Abstract
Background
Despite the substantial overlap of obesity and metabolic disease, there is hetereogeneity with respect to cardiovascular risk. We sought to investigate preclinical differences in systolic and diastolic function in obesity, and specifically compare obese individuals with and without metabolic syndrome (MS).
Methods and Results
Obese individuals without cardiac disease with (OB/MS+, n=124) and without MS (OB/MS−, n=37) were compared to non-obese controls (n=29). Diastolic function was assessed by transmitral and tissue Doppler. Global longitudinal strain (LS) and time-based dyssynchrony were assessed by speckle tracking. Both Ob/MS− and OB/MS+ groups had similar ejection fraction but worse systolic mechanics as assessed by LS and dyssynchrony compared with non-obese controls. Specifically, OB/MS− had 2.5% lower LS (s.e. 0.7%, P=0.001 in multivariable-adjusted analyses) and 10.8 ms greater dyssynchrony (s.e. 3.3, P=0.002), and OB/MS+ had 1.0% lower LS (s.e. 0.3%, P<0.001) and 7.8 ms greater dyssynchrony (s.e. 1.5, P<0.001) compared with controls. Obesity was associated with impaired diastolic function regardless of MS status, as evidenced by greater left atrial diameter and left ventricular mass, though diastolic dysfunction was more pronounced in OB/MS+ compared with OB/MS− individuals.
Conclusions
Obesity is associated with subclinical differences in both systolic and diastolic function regardless of the presence or absence of MS, although MS appears to be associated with worse diastolic dysfunction. Compared to controls, ‘metabolically healthy’ obese had lower LS, greater dyssynchrony, and early diastolic dysfunction, supporting the notion that obesity per se may have adverse cardiovascular effects regardless of metabolic disease.
Keywords: obesity, metabolic syndrome, cardiac function
The prevalence of obesity has increased in Western countries over the last decades, reaching more than thirty percent among the whole population.1 Obesity is associated with multiple metabolic abnormalities, and worsening obesity is accompanied by increased prevalence of metabolic syndrome.2 Both obesity and metabolic syndrome have been linked to a wide spectrum of cardiovascular morbidity and mortality, including increased risk of heart failure.3–5 Individuals with obesity and/or metabolic syndrome are known to have changes in cardiac structure and function, including left ventricular (LV) hypertrophy and diastolic dysfunction.6–9 Previous studies have demonstrated that subclinical changes in diastolic function and LV mass both precede the development of clinical heart failure, and specifically increase the risk of heart failure with preserved ejection fraction.10–13 The effect of obesity and metabolic disease on systolic function and mechanics have been less well studied.14
Despite the substantial overlap of obesity and metabolic disease, there is hetereogeneity with respect to cardiovascular risk, with some studies showing favorable prognosis for ‘metabolically healthy’ obese individuals.15, 16 The notion of metabolically healthy obesity has recently been questioned, however, and it appears that obesity even in the absence of metabolic disease may be associated with cardiovascular risk and adverse outcomes.17, 18 We sought to investigate preclinical differences in systolic and diastolic function in obesity, and specifically compare obese individuals with and without metabolic syndrome, in a cohort of individuals without existing cardiovascular disease.
Methods
Study population
We recruited consecutive participants with obesity, defined as body-mass index (BMI) ≥30 kg/m2, without existing cardiovascular disease, who attended outpatient clinic visits at Boston Medical Center. Metabolic syndrome was defined as meeting three or more of the following criteria: (a) increased waist circumference (≥102 cm in men, ≥88 cm in women); (b) increased fasting triglyceride (≥150 mg/dL); (c) high blood pressure (BP: ≥130/85 mmHg) or receiving anti-hypertensive therapy; (d) decreased high-density lipoprotein cholesterol (HDL: <40 mg/dL in men, <50 mg/dL in women); (e) impaired fasting glucose (≥100 mg/dL).19 Participants were grouped either into those with obesity and metabolic syndrome (OB/MS+), or ‘metabolically healthy’ obese, defined as obese individuals meeting ≤1 of the metabolic syndrome criteria with exception of increased waist circumference (OB/MS−). We also recruited non-obese controls with BMI<30 kg/m2 who had no other major comorbidities (hypertension, cardiovascular disease, diabetes mellitus, hyperlipidemia). Participants with known cardiac related signs/symptoms, LV ejection fraction <50%, or existing cardiovascular disease such as heart failure, ischemic heart disease, or valvular heart disease were excluded from the study.
Clinical assessment
All participants underwent a comprehensive medical history and physical examination, including anthropometrics, resting blood pressure (average of three consecutive measurements), and fasting blood work. Hypertension was defined as current treatment with anti-hypertensive therapies, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Diabetes was defined as a fasting serum glucose level ≥126 mg/dL and/or current treatment for diabetes. The study was approved by the Boston University Medical Center Institutional Review Board and all participants provided informed consent.
Transthoracic echocardiography
All participants underwent standard transthoracic echocardiography including tissue Doppler imaging and speckle tracking (iE33, Philips; Andover, MA) with a 1- to 5-MHz transducer. Chamber dimensions including left atrial (LA), LV wall and chamber sizes, and relative wall thickness (RWT) were measured. Left atrial volumes were measured using the biplane area-length method, and indexed to body-surface area. LV ejection fraction was estimated by the modified Simpson’s rule. LV mass (g) was calculated by the formula: 1.04 × [(LV end-diastole dimension + posterior wall thickness + interventricular septum thickness)3 − (LV end-diastole dimension)3] − 13.6, and indexed to height to the power of 2.7 (LV mass/Ht2.7) to correct for body habitus.20 Measured Doppler parameters included isovolumic relaxation time (IVRT), mitral E and A wave velocities, deceleration time. Tissue Doppler measures included mitral annular early (e’) and late (a’) diastolic velocities, calculated as the mean of septal and lateral velocities, respectively. Speckle-tracking analysis was performed offline using commercially available software (QLAB Software version 9 Cardiac Motion/Mechanics Quantification, Philips). In brief, global longitudinal systolic strain (LS) was the average of the negative peak longitudinal strain from 17 ventricular segments obtained from the apical 4-chamber, 3-chamber, and 2-chamber views. Time-based dyssynchrony was defined as the standard deviation of the electromechanical time delay from the QRS onset to peak systolic strain of the 17 LV segments. All strain analyses were performed by a single observer blinded to clinical status (YCW). Of 222 participants who underwent strain imaging, 32 were excluded due to technical issues precluding analysis (18 had limited views, and 14 had poor quality images), leaving 190 participants for analysis. Intra-observer reproducibility was estimated in 24 randomly selected participants. The coefficient of variation was 2.2% for strain, and 3.7% for time-based dyssynchrony, with intraclass correlation coefficients of 0.93 and 0.98, respectively.
Statistical analysis
Descriptive data are listed as mean ± standard deviation (SD) for continuous variables and as a percentage for discrete variables. Differences among the three groups for baseline characteristics were compared using the one-way analysis of variance (ANOVA). Pairwise comparisons were evaluated using two sample t-tests for continuous variables and a chi-squared test for discrete variables, with Bonferroni correction for multiple testing. For echocardiographic variables that were significantly different between groups by ANOVA, we further investigated the association of obesity and metabolic syndrome using multivariable linear regression, comparing study groups after adjusting for potential confounders that were selected a priori including age, sex, race, and heart rate. We additionally adjusted for individual components of the metabolic syndrome including systolic blood pressure, fasting glucose, waist circumference, and the triglyceride-to-HDL cholesterol ratio, and examined each component individually in exploratory analyses. For all tests, a two-tailed P-value < 0.05 was considered statistically significant.
In sensitivity analyses, multivariable regression models were repeated after exclusion of individuals with hypertension or diabetes. In exploratory analyses, we used forward and backward selection models to investigate the association of metabolic risk factors as continuous variables (systolic blood pressure, waist circumference, fasting glucose, HDL cholesterol, log-triglyceride level) with strain measures among obese individuals. The linear regression model was fitted with longitudinal strain as the response variable, and age and sex were retained. Backward selection was performed with eligible covariates removed sequentially based on the least significant term using a significance level of P≥0.05, and the model reestimated after removal. We repeated the same models using forward selection. Specifically, eligible covariates were entered sequentially using a significance level of P<0.05, with the most significant term added first and reestimated. All analyses were performed using the Stata 11.2, and forward and backward selection models were fitted using the ‘stepwise’ command (StataCorp, College Station, TX).
Results
A total of 124 obese individuals with metabolic syndrome (OB/MS+, 45±11 years, 77% women), and 37 ‘metabolically healthy’ obese participants (OB/MS−, 39±11 years, 86% women) were compared with 29 non-obese controls (43±12 years, 69% women). Baseline clinical characteristics are displayed in Table 1. Among obese individuals, 42% were severely obese (BMI >40 kg/m2), and there was no significant difference in BMI between participants with or without metabolic syndrome (P=0.54). Among non-obese individuals, half were overweight (52% with BMI ≥ 25 but < 30 kg/m2). Within ‘metabolically healthy’ obese individuals, none had diabetes mellitus, and the triglyceride/HDL ratio was similar to that of healthy controls (1.8±1.0 versus 1.6±1.0, respectively, P>0.99), whereas the triglyceride/HDL ratio in the OB/MS+ group was more than 2.5-fold higher (4.2±3.7, P<0.001 compared with OB/MS−). Traditional cardiovascular risk factors were most prevalent in OB/MS+, followed by OB/MS−, and absent in healthy controls (Table 1).
Table 1.
Clinical characteristics in controls and obese with and without metabolic syndrome
| Control (n=29) | OB/MS− (n=37) | OB/MS+ (n=124) | P for ANOVA | |
|---|---|---|---|---|
| Age, years | 43±12 | 39±11 | 45±11† | 0.01 |
| Women, n (%) | 20 (69) | 32 (86) | 96 (77) | 0.23 |
| White, n (%) | 18 (62) | 6 (16)* | 38 (31)* | <0.001 |
| Systolic BP, mmHg | 109±13 | 119±11 | 126±16*† | <0.001 |
| Diastolic BP (mmHg) | 69±8 | 75±8 | 78±12* | <0.001 |
| Hypertension treatment, n (%) | 0 (0) | 9 (24)* | 82 (66)*† | <0.001 |
| Heart rate, beats per minute | 64±11 | 66±8 | 71±11*† | <0.001 |
| Current smoker, n (%) | 1 (3) | 3 (8) | 14 (11) | 0.41 |
| Body mass index, kg/m2 | 24.4±2.3 | 37.8±7.1* | 40.0±7.1* | <0.001 |
| Waist circumference, cm | 83±14 | 108±19* | 120±18*† | <0.001 |
| Fasting glucose, mg/dl | 84±11 | 89±10 | 117±54*† | <0.001 |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | 57 (46)*† | <0.001 |
| Total cholesterol, mg/dl | 191±31 | 179±34 | 187±42 | 0.40 |
| HDL cholesterol, mg/dl | 57±13 | 49±10* | 44±11* | <0.001 |
| Triglycerides (mg/dl) | 81±35 | 86±42 | 169±126*† | <0.001 |
| Triglyceride/HDL ratio | 1.6±1.0 | 1.8±1.0 | 4.2±3.7*† | <0.001 |
| Creatinine, mg/dl | 0.85±0.13 | 0.85±0.15 | 0.82±0.16 | 0.47 |
Values represent means and standard deviations unless otherwise noted.
BP, blood pressure; HDL, high-density lipoprotein; MS, metabolic syndrome; OB, obese;
P < 0.05 vs. control;
P < 0.05 Ob/MS− vs. Ob/MS+
Measures of systolic and diastolic function in obesity with or without metabolic syndrome
While LV dimensions were similar between groups, obesity was associated with significantly greater LV mass and LA diameter regardless of metabolic syndrome status, and there was no difference in LV mass or LA diameter in OB/MS− versus OB/MS+ groups (Table 2). For measures of diastolic function, E/A ratio and mitral e’ were lowest, and E/e’ ratio highest in the OB/MS+ group, while these features were largely intermediate or similar to healthy controls in the OB/MS− group (Table 2). For example, mitral e’ was lowest in OB/MS+ (9.2±2.3 cm/s, P<0.001 compared with OB/MS−) whereas there was no significant difference between OB/MS− and healthy controls (11.4±2.4 and 11.4±3.0 cm/s, respectively, P>0.99 for OB/MS− vs. controls).
Table 2.
Echocardiographic characteristics in controls and obese with and without metabolic syndrome
| Control (n=29) | OB/MS− (n=37) | OB/MS+ (n=124) | P for ANOVA | |
|---|---|---|---|---|
| Left atrial diameter, mm | 32±3 | 38±5* | 38±4* | <0.001 |
| Left atrial volume index, ml/m2 | 30±6 | 37±9* | 30±8† | <0.001 |
| LVEDD, mm | 45±4 | 48±4* | 45±5† | 0.007 |
| LVESD, mm | 29±4 | 32±3 | 29±5† | 0.02 |
| Septal thickness, mm | 8±1 | 9±2* | 10±1*† | <0.001 |
| Posterior wall thickness, mm | 8±1 | 9±2* | 10±2* | <0.001 |
| Relative wall thickness | 0.36±0.08 | 0.40±0.09 | 0.44±0.08*† | <0.001 |
| LV mass/height2.7, g/m2.7 | 28±6 | 40±10* | 40±9* | <0.001 |
| LV ejection fraction, % | 64±6 | 62±5 | 63±6 | 0.54 |
| Mitral E-flow, cm/s | 74±15 | 82±16 | 79±18 | 0.23 |
| Mitral A-flow, cm/s | 50±14 | 59±13* | 68±14*† | <0.001 |
| Mitral E/A ratio | 1.6±0.5 | 1.4±0.3 | 1.2±0.3*† | <0.001 |
| Deceleration time, ms | 205±32 | 193±27 | 201±35 | 0.31 |
| Isovolumic relaxation time, ms | 75±11 | 78±15 | 85±15*† | <0.001 |
| Mitral e’, cm/s | 11.4±3.0 | 11.4±2.4 | 9.2±2.3*† | <0.001 |
| E/e’ ratio | 6.9±2.2 | 7.4±2.0 | 8.9±2.5*† | <0.001 |
| Mitral a’, cm/s | 8.2±2.0 | 8.0±1.5 | 9.0±1.6† | 0.002 |
| Mitral s’, cm/s | 8.1±1.7 | 7.7±0.9 | 7.4±0.9* | 0.006 |
| Global longitudinal strain, % | −20.8±2.5 | −19.1±2.6* | −18.5±2.8* | <0.001 |
| Longitudinal strain rate, 1/s | −1.02±0.14 | −0.96±0.15 | −0.98±0.16* | 0.20 |
| Time-based dyssynchrony, ms | 40±11 | 49±13* | 55±14*† | <0.001 |
LVEDD, left ventricular end-diastole dimension; LVESD, left ventricular end-systole dimension; MS, metabolic syndrome; Ob, obese; dyssynchrony index, standard deviation of the time delay between QRS onset and peak strain of 17 LV segments.
P < 0.05 vs. control;
P < 0.05 Ob/MS− vs. Ob/MS+.
With regard to systolic function, there was no detectable difference in LV systolic function as measures by LV ejection fraction between groups (P for ANOVA = 0.54). However, systolic myocardial mechanics were impaired in obese individuals as evidenced by a graded decrease of longitudinal strain, with the lowest LS in the OB/MS+ group. Specifically, longitudinal strain was −18.5±2.8% in OB/MS+, −19.1±2.6% in OB/MS−, and −20.8±2.5% in controls (P ANOVA <0.001). Similarly, time-based dyssynchrony appeared worse in obese individuals with and without MS compared with healthy controls (55±14 ms in OB/MS+; 49±13 in OB/MS−; 40+11 in controls, P<0.001, Table 2). Lastly, mitral annular plane systolic excursion (s’) as measured by tissue Doppler was significantly different between groups (P ANOVA=0.006, Table 2).
Differences in systolic and diastolic function in obesity with and without metabolic syndrome are independent of potential clinical confounders
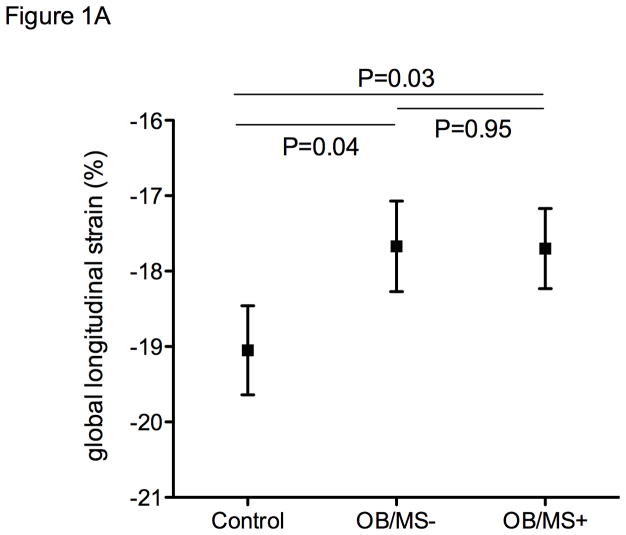
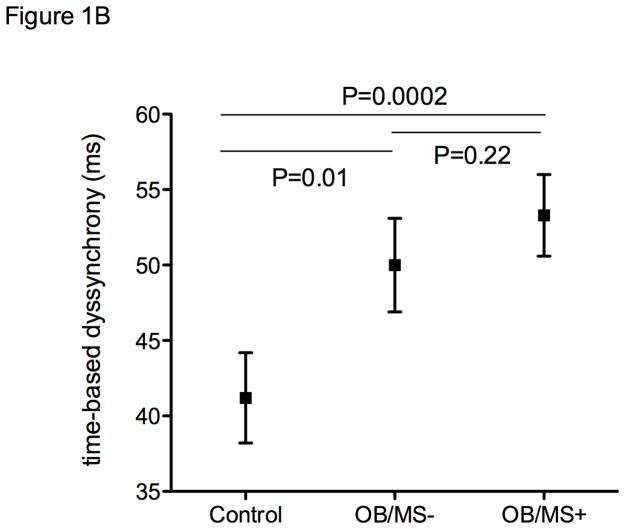
Longitudinal strain remained worse in the obese regardless of metabolic disease even after accounting for age, sex, systolic blood pressure, and use of antihypertensive medications (adjusted mean −17.7%, s.e. 0.5 in OB/MS+, −17.7%, s.e. 0.6 in OB/MS−, −19.0%, s.e. 0.6 in controls, with pairwise comparison P=0.03 OB/MS+ vs controls, and P=0.04 OB/MS− vs controls, Figure 1A). Similarly, mitral s’ also remained significantly different between groups after accounting for blood pressure in multivariable models (adjusted mean 7.8 cm/s, s.e. 0.2 in OB/MS+, 7.9 cm/s, s.e. 0.3 in OB/MS−, and 8.4 cm/s, s.e. 0.2 in controls, with pairwise comparison P=0.02 OB/MS+ vs controls, and P=0.07 OB/MS− vs controls, Figure 1B).
Figure 1.
Strain measures by study group, including non-obese controls, obese individuals without metabolic syndrome (OB/MS−), and obese individuals with metabolic syndrome (OB/MS+). Panel A shows differences in global longitudinal strain, and panel B shows time-based dyssynchrony. Values represent adjusted means and standard errors, after accounting for age, sex, systolic blood pressure, and the use of anti-hypertensive medications.
Evidence of LV remodeling and differences in both systolic and diastolic function in ‘metabolically healthy’ obese individuals compared with healthy controls persisted after adjusting for other potential confounders, including age, sex, race, and heart rate (Table 3). Specifically, obesity in the absence of metabolic syndrome was associated with greater LA diameter, LV end-diastolic dimension, LV end-systolic dimension, LV mass/Ht2.7, mitral A-flow, and dyssynchrony, and lower mitral E/A and global longitudinal strain. With regards to systolic mechanics, ‘metabolically healthy’ obese individuals had a 2.5% lower longitudinal strain, and 11 ms greater time-based dyssychrony compared with non-obese controls. Further, when comparing obese individuals with and without metabolic syndrome, those with metabolic syndrome had greater relative wall thickness, lower mitral e’, and higher mitral E/e’ compared with ‘metabolically healthy’ obese individuals (Table 3).
Table 3.
Differences in cardiac structure and function in obesity with or without metabolic syndrome compared with healthy controls
| OB/MS− vs. controls | OB/MS+ vs. controls | OB/MS+ vs. OB/MS− | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| β (s.e)* | P | β (s.e)* | P | β (s.e)* | P | |
| Left atrial diameter | 5.7 (1.2) | <0.001 | 3.3 (0.4) | <0.001 | ||
| Left atrial volume index | 7.0 (2.0) | 0.001 | −6.6 (1.6) | <0.001 | ||
| Left ventricular end-diastolic dimension | 3.7 (1.1) | 0.002 | −2.0 (0.8) | 0.02 | ||
| Left ventricular end-systolic dimension | 2.8 (1.0) | 0.008 | −1.9 (0.1) | 0.03 | ||
| Relative wall thickness | 0.03 (0.01) | <0.001 | 0.04 (0.02) | 0.03 | ||
| LV mass/height2.7 | 11.2 (2.5) | <0.001 | 6.4 (0.9) | <0.001 | ||
| Mitral A-flow | 8.1 (3.2) | 0.01 | 7.4 (1.3) | <0.001 | 5.0 (2.4) | 0.04 |
| Mitral E/A ratio | −0.13 (0.03) | <0.001 | ||||
| Isovolumic relaxation time | 6.0 (1.6) | <0.001 | ||||
| Mitral e’ (cm/s) | −0.9 (0.2) | <0.001 | −1.2 (0.4) | 0.001 | ||
| E/e’ ratio | 1.0 (0.5) | 0.04 | 1.0 (0.3) | <0.001 | 1.2 (0.5) | 0.008 |
| Global longitudinal strain | 2.5 (0.7) | 0.001 | 1.0 (0.3) | 0.001 | ||
| Time-based dyssynchrony | 10.8 (3.3) | 0.002 | 7.8 (1.5) | <0.001 | 5.3 (2.6) | 0.04 |
β estimate represents difference in echocardiographic characteristic between two groups after adjustment for age, sex, race, and heart rate. Regression models with P>0.05 for differences between groups are not displayed. MS, metabolic syndrome; OB, obese; s.e, standard error
After additionally accounting for individual components of metabolic syndrome, including systolic blood pressure, fasting glucose, waist circumference, and triglyceride/HDL ratio, the association of obesity with greater LA diameter, LV mass, and time-based dyssynchrony remained robust, although differences in strain were attenuated (Table 4). In obese individuals with metabolic syndrome, similar differences in systolic mechanics and diastolic function were seen as in the OB/MS− group when compared with controls after accounting for potential clinical confounders. In addition, further evidence of differences in other diastolic parameters and LV remodeling were apparent, including greater relative-wall thickness, longer IVRT, lower mitral e’, and higher mitral E/e’. Most differences in systolic and diastolic function between obese individuals with metabolic syndrome and controls persisted, including LA diameter, LV mass, E/A ratio, mean e’, and dyssynchrony. When comparing obese individuals with and without metabolic syndrome, differences in LV dimensions and mean e’ persisted (Table 4).
Table 4.
Differences in cardiac structure and function in obesity with or without metabolic syndrome compared with healthy controls after additional adjustment for metabolic factors
| OB/MS− vs. controls | OB/MS+ vs. controls | OB/MS+ vs. OB/MS− | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| β (s.e)* | P | β (s.e)* | P | β (s.e)* | P | |
| Left atrial diameter | 3.8 (1.4) | 0.008 | 1.7 (0.5) | 0.001 | ||
| Left ventricular end-diastolic dimension | −3.3 (0.9) | 0.001 | ||||
| Left ventricular end-systolic dimension | −2.8 (1.0) | 0.004 | ||||
| Relative wall thickness | 0.03 (0.01) | <0.001 | ||||
| LV mass/height2.7 | 6.9 (2.9) | 0.02 | 3.0 (1.2) | 0.01 | ||
| Mitral A-flow | 5.5 (1.8) | 0.002 | ||||
| Mitral E/A ratio | −0.12 (0.04) | 0.003 | ||||
| Isovolumic relaxation time | 5.4 (2.2) | 0.02 | ||||
| Mitral e’ (cm/s) | −0.6 (0.3) | 0.04 | −0.9 (0.4) | 0.03 | ||
| E/e’ ratio | ||||||
| Global longitudinal strain | ||||||
| Time-based dyssynchrony | 8.7 (4.1) | 0.04 | 5.4 (2.0) | 0.007 | ||
β estimate represents difference in echocardiographic characteristic between two groups after adjustment for age, sex, race, heart rate, systolic blood pressure, fasting glucose, waist circumference, TG/HDL ratio. Regression models with P>0.05 for differences between groups are not displayed.
In exploratory analyses examining individual components of metabolic syndrome and their effect on differences in global longitudinal strain, we found the greatest attenuation with the addition of waist circumference when comparing OB/MS+ and non-obese control groups. For OB/MS− compared with controls, differences in strain were attenuated with both the addition of waist circumference and systolic blood pressure (Supplemental Table 1).
Alterations in systolic and diastolic function persist in the absence of hypertension or diabetes mellitus
In sensitivity analyses, we excluded individuals with hypertension (n = 96) from the analysis (Supplemental Table 2). In the absence of hypertension, both diastolic function and systolic mechanics as assessed by time-based dyssynchrony remained impaired in obese individuals regardless of metabolic syndrome status when compared with controls, even after multivariable adjustment. Similarly, we conducted a sensitivity analysis after excluding individuals with diabetes mellitus (n = 57), (Supplemental Table 3) and found persistent alterations in longitudinal strain, dyssynchrony, and diastolic function parameters in the obese regardless of metabolic syndrome status when compared with controls.
Correlates of strain and dyssynchrony
In exploratory analyses among obese individuals, we used stepwise models to examine metabolic risk factors as continuous variables in relation to strain parameters, with both forward and backward selection yielding the same results. Higher systolic blood pressure, male sex, and higher waist circumference were associated with lower global longitudinal strain. Specifically, every 15cm increase in waist circumference was associated with a 0.5% lower longitudinal strain (β 0.5, s.e. 0.1, P=0.001). Similarly, age, fasting glucose, waist circumference, and systolic blood pressure were all associated with greater time-based dyssynchrony. Every 15cm increase in waist circumference was associated with a 2.8 ms increase in dyssynchrony (β 2.8 s.e. 0.7, P<0.001).
Discussion
We demonstrated that obesity per se was associated with impaired systolic mechanics as reflected by longitudinal strain and dyssynchrony, regardless of the presence or absence of metabolic syndrome. Further, subclinical changes in systolic mechanics were detected in the absence of any impairment in LV ejection fraction. In individuals with metabolic syndrome, this subclinical impairment of systolic function occurred concomitantly with altered diastolic function. In contrast, diastolic dysfunction appeared to be less pronounced in the metabolically healthy obese. These subclinical alterations in systolic and diastolic function suggest that cardiovascular effects of obesity and metabolic syndrome are manifest even in the absence of overt cardiovascular disease. Detection of early myocardial dysfunction may help identify individuals at risk for development of subsequent clinical heart failure,21 and may guide future preventive strategies.
Few studies have examined systolic mechanics in obesity. Severe obesity was previously associated with lower regional myocardial systolic strain and strain rate compared with healthy controls.22 Obesity was not further subclassified into those with and without metabolic syndrome in this study, and not unexpectedly, the obese group had significantly higher insulin resistance compared with controls. Interestingly, insulin resistance was strongly correlated with systolic mechanics.22 Similarly, metabolic syndrome was associated with lower longitudinal strain and strain rate, as well as LV dyssynchrony as assessed by speckle tracking in non-diabetic participants of the RESOLVE study. Differences in systolic mechanics correlated strongly with abdominal obesity and markers of systemic inflammation.14, 23 More recently, obesity in early adulthood was associated with LV strain measures in the Coronary Artery Risk Development in Young Adults Study.24 Our findings are consistent with these prior studies, confirming both lower longitudinal strain and greater dyssynchrony in obese individuals with metabolic syndrome. Importantly, we now extend these findings to the ‘metabolically healthy’ obese in our carefully phenotyped cohort, demonstrating worse strain and greater dyssynchrony in obesity even in the absence of metabolic syndrome. A recent small study in 10 obese middle-aged non-diabetic men demonstrated improvement in systolic strain and dyssynchrony measures as well as diastolic function with exercise training, suggesting that these functional changes are potentially reversible.25 Notably, after accounting for individual components of metabolic syndrome, the association with longitudinal strain was attenuated, however the association of obesity and greater dyssynchrony persisted. These findings suggest that the observed differences in strain could be explained by components of metabolic syndrome, however that metabolic syndrome in and of itself does not have a discernible effect beyond its individual components.
Numerous studies have demonstrated diastolic dysfunction in metabolic disease, which appears to be independent of LV hypertrophy,8, 26–28 and our results support these observations. Fewer studies have examined the effect of ‘metabolically healthy’ obesity on diastolic function - Pascual et al studied 31 obese individuals (mean age<30 years) without hypertension, diabetes, or dyslipidemia.29 Although there was no difference in mitral E/A between the obese and the normal group, the obese had a relatively higher A-flow velocity. Another study that examined 29 obese participants (mean age 49 years) without metabolic syndrome showed that isolated obesity was associated with lower e’ and higher E/e’ compared with the controls.30 We did not find a significant difference in e’ or E/e’ ratio between ‘metabolically healthy’ obese and controls, although we did find greater LA diameter, LV mass, and lower E/A ratio, indicating early changes in diastolic function in the obese even in the absence of metabolic syndrome. This suggests a potential intermediate phenotype of subclinical cardiovascular disease in the ‘metabolically healthy’ obese. Because age may not only influence diastolic function but also serve as a potential indicator of the duration of obesity, age differences could contribute to the variable spectrum of diastolic dysfunction in these previous studies.31
While earlier studies supported the concept of ‘metabolically healthy’ obesity,15, 16 more recent studies have demonstrated higher prevalence of subclinical coronary atherosclerosis,17 greater risk of cardiovascular events,18 and specifically increased risk of future heart failure32 in ‘metabolically healthy’ obese when compared with metabolically healthy normal weight individuals. Our findings further support preclinical changes in both systolic and diastolic function even in the ‘metabolically healthy’ obese, contributing to the mounting evidence that “healthy obesity is ... a myth”,33 and that obesity per se, in the absence of metabolic disease, may have adverse cardiovascular effects. Interestingly, weight gain has been associated with worsening LV diastolic stiffness, and conversely, weight loss with improved reduced arterial stiffness, suggesting that modulation of weight may impact cardiovascular disease.34, 35
A number of limitations deserve mention. First, the definition of ‘metabolically healthy’ obese has not been consistent in the literature.17, 18, 32, 36 Our definition was consistent with a more conservative approach used in some prior studies,37 and included individuals with 0–1 traditional metabolic syndrome risk factors in the ‘metabolically healthy’ obese group. The triglyceride/HDL ratio in the ‘metabolically healthy’ obese was similar to healthy controls, suggesting that this group did represent obesity largely in the absence of metabolic disease, however it is possible that alterations in systolic mechanics are confounded by other risk factors in the obese. We adjusted for potential clinical confounders and results were also relatively robust in sensitivity analyses excluding hypertension and diabetes, however residual confounding remains a possibility. One such confounder may be physical activity and fitness, which were not assessed in our study, and may account for differences between metabolically healthy and unhealthy individuals.38 In the setting of our modest sample size, multivariable adjustment may be limited due to concerns of overfitting statistical models. Given that ours is an observational cross-sectional study, causal inferences cannot be drawn, and the clinical implications of these subclinical changes in cardiac structure and function are not known. Lastly, epicardial adipose tissue was not measured and may lend further insights into the cardiovascular effects of obesity, metabolic disease, and adiposity in future studies.
In summary, we found that obesity was associated with subclinical differences in both systolic and diastolic function regardless of the presence or absence of metabolic syndrome. While abnormalities were more pronounced in those with metabolic syndrome, we specifically observed lower global longitudinal strain, greater dyssynchrony, and early diastolic dysfunction in the ‘metabolically healthy’ obese, supporting the notion that obesity may have adverse cardiovascular effects regardless of metabolic disease. Future studies are needed to confirm these findings, and to examine the impact of obesity and potential weight management strategies on cardiovascular risk, and specifically, the development of heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health, including grant number NO1-HV-00239 (Dr. Colucci), and K23-HL116780 (Dr. Ho). Dr. Ho is supported by a Boston University School of Medicine, Department of Medicine Career Investment award.
Footnotes
Disclosures
None.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies C. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundström J, Risérus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1992;19:130–134. doi: 10.1016/0735-1097(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 7.Hwang Y-C, Jee JH, Kang M, Rhee E-J, Sung J, Lee M-K. Metabolic syndrome and insulin resistance are associated with abnormal left ventricular diastolic function and structure independent of blood pressure and fasting plasma glucose level. Int J Cardiol. 2012;159:107–111. doi: 10.1016/j.ijcard.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messerli FH, Sundgaard-Riise K, Reisin ED, Dreslinski GR, Ventura HO, Oigman W, Frohlich ED, Dunn FG. Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med. 1983;99:757–61. doi: 10.7326/0003-4819-99-6-757. [DOI] [PubMed] [Google Scholar]
- 10.Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–33. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 11.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, Lam PK, Sanderson JE. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41:820–6. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 13.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–93. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 14.Crendal E, Walther G, Vinet A, Dutheil F, Naughton G, Lesourd B, Chapier R, Rupp T, Courteix D, Obert P. Myocardial deformation and twist mechanics in adults with metabolic syndrome: Impact of cumulative metabolic burden. Obesity. 2013;21:E679–E686. doi: 10.1002/oby.20537. [DOI] [PubMed] [Google Scholar]
- 15.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–12. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 16.St-Pierre AC, Cantin B, Mauriege P, Bergeron J, Dagenais GR, Despres JP, Lamarche B. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172:1301–5. doi: 10.1503/cmaj.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y, Kim B-K, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung H-S, Choi Y, Ahn J, Lima JAC, Shin H, Guallar E, Ryu S. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–2686. doi: 10.1016/j.jacc.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–69. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F, Association AH Institute NHLaB. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MSJ, Stewart WJ. Recommendations for chamber quantification. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Bello V, Santini F, Di Cori A, Pucci A, Palagi C, Delle Donne MG, Giannetti M, Talini E, Nardi C, Pedrizzetti G, Fierabracci P, Vitti P, Pinchera A, Balbarini A. Relationship between preclinical abnormalities of global and regional left ventricular function and insulin resistance in severe obesity: a Color Doppler Imaging Study. Int J Obes. 2006;30:948–956. doi: 10.1038/sj.ijo.0803206. [DOI] [PubMed] [Google Scholar]
- 23.Crendal E, Walther G, Dutheil F, Courteix D. Left ventricular myocardial dyssynchrony is already present in non-diabetic patients with metabolic syndrome. Can J Cardiol. 2014;30:320–324. doi: 10.1016/j.cjca.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR, Jr, Carr JJ, Terry JG, Liu K, Goff DC, Jr, Lima JA. Association of Obesity in Early Adulthood and Middle Age With Incipient Left Ventricular Dysfunction and Structural Remodeling: The CARDIA Study (Coronary Artery Risk Development in Young Adults) JACC Heart Fail. 2014;2:500–8. doi: 10.1016/j.jchf.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster I, Vinet A, Karpoff L, Startun A, Jourdan N, Dauzat M, Nottin S, Perez-Martin A. Diastolic dysfunction and intraventricular dyssynchrony are restored by low intensity exercise training in obese men. Obesity. 2012;20:134–140. doi: 10.1038/oby.2011.270. [DOI] [PubMed] [Google Scholar]
- 26.Masugata H, Senda S, Goda F, Yoshihara Y, Yoshikawa K, Fujita N, Daikuhara H, Nakamura H, Taoka T, Kohno M. Left ventricular diastolic dysfunction as assessed by echocardiography in metabolic syndrome. Hypertens Res. 2006;29:897–903. doi: 10.1291/hypres.29.897. [DOI] [PubMed] [Google Scholar]
- 27.de las Fuentes L, Brown AL, Mathews SJ, Waggoner AD, Soto PF, Gropler RJ, Davila-Roman VG. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–559. doi: 10.1093/eurheartj/ehl526. [DOI] [PubMed] [Google Scholar]
- 28.Ayalon N, Gopal DM, Mooney DM, Simonetti JS, Grossman JR, Dwivedi A, Donohue C, Perez AJ, Downing J, Gokce N, Miller EJ, Liang CS, Apovian CM, Colucci WS, Ho JE. Preclinical Left Ventricular Diastolic Dysfunction in Metabolic Syndrome. Am J Cardiol. 2014;114:838–842. doi: 10.1016/j.amjcard.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascual M, Pascual DA, Soria F, Vicente T, Hernández AM, Tébar FJ, Valdés M. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orhan AL, Uslu N, Dayi SU, Nurkalem Z, Uzun F, Erer HB, Hasdemir H, Emre A, Karakus G, Soran O, Gorcsan J, Eren M. Effects of isolated obesity on left and right ventricular function: a tissue Doppler and strain rate imaging study. Echocardiography. 2010;27:236–243. doi: 10.1111/j.1540-8175.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 31.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–8. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Puri R. Is it finally time to dispel the concept of metabolically-healthy obesity? J Am Coll Cardiol. 2014;63:2687–2688. doi: 10.1016/j.jacc.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 34.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, Borlaug BA. Impact of General and Central Adiposity on Ventricular-Arterial Aging in Women and Men. JACC Heart Fail. 2014;2:489–99. doi: 10.1016/j.jchf.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavie CJ, Milani RV, Ventura HO. Effects of obesity and weight changes on cardiac and vascular structure and function: does the clinical impact carry any weight? JACC Heart Fail. 2014;2:509–11. doi: 10.1016/j.jchf.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32:2297–9. doi: 10.2337/dc09-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 38.Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–97. doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.