseline predicted changes in their depressive symptoms over the two-year period. Results indicated no significant effects for children’s peak pupil dilation to happy or angry faces being related to the CDRS-R intercept (angry: t(44) = 1.79, p = .08, r effect size = 26; happy: t(44) = −0.53, p = .60, r effect size = .08), linear change in depressive symptoms (angry: t(44) = 0.01, p = .99, r effect size = .00; happy: t(44) = 1.29, p = .19, r effect size = .19), or quadratic change in depressive symptoms (angry: t(44) = −0.23, p = .82, r effect size = .03 ; happy: t(44) = −1.32, p = .19, r effect size = .19) across the follow-up period.
Does children’s peak pupil dilation to sad faces predict the development of new depressive episodes over two years?
Using survival analyses, we tested the hypothesis that pupil dilation to sad faces would predict prospective onsets of depressive episodes. One child who met criteria for current MDD at T1 was excluded from these analyses. Therefore, the final sample consisted of 46 children, of whom 11 (24%) experienced a depressive episode during the follow-up. In the survival analysis, children’s peak pupil dilation to sad faces significantly predicted a shorter time to depression onset, β = 14.29, Wald statistic = 5.44, p = .02. To visually depict these findings, we repeated the analysis, using upper and lower quartiles of children’s pupil dilation (see Figure 3). As seen in the figure, higher peak pupil dilation to sad faces predicted a shorter time to depression onset. The predictive power of peak pupil dilation to sad faces was maintained when we statistically controlled for the influence of family income and children’s baseline depressive symptoms, gender, age, and pubertal status, β = 14.09, Wald = 5.22, p = .03, suggesting that the predictive validity of pupil dilation is at least partially independent of these other known risk factors for depression. Importantly, our results were specific to children’s pupil dilation to sad faces. That is, consistent with our prediction, pupil dilation to angry, β = −5.06, Wald = .26, p = .61, and happy, β = −0.72, Wald = .01, p = .95, faces did not predict new depressive diagnoses among children of depressed mothers.
Figure 3.
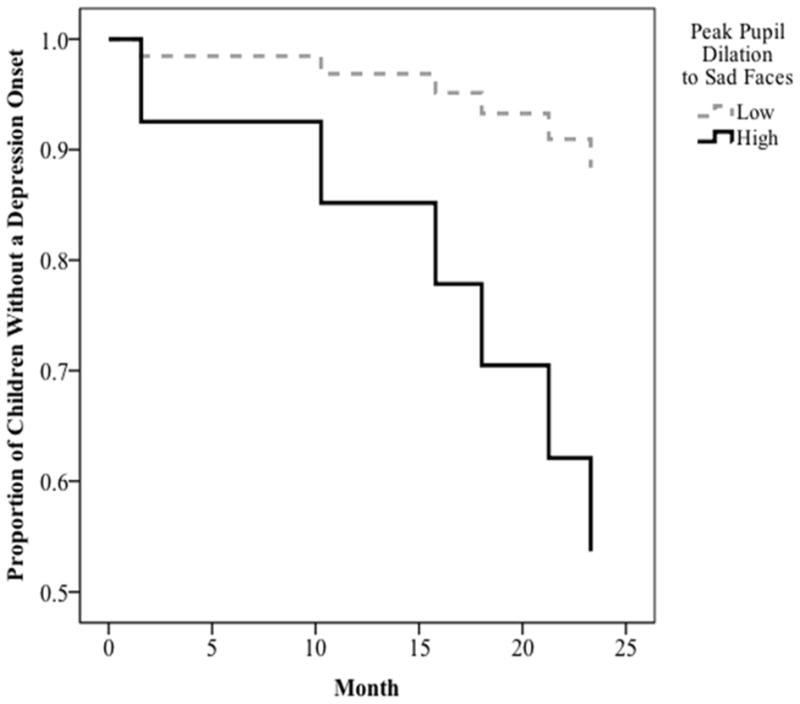
Results of survival analysis predicting time to depression onset among children of depressed mothers as a function of peak pupil dilation to sad faces.
Finally, we examined the rate at which depression onset occurred as a function of low (lower quartile) and high (upper quartile) peak pupil dilation to sad faces. Among those who experienced a depression onset, the mean time to depression onset among children with high pupil dilation to sad faces was 9.79 months versus 12.88 months for children with low pupil dilation. We then used Receiver Operating Characteristic (ROC) curve analysis to examine the predictive validity (sensitivity and specificity) of peak pupil dilation to sad faces predicting onset of depression (yes, no). The area under the curve for this analysis was significant, .74, p = .02, and a pupil dilation value of .06mm maximized sensitivity (.55) and specificity (.77) in predicting onsets of depression.
Discussion
The primary aim of the current study was to examine whether physiological reactivity to depression-relevant stimuli, measured via pupillometry, serves as a biomarker of depression risk among children of depressed mothers. In a previous study, we found that children of depressed mothers exhibited increased pupil dilation specifically to sad, but not angry or happy, facial stimuli relative to children of nondepressed mothers (Burkhouse, Siegle, & Gibb, 2014). In the current study, we followed these high-risk children longitudinally over two years to determine whether pupillary reactivity to sad faces prospectively predicted future depressive symptom trajectories and the onset of depressive episodes in children. Consistent with our hypothesis, children exhibiting relatively greater pupil dilation to sad stimuli experienced (i) elevated trajectories of children’s depressive symptoms across the 2-year follow up and (ii) a shorter time to the onset of a depressive episode among children of depressed mothers. Importantly, we found that the predictive validity of children’s pupil dilation was specific to sad faces, suggesting that it does not reflect higher reactivity to emotional stimuli broadly. In addition, the significant results for pupil dilation to sad faces were maintained even when we statistically controlled for the influence of children’s baseline depressive symptoms, sex, pubertal status, age, and family income, suggesting that individual differences in pupil dilation contribute unique risk to depression beyond these other well-known risk factors.
Notably, the ROC analysis suggested that pupil dilation exhibits promising specificity and sensitivity in predicting depression onset among children of depressed mothers. However, given the small sample size, caution should be used when interpreting these findings and future studies are needed to establish true cut scores that could be used as a biomarker of risk. This said, if replicated, the current findings could have exciting clinical implications. The identification of biomarkers for this population (i.e., children of depressed mothers) has the potential to aid clinicians in objectively identifying risk early in development, even in the absence of overt behavioral signs. The current findings suggest that physiological reactivity to depression-relevant stimuli, assessed by pupil dilation, may be one promising biomarker of risk in this population. Pupillometry is an inexpensive tool that could be administered in research or clinical settings, such as pediatricians’ offices. As a result, it could be a promising way of identifying which children are at highest risk for developing depression. In turn, this could lead to intervention efforts for this population. One promising intervention could be training to reduce reactivity to negative cues. A bottom-up model suggests that disrupted reactivity to emotional stimuli can lend itself to difficulty interpreting and regulating emotions (Disner et al., 2011). Therefore, training efforts that focus on improving reactivity to emotional stimuli may prove to be most beneficial. For example, recent studies have utilized real-time fMRI to train depressed adults to decrease activity in the subgenual cingulate (Hamilton, Glover, Hsu, Johnson, & Gotlib, 2011) and amygdala (Johnston, Boehm, Healy, Goebel, & Linden, 2010). Similar methods could be used with this high-risk population to reduce pupillary reactivity to depression-relevant cues. However, before training methods can be used with this population, a set of established norms for pupil dilation to emotional stimuli is needed.
The current findings also add to an emerging picture of broad disruptions in affective processing in children of depressed mothers. Specifically, findings from our lab and others suggest that children of depressed mothers exhibit heightened neural and physiological reactivity during a negative mood induction (Joormann, Cooney, Henry, & Gotlib, 2012) and in response to salient emotional stimuli, including sad facial stimuli (Burkhouse, Siegle, & Gibb, 2014), which they may attempt to regulate by averting their gaze from these stimuli (Gibb, Benas, Grassia, & McGeary, 2009; see also Harrison & Gibb, in press), particularly if they carry genotypes associated with heightened HPA axis reactivity (Owens et al., in press). These processing biases may also impact other forms of information processing including higher levels of brooding rumination (Gibb, Grassia, Stone, Uhrlass, & McGeary, 2011; Woody et al., in press), overgeneral memory for negative stimuli (Woody, Burkhouse, & Gibb, 2015), and heightened sensitivity for detecting facial displays of sadness (Burkhouse et al., 2015; Lopez-Duran, Kuhlman, George, & Kovacs, 2013). The biases are all linked to disruptions in cortico-limbic circuitry, driven by heightened reactivity in limbic areas and/or reduced prefrontal control of these limbic inputs (Disner, Beevers, Haigh, & Beck, 2011), suggesting a core deficit that may bias the processing of emotional stimuli. Future studies are needed to more definitively determine the links between pupil dilation and each of these other processes.
The current study demonstrated several strengths including the prospective design and focus on a promising biological marker for depression suggested by a line of previous research. In addition, the age range of the children in the sample allowed us to examine mechanisms of risk in a key period of risk – the transition to adolescence – when rates of depression skyrocket (for a review, see Avenevoli et al., 2008). The current findings, therefore, provide promising evidence that pupil dilation to sad facial stimuli may serve as a marker of risk among youth and may help to determine which children of depressed mothers are at greatest risk for depression themselves.
Despite the strengths of this study, there were limitations as well, which provide important avenues for future research. First, the sample size of this study was small; therefore, caution should be granted when interpreting these findings until replication is completed. The size of the sample also precluded us from examining first onsets versus recurrences of MDD specifically. Rather, due to the limited number of new depressive episodes in children, the current study examined onsets of both major and minor depression. Although the predictive power of pupil dilation to sad faces was maintained when focusing solely on the onset of MDD, future studies with a larger sample size are needed to determine if our findings with MDD replicate. Nevertheless, we believe that the inclusion of minor depression in future studies is valuable as children are more likely to present with minor depression than major depression (Angold, Costello, & Worthman, 1998a). Given that minor depression is a significant risk factor for the subsequent onset of major depression (Lewinsohn, Solomon, Seeley, & Zeiss, 2000), has a similar course to major depression (Kessler, Zhao, Blazer, & Swartz, 1997), and is associated with both current and lifetime role impairments (Kessler et al., 1997), the identification of biomarkers for minor depression is also important. This approach is also consistent with the NIMH Research Domain Criteria (RDoC) initiative, as well as core tenets of developmental psychopathology, which emphasize the study of the full range of functioning from typical to atypical and suggest that studies include milder forms of the disorder (Cuthbert & Insel, 2013). This said, it will be important for future, larger studies to examine whether pupil dilation predicts risk for the development of both mild and severe forms of depression. Next, because the base rate of depression among children of nondepressed mothers was so low, we could not examine whether differences in pupil dilation also predicted risk for depression onset in these children. Future research including a large comparison group will be necessary to prospectively examine whether this potential biological marker is unique to children of depressed mothers. The current study also was unable to examine potential moderators of the effects of pupil dilation such as age or pubertal status due to the sample size. Previous research shows that post-pubertal children exhibit heightened reactivity to emotional stimuli compared to pre-pubertal children (Silk et al., 2009). Therefore, future studies are needed to address whether the predictive validity of pupil dilation changes across development (e.g., from pre- to post-puberty) and, if so, how. It will also be important to identify whether pupil dilation is a trait-like marker of risk and, if so, what is the earliest age at which pupillary reactivity to affective stimuli can be assessed to determine future risk.
A second limitation is that, because pupil dilation is influenced by both cortical and subcortical inputs (Critchley et al., 2005; Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003b; Urry et al., 2006), we cannot draw any firm conclusions regarding the specific neural mechanisms driving the effects observed in this study. Although we have interpreted children’s relatively increased pupil dilation to sad faces as a form of emotional reactivity, children’s peak pupillary responses closely coincided with their response times in which they were required to make a decision regarding the valence of the face. Therefore, we cannot rule out the possibility of pupil dilation being influenced by cognitive effort. This being said, as reported in our previous study (Burkhouse, Siegle, & Gibb, 2014), children of depressed mothers did not differ from children of non-depressed mothers in their pupillary responses to sad faces at lower or medium levels of emotional intensity. Therefore, if differences in pupil dilation between the two groups were purely a result of increasing cognitive effort, we would have expected to see differences at these lower levels of emotional intensity. However, future studies would benefit from a passive viewing task in which stimuli are presented at full emotional intensity to remove the possibility of increased pupil dilation being a result of increased cognitive effort.
A third potential limitation is that the current study focused only on pupillary reactivity to facial displays of emotion so it is unclear whether the predictive validity of children’s pupil dilation is specific to responses to sad faces or whether it would also be observed for other types of stimuli (e.g., words). In this regard, we should note that studies with currently depressed populations have typically used emotional words in their paradigms (Siegle et al., 2003a; Silk et al., 2007) and differences in the type of stimuli used in pupil dilation paradigms may yield different patterns of reactivity among youth. For example, Silk and colleagues (2007) found that currently depressed adolescents exhibited a blunted, rather than an increased, pupillary response to negative words. Therefore, future studies are needed to directly compare responses to pictorial versus word stimuli to determine which may hold the greatest promise as a biomarker of risk.
Finally, our task only allowed us to examine whether pupil dilation to sad faces within the first three seconds following stimulus onset predicts depression risk. However, other research has suggested that pupillary reactivity differences in depressed populations persist well after the stimulus has disappeared from the screen, indicating an emotional ruminative response (e.g., Siegle et al., 2003a). Future research is needed to determine whether children of depressed versus nondepressed mothers exhibit differences in sustained pupillary reactivity and whether heightened initial versus sustained pupil dilation to sad stimuli may be a stronger predictor of depression onset among children.
In summary, the current study contributes to existing research on the intergenerational transmission of depression by providing promising evidence for one promising biological marker of risk: pupillary reactivity to sad facial stimuli. Notably, this is the first study of which we are aware to examine whether disrupted physiological reactivity prospectively predicts future depressive symptom trajectories and diagnoses in children of depressed mothers. These findings may help to pave the way for future intervention efforts designed to reduce depression risk among children and adolescents.
Lay Summary.
Physiological reactivity to depression-relevant cues has been proposed as a potential mechanism involved in the intergenerational transmission of depression. This study confirms this hypothesis by showing that children (ages 8–14) of depressed mothers exhibiting relatively higher pupil dilation to sad faces experienced elevated trajectories of depressive symptoms across a two-year follow up and a shorter time to depression onset.
Acknowledgments
This project was supported by National Institute of Child Health and Human Development grant HD057066 and National Institute of Mental Health grant MH098060 awarded to B. E. Gibb. We would like to thank Ashley Johnson, Lindsey Stone, Andrea Hanley, Sydney Meadows, Michael Van Wie, Devra Alper, Cope Feurer, Eric Funk, and Effua Sosoo for their help in conducting assessments for this project.
Footnotes
The sample reported in Burkhouse, Siegle, and Gibb (2014) included 53 mothers with a history of depression and their children. However, only 47 out of 53 families participated in at least 1 follow-up assessment; therefore, the current study focuses on the 47 families with follow-up data.
Consistent with Research Diagnostic Criteria (RDC; Spitzer, Endicott, & Robins, 1978), as well as past research studies of youth focusing on diagnoses of minor depression (e.g., Burkhouse, Uhrlass, Stone, Knopik, & Gibb, 2012; Gibb, Grassia, Stone, Uhrlass, & McGeary, 2012; Stone, Hankin, Gibb, & Abela, 2011), criteria for minor depression included the presence of a criterion A symptom plus at least one symptom from criterion B, which lasted for at least two weeks and resulted in clinically significant impairment. We should note, however, that all of the results in this study were maintained even when excluding children with minor depression and focusing solely on diagnoses of MDD.
Children’s lifetime history of anxiety and behavioral disorders were not significantly related to their peak pupillary responses at the baseline assessment and the study’s findings were maintained when controlling for these variables.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status, and pubertal timing. Psychological Medicine. 1998a;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Knight E, Kessler RC, Merikangas KR. Epidemiology of depression in children and adolescents. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. New York, NY: Guilford Press; 2008. pp. 6–32. [Google Scholar]
- Birmaher B, Williamson DE, Dahl RE, Axelson DA, Kaufman J, Dorn LD, Ryan ND. Clinical presentation and course of depression in youth: does onset in childhood differ from onset in adolescence? Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(1):63–70. doi: 10.1097/00004583-200401000-00015. [DOI] [PubMed] [Google Scholar]
- Bistricky SL, Ingram RE, Siegle GJ, Short M. Parental depression risk and reduced physiological responses during a valence identification task. Cognitive Therapy and Research. 2015;39:318–331. [Google Scholar]
- Burkhouse KL, Woody ML, Owens M, McGeary JE, Knopik VS, Gibb BE. Sensitivity in detecting facial displays of emotion: Impact of maternal depression and oxytocin receptor genotype. Cognition and Emotion. doi: 10.1080/02699931.2014.996531. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Gibb BE. Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. Journal of Child Psychology and Psychiatry. 2014;55:1009–1016. doi: 10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Uhrlass DJ, Stone LB, Knopik VS, Gibb BE. Expressed emotion-criticism and risk of depression onset in children. Journal of Clinical Child and Adolescent Psychology. 2012;41:771–777. doi: 10.1080/15374416.2012.703122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine. 2013;11(1):126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Medicine. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM Disorders – Patient Edition (SCID-I/P) New York: Biometrics Research Department, NY State Psychiatric Institute; 1995. [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Dahl RE. Positive and negative affect in depression: Influence of sex and puberty. Annals of the New York Academy of Sciences. 2004;1021:341–347. doi: 10.1196/annals.1308.042. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: potential mechanisms linking mother and child depression. Journal of Clinical Child & Adolescent Psychology. 2009;38(3):415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Grassia M, Stone LB, Uhrlass DJ, McGeary JE. Brooding rumination and risk for depressive disorders in children of depressed mothers. Journal of Abnormal Child Psychology. 2012;40(2):317–326. doi: 10.1007/s10802-011-9554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Reviews of Clinical Psycholology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Human Brain Mapping. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Children of depressed parents. In: Gotlib IH, Hammen C, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 275–297. [Google Scholar]
- Harrison AJ, Gibb BE. Attentional Biases in Currently Depressed Children: An Eye-Tracking Study of Biases in Sustained Attention to Emotional Stimuli. Journal of Clinical Child & Adolescent Psychology. doi: 10.1080/15374416.2014.930688. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RR, Miranda J, Segal Z. Cognitive vulnerability to depression. Cognitive vulnerability to emotional disorders. 2006:63–91. [Google Scholar]
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DEJ. Neurofeedback: A promising tool for the self-regulation of emotion networks. NeuroImage. 2010;49(1):1066–1072. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of mood regulation in girls at high risk for depression. Journal of Abnormal Psychology. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for affective disorders and schizophrenia for school-age children - present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Zhao S, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. Journal of Affective Disorders. 1997;45:19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. Journal of Abnormal Psychology. 2000;109(2):345–351. [PubMed] [Google Scholar]
- Lopez-Duran NL, Kuhlman KR, George C, Kovacs M. Facial emotion expression recognition by children at familial risk for depression: high-risk boys are oversensitive to sadness. Journal of Child Psychology and Psychiatry. 2013;54(5):565–574. doi: 10.1111/jcpp.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion. San Francisco State University; San Francisco: 1988. [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children’s Depression Rating Scale–Revised in adolescents. Journal of child and adolescent psychopharmacology. 2010;20(6):513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naicker K, Galambos NL, Zeng Y, Senthilselvan A, Colman I. Social, demographic, and health outcomes in the 10 years following adolescent depression. Journal of Adolescent Health. 2013;52(5):533–538. doi: 10.1016/j.jadohealth.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Owens M, Johnson AL, Burkhouse KL, McGeary JE, Knopik VS, Palmer RHC, Gibb BE. Eye-tracking indices of attentional biases in children of depressed mothers: Polygenic influences help to clarify previous mixed findings. Development and Psychopathology. doi: 10.1017/S0954579415000462. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children’s depression rating scale, revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon R. HLM 6: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Siegle GJ, Ichikawa N, Steinhauer S. Blink before and after you think: blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology. 2008;45(5):679–687. doi: 10.1111/j.1469-8986.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003a;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fmri data. NeuroImage. 2003b;20:114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21:7–26. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Brimaher B, Siegle GJ. Pupillary reactivity to emotional stimuli in child and adolescent depression: Links to clinical and ecological measures. American Journal of Psychiatry. 2007;164:1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: Rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Steidtmann D, Ingram RE, Siegle GJ. Pupil response to negative emotional information in individuals at risk for depression. Cognition and Emotion. 2010;24(3):480–496. [Google Scholar]
- Stone LB, Hankin BL, Gibb BE, Abela JR. Co-rumination predicts the onset of depressive disorders during adolescence. Journal of Abnormal Psychology. 2011;120:752–757. doi: 10.1037/a0023384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Burkhouse KL, Gibb BE. Overgeneral autobiographical memory in children of depressed mothers. Cognition and Emotion. 2015;29:130–137. doi: 10.1080/02699931.2014.891972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Kudinova AY, McGeary JE, Knopik VS, Palmer RHC, Gibb BE. Influence of maternal depression on children’s brooding rumination: Moderation by CRHR1 TAT haplotype. Cognition and Emotion. doi: 10.1080/02699931.2014.998631. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


